The Effect of CaO on the CO and NOx Emission Characteristics of Fast-Growing Grass Combustion
Abstract
1. Introduction
2. Materials and Methods
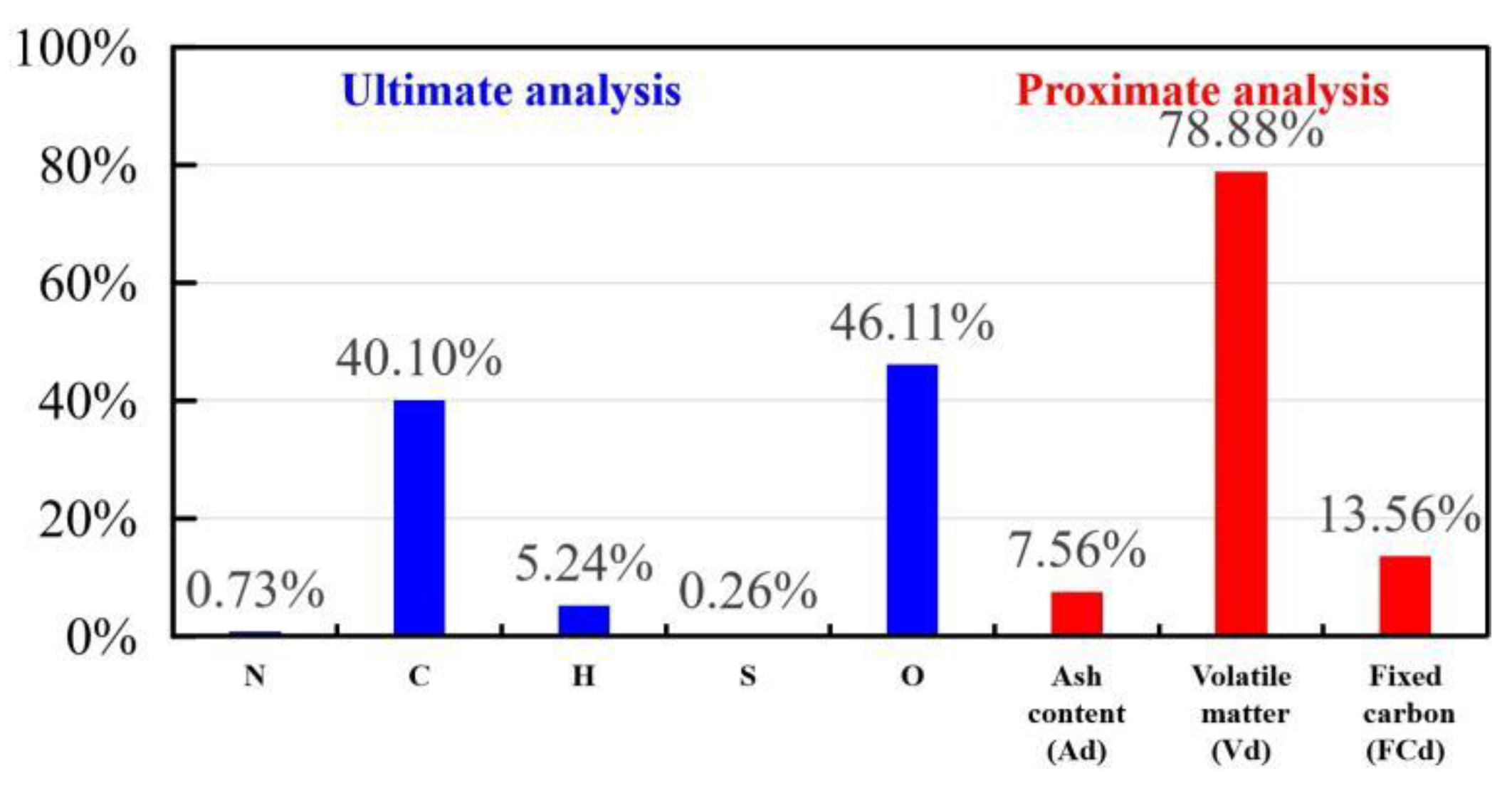
2.1. Materials
2.2. Apparatus and Methods
2.3. Calculation Method
3. Results and Discussion
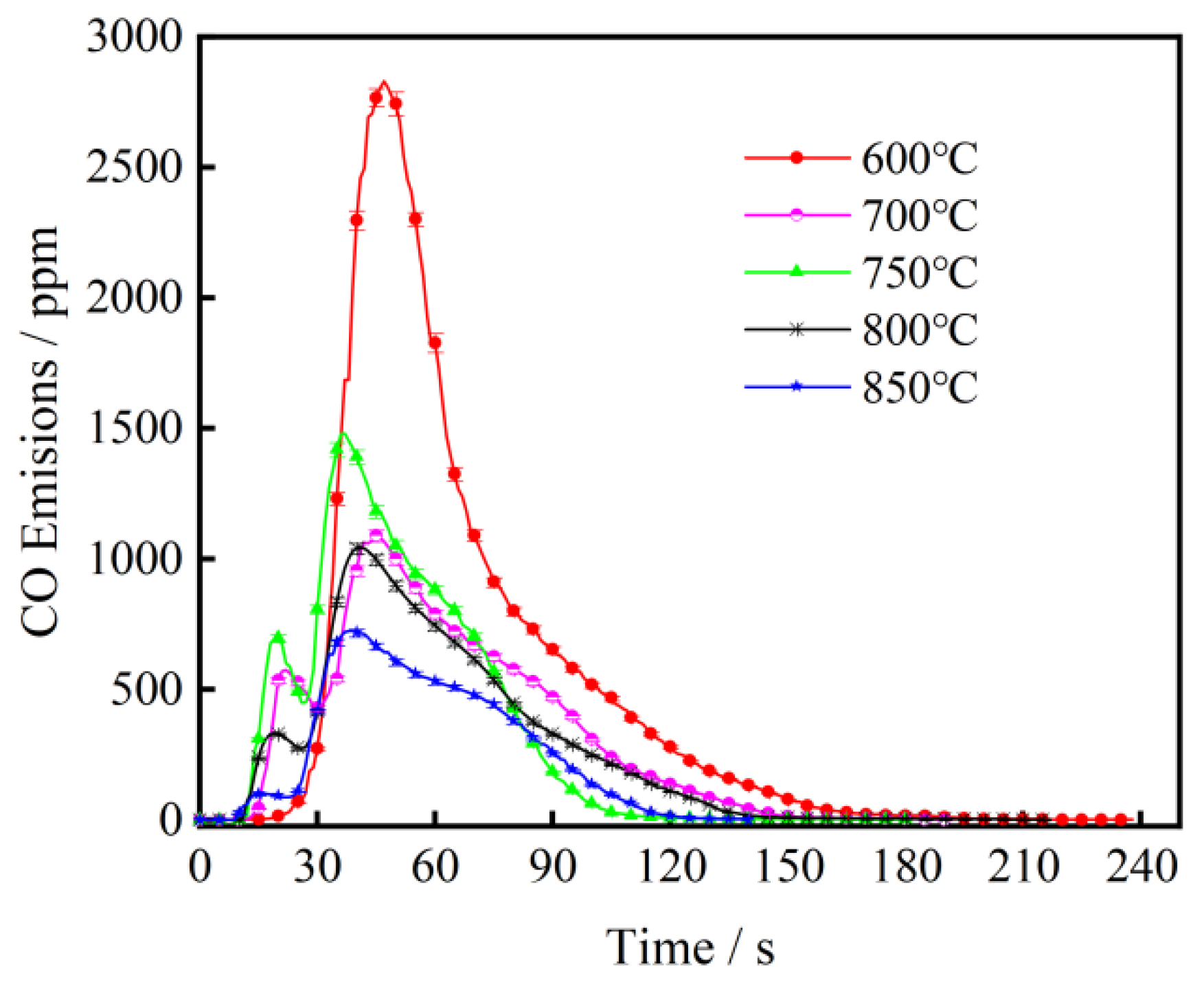
