Effect of Addition of Zero-Valent Iron (Fe) and Magnetite (Fe3O4) on Methane Yield and Microbial Consortium in Anaerobic Digestion of Food Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methane Production Potential
2.3. Analysis of Microbial Consortium
2.3.1. DNA Extraction and Quantification
2.3.2. Library Construction and Sequencing
2.4. Chemical Analysis
2.5. Statistical Analysis
3. Results and Discussion
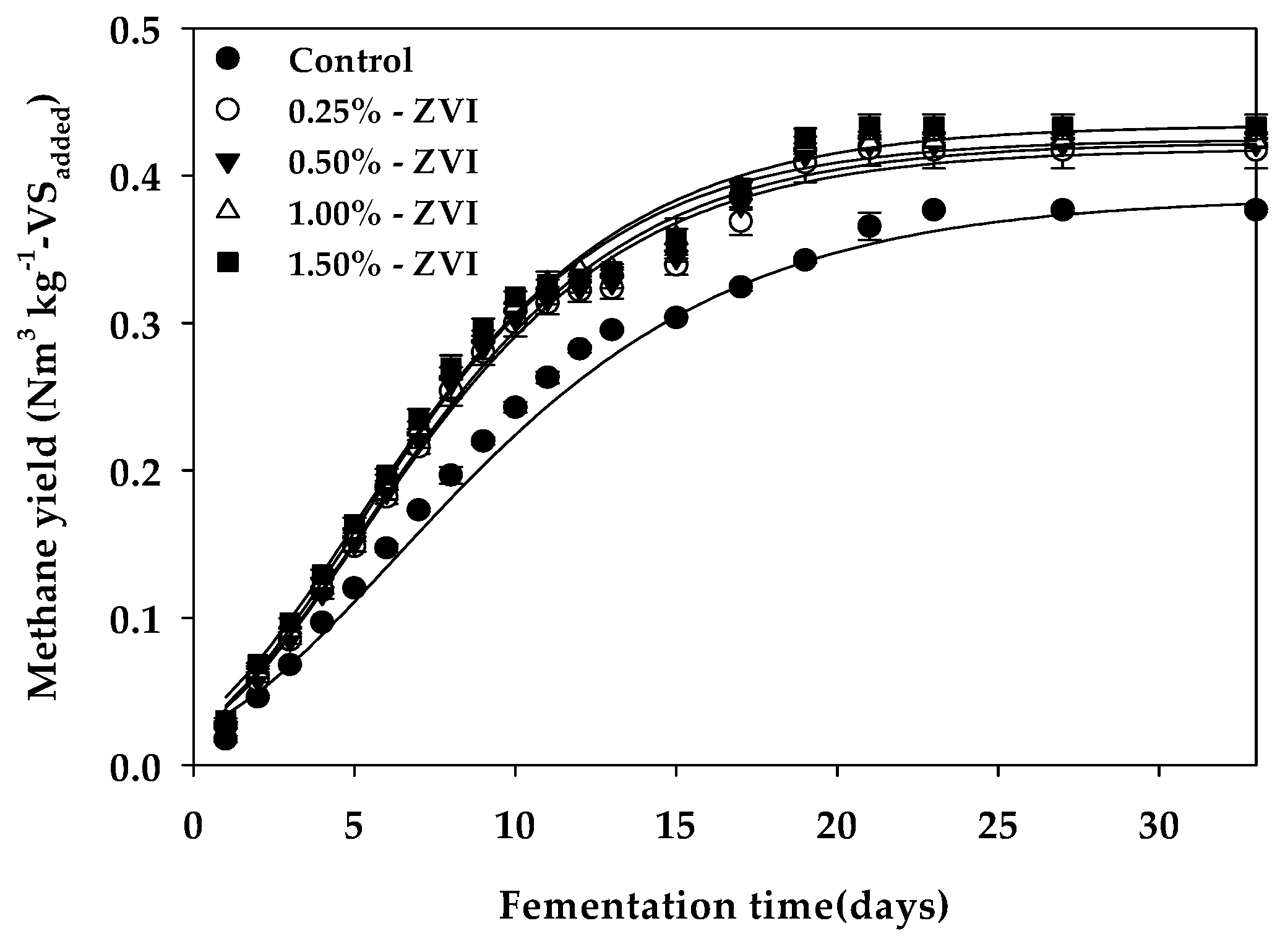
3.1. Methane Yield and Reaction Kinetics
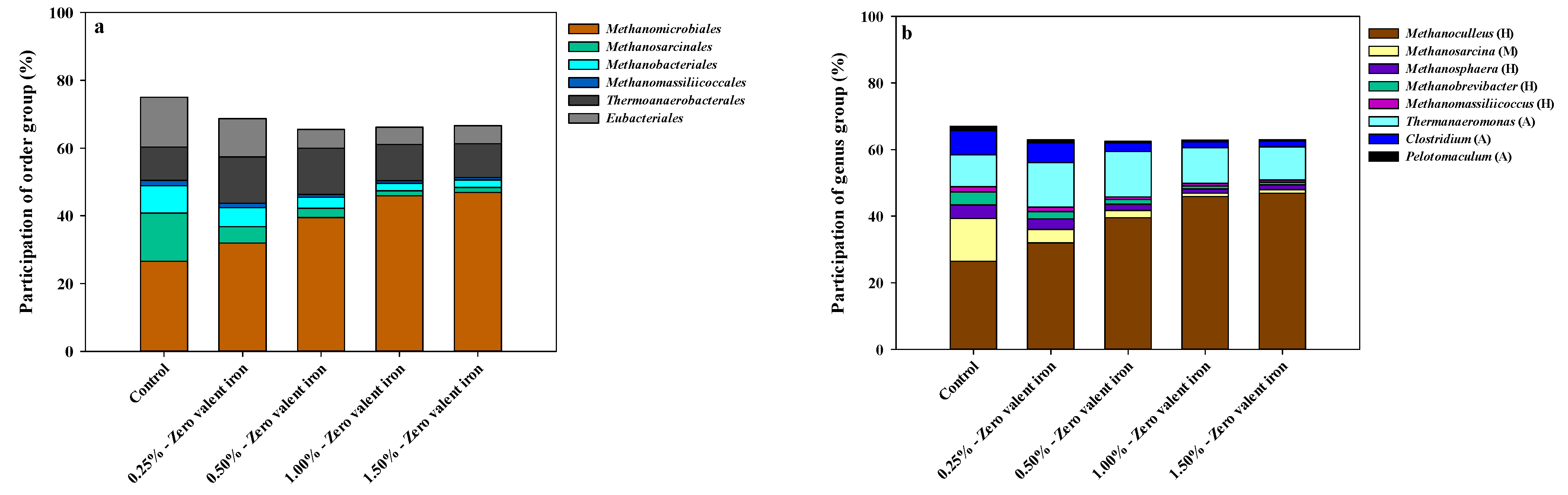
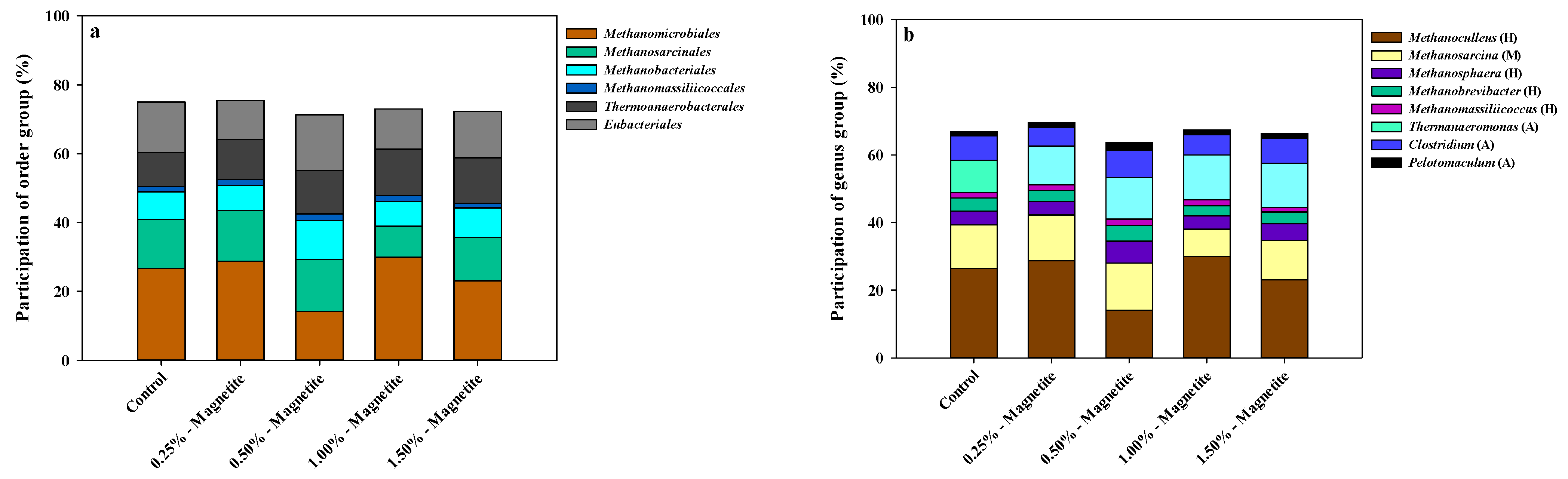
3.2. Microbial Community
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ME [Ministry of Environment]. National Waste Generation and Treatment Status [2020]; Ministry of Environment: Sejong, Republic of Korea, 2021.
- KEITI [Korea Environmental Industry & Technology Institute]. Trends in Land Treatment of Food Wastewater; Korea Environmental Industry & Technology Institute: Seoul, Republic of Korea, 2016.
- Akindele, A.A.; Sartaj, M. The toxicity effects of ammonia on anaerobic digestion of organic fraction of municipal solid waste. Waste Manag. 2018, 71, 757–766. [Google Scholar] [CrossRef]
- Li, L.; Xu, Y.; Dai, X.; Dai, L. Principles and advancements in improving anaerobic digestion of organic waste via direct interspecies electron transfer. Renew. Sustain. Energy Rev. 2021, 148, 111367. [Google Scholar] [CrossRef]
- Müller, N.; Worm, P.; Schink, B.; Stams, A.J.; Plugge, C.M. Syntrophic butyrate and propionate oxidation processes: From genomes to reaction mechanisms. Environ. Microbiol. Rep. 2010, 2, 489–499. [Google Scholar] [CrossRef]
- Morita, M.; Malvankar, N.S.; Franks, A.E.; Summers, Z.M.; Giloteaux, L.; Rotaru, A.E.; Rotaru, C.; Lovley, D.R. Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. MBio 2011, 2, e00111–e00159. [Google Scholar] [CrossRef]
