Efficacious Utilization of Food Waste for Bioenergy Generation through the Anaerobic Digestion Method
Abstract
1. Introduction
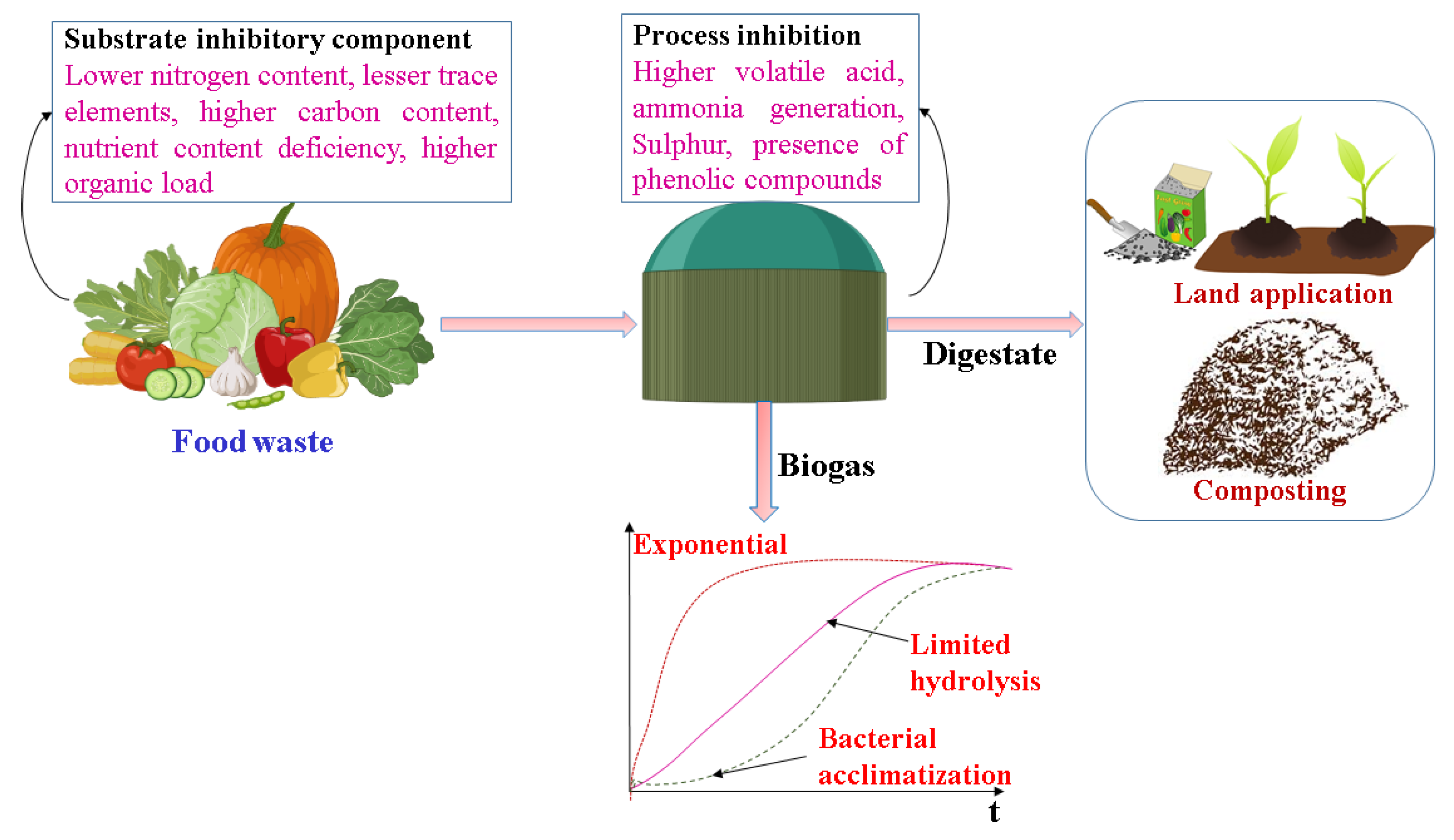
2. Food Waste and Its Characterization
| Waste Type | Composition | Reference |
|---|---|---|
| Food waste | Fat: 8.79% of VS Protein: 17.17% of VS Carbohydrate: 74.04% of VS | [20] |
| Cafeteria food waste | Total carbohydrate: 277.2 ± 0.1 g/L Total protein: 114.3 ± 0.4 g/L | [21] |
| Raw fresh food waste | Carbohydrate: 37.6% Crude protein: 19.2% Crude fat: 32.5% Cellulose: 16.8% Hemicellulose: 8.3% Lignin: 8.7% | [22] |
| Food waste | Cellulose: 2.0 wt% Hemicellulose: 1.2 wt% Lignin: 0.1 wt% Extractives: 96.7 wt% | [23] |
| Dried food waste | Protein: 7 wt% Lipid: 10 wt% Carbohydrate: 67 wt% Insoluble dietary fiber: 5 wt% Salt: >0.65 wt% | [24] |
| Food waste | Carbohydrate: 48% Protein: 15.1% Lipid: 10.6% | [25] |
| Food waste | Carbohydrate: 31% Protein: 14.1% Cellulose: 13.9 g/L | [26] |
| Food waste | Carbohydrate: 43.5% VS Protein: 18.4% VS | [27] |
| Restaurant food waste | Crude fat: 31.8% TS Cellulose: 4.70% TS Hemicellulose: 10.05% TS Lignin: 2.12% TS Crude protein: 15.5% TS Carbohydrate: 41.6% TS | [28] |
3. Impeding Components in Food Waste
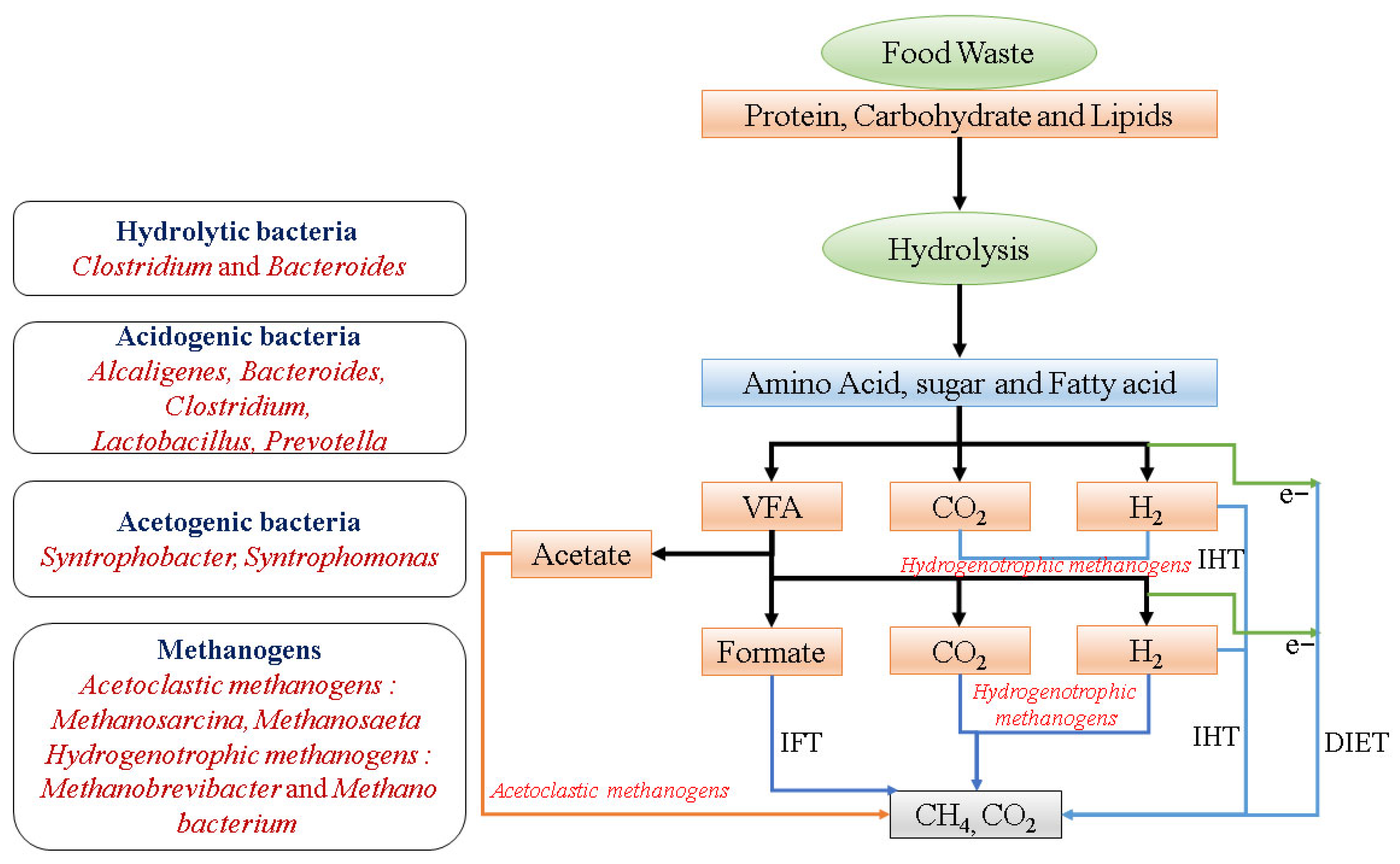
4. Anaerobic Digestion of Food Waste
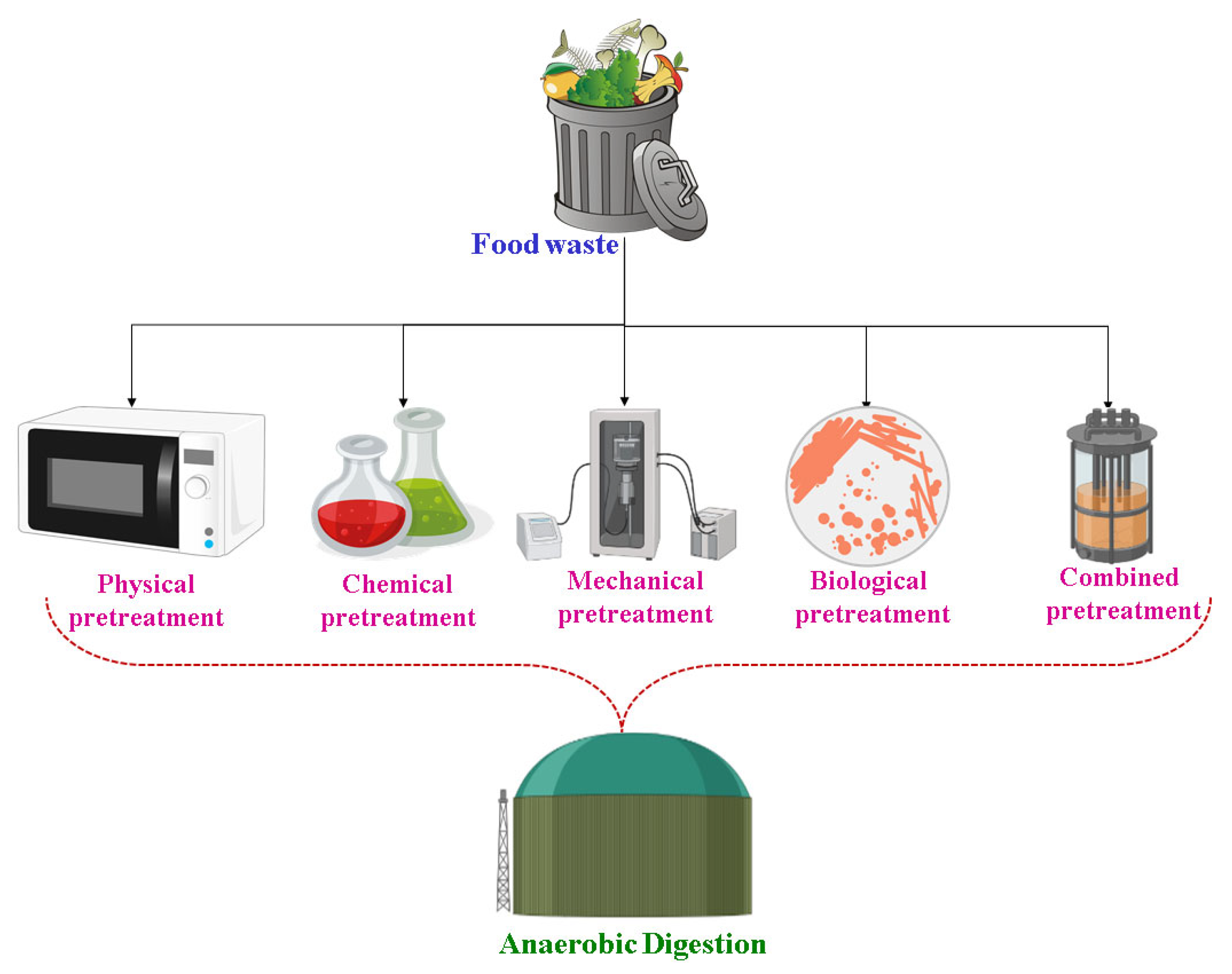
5. Existing Pretreatment Methods
5.1. Physical Pretreatment
5.2. Chemical Pretreatment
5.3. Mechanical Pretreatment
5.4. Biological Pretreatment
5.5. Combined Pretreatment
6. Advantages and Limitations of the Pretreatment Method
7. Energy and Cost Assessment of the Pretreatment Sector
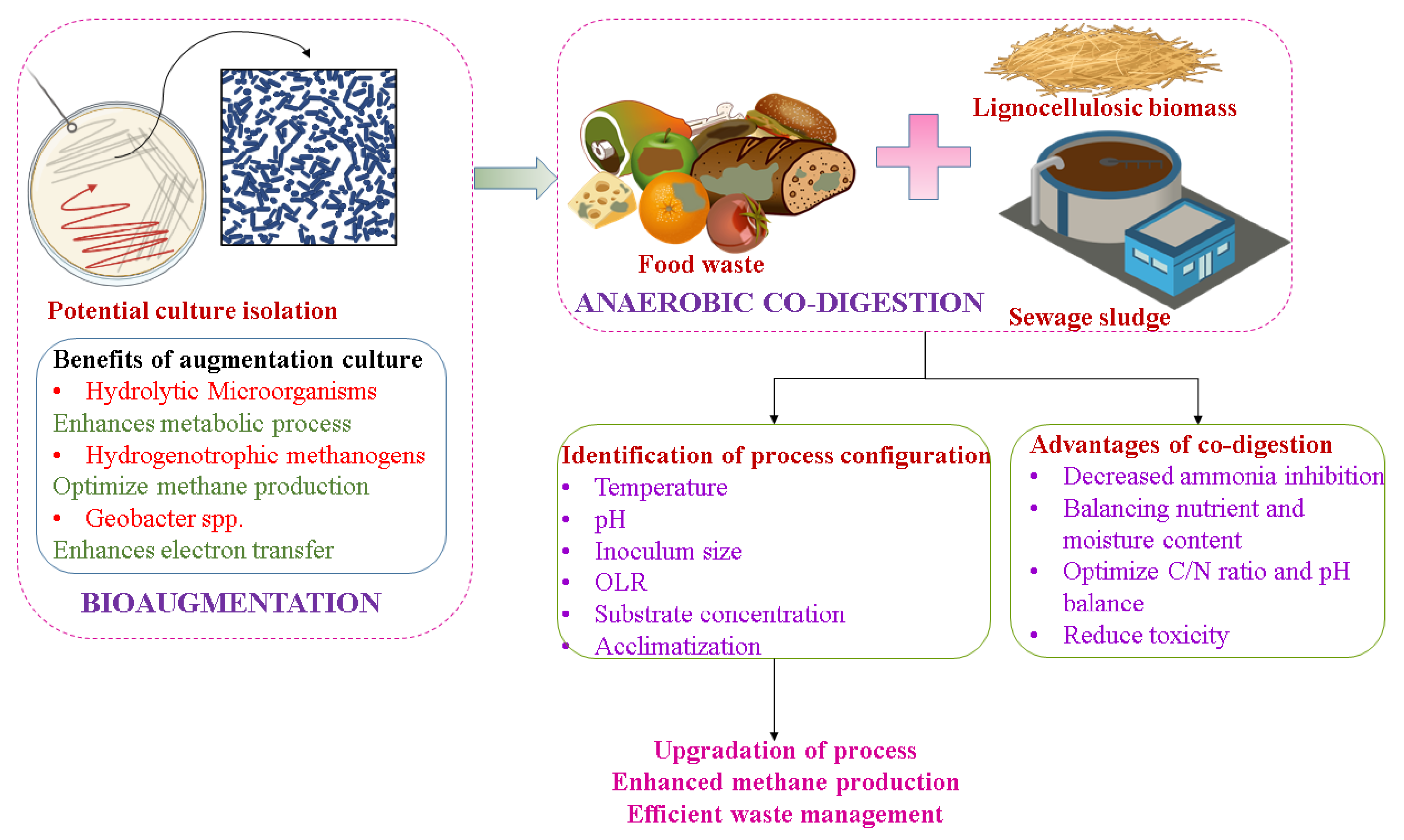
8. Different Innovative Approaches in the Field of Bioenergy Generation
9. Challenges Encumbering the Digestion Process
10. Future Prospects and Recommendation
- The sorting of FW from municipal solid waste is the major obstacle and it is not practiced in many countries. In the future, proper steps will be needed to separate FW from municipal solid waste to enhance energy production;
- The AD efficiency is enhanced and FW degradation is enhanced by different pretreatment methods. The optimization of the process is needed in most research but is also essential to characterize the FW structure. It is perceived that most of the pretreatment studies are narrowed to the lab scale and not to the pilot scale. From a long-term development point of view, future research must be more focused on a full-scale application to optimize the results based on energy and mass balance analysis;
- The stability of the AD of FW is an essential concern. The co-digestion method is now focused on by many researchers for biogas production from FW. Moreover, the feeding of FW as a co-substrate helps in eradicating the higher capital cost in existing industrial plants. Thus, the optimization of the co-digestion of FW is necessary. The adoption of the biorefinery concept by producing valued product along with biogas simultaneously is conceptual research and this topic needs future attention [49];
- Physical pretreatment methods are applied in large-scale applications whereas the energy requirements and maintenance charges are high and generate inhibitory components. These methods are present in large-scale applications whereas there are no clear data about economic analysis. In the chemical method, the biogas yield can be improved whereas it is widely applied in bioethanol generation and information is lacking about the AD process, and it is needed in further studies [11]. The combination of the pretreatment process shows many advantages and thus contributes to the favorable field of research to explore. Research must progress to understand the mechanism of combined pretreatment and a techno-economic analysis is needed to evaluate these treatment methods on an industrial scale.
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Srisowmeya, G.; Chakravarthy, M.; Devi, G.N. Critical considerations in two-stage anaerobic digestion of food waste—A review. Renew. Sustain. Energy Rev. 2020, 119, 109587. [Google Scholar] [CrossRef]
- Malav, L.C.; Yadav, K.K.; Gupta, N.; Kumar, S.; Sharma, G.K.; Krishnan, S.; Rezania, S.; Kamyab, H.; Pham, Q.B.; Yadav, S.; et al. A review on municipal solid waste as a renewable source for waste-to-energy project in India: Current practices, challenges, and future opportunities. J. Clean. Prod. 2020, 277, 123227. [Google Scholar] [CrossRef]
- Rafey, A.; Prabhat, K.; Samar, M. Comparison of technologies to serve waste to energy conversion. Int. J. Waste Resour. 2020, 10, 372. [Google Scholar]