3.1. The Effect of Combustion Temperature on CO Emission from Fast-Growing Grass Combustion
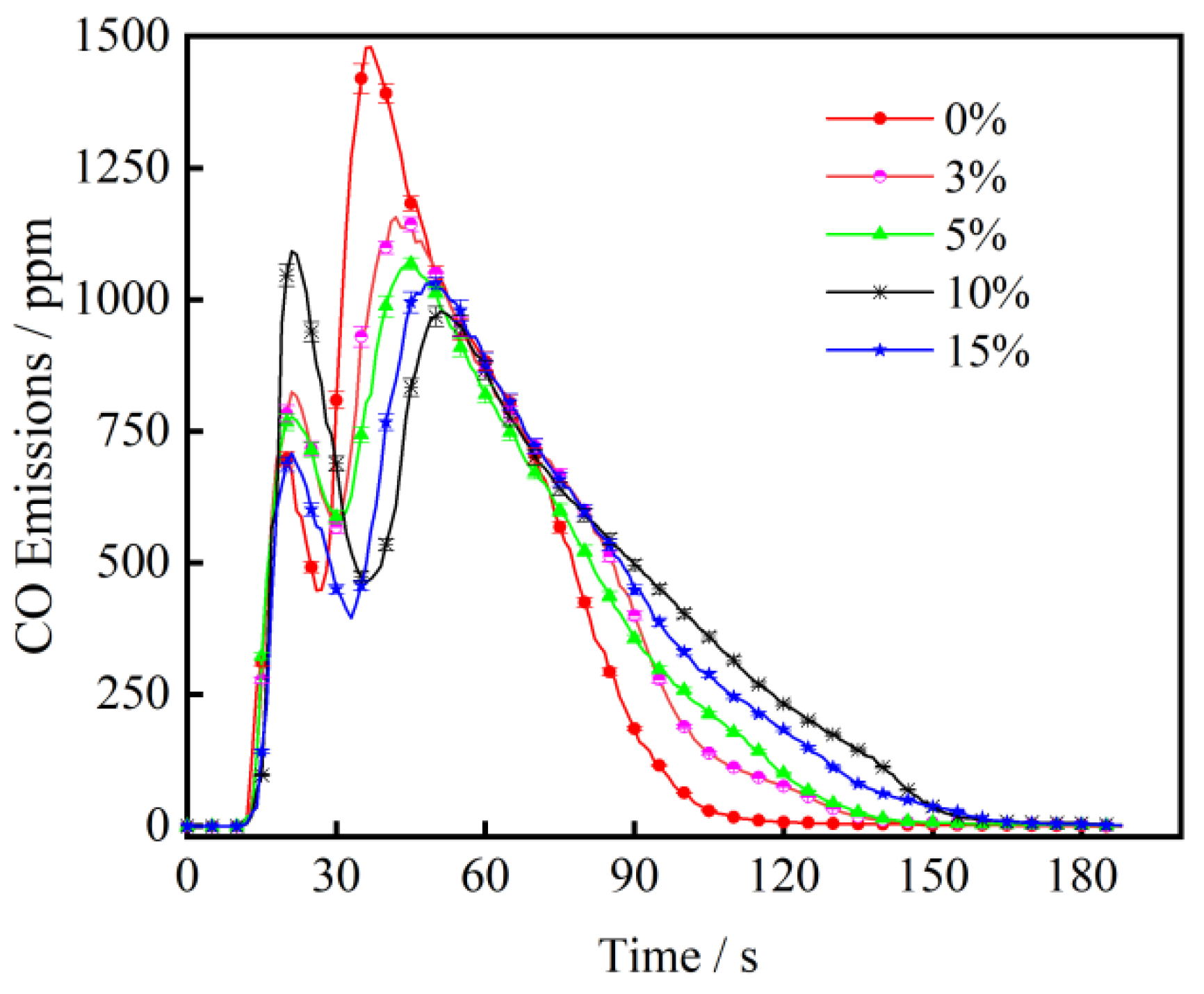