- Rotaru, A.-E.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef] [PubMed]
- Lauderdale, J.M.; Braakman, R.; Forget, G.; Dutkiewicz, S.; Follows, M.J. Microbial feedbacks optimize ocean iron availability. Proc. Natl. Acad. Sci. USA 2020, 117, 4842–4849. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Lee, C. A review of the effects of iron compounds on methanogenesis in anaerobic environments. Renew. Sustain. Energy Rev. 2019, 113, 109282. [Google Scholar] [CrossRef]
- Seedorf, H.; Hagemeier, C.H.; Shima, S.; Thauer, R.K.; Warkentin, E.; Ermler, U. Structure of coenzyme F420H2 oxidase (FprA), a di-iron flavoprotein from methanogenic Archaea catalyzing the reduction of O2 to H2O. FEBS J. 2007, 274, 1588–1599. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, C.A.; Morgado, L.; Silva, M.A.; Ferreira, M.R.; Fernandes, T.M.; Portela, P.C. From iron to bacterial electroconductive filaments: Exploring cytochrome diversity using Geobacter bacteria. Coord. Chem. Rev. 2022, 452, 214284. [Google Scholar] [CrossRef]
- Charalambous, P.; Vyrides, I. In situ biogas upgrading and enhancement of anaerobic digestion of cheese whey by addition of scrap or powder zero-valent iron (ZVI). J. Environ. Manag. 2021, 280, 111651. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, F.; Huang, W.; Huang, W.; Li, F.; Lei, Z.; Zhang, Z. Enhanced anaerobic digestion of ammonia-rich swine manure by zero-valent iron: With special focus on the enhancement effect on hydrogenotrophic methanogenesis activity. Bioresour. Technol. 2018, 270, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-Y.; He, Z.-W.; Ren, Y.-X.; Tang, C.-C.; Zhou, A.-J.; Liu, W.; Liang, B.; Li, Z.-H.; Wang, A. Current advances and challenges for direct interspecies electron transfer in anaerobic digestion of waste activated sludge. Chem. Eng. J. 2022, 450, 137973. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and potential of direct interspecies electron transfer in anaerobic digestion. Energies 2018, 11, 107. [Google Scholar] [CrossRef]