- Sharma, P.; Gaur, V.K.; Kim, S.-H.; Pandey, A. Microbial strategies for bio-transforming food waste into resources. Bioresour. Technol. 2020, 299, 122580. [Google Scholar] [CrossRef]
- Ng, H.S.; Kee, P.E.; Yim, H.S.; Chen, P.-T.; Wei, Y.-H.; Lan, J.C.-W. Recent advances on the sustainable approaches for conversion and reutilization of food wastes to valuable bioproducts. Bioresour. Technol. 2020, 302, 122889. [Google Scholar] [CrossRef]
- Sakaguchi, L.; Pak, N.; Potts, M.D. Tackling the issue of food waste in restaurants: Options for measurement method, reduction and behavioral change. J. Clean. Prod. 2018, 180, 430–436. [Google Scholar] [CrossRef]
- Chew, K.R.; Leong, H.Y.; Khoo, K.S.; Vo, D.-V.N.; Anjum, H.; Chang, C.-K.; Show, P.L. Effects of anaerobic digestion of food waste on biogas production and environmental impacts: A review. Environ. Chem. Lett. 2021, 19, 2921–2939. [Google Scholar] [CrossRef]
- Zhongming, Z.; Linong, L.; Xiaona, Y.; Wangqiang, Z.; Wei, L. UNEP Food Waste Index Report 2021; United Nations Environment Programme: Nairobi, Kenya, 2021. [Google Scholar]
- Baiano, A. Recovery of Biomolecules from Food Wastes—A Review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef]
- Pour, F.H.; Makkawi, Y.T. A review of post-consumption food waste management and its potentials for biofuel production. Energy Rep. 2021, 7, 7759–7784. [Google Scholar] [CrossRef]
- Dahiya, S.; Kumar, A.N.; Shanthi Sravan, J.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef]
- Ouadi, M.; Bashir, M.A.; Speranza, L.G.; Jahangiri, H.; Hornung, A. Food and Market Waste—A Pathway to Sustainable Fuels and Waste Valorization. Energy Fuels 2019, 33, 9843–9850. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste—Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Bong, C.P.C.; Lim, L.Y.; Lee, C.T.; Klemeš, J.J.; Ho, C.S.; Ho, W.S. The characterisation and treatment of food waste for improvement of biogas production during anaerobic digestion—A review. J. Clean. Prod. 2018, 172, 1545–1558. [Google Scholar] [CrossRef]
- Kannah, R.Y.; Merrylin, J.; Devi, T.P.; Kavitha, S.; Sivashanmugham, P.; Kumar, G.; Banu, J.R. Food waste valorization: Biofuels and value added product recovery. Bioresour. Technol. Rep. 2020, 11, 100524. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Mofijur, M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An Overview of Recent Developments in Biomass Pyrolysis Technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Zaman, C.Z.; Pal, K.; Yehye, W.A.; Sagadevan, S.; Shah, S.T.; Adebisi, G.A.; Marliana, E.; Rafique, R.F.; Bin Johan, R. Pyrolysis: A Sustainable Way to Generate Energy from Waste; IntechOpen: Rijeka, Croatia, 2017; Volume 1. [Google Scholar]
- Opatokun, S.A.; Kan, T.; Al Shoaibi, A.; Srinivasakannan, C.; Strezov, V. Characterization of Food Waste and Its Digestate as Feedstock for Thermochemical Processing. Energy Fuels 2016, 30, 1589–1597. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Sustainable bioconversion of food waste into high-value products by immobilized enzymes to meet bio-economy challenges and opportunities—A review. Food Res. Int. 2019, 123, 226–240. [Google Scholar] [CrossRef]
- Aguilar, M.C.; Wang, Y.D.; Roskilly, T.; Pathare, P.B.; Lamidi, R.O. Biogas from anaerobic co-digestion of food waste and primary sludge for cogeneration of power and heat. Energy Procedia 2017, 142, 70–76. [Google Scholar] [CrossRef]
- Fadzil, F.; Sulaiman, S.; Shaharoshaha, A.; Seswoya, R. Mild Thermal Pre-treatment as a Method for Increasing the Methane Potential of Food Waste. J. Homepage 2020, 15, 425–430. [Google Scholar] [CrossRef]
- Liu, X.; Lee, C.; Kim, J.Y. Thermal hydrolysis pre-treatment combined with anaerobic digestion for energy recovery from organic wastes. J. Mater. Cycles Waste Manag. 2020, 22, 1370–1381. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.; Lee, J. Co-pyrolysis for the valorization of food waste and oriental herbal medicine byproduct. J. Anal. Appl. Pyrolysis 2021, 154, 105016. [Google Scholar] [CrossRef]
- Chen, J.; Fan, Y.; E, J.; Cao, W.; Zhang, F.; Gong, J.; Liu, G.; Xu, W. Effects analysis on the gasification kinetic characteristics of food waste in supercritical water. Fuel 2019, 241, 94–104. [Google Scholar] [CrossRef]
