Effect of Air-Drying and Freeze-Drying Temperature on the Process Kinetics and Physicochemical Characteristics of White Mulberry Fruits (Morus alba L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Dry Matter Content
2.3. Drying Process
2.4. Modeling of Drying Curves
2.5. Color Coordinates
2.6. Total Ascorbic Acid Content
2.7. Total Phenolics Content and Antioxidant Capacity
2.8. Statistical Analysis of Data
3. Results and Discussion
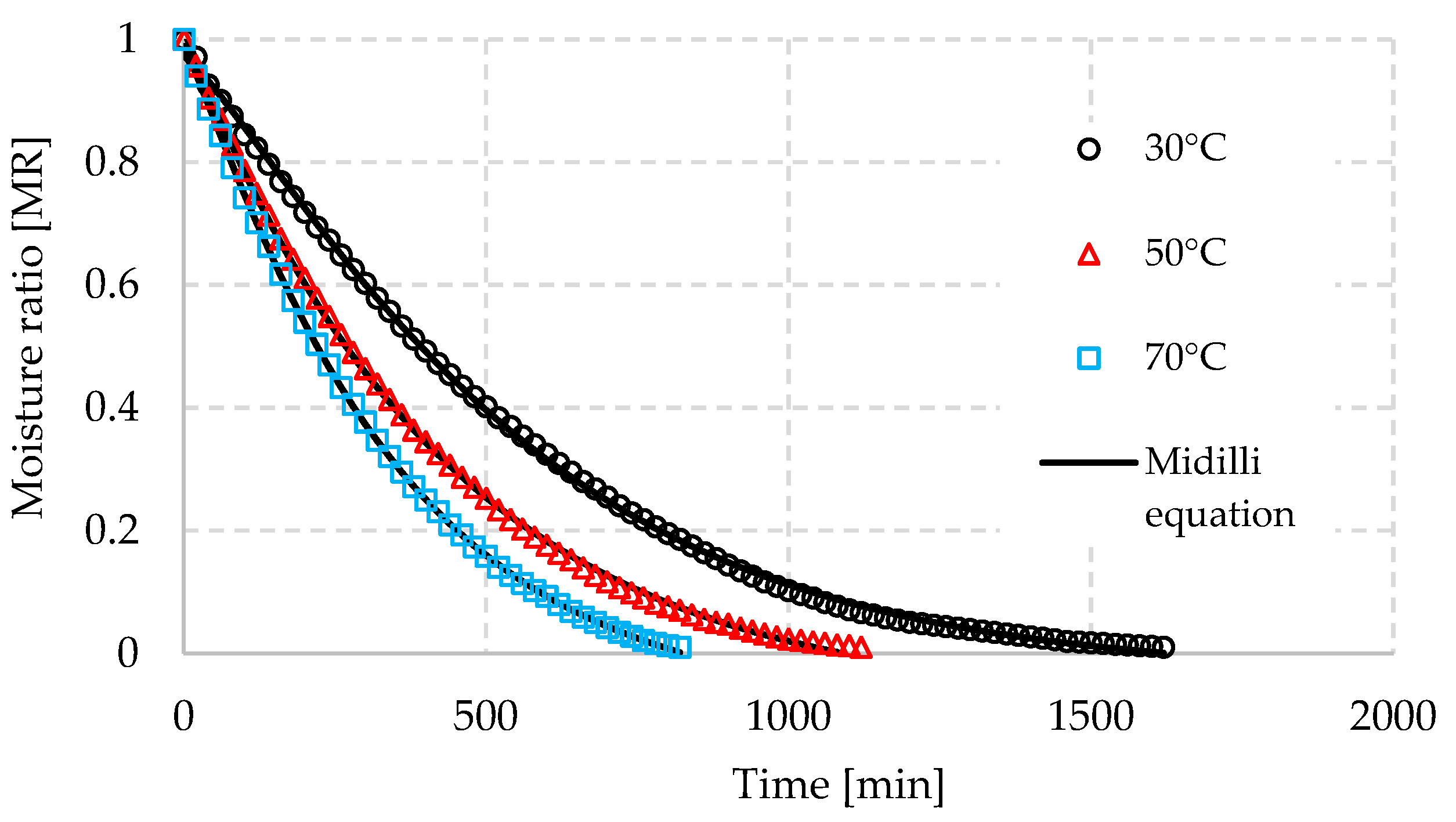
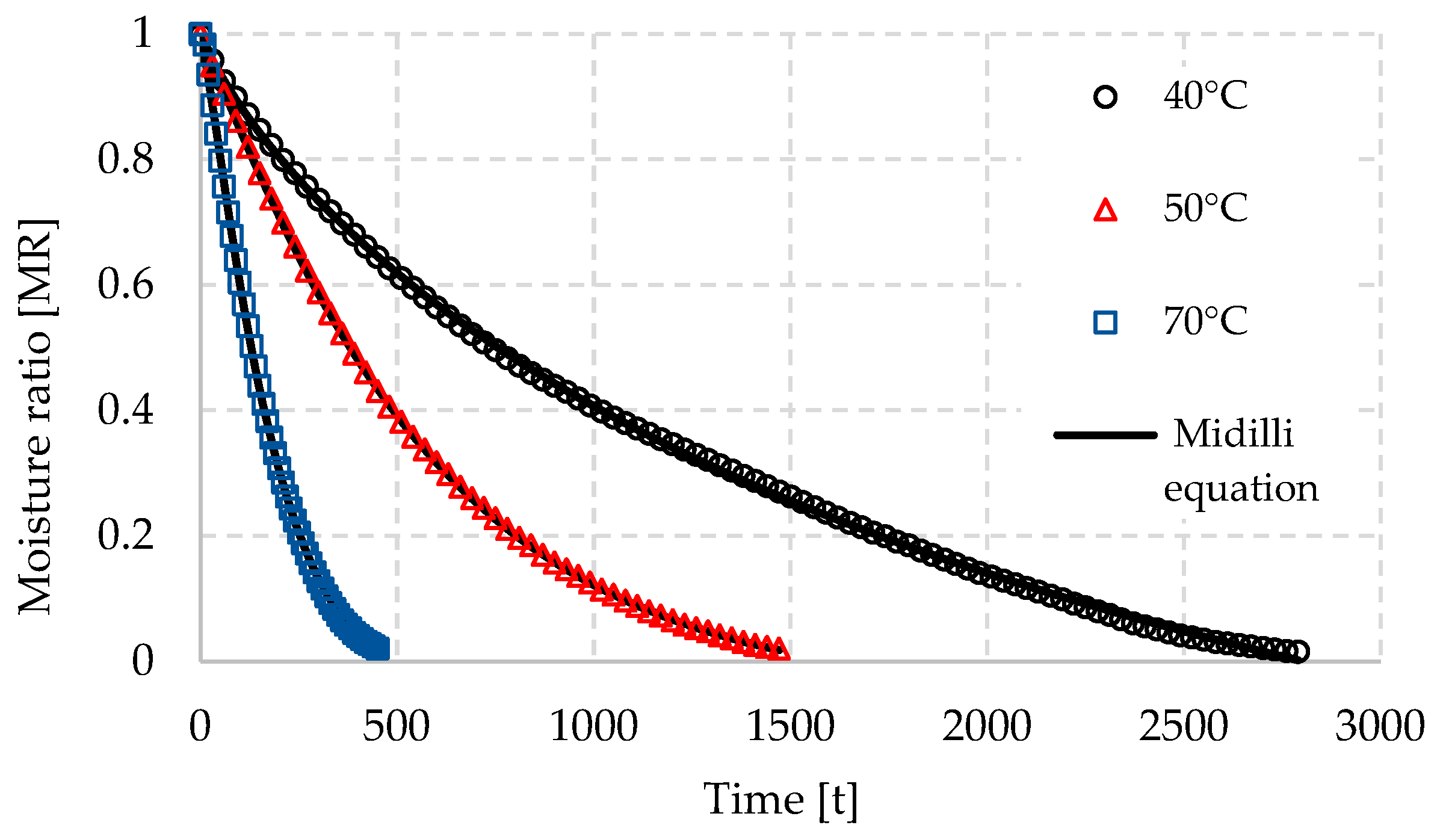
3.1. Drying Kinetics
3.2. Color Coordinates
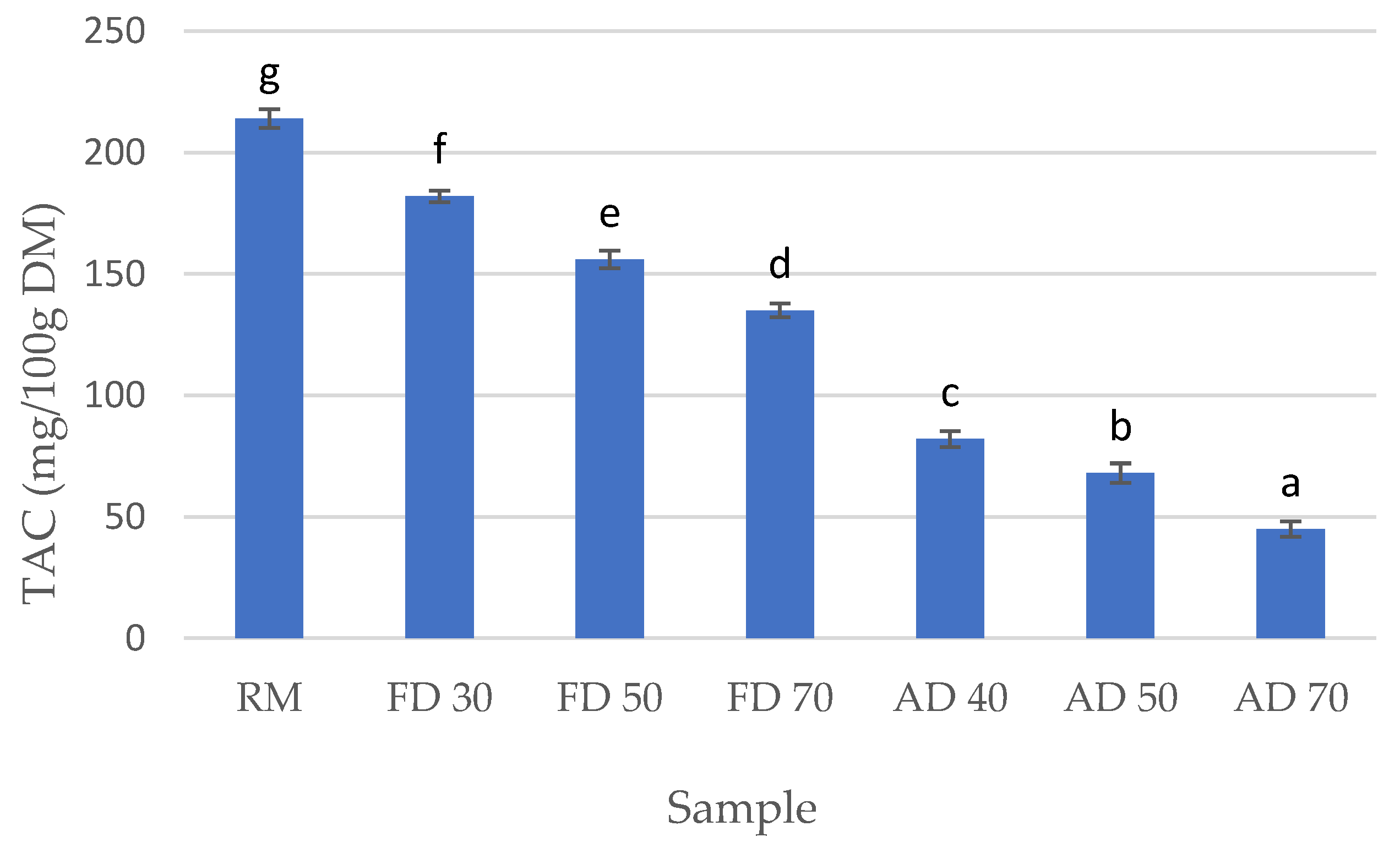
3.3. Total Ascorbic Acid Content
3.4. Phenolics Content and Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, J.M.; Kim, Y.B.; Kum, J.S.; Choi, Y.S.; Seo, D.H.; Choi, H.W.; Park, J.D. Effects