- Kato, S.; Hashimoto, K.; Watanabe, K. Methanogenesis facilitated by electric syntrophy via (semi) conductive iron-oxide minerals. Environ. Microbiol. 2012, 14, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wang, J.; Xue, J.; Liu, S.-Y.; Peng, S.-C.; Ma, D.; Chen, T.-H.; Yue, Z. Methane production and microbial community analysis in the goethite facilitated anaerobic reactors using algal biomass. Fuel 2015, 145, 196–201. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Dai, L.; Dong, B.; Dai, X. Magnetite triggering enhanced direct interspecies electron transfer: A scavenger for the blockage of electron transfer in anaerobic digestion of high-solids sewage sludge. Environ. Sci. Technol. 2018, 52, 7160–7169. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Woodard, T.; Nevin, K.; Lovley, D. Enhancing syntrophic metabolism in up-flow anaerobic sludge blanket reactors with conductive carbon materials. Bioresour. Technol. 2015, 191, 140–145. [Google Scholar] [CrossRef]
- Zhuang, L.; Tang, J.; Wang, Y.; Hu, M.; Zhou, S. Conductive iron oxide minerals accelerate syntrophic cooperation in methanogenic benzoate degradation. J. Hazard. Mater. 2015, 293, 37–45. [Google Scholar] [CrossRef]
- Aguilar-Moreno, G.S.; Navarro-Cerón, E.; Velázquez-Hernández, A.; Hernández-Eugenio, G.; Aguilar-Méndez, M.Á.; Espinosa-Solares, T. Enhancing methane yield of chicken litter in anaerobic digestion using magnetite nanoparticles. Renew. Energy 2020, 147, 204–213. [Google Scholar] [CrossRef]