- Shi, X.; Guo, X.; Zuo, J.; Wang, Y.; Zhang, M. A comparative study of thermophilic and mesophilic anaerobic co-digestion of food waste and wheat straw: Process stability and microbial community structure shifts. Waste Manag. 2018, 75, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-J.; Kobayashi, T.; Kuramochi, H.; Li, Y.-Y.; Xu, K.-Q. Improved biogas production from food waste by co-digestion with de-oiled grease trap waste. Bioresour. Technol. 2016, 201, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.M.; Mahler, C.F.; Oliveira, L.B.; Bassin, J.P. Hydrogen and methane production in a two-stage anaerobic digestion system by co-digestion of food waste, sewage sludge and glycerol. Waste Manag. 2018, 76, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lang, Q.; Fang, M.; Li, X.; Bah, H.; Dong, H.; Dong, R. Combined effect of crude fat content and initial substrate concentration on batch anaerobic digestion characteristics of food waste. Bioresour. Technol. 2017, 232, 304–312. [Google Scholar] [CrossRef]
- Isha, A.; Silva, T.C.D.; Subbarao, P.M.; Chandra, R.; Vijay, V.K. Stabilization of anaerobic digestion of kitchen wastes using protein-rich additives: Study of process performance, kinetic modelling and energy balance. Bioresour. Technol. 2021, 337, 125331. [Google Scholar] [CrossRef]
- Ahn, K.-H.; Shin, D.-C.; Jung, J.; Jeong, Y.; Lee, Y.-E.; Kim, I.-T. Physicochemical Properties of Torrefied and Pyrolyzed Food Waste Biochars as Fuel: A Pilot-Scale Study. Energies 2022, 15, 333. [Google Scholar] [CrossRef]
- Giwa, A.S.; Xu, H.; Chang, F.; Wu, J.; Li, Y.; Ali, N.; Ding, S.; Wang, K. Effect of biochar on reactor performance and methane generation during the anaerobic digestion of food waste treatment at long-run operations. J. Environ. Chem. Eng. 2019, 7, 103067. [Google Scholar] [CrossRef]
- Ugwu, S.N.; Enweremadu, C.C. Enhancing anaerobic digestion of okra waste with the addition of iron nanocomposite (Ppy/Fe3O4). Biofuels 2020, 11, 503–512. [Google Scholar] [CrossRef]
- Ali, A.; Mahar, R.B.; Sherazi, S.T.H. Methane Augmentation of Anaerobic Digestion of Food Waste in the Presence of Fe3O4 and Carbamide Capped Fe3O4 Nanoparticles. Waste Biomass Valorization 2020, 11, 4093–4107. [Google Scholar] [CrossRef]
- Orangun, A.; Kaur, H.; Kommalapati, R. Batch Anaerobic Co-Digestion and Biochemical Methane Potential Analysis of Goat Manure and Food Waste. Energies 2021, 14, 1952. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Borrion, A.; Li, H.; Li, J. Effects of organic composition on mesophilic anaerobic digestion of food waste. Bioresour. Technol. 2017, 244, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Akindele, A.A.; Sartaj, M. The toxicity effects of ammonia on anaerobic digestion of organic fraction of municipal solid waste. Waste Manag. 2018, 71, 757–766. [Google Scholar] [CrossRef]
- Choong, Y.Y.; Norli, I.; Abdullah, A.Z.; Yhaya, M.F. Impacts of trace element supplementation on the performance of anaerobic digestion process: A critical review. Bioresour. Technol. 2016, 209, 369–379. [Google Scholar] [CrossRef]
- Atelge, M.R.; Krisa, D.; Kumar, G.; Eskicioglu, C.; Nguyen, D.D.; Chang, S.W.; Atabani, A.E.; Al-Muhtaseb, A.H.; Unalan, S. Biogas Production from Organic Waste: Recent Progress and Perspectives. Waste Biomass Valorization 2020, 11, 1019–1040. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Suja, F.B.; Zain, S.M.; Pramanik, B.K. The anaerobic digestion process of biogas production from food waste: Prospects and constraints. Bioresour. Technol. Rep. 2019, 8, 100310. [Google Scholar] [CrossRef]
- Morales-Polo, C.; del Mar Cledera-Castro, M.; Soria, B.Y.M. Reviewing the Anaerobic Digestion of Food Waste: From Waste Generation and Anaerobic Process to Its Perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef]
- Kumar, P.; Chandrasekhar, K.; Kumari, A.; Sathiyamoorthi, E.; Kim, B.S. Electro-Fermentation in Aid of Bioenergy and Biopolymers. Energies 2018, 11, 343. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Tabatabaei, M.; Aghbashlo, M. Biogas production from food wastes: A review on recent developments and future perspectives. Bioresour. Technol. Rep. 2019, 7, 100202. [Google Scholar] [CrossRef]
- Caruso, M.C.; Braghieri, A.; Capece, A.; Napolitano, F.; Romano, P.; Galgano, F.; Altieri, G.; Genovese, F. Recent Updates on the Use of Agro-Food Waste for Biogas Production. Appl. Sci. 2019, 9, 1217. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Awasthi, M.K.; Taherzadeh, M. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and potential of direct interspecies electron transfer in anaerobic digestion. Energies 2018, 11, 107. [Google Scholar] [CrossRef]