of freeze-dried mulberry on antioxidant activities and fermented characteristics of yogurt during refrigerated storage. Korean J. Food Sci. Anim. 2015, 35, 807–814. [Google Scholar] [CrossRef] [Green Version]
- Machii, H.; Koyama, A.; Yamanouchi, H. FAO Electronic Conference: Mulberry for Animal Production. 2000. Available online: http://www.fao.org/livestock/agap/frg/mulberry/ (accessed on 20 December 2022).
- Grześkowiak, J.; Łochyńska, M. Bioactive Compounds in White Mulberry (Morus alba L.) and Their Therapeutic Activity. Post. Fitoter. 2017, 18, 31–35. Available online: http://www.postepyfitoterapii.pl/wp-content/uploads/2017/06/pf_2017_031-035.pdf (accessed on 15 January 2023).
- Ali, M.A.; Memon, N.; Luthria, D.; Bhanger, M.I.; Pitafi, A.A. Phenolic Acid Profiling and Antioxidant Potential of Mulberry Leaves and Fruits Grow in Pakistan. Pol. J. Food Nutr. Sci. 2010, 60, 25–32. Available online: http://journal.pan.olsztyn.pl/pdf-98237-30952?filename=PHENOLIC%20ACIDS%20PROFILING.pdf (accessed on 12 January 2023).
- Ercisli, S.; Orhan, E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. J. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Khattak, M.K.; Hanif, M.; Khan, M.; Ramzan, M.; Abdurab, K. Comparison of Drying Process and Preservatives on Drying Kinetics, Texture and Antioxidants Retention in Mulberry Fruits. J. Anim. Plant Sci. 2019, 29, 803–808. Available online: http://www.thejaps.org.pk/docs/v-29-03/21.pdf (accessed on 3 January 2023).