- Altamirano-Corona, M.F.; Anaya-Reza, O.; Durán-Moreno, A. Biostimulation of food waste anaerobic digestion supplemented with granular activated carbon, biochar and magnetite: A comparative analysis. Biomass Bioenergy 2021, 149, 106105. [Google Scholar] [CrossRef]
- Li, D.; Song, L.; Fang, H.; Li, P.; Teng, Y.; Li, Y.-Y.; Liu, R.; Niu, Q. Accelerated bio-methane production rate in thermophilic digestion of cardboard with appropriate biochar: Dose-response kinetic assays, hybrid synergistic mechanism, and microbial networks analysis. Bioresour. Technol. 2019, 290, 121782. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhang, L.; Wijaya, S.M.; Zhou, Y. Unveiling the role of activated carbon on hydrolysis process in anaerobic digestion. Bioresour. Technol. 2020, 296, 122366. [Google Scholar] [CrossRef] [PubMed]
- Lizama, A.C.; Figueiras, C.C.; Pedreguera, A.Z.; Espinoza, J.E.R. Enhancing the performance and stability of the anaerobic digestion of sewage sludge by zero valent iron nanoparticles dosage. Bioresour. Technol. 2019, 275, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Barrena, R.; del Carmen Vargas-García, M.; Capell, G.; Barańska, M.; Puntes, V.; Moral-Vico, J.; Sánchez, A.; Font, X. Sustained effect of zero-valent iron nanoparticles under semi-continuous anaerobic digestion of sewage sludge: Evolution of nanoparticles and microbial community dynamics. Sci. Total Environ. 2021, 777, 145969. [Google Scholar] [CrossRef] [PubMed]
- Cerrillo, M.; Burgos, L.; Ruiz, B.; Barrena, R.; Moral-Vico, J.; Font, X.; Sanchez, A.; Bonmati, A. In-situ methane enrichment in continuous anaerobic digestion of pig slurry by zero-valent iron nanoparticles addition under mesophilic and thermophilic conditions. Renew. Energy 2021, 180, 372–382. [Google Scholar] [CrossRef]
- ME [Ministry of Environment]. Techical Guidelines for Biogasification Facilities of Food Waste; Ministry of Environment: Sejong, Republic of Korea, 2017.