- Stams, A.J.M.; Plugge, C.M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Microbiol. 2009, 7, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Syntrophy Goes Electric: Direct Interspecies Electron Transfer. Annu. Rev. Microbiol. 2017, 71, 643–664. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.-J. Direct interspecies electron transfer mechanism in enhanced methanogenesis: A mini-review. Bioresour. Technol. 2021, 330, 124980. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A comprehensive review on food waste anaerobic digestion: Research updates and tendencies. Bioresour. Technol. 2018, 247, 1069–1076. [Google Scholar] [CrossRef]
- Pandey, S.; Singh, N.K.; Rao, K.N.S.; Yadav, T.C.; Sanghavi, G.; Yadav, M.; Bansal, A.K.; Thanki, A.; Nayak, J. Bacterial production of organic acids and subsequent metabolism. In Engineering of Microbial Biosynthetic Pathways; Springer: Singapore, 2020; pp. 153–173. [Google Scholar]
- Goswami, R.; Chattopadhyay, P.; Shome, A.; Banerjee, S.N.; Chakraborty, A.K.; Mathew, A.K.; Chaudhury, S. An overview of physico-chemical mechanisms of biogas production by microbial communities: A step towards sustainable waste management. 3 Biotech 2016, 6, 72. [Google Scholar] [CrossRef]
- Ros, M.; Franke-Whittle, I.; Morales, A.; Insam, H.; Ayuso, M.; Pascual, J. Archaeal community dynamics and abiotic characteristics in a mesophilic anaerobic co-digestion process treating fruit and vegetable processing waste sludge with chopped fresh artichoke waste. Bioresour. Technol. 2013, 136, 1–7. [Google Scholar] [CrossRef]
- Zou, L.; Wan, Y.; Zhang, S.; Luo, J.; Li, Y.-Y.; Liu, J. Valorization of food waste to multiple bio-energies based on enzymatic pretreatment: A critical review and blueprint for the future. J. Clean. Prod. 2020, 277, 124091. [Google Scholar] [CrossRef]
- Li, W.; Loh, K.-C.; Zhang, J.; Tong, Y.W.; Dai, Y. Two-stage anaerobic digestion of food waste and horticultural waste in high-solid system. Appl. Energy 2018, 209, 400–408. [Google Scholar] [CrossRef]
- Matheri, A.N.; Belaid, M.; Seodigeng, T.; Ngila, C.J. Modelling the kinetic of biogas production from co-digestion of pig waste and grass clippings. In Proceedings of the 24th World Congress on Engineering (WCE 2016), London, UK, 29 June–1 July 2016. [Google Scholar]
- Cesaro, A.; Belgiorno, V. Pretreatment methods to improve anaerobic biodegradability of organic municipal solid waste fractions. Chem. Eng. J. 2014, 240, 24–37. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K.; Kumar, R. Cellulose solvent-based pretreatment for enhanced second-generation biofuel production: A review. Sustain. Energy Fuels 2019, 3, 11–62. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Li, J.; Li, H.; Yu, Z.; Nie, Y. Effects of thermal pretreatment on degradation kinetics of organics during kitchen waste anaerobic digestion. Energy 2017, 118, 377–386. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Trably, E.; Mehariya, S.; Bernet, N.; Wong, J.W.; Carrere, H. Pretreatment of food waste for methane and hydrogen recovery: A review. Bioresour. Technol. 2018, 249, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Ushani, U.; Banu, J.R.; Tamilarasan, K.; Kavitha, S.; Yeom, I.T. Surfactant coupled sonic pretreatment of waste activated sludge for energetically positive biogas generation. Bioresour. Technol. 2017, 241, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Banu, J.R.; Kannah, R.Y.; Kavitha, S.; Gunasekaran, M.; Yeom, I.T.; Kumar, G. Disperser-induced bacterial disintegration of partially digested anaerobic sludge for efficient biomethane recovery. Chem. Eng. J. 2018, 347, 165–172. [Google Scholar] [CrossRef]
- Eswari, A.P.; Kavitha, S.; Banu, J.R.; Karthikeyan, O.P.; Yeom, I.-T. H2O2 induced cost effective microwave disintegration of dairy waste activated sludge in acidic environment for efficient biomethane generation. Bioresour. Technol. 2017, 244, 688–697. [Google Scholar] [CrossRef]
- Kavitha, S.; Subbulakshmi, P.; Banu, J.R.; Gobi, M.; Yeom, I.T. Enhancement of biogas production from microalgal biomass through cellulolytic bacterial pretreatment. Bioresour. Technol. 2017, 233, 34–43. [Google Scholar] [CrossRef]
- Kannah, R.Y.; Kavitha, S.; Sivashanmugam, P.; Kumar, G.; Banu, J.R. Ultrasonic induced mechanoacoustic effect on delignification of rice straw for cost effective biopretreatment and biomethane recovery. Sustain. Energy Fuels 2021, 5, 1832–1844. [Google Scholar] [CrossRef]
- Kavitha, S.; Kannah, R.Y.; Banu, J.R.; Kaliappan, S.; Johnson, M. Biological disintegration of microalgae for biomethane recovery-prediction of biodegradability and computation of energy balance. Bioresour. Technol. 2017, 244, 1367–1375. [Google Scholar] [CrossRef]
- Ma, C.; Liu, J.; Ye, M.; Zou, L.; Qian, G.; Li, Y.-Y. Towards utmost bioenergy conversion efficiency of food waste: Pretreatment, co-digestion, and reactor type. Renew. Sustain. Energy Rev. 2018, 90, 700–709. [Google Scholar] [CrossRef]