- Afrin, S.; Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Amici, A.; Cianciosi, D. Manuka honey synergistically enhances the chemopreventive effect of 5-fluorouracil on human colon cancer cells by inducing oxidative stress and apoptosis, altering metabolic phenotypes and suppressing metastasis ability. Free Radic. Biol. Med. 2018, 126, 41–54. [Google Scholar] [CrossRef]
- Cianciosi, D.; Varela-Lopez, A.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Reboredo-Rodriguez, P.; Zhang, J.; Quiles, J.L.; Nabavi, S.F.; Battino, M.; et al. Targeting molecular pathways in cancer stem cells by natural bioactive compounds. Pharmacol. Res. 2018, 135, 150–165. [Google Scholar] [CrossRef]
- Pan, P.; Huang, Y.W.; Oshima, K.; Yearsley, M.; Zhang, J.; Yu, J.; Arnold, M.; Wang, L.S. An immunological perspective for preventing cancer with berries. J. Berry Res. 2018, 8, 163–175. [Google Scholar] [CrossRef]
- Sharma, A.; Kashyap, D.; Sak, K.; Tuli, H.S.; Sharma, A.K. Therapeutic charm of quercetin and its derivatives: A review of research and patents. Pharm. Pat. Anal. 2018, 7, 15–32. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.G.; Linhardt, R.J.; Liao, S.T.; Wu, H.; Zou, Y.X. Mulberry: A Review of Bioactive Compounds and Advanced Processing Technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Doymaz, I. Drying Kinetics of White Mulberry. J. Food Eng. 2004, 61, 341–346. [Google Scholar] [CrossRef]
- Maskan, M.; Gogus, F. Sorption Isotherms and drying characteristics of mulberry (Morus alba). J. Food Eng. 1998, 37, 437–449. [Google Scholar] [CrossRef]
- Akbulut, A.; Durmus, A. Energy and exergy analyses of thin layer drying of mulberry in a forced solar dryer. Energy 2010, 35, 1754–1763. [Google Scholar] [CrossRef]
- Ali, M.; Durrani, Y.; Ayub, M. Effect of Drying Techniques and Storage on Mulberry (Morus alba) Quality. Sarhad J. Agric. 2016, 32, 80–88. [Google Scholar] [CrossRef]
- Akbulut, A.; Durmus, A. Thin layer solar drying and mathematical modelling of mulberry. Int. J. Energy Res. 2009, 33, 687–695. [Google Scholar] [CrossRef]
- Karaaslan, S.; Ekinci, K.; Akbolat, D.; Kumbul, B.S. Mathematical MOdeling of Thin Layer Drying Kinetics of White Mulberry (Morus alba L.) in Solar Tunnel Drier. Agronomy 2017, 60, 486–490. Available online: https://agronomyjournal.usamv.ro/pdf/2017/Art80.pdf (accessed on 20 December 2022).
- Chottamom, P.; Kongmanee, R.; Manklang, C.; Soponronnarit, S. Effect of osmotic treatment on drying kinetics and antioxidant properties of dried mulberry. Dry. Technol. 2012, 30, 80–87. [Google Scholar] [CrossRef]
- Doymaz, I.; Pala, M. Effect of ethyl oleate on drying characteristics of mulberries. Nahr. Food 2003, 47, 304–308. [Google Scholar] [CrossRef]
- Esmaeili, A.M.; Nikbakht, A.M.; Motevali, A.; Mousavi, S.S. Investigation of Black Mulberry Drying Kinetics Applying Different Pretreatments. J. Agric. Sci. Technol. 2013, 15, 23–34. Available online: https://jast.modares.ac.ir/article-23-1970-en.pdf (accessed on 12 December 2022).
- Zhou, M.; Chen, Q.; Bi, J.; Wang, Y.; Wu, X. Degradation kinetics of cyanidin 3-O-glucoside and cyanidin 3-Orutinoside during hot air and vacuum drying in mulberry (Morus alba L.) fruit: A comparative study based on solid food system. Food Chem. 2017, 229, 574–579. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Z.; Bi, J.; Zhou, L.; Yi, J.; Wu, X. Effect of hybrid drying methods on physicochemical, nutritional and anti-oxidant properties of dried black mulberry. LWT-Food Sci. Technol. 2017, 80, 178–184. [Google Scholar] [CrossRef]
- Darvishi, H.; Zarein, M.; Minaei, S.; Khafajeh, H. Exergy and energy analysis, drying kinetics and mathematical modeling of white mulberry drying process. Int. J. Food Eng. 2014, 10, 269–280. [Google Scholar] [CrossRef]
- Wojdyło, A.; Figiel, A.; Oszmiański, J. Effect of drying methods with the application of vacuum microwaves on the bioactive compounds, color, and antioxidant activity of strawberry fruits. J. Agric. Food Chem. 2009, 57, 1337–1343. [Google Scholar] [CrossRef]
- Rayaguru, K.; Routray, W. Mathematical Modeling of Thin Layer Drying Kinetics of Stone Apple Slices. Int. Food Res. J. 2012, 19, 1503–1510. Available online: http://www.ifrj.upm.edu.my/19%20(04)%202012/30%20IFRJ%2019%20(04)%202012%20Winny%20(397).pdf (accessed on 28 January 2023).