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Yoon, Y.-M. Energy recovery efficiency of poultry slaughterhouse sludge cake by hydrothermal carbonization. Energies 2017, 10, 1876. [Google Scholar] [CrossRef]
- Lay, J.-J.; Li, Y.-Y.; Noike, T. Mathematical model for methane production from landfill bioreactor. J. Environ. Eng. 1998, 124, 730–736. [Google Scholar] [CrossRef]
- Luna-deRisco, M.; Normak, A.; Orupõld, K. Biochemical methane potential of different organic wastes and energy crops from Estonia. Agron. Res. 2011, 9, 331–342. [Google Scholar]
- Sørensen, A.H.; Winther-Nielsen, M.; Ahring, B.K. Kinetics of lactate, acetate and propionate in unadapted and lactate-adapted thermophilic, anaerobic sewage sludge: The influence of sludge adaptation for start-up of thermophilic UASB-reactors. Appl. Microbiol. Biotechnol. 1991, 34, 823–827. [Google Scholar] [CrossRef]
- Rice, E.; Baird, R.; Eaton, A.; Clesceri, L. APHA (American Public Health Association): Standard Method for the Examination of Water and Wastewater; AWWA (American Water Works Association) and WEF (Water Environment Federation): Washington, DC, USA, 2012. [Google Scholar]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Yuan, T.; Bian, S.; Ko, J.H.; Liu, J.; Shi, X.; Xu, Q. Exploring the roles of zero-valent iron in two-stage food waste anaerobic digestion. Waste Manag. 2020, 107, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, Y.; Li, Q.; Quan, X. Adding Fe0 powder to enhance the anaerobic conversion of propionate to acetate. Biochem. Eng. J. 2013, 73, 80–85. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Fang, H. Comparison of enhancement of anaerobic digestion of waste activated sludge through adding nano-zero valent iron and zero valent iron. RSC Adv. 2018, 8, 27181–27190. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Lu, T.; Liu, J.; Wang, Y.; Shen, P.; Wei, Y. Response and mechanisms of the performance and fate of antibiotic resistance genes to nano-magnetite during anaerobic digestion of swine manure. J. Hazard. Mater. 2019, 366, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Lu, J.; Ye, J.; Zhou, Y. The effects and mechanisms of zero-valent iron on anaerobic digestion of solid waste: A mini-review. J. Clean. Prod. 2021, 278, 123567. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.; Hu, Z. Impact of nano zero valent iron (NZVI) on methanogenic activity and population dynamics in anaerobic digestion. Water Res. 2013, 47, 6790–6800. [Google Scholar] [CrossRef]
- Kong, X.; Wei, Y.; Xu, S.; Liu, J.; Li, H.; Liu, Y.; Yu, S. Inhibiting excessive acidification using zero-valent iron in anaerobic digestion of food waste at high organic load rates. Bioresour. Technol. 2016, 211, 65–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Xu, R.; Xiang, Y.; Jia, M.; Hu, J.; Zheng, Y.; Xiong, W.; Cao, J. Enhanced mesophilic anaerobic digestion of waste sludge with the iron nanoparticles addition and kinetic analysis. Sci. Total Environ. 2019, 683, 124–133. [Google Scholar] [CrossRef]