- Kavitha, S.; Banu, J.R.; Priya, A.A.; Uan, D.K.; Yeom, I.T. Liquefaction of food waste and its impacts on anaerobic biodegradability, energy ratio and economic feasibility. Appl. Energy 2017, 208, 228–238. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Gao, X.; Zhou, Y.; Shen, R. Effect of thermal pretreatment on the physical and chemical properties of municipal biomass waste. Waste Manag. 2012, 32, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Ariunbaatar, J.; Panico, A.; Frunzo, L.; Esposito, G.; Lens, P.N.; Pirozzi, F. Enhanced anaerobic digestion of food waste by thermal and ozonation pretreatment methods. J. Environ. Manag. 2014, 146, 142–149. [Google Scholar] [CrossRef] [PubMed]
- EL Gnaoui, Y.; Karouach, F.; Bakraoui, M.; Barz, M.; EL Bari, H. Mesophilic anaerobic digestion of food waste: Effect of thermal pretreatment on improvement of anaerobic digestion process. Energy Rep. 2020, 6, 417–422. [Google Scholar] [CrossRef]
- Saragih, F.N.A.; Priadi, C.R.; Adityosulindro, S.; Abdillah, A.; Islami, B.B. The effectiveness of anaerobic digestion process by thermal pre-treatment on food waste as a substrate. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 251, p. 12014. [Google Scholar]
- Yang, Q.; Yi, J.; Luo, K.; Jing, X.; Li, X.; Liu, Y.; Zeng, G. Improving disintegration and acidification of waste activated sludge by combined alkaline and microwave pretreatment. Process Saf. Environ. Prot. 2013, 91, 521–526. [Google Scholar] [CrossRef]
- Banu, J.R.; Merrylin, J.; Usman, T.M.; Kannah, R.Y.; Gunasekaran, M.; Kim, S.-H.; Kumar, G. Impact of pretreatment on food waste for biohydrogen production: A review. Int. J. Hydrogen Energy 2020, 45, 18211–18225. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, C.; Tong, J.; Liu, J.; Liu, J.; Yu, D.; Wang, Y.; Chen, M.; Wei, Y. Optimization and microbial community analysis of anaerobic co-digestion of food waste and sewage sludge based on microwave pretreatment. Bioresour. Technol. 2016, 200, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xie, Y.; Hou, F.; Yu, Q.; Wang, Y.; Wang, X.; Miao, C.; Ma, J.; Ge, W.; Zhang, T.; et al. Enhancement on methane production and anaerobic digestion stability via co-digestion of microwave-Ca(OH)2 pretreated sugarcane rind slurry and kitchen waste. J. Clean. Prod. 2020, 264, 121731. [Google Scholar] [CrossRef]
- Shahriari, H.; Warith, M.; Hamoda, M.; Kennedy, K. Evaluation of single vs. staged mesophilic anaerobic digestion of kitchen waste with and without microwave pretreatment. J. Environ. Manag. 2013, 125, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, Y.; Dong, Y. Pretreatment for biogas production by anaerobic fermentation of mixed corn stover and cow dung. Energy 2012, 46, 644–648. [Google Scholar] [CrossRef]
- Song, Z.; Yang, G.; Guo, Y.; Zhang, T. Comparison of two chemical pretreatments of rice straw for biogas production by anaerobic digestion. BioResources 2012, 7, 3223–3236. [Google Scholar]
- Rodriguez, C.; Alaswad, A.; Benyounis, K.; Olabi, A. Pretreatment techniques used in biogas production from grass. Renew. Sustain. Energy Rev. 2017, 68, 1193–1204. [Google Scholar] [CrossRef]
- Gayathri, T.; Kavitha, S.; Kumar, S.A.; Kaliappan, S.; Yeom, I.T.; Banu, J.R. Effect of citric acid induced deflocculation on the ultrasonic pretreatment efficiency of dairy waste activated sludge. Ultrason. Sonochem. 2015, 22, 333–340. [Google Scholar] [CrossRef]
- Vavouraki, A.I.; Angelis, E.M.; Kornaros, M. Optimization of thermo-chemical hydrolysis of kitchen wastes. Waste Manag. 2013, 33, 740–745. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Panigrahi, S.; Dubey, B.K. A critical review on operating parameters and strategies to improve the biogas yield from anaerobic digestion of organic fraction of municipal solid waste. Renew. Energy 2019, 143, 779–797. [Google Scholar] [CrossRef]
- Karthikeyan, O.; Hao, H.N.; Razaghi, A.; Heimann, K. Recycling of food waste for fuel precursors using an integrated bio-refinery approach. Bioresour. Technol. 2018, 248, 194–198. [Google Scholar] [CrossRef]
- Kavitha, S.; Preethi, J.; Banu, J.R.; Yeom, I.T. Low temperature thermochemical mediated energy and economically efficient biological disintegration of sludge: Simulation and prediction studies for anaerobic biodegradation. Chem. Eng. J. 2017, 317, 481–492. [Google Scholar] [CrossRef]