- El-Beltagy, A.; Gamea, G.R.; Amer Essa, A.H. Solar drying characteristics of strawberry. J. Food Eng. 2007, 78, 456–464. [Google Scholar] [CrossRef]
- Özdemir, M.; Onur Devres, Y. Thin layer drying characteristics of hazelnuts during roasting. J. Food Eng. 1999, 42, 225–233. [Google Scholar] [CrossRef]
- Sarimeseli, A. Microwave drying characteristics of coriander (Coriandrum sativum L.) leaves. Energy Convers. Manag. 2011, 52, 1449–1453. [Google Scholar] [CrossRef]
- Motevali, A.; Younji, S.; Chayjan, R.A.; Aghilinategh, N.; Banakar, A. Drying kinetics of dill leaves in a convective dryer. Int. Agrophysics 2013, 27, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Midilli, A.; Kucuk, H.; Yapar, Z. A new model for single-layer drying. Dry. Technol. 2007, 20, 1503–1513. [Google Scholar] [CrossRef]
- Diamante, L.M.; Munro, P.A. Mathematical modelling of the thin layer solar drying of sweet potato slices. Sol. Energy 1993, 51, 271–276. [Google Scholar] [CrossRef]
- AOAC International. Method 934.06 moisture in dried fruits. In Official Methods of the Association of Analytical Chemists, 15th ed.; AOAC: Virginia, VA, USA, 1990. [Google Scholar]
- Dziki, D.; Polak, R.; Rudy, S.; Krzykowski, A.; Gawlik-Dziki, U.; Rózyło, R.; Miś, A.; Combrzyński, M. Simulation of the process kinetics and analysis of physicochemical properties in the freeze drying of kale. Int. Agrophysics 2018, 32, 49–56. [Google Scholar] [CrossRef]
- Krzykowski, A.; Dziki, D.; Rudy, S.; Gawlik-Dziki, U.; Janiszewska-Turak, E.; Biernacka, B. Wild strawberry Fragaria vesca L.: Kinetics of fruit drying and quality characteristics of the dried fruits. Processes 2020, 8, 1265. [Google Scholar] [CrossRef]
- Krzykowski, A.; Dziki, D.; Rudy, S.; Gawlik-Dziki, U.; Polak, R.; Biernacka, B. Effect of pre-treatment conditions and freeze-drying temperature on the process kinetics and physicochemical properties of pepper. LWT Food Sci. Technol. 2018, 98, 25–30. [Google Scholar] [CrossRef]
- Mazurek, A.; Jamroz, J. Precision of dehydroascorbic acid quantitation with the use of the subtraction method—Validation of HPLC–DAD method for determination of total vitamin C in food. Food Chem. 2015, 173, 543–550. [Google Scholar] [CrossRef]
- Romankiewicz, D.; Hassoon, W.H.; Cacak-Pietrzak, G.; Sobczyk, M.; Wirkowska-Wojdyła, M.; Ceglińska, A.; Dziki, D. The effect of chia seeds (Salvia hispanica L.) addition on quality and nutritional value of wheat bread. J. Food Qual. 2017, 2017, 7352631. [Google Scholar] [CrossRef] [Green Version]
- Sujka, K.; Cacak-Pietrzak, G.; Sułek, A.; Murgrabia, K.; Dziki, D. Buckwheat hull-enriched pasta: Physicochemical and sensory properties. Molecules 2022, 27, 4065. [Google Scholar] [CrossRef]
- Singh, A.; Raju, R.; Mrad, M.; Reddell, P.; Münch, G. The reciprocal EC50 value as a convenient measure of the potency of a compound in bioactivity-guided purification of natural products. Fitoterapia 2020, 143, 104598. [Google Scholar] [CrossRef]
- Royen, M.J.; Noori, A.W.; Haydary, J. Experimental study and mathematical modeling of convective thin-layer drying of apple slices. Processes 2020, 8, 1562. [Google Scholar] [CrossRef]
- Macedo, L.L.; Vimercati, W.C.; Araújo, C.S.; Saraiva, S.H.; Teixeira, L.J.Q. Effect of drying air temperature on drying kinetics and physicochemical characteristics of dried banana. J. Food Process Eng. 2020, 43, e13451. [Google Scholar] [CrossRef]