- Jing, Y.; Wan, J.; Angelidaki, I.; Zhang, S.; Luo, G. iTRAQ quantitative proteomic analysis reveals the pathways for methanation of propionate facilitated by magnetite. Water Res. 2017, 108, 212–221. [Google Scholar] [CrossRef]
- Yin, Q.; Miao, J.; Li, B.; Wu, G. Enhancing electron transfer by ferroferric oxide during the anaerobic treatment of synthetic wastewater with mixed organic carbon. Int. Biodeterior. Biodegrad. 2017, 119, 104–110. [Google Scholar] [CrossRef]
- Akturk, A.S.; Demirer, G.N. Improved food waste stabilization and valorization by anaerobic digestion through supplementation of conductive materials and trace elements. Sustainability 2020, 12, 5222. [Google Scholar] [CrossRef]
- Straub, K.L.; Benz, M.; Schink, B. Iron metabolism in anoxic environments at near neutral pH. FEMS Microbiol. Ecol. 2001, 34, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.-z.; Fang, S.; Zhang, L.; Huang, W.; Shao, Q.; Fang, F.; Feng, Q.; Cao, J.; Luo, J. Distribution patterns of functional microbial community in anaerobic digesters under different operational circumstances: A review. Bioresour. Technol. 2021, 341, 125823. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.; Belay, N.; Rajagopal, B.S.; Weimer, P.J. Bacterial methanogenesis and growth from CO2 with elemental iron as the sole source of electrons. Science 1987, 237, 509–511. [Google Scholar] [CrossRef]
- Dinh, H.T.; Kuever, J.; Mußmann, M.; Hassel, A.W.; Stratmann, M.; Widdel, F. Iron corrosion by novel anaerobic microorganisms. Nature 2004, 427, 829–832. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, H.; Cao, H.; Zhang, Y.; Sheng, Y.; Li, T.; Zhou, S.; Li, H. New insights of enhanced anaerobic degradation of refractory pollutants in coking wastewater: Role of zero-valent iron in metagenomic functions. Bioresour. Technol. 2020, 300, 122667. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, F.; Huang, W.; Lei, Z.; Zhang, Z.; Huang, W. Combined effect of zero valent iron and magnetite on semi-dry anaerobic digestion of swine manure. Bioresour. Technol. 2022, 346, 126438. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Yu, Q.; Zhang, Y. Ferroferric oxide triggered possible direct interspecies electron transfer between Syntrophomonas and Methanosaeta to enhance waste activated sludge anaerobic digestion. Bioresour. Technol. 2018, 250, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.Y.; Tian, H.; Chen, Y.; Ni, K.; Zhang, J.; Tong, Y.W. Methanogenic pathway and microbial succession during start-up and stabilization of thermophilic food waste anaerobic digestion with biochar. Bioresour. Technol. 2020, 314, 123751. [Google Scholar] [CrossRef]
- Jang, H.M.; Kim, J.H.; Ha, J.H.; Park, J.M. Bacterial and methanogenic archaeal communities during the single-stage anaerobic digestion of high-strength food wastewater. Bioresour. Technol. 2014, 165, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, E.; Han, G.; Tongco, J.V.; Shin, S.G.; Hwang, S. Microbial communities underpinning mesophilic anaerobic digesters treating food wastewater or sewage sludge: A full-scale study. Bioresour. Technol. 2018, 259, 388–397. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Food Wastewater | |
|---|---|---|