- Kavitha, S.; Kaliappan, S.; Kumar, S.A.; Yeom, I.T.; Banu, J.R. Effect of NaCl induced floc disruption on biological disintegration of sludge for enhanced biogas production. Bioresour. Technol. 2015, 192, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qin, Y.; Chen, B.; Wu, C.; Zheng, S.; Chen, R.; Yang, S.; Yang, L.; Liu, Z. Enhancing degradation and biogas production during anaerobic digestion of food waste using alkali pretreatment. Environ. Res. 2020, 188, 109743. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Atelge, M.R.; Atabani, A.E.; Banu, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.Ş.; Unalan, S.; et al. A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Sethupathy, A.; Pathak, P.K.; Sivashanmugam, P.; Arun, C.; Banu, J.R.; Ashokkumar, M. Enrichment of hydrogen production from fruit waste biomass using ozonation assisted with citric acid. Waste Manag. Res. 2021, 40, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Atay, Ş.; Akbal, F. Classification and Effects of Sludge Disintegration Technologies Integrated into Sludge Handling Units: An Overview. CLEAN-Soil Air Water 2016, 44, 1198–1213. [Google Scholar] [CrossRef]
- Magare, M.E.; Sahu, N.; Kanade, G.S.; Chanotiya, C.S.; Thul, S.T. An Integrated Process of Value Addition to Citrus Waste and Performance of Fenton Process for Its Conversion to Biogas. Waste Biomass Valorization 2020, 11, 165–172. [Google Scholar] [CrossRef]
- Tamilarasan, K.; Banu, J.R.; Kumar, M.D.; Sakthinathan, G.; Park, J.-H. Influence of Mild-Ozone Assisted Disperser Pretreatment on the Enhanced Biogas Generation and Biodegradability of Green Marine Macroalgae. Front. Energy Res. 2019, 7, 89. [Google Scholar] [CrossRef]
- Gadhe, A.; Sonawane, S.S.; Varma, M.N. Ultrasonic pretreatment for an enhancement of biohydrogen production from complex food waste. Int. J. Hydrogen Energy 2014, 39, 7721–7729. [Google Scholar] [CrossRef]
- Rasapoor, M.; Adl, M.; Baroutian, S.; Iranshahi, Z.; Pazouki, M. Energy performance evaluation of ultrasonic pretreatment of organic solid waste in a pilot-scale digester. Ultrason. Sonochem. 2019, 51, 517–525. [Google Scholar] [CrossRef]
- Li, X.; Mettu, S.; Martin, G.J.; Ashokkumar, M.; Lin, C.S.K. Ultrasonic pretreatment of food waste to accelerate enzymatic hydrolysis for glucose production. Ultrason. Sonochem. 2019, 53, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Cheng, J.; Tang, S.; An, X.; Hua, J.; Dong, H.; Zhou, J. Ultrasound and microwave pretreatments promote methane production potential and energy conversion during anaerobic digestion of lipid and food wastes. Energy 2021, 228, 120525. [Google Scholar] [CrossRef]
- Kavitha, S.; Saranya, T.; Kaliappan, S.; Kumar, S.A.; Yeom, I.T.; Banu, J.R. Accelerating the sludge disintegration potential of a novel bacterial strain Planococcus jake 01 by CaCl2 induced deflocculation. Bioresour. Technol. 2015, 175, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, S.; Kumar, S.A.; Kaliappan, S.; Yeom, I.T.; Banu, J.R. Achieving profitable biological sludge disintegration through phase separation and predicting its anaerobic biodegradability by non linear regression model. Chem. Eng. J. 2015, 279, 478–487. [Google Scholar] [CrossRef]
- Han, W.; Yan, Y.; Shi, Y.; Gu, J.; Tang, J.; Zhao, H. Biohydrogen production from enzymatic hydrolysis of food waste in batch and continuous systems. Sci. Rep. 2016, 6, 38395. [Google Scholar] [CrossRef]
- Han, W.; Liu, Y.; Xu, X.; Huang, J.; He, H.; Chen, L.; Qiu, S.; Tang, J.; Hou, P. Bioethanol production from waste hamburger by enzymatic hydrolysis and fermentation. J. Clean. Prod. 2020, 264, 121658. [Google Scholar] [CrossRef]
- Liu, Y.; Han, W.; Xu, X.; Chen, L.; Tang, J.; Hou, P. Ethanol production from waste pizza by enzymatic hydrolysis and fermentation. Biochem. Eng. J. 2020, 156, 107528. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and Physicochemical Pretreatment of Lignocellulosic Biomass: A Review. Enzym. Res. 2011, 2011, 787532. [Google Scholar] [CrossRef]
- Ye, M.; Liu, J.; Ma, C.; Li, Y.-Y.; Zou, L.; Qian, G.; Xu, Z.P. Improving the stability and efficiency of anaerobic digestion of food waste using additives: A critical review. J. Clean. Prod. 2018, 192, 316–326. [Google Scholar] [CrossRef]
- Meng, Y.; Luan, F.; Yuan, H.; Chen, X.; Li, X. Enhancing anaerobic digestion performance of crude lipid in food waste by enzymatic pretreatment. Bioresour. Technol. 2017, 224, 48–55. [Google Scholar] [CrossRef]
- Snehya, A.; Sundaramahalingam, M.; Rajeshbanu, J.; Anandan, S.; Sivashanmugam, P. Studies on evaluation of surfactant coupled sonication pretreatment on Ulva fasciata (marine macroalgae) for enhanced biohydrogen production. Ultrason. Sonochem. 2021, 81, 105853. [Google Scholar] [CrossRef] [PubMed]