- Soysal, Y.; Öztekin, S.; Eren, Ö. Microwave drying of parsley: Modeling, kinetics, and energy aspects. Biosyst. Eng. 2006, 93, 403–413. [Google Scholar] [CrossRef]
- Artnaseaw, A.; Theerakulpisut, S.; Benjapiyaporn, C. Drying characteristic of shiitake mushroom and jinda chili during vacuum heat pump drying. Food Bioprod. Process. 2010, 88, 105–114. [Google Scholar] [CrossRef]
- Dziki, D. Recent trends in pretreatment of food before freeze-drying. Processes 2020, 8, 1661. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, Á.A.; Figiel, A. Comparison of traditional and novel drying techniques and its effect on quality of fruits, vegetables and aromatic herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef]
- Biernacka, B.; Dziki, D.; Rudy, S.; Krzykowski, A.; Polak, R.; Dziki, L. Influence of pretreatments and freeze-drying conditions of strawberries on drying kinetics and physicochemical properties. Processes 2022, 10, 1588. [Google Scholar] [CrossRef]
- Rudy, S.; Dziki, D.; Krzykowski, A.; Gawlik-Dziki, U.; Polak, R.; Różyło, R.; Kulig, R. Influence of pre-treatments and freeze-drying temperature on the process kinetics and selected physico-chemical properties of cranberries (Vaccinium macrocarpon Ait.). LWT-Food Sci. Technol. 2015, 63, 497–503. [Google Scholar] [CrossRef]
- Karabulut, I.; Topcu, A.; Duran, A.; Turan, S.; Ozturb, B. Effect of hot air drying and sun drying on color values and β-carotene content of apricot (Prunus armenica L.). LWT-Food Sci. Technol. 2007, 40, 753–758. [Google Scholar] [CrossRef]
- Nowosad, K.; Sujka, M.; Zielińska, E.; Pankiewicz, U. Accumulation of vitamin c in yeast under pulsed electric field (PEF) conditions. Appl. Sci. 2022, 12, 10206. [Google Scholar] [CrossRef]
- McCall, S.J.; Clark, A.B.; Luben, R.N.; Wareham, N.J.; Khaw, K.T.; Myint, P.K. Plasma vitamin C levels: Risk factors for deficiency and association with self-reported functional health in the European Prospective Investigation into Cancer-Norfolk. Nutrients 2019, 11, 1552. [Google Scholar] [CrossRef] [Green Version]
- Young, J.I.; Züchner, S.; Wang, G. Regulation of the epigenome by vitamin C. Annu. Rev. Nutr. 2015, 35, 545–564. [Google Scholar] [CrossRef] [Green Version]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Akar, G.; Mazi, I.B. Color change, ascorbic acid degradation kinetics, and rehydration behavior of kiwifruit as affected by different drying methods. J. Food Process Eng. 2019, 42, e13011. [Google Scholar] [CrossRef]
- Sernikli, C.; Kadakal, C. Thermal degradation kinetics of ascorbic acid and some b-complex vitamins in black mulberry (Morus nigra) juice. Celal Bayar Univ. J. Sci. 2020, 16, 379–386. [Google Scholar] [CrossRef]
- Butkhup, L.; Samappito, W.; Samappito, S. Phenolic composition and antioxidant activity of white mulberry (Morus alba L.) fruits. Int. J. Food Sci. Technol. 2013, 48, 934–940. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Djomech, Z.E.; Askari, G. Encapsulation and delivery of bioactive compounds using spray and freeze-drying techniques: A review. Dry. Technol. 2020, 38, 235–258. [Google Scholar] [CrossRef]
- Song, X.D.; Mujumdar, A.S.; Law, C.L.; Fang, X.M.; Peng, W.J.; Deng, L.Z.; Wang, J.; Xiao, H.W. Effect of drying air temperature on drying kinetics, color, carotenoid content, antioxidant capacity and oxidation of fat for lotus pollen. Dry. Technol. 2020, 38, 1151–1164. [Google Scholar] [CrossRef]