| pH (-) | 3.53 | |
| TS 1 (mg/L) | 102,422 | |
| VS 2 (mg/L) | 89,756 | |
| TKN 3 (mg/L) | 3870 | |
| NH4+-N 4 (mg/L) | 381 | |
| CODCr 5 (mg/L) | 166,333 | |
| SCODCr 6 (mg/L) | 5474 | |
| TVFAs 7 (mg/L as acetate) | 7657 | |
| Elemental composition (wt.%, d.b. 8) | C | 46.70 |
| H | 6.53 | |
| O | 3.27 | |
| N | 34.60 | |
| S | 0.00 | |
| Parameters | pH (-) | TS 1 | VS 2 | TKN 3 | NH4+-N 4 | CODCr 5 | SCODCr 6 | Alkalinity (mg L−1 as CaCO3) | TVFAs 7 (mg L−1 as Acetate) |
|---|---|---|---|---|---|---|---|---|---|
| (mg L−1) | |||||||||
| Inoculum | 8.36 | 20,767 | 9511 | 3467 | 2746 | 6017 | 4930 | 12,975 | 41 |
| Parameters | Control | Zero-Valent Iron Concentration (%) | |||
|---|---|---|---|---|---|
| 0.25 | 0.50 | 1.00 | 1.50 | ||
| Bu 1 (Nm3 kg−1-VSadded) | 0.380 c | 0.418 b | 0.423 ab | 0.425 ab | 0.434 a |
| Rm 2 (mL day−1) | 15.73 c | 19.09 b | 19.53 ab | 20.32 a | 19.63 ab |
| λ 3 (days) | 0.541 a | 0.352 b | 0.395 b | 0.364 b | 0.065 c |
| Bth 4 (Nm3 kg−1-VSadded) | 0.437 | 0.437 | 0.437 | 0.437 | 0.437 |
| VSr 5 (%) | 86.95 | 95.72 | 96.73 | 97.28 | 99.45 |
| Parameters | Control | Magnetite Concentration (%) | |||
|---|---|---|---|---|---|
| 0.25 | 0.50 | 1.00 | 1.50 | ||
| Bu 1 (Nm3 kg−1-VSadded) | 0.380 c | 0.411 b | 0.412 b | 0.431 a | 0.419 b |
| Rm 2 (mL day−1) | 15.73 b | 15.94 b | 16.48 b | 18.44 a | 18.26 a |
| λ 3 (days) | 0.541 ab | 0.469 b | 0.600 ab | 0.673 a | 0.650 a |
| Bth 4 (Nm3 kg−1-VSadded) | 0.437 | 0.437 | 0.437 | 0.437 | 0.437 |
| VSr 5 (%) | 86.95 | 94.05 | 94.28 | 98.65 | 95.95 |
| Treatments | Solubilized Fe | ||
|---|---|---|---|
| Soluble 1 Forms | Total 2 Forms | ||
| ZVI concentration (%) | 0.25 | 27.0 (0.1) 3 | 530.4 (3.3) |
| 0.50 | 38.0 (0.3) | 690.1 (1.4) | |
| 1.00 | 30.1 (0.0) | 606.3 (1.3) | |
| 1.50 | 34.3 (0.0) | 703.6 (1.1) | |
| Magnetite concentration (%) | 0.25 | 24.9 (0.1) | 175.0 (0.1) |
| 0.50 | 23.5 (0.2) | 182.0 (0.8) | |
| 1.00 | 24.9 (0.1) | 254.6 (0.8) | |
| 1.50 | 25.3 (0.0) | 214.9 (0.9) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Lee, J.-H.; Kim, S.-Y.; Yoon, Y.-M. Effect of Addition of Zero-Valent Iron (Fe) and Magnetite (Fe3O4) on Methane Yield and Microbial Consortium in Anaerobic Digestion of Food Wastewater. Processes 2023, 11, 759. https://doi.org/10.3390/pr11030759
Lee J-H, Lee J-H, Kim S-Y, Yoon Y-M. Effect of Addition of Zero-Valent Iron (Fe) and Magnetite (Fe3O4) on Methane Yield and Microbial Consortium in Anaerobic Digestion of Food Wastewater. Processes. 2023; 11(3):759. https://doi.org/10.3390/pr11030759
Chicago/Turabian StyleLee, Jun-Hyeong, Jae-Hyuk Lee, Sang-Yoon Kim, and Young-Man Yoon. 2023. "Effect of Addition of Zero-Valent Iron (Fe) and Magnetite (Fe3O4) on Methane Yield and Microbial Consortium in Anaerobic Digestion of Food Wastewater" Processes 11, no. 3: 759. https://doi.org/10.3390/pr11030759
APA StyleLee, J.-H., Lee, J.-H., Kim, S.-Y., & Yoon, Y.-M. (2023). Effect of Addition of Zero-Valent Iron (Fe) and Magnetite (Fe3O4) on Methane Yield and Microbial Consortium in Anaerobic Digestion of Food Wastewater. Processes, 11(3), 759. https://doi.org/10.3390/pr11030759