- Shanthi, M.; Banu, R.; Sivashanmugam, P. Synergistic effect of combined pretreatment in solubilizing fruits and vegetable residue for biogas production: Hydrolysis, energy assessment. Fuel 2019, 250, 194–202. [Google Scholar] [CrossRef]
- Shanthi, M.; Banu, J.R.; Sivashanmugam, P. Effect of surfactant assisted sonic pretreatment on liquefaction of fruits and vegetable residue: Characterization, acidogenesis, biomethane yield and energy ratio. Bioresour. Technol. 2018, 264, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Wainaina, S.; Awasthi, M.K.; Sarsaiya, S.; Chen, H.; Singh, E.; Kumar, A.; Ravindran, B.; Awasthi, S.K.; Liu, T.; Duan, Y.; et al. Resource recovery and circular economy from organic solid waste using aerobic and anaerobic digestion technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef] [PubMed]
- Ravi, Y.K.; Zhang, W.; Liang, Y. Effect of surfactant assisted ultrasonic pretreatment on production of volatile fatty acids from mixed food waste. Bioresour. Technol. 2023, 368, 128340. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Alriols, M.G.; Labidi, J. Evaluation of different lignocellulosic raw materials as potential alternative feedstocks in biorefinery processes. Ind. Crops Prod. 2014, 53, 102–110. [Google Scholar] [CrossRef]
- Barakat, A.; de Vries, H.; Rouau, X. Dry fractionation process as an important step in current and future lignocellulose biorefineries: A review. Bioresour. Technol. 2013, 134, 362–373. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Zhang, X.; Zhang, G. Digestion of thermally hydrolyzed sewage sludge by anaerobic sequencing batch reactor. J. Hazard. Mater. 2009, 162, 799–803. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, M.; Lv, C.; Yue, P. The effect of microwave pretreatment on anaerobic co-digestion of sludge and food waste: Performance, kinetics and energy recovery. Environ. Res. 2020, 189, 109856. [Google Scholar] [CrossRef]
- Panigrahi, S.; Sharma, H.B.; Dubey, B.K. Anaerobic co-digestion of food waste with pretreated yard waste: A comparative study of methane production, kinetic modeling and energy balance. J. Clean. Prod. 2020, 243, 118480. [Google Scholar] [CrossRef]
- Ma, Y.; Yin, Y.; Liu, Y. A holistic approach for food waste management towards zero-solid disposal and energy/resource recovery. Bioresour. Technol. 2017, 228, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Patinvoh, R.J.; Osadolor, O.A.; Chandolias, K.; Horváth, I.S.; Taherzadeh, M.J. Innovative pretreatment strategies for biogas production. Bioresour. Technol. 2017, 224, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Shah, G.; Sahota, S.; Singh, L.; Vijay, V.K. Biogas production from waste: Technical overview, progress, and challenges. In Bioreactors: Sustainable Design and Industrial Applications in Mitigation of GHG Emission; Elsevier: Amsterdam, The Netherlands, 2020; pp. 89–104. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Zhou, X.; Wang, Q.; Wu, Y.; Saino, M.; Bai, X. Study on the bio-methane yield and microbial community structure in enzyme enhanced anaerobic co-digestion of cow manure and corn straw. Bioresour. Technol. 2016, 219, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Zeshan; Karthikeyan, O.P.; Visvanathan, C. Effect of C/N ratio and ammonia-N accumulation in a pilot-scale thermophilic dry anaerobic digester. Bioresour. Technol. 2012, 113, 294–302. [Google Scholar] [CrossRef]
- Kasinath, A.; Fudala-Ksiazek, S.; Szopinska, M.; Bylinski, H.; Artichowicz, W.; Remiszewska-Skwarek, A.; Luczkiewicz, A. Biomass in biogas production: Pretreatment and codigestion. Renew. Sustain. Energy Rev. 2021, 150, 111509. [Google Scholar] [CrossRef]
- Shah, F.A.; Mahmood, Q.; Rashid, N.; Pervez, A.; Raja, I.A.; Shah, M.M. Co-digestion, pretreatment and digester design for enhanced methanogenesis. Renew. Sustain. Energy Rev. 2015, 42, 627–642. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Dosta, J.; Romero-Güiza, M.; Fonoll, X.; Peces, M.; Astals, S. A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- Pan, Y.; Zhi, Z.; Zhen, G.; Lu, X.; Bakonyi, P.; Li, Y.-Y.; Zhao, Y.; Banu, R. Synergistic effect and biodegradation kinetics of sewage sludge and food waste mesophilic anaerobic co-digestion and the underlying stimulation mechanisms. Fuel 2019, 253, 40–49. [Google Scholar] [CrossRef]
- Zamanzadeh, M.; Hagen, L.H.; Svensson, K.; Linjordet, R.; Horn, S.J. Biogas production from food waste via co-digestion and digestion- effects on performance and microbial ecology. Sci. Rep. 2017, 7, 17664. [Google Scholar] [CrossRef]