| Model Number | Model Name | Model Equation | References |
|---|---|---|---|
| 1 | Newton | [25] | |
| 2 | Page | [26] | |
| 3 | Henderson and Pabis | [27] | |
| 4 | Logarithmic | [28] | |
| 5 | Wang and Singh | [29] | |
| 6 | Midilli | [30] | |
| 7 | Logistic | [31] |
| Model Name | Sample | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 °C | 50 °C | 70 °C | |||||||
| R2 | RMSE | χ2 | R2 | RMSE | χ2 | R2 | RMSE | χ2 | |
| Newton | 0.991 | 0.006 | 3.503 × 10−5 | 0.991 | 0.006 | 3.508 × 10−5 | 0.987 | 0.007 | 5.094 × 10−5 |
| Page | 0.998 | 0.001 | 2.154 × 10−6 | 0.998 | 0.001 | 1.000 × 10−6 | 0.998 | 0.001 | 1.787 × 10−6 |
| Henderson and Pabis | 0.991 | 0.007 | 5.126 × 10−5 | 0.993 | 0.004 | 1.828 × 10−5 | 0.990 | 0.005 | 2.878 × 10−5 |
| Logarithmic | 0.998 | 0.001 | 2.298 × 10−6 | 0.999 | 0.000 | 1.536 × 10−7 | 1.000 | 0.000 | 7.188 × 10−8 |
| Wang and Singh | 0.997 | 0.002 | 3.677 × 10−6 | 0.996 | 0.003 | 7.326 × 10−6 | 0.998 | 0.001 | 1.450 × 10−6 |
| Logistic | 0.999 | 0.001 | 3.145 × 10−7 | 0.999 | 0.001 | 2.738 × 10−7 | 0.999 | 0.001 | 5.319 × 10−7 |
| Midilli | 0.999 | 0.000 | 2.185 × 10−7 | 1.000 | 0.000 | 1.890 × 10−8 | 1.000 | 0.000 | 7.350 × 10−9 |
| Model Name | Sample | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 40 °C | 50 °C | 70 °C | |||||||
| R2 | RMSE | χ2 | R2 | RMSE | χ2 | R2 | RMSE | χ2 | |
| Newton | 0.989 | 0.007 | 5.347 × 10−5 | 0.993 | 0.004 | 1.744 × 10−5 | 0.987 | 0.007 | 1.435 × 10−4 |
| Page | 0.990 | 0.007 | 4.631 × 10−5 | 0.999 | 0.001 | 2.911 × 10−7 | 0.998 | 0.001 | 3.471 × 10−7 |
| Henderson and Pabis | 0.989 | 0.007 | 5.380 × 10−5 | 0.995 | 0.007 | 5.381 × 10−5 | 0.990 | 0.005 | 4.679 × 10−5 |
| Logarithmic | 0.998 | 0.001 | 2.145 × 10−6 | 0.906 | 0.054 | 3.055 × 10−3 | 1.000 | 0.000 | 8.919 × 10−7 |
| Wang and Singh | 0.983 | 0.012 | 1.473 × 10−4 | 0.983 | 0.002 | 5.920 × 10−6 | 0.998 | 0.001 | 1.694 × 10−7 |
| Logistic | 0.993 | 0.005 | 2.337 × 10−5 | 0.999 | 0.000 | 1.015 × 10−7 | 0.999 | 0.001 | 1.679 × 10−7 |
| Midilli | 1.000 | 0.000 | 8.305 × 10−8 | 1.000 | 0.000 | 5.109 × 10−10 | 1.000 | 0.000 | 1.586 × 10−8 |
| Temperature | Equation | Coefficient | |||
|---|---|---|---|---|---|
| a | k (min−1) | n | b | ||
| 30 °C | Newton | 0.001970 | |||
| Page | 0.000511 | 1.210111 | |||
| Henderson and Pabis | 0.000511 | 1.210111 | |||
| Logarithmic | 1.111092 | 0.003301 | −0.1964 | ||
| Wang and Singh | −0.001426 | 0.000001 | |||
| Logistic | 1.660363 | 0.700757 | 0.003019 | ||
| Midilli | 0.973077 | 0.000475 | 1.210584 | −0.000015 | |
| 50 °C | Newton | 0.005995 | |||
| Page | 0.000991 | 1.169959 | |||
| Henderson and Pabis | 1.050253 | 0.002918 | |||
| Logarithmic | 1.098745 | 0.001668 | −0.088077 | ||
| Wang and Singh | −0.002023 | 0.000001 | |||
| Logistic | 1.924220 | 0.956935 | 0.003966 | ||
| Midilli | 1.001843 | 0.001910 | 1.206081 | −0.000080 | |
| 70 °C | Newton | 0.008463 | |||
| Page | 0.001087 | 1.198783 | |||
| Henderson and Pabis | 1.053648 | 0.003647 | |||
| Logarithmic | 1.136264 | 0.002737 | −0.124730 | ||
| Wang and Singh | −0.002552 | 0.000002 | |||
| Logistic | 1.736335 | 0.771779 | 0.005216 | ||
| Midilli | 0.988800 | 0.001573 | 1.117076 | −0.000068 | |
| Temperature | Equation | Coefficient | |||
|---|---|---|---|---|---|
| a | k (min−1) | n | b | ||
| 40 °C | Newton | 0.000959 | |||
| Page | 0.000691 | 1.046169 | |||
| Henderson and Pabis | 0.995797 | 0.000955 | |||
| Logarithmic | 1.094992 | 0.000685 | −0.144593 | ||
| Wang and Singh | −0.000716 | 0.000001 | |||
| Logistic | 2.211470 | 1.342309 | 0.009708 | ||
| Midilli | 1.005795 | 0.002944 | 0.804393 | −0.000063 | |
| 50 °C | Newton | 0.001956 | |||
| Page | 0.000762 | 1.147366 | |||
| Henderson and Pabis | 1.045217 | 0.002044 | |||
| Logarithmic | 1.0283351 | 0.002643 | −0.026413 | ||
| Wang and Singh | −0.001459 | 0.000001 | |||
| Logistic | 2.210854 | 1.230381 | 0.002661 | ||
| Midilli | 0.994949 | 0.001019 | 1.091671 | −0.000024 | |
| 70 °C | Newton | 0.005995 | |||
| Page | 0.001388 | 1.278262 | |||
| Henderson and Pabis | 1.087555 | 0.006512 | |||
| Logarithmic | 1.179409 | 0.004836 | −0.136079 | ||
| Wang and Singh | −0.004461 | 0.000005 | |||
| Logistic | 1.653568 | 0.648026 | 0.009708 | ||
| Midilli | 1.001843 | 0.001910 | 1.206082 | −0.000080 | |
| MD * | DT (°C) | Parameter | |||
|---|---|---|---|---|---|
| Lightness | Redness | Yellowness | ΔE | ||
| FD | 30 | 62.67 ± 0.17 d** | 12.82 ± 0.03 b | 34.84 ± 0.13 d | 44.53 ± 0.20 d |
| 50 | 63.19 ± 0.67 d | 14.19 ± 0.04 c | 34.43 ± 0.12 d | 45.54 ± 0.59 e | |
| 70 | 75.24 ± 0.62 e | 6.02 ± 0.11 a | 27.32 ± 0.43 b | 51.21 ± 0.45 f | |
| AD | 40 | 39.55 ± 0.52 a | 15.22 ± 0.28 d | 26.99 ± 0.35 b | 26.89 ± 0.55 b |
| 50 | 56.67 ± 0.26 c | 15.34 ± 0.11 d | 30.51 ± 0.11 c | 38.59 ± 0.27 c | |
| 70 | 42.73 ± 1.02 b | 14.98 ± 0.30 d | 24.59 ± 0.54 a | 23.82 ± 0.75 a | |
| MD * | DT (°C) | TP (mg GAE/g DM) | EC50DPPH (mg DM/mL) | EC50ABTS (mg DM/mL) |
|---|---|---|---|---|
| FD | 30 | 140.4 ± 2.6 d** | 6.02 ± 0.11 a | 24.59 ± 0.54 a |
| 50 | 118.1 ± 3.7 c | 12.82 ± 0.03 b | 26.99 ± 0.35 b | |
| 70 | 93.6 ± 5.2 b | 14.19 ± 0.04 c | 32.51 ± 0.11 c | |
| AD | 40 | 122.6 ± 2.6 c | 15.22 ± 0.28 d | 27.32 ± 0.43 b |
| 50 | 98.1 ± 3.8 b | 15.34 ± 0.11 d | 34.84 ± 0.13 d | |
| 70 | 88.5 ± 4.6 a | 17.65 ± 0.30 e | 34.43 ± 0.12 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzykowski, A.; Dziki, D.; Rudy, S.; Polak, R.; Biernacka, B.; Gawlik-Dziki, U.; Janiszewska-Turak, E. Effect of Air-Drying and Freeze-Drying Temperature on the Process Kinetics and Physicochemical Characteristics of White Mulberry Fruits (Morus alba L.). Processes 2023, 11, 750. https://doi.org/10.3390/pr11030750
Krzykowski A, Dziki D, Rudy S, Polak R, Biernacka B, Gawlik-Dziki U, Janiszewska-Turak E. Effect of Air-Drying and Freeze-Drying Temperature on the Process Kinetics and Physicochemical Characteristics of White Mulberry Fruits (Morus alba L.). Processes. 2023; 11(3):750. https://doi.org/10.3390/pr11030750
Chicago/Turabian StyleKrzykowski, Andrzej, Dariusz Dziki, Stanisław Rudy, Renata Polak, Beata Biernacka, Urszula Gawlik-Dziki, and Emilia Janiszewska-Turak. 2023. "Effect of Air-Drying and Freeze-Drying Temperature on the Process Kinetics and Physicochemical Characteristics of White Mulberry Fruits (Morus alba L.)" Processes 11, no. 3: 750. https://doi.org/10.3390/pr11030750
APA StyleKrzykowski, A., Dziki, D., Rudy, S., Polak, R., Biernacka, B., Gawlik-Dziki, U., & Janiszewska-Turak, E. (2023). Effect of Air-Drying and Freeze-Drying Temperature on the Process Kinetics and Physicochemical Characteristics of White Mulberry Fruits (Morus alba L.). Processes, 11(3), 750. https://doi.org/10.3390/pr11030750