- Li, K.; Liu, R.; Cui, S.; Yu, Q.; Ma, R. Anaerobic co-digestion of animal manures with corn stover or apple pulp for enhanced biogas production. Renew. Energy 2018, 118, 335–342. [Google Scholar] [CrossRef]
- Da Silva, T.L.; Gouveia, L.; Reis, A. Integrated microbial processes for biofuels and high value-added products: The way to improve the cost effectiveness of biofuel production. Appl. Microbiol. Biotechnol. 2014, 98, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Li, C.; Zhou, Y.; Lu, W.; Nges, I.A. Enhancement of the solid-state anaerobic digestion of rice straw by liquor supplementation. Bioresour. Technol. Rep. 2019, 5, 59–65. [Google Scholar] [CrossRef]
- Algapani, D.E.; Qiao, W.; Ricci, M.; Bianchi, D.; Wandera, S.M.; Adani, F.; Dong, R. Bio-hydrogen and bio-methane production from food waste in a two-stage anaerobic digestion process with digestate recirculation. Renew. Energy 2019, 130, 1108–1115. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Q.; Qu, Y.; Dai, Y.; He, Y.; Wang, C.-H.; Tong, Y.W. Integrating food waste sorting system with anaerobic digestion and gasification for hydrogen and methane co-production. Appl. Energy 2020, 257, 113988. [Google Scholar] [CrossRef]
- Kim, W.; Cho, K.; Lee, S.; Hwang, S. Comparison of methanogenic community structure and anaerobic process performance treating swine wastewater between pilot and optimized lab scale bioreactors. Bioresour. Technol. 2013, 145, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Raper, E.; Stephenson, T.; Anderson, D.; Fisher, R.; Soares, A. Industrial wastewater treatment through bioaugmentation. Process Saf. Environ. Prot. 2018, 118, 178–187. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, D.-H.; Yun, Y.-M. Effect of operation temperature on anaerobic digestion of food waste: Performance and microbial analysis. Fuel 2017, 209, 598–605. [Google Scholar] [CrossRef]
- Jiang, J.; Li, L.; Li, Y.; He, Y.; Wang, C.; Sun, Y. Bioaugmentation to enhance anaerobic digestion of food waste: Dosage, frequency and economic analysis. Bioresour. Technol. 2020, 307, 123256. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Sun, Y.; Yuan, Z. Bioaugmentation strategy for enhancing anaerobic digestion of high C/N ratio feedstock with methanogenic enrichment culture. Bioresour. Technol. 2018, 261, 188–195. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.; Vasileiou, S.; Treu, L.; Campanaro, S.; Lyberatos, G.; Angelidaki, I. Bioaugmentation with hydrolytic microbes to improve the anaerobic biodegradability of lignocellulosic agricultural residues. Bioresour. Technol. 2017, 234, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
| Food Waste Type | Proximate Analysis | Ultimate Analysis | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | Moisture (%) | TS (%) | VS (%) | C (%) | H (%) | N (%) | O (%) | S (%) | C/N Ratio | ||
| Kitchen waste—highly acidic | 2–5 | 86 | 14 | 88 | 41.8 | 5.06 | 2.01 | 20.8 | [29] | ||
| Kitchen waste—medium acidic | 5–7 | 89 | 11 | 89 | 40.3 | 5.14 | 1.92 | 21 | |||
| Kitchen waste—lesser alkaline | 7–8 | 90 | 10 | 95 | 42.1 | 5.21 | 1.86 | 22.6 | |||
| Dried food waste | 9.91 | 70.2 | 43.1 | 6.91 | 3.14 | 38.45 | 0.62 | [30] | |||
| Food waste | 5.4 | 0.97 | 20 | 18.01 | 50.69 | 7.35 | 3.51 | 0.28 | 0.42 | [31] | |
| Okra waste | 7 | 15.15 | 13.11 | 39.3 | 5.39 | 3.21 | 35.74 | 12.2 | [32] | ||
| Food waste | 93.74 | 6.42 | 5.85 | 48.04 | 5.71 | 2.95 | 0.44 | [33] | |||
| Food waste | 3.3% | 71.3 | 47.5 | 6.6 | 3.9 | 31.3 | 0.4 | [23] | |||
| Dried food waste | 6.09% | 80.9 | 41.5 | 5.76 | 1.55 | 51.04 | 0.12 | [24] | |||
| Food waste | 5.1 | 14.8 ± 0.6 | 85.2 ± 0.6 | 44.3 ± 2.8 | 47.0 ± 2.4 | 7.3 ± 0.4 | 2.7 ± 0.4 | 42.9 ± 3.3 | 0.2 | 18:1 | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthu, P.; Muniappan, G.; Jeyakumar, R.B. Efficacious Utilization of Food Waste for Bioenergy Generation through the Anaerobic Digestion Method. Processes 2023, 11, 702. https://doi.org/10.3390/pr11030702
Muthu P, Muniappan G, Jeyakumar RB. Efficacious Utilization of Food Waste for Bioenergy Generation through the Anaerobic Digestion Method. Processes. 2023; 11(3):702. https://doi.org/10.3390/pr11030702
Chicago/Turabian StyleMuthu, Preethi, Gunasekaran Muniappan, and Rajesh Banu Jeyakumar. 2023. "Efficacious Utilization of Food Waste for Bioenergy Generation through the Anaerobic Digestion Method" Processes 11, no. 3: 702. https://doi.org/10.3390/pr11030702
APA StyleMuthu, P., Muniappan, G., & Jeyakumar, R. B. (2023). Efficacious Utilization of Food Waste for Bioenergy Generation through the Anaerobic Digestion Method. Processes, 11(3), 702. https://doi.org/10.3390/pr11030702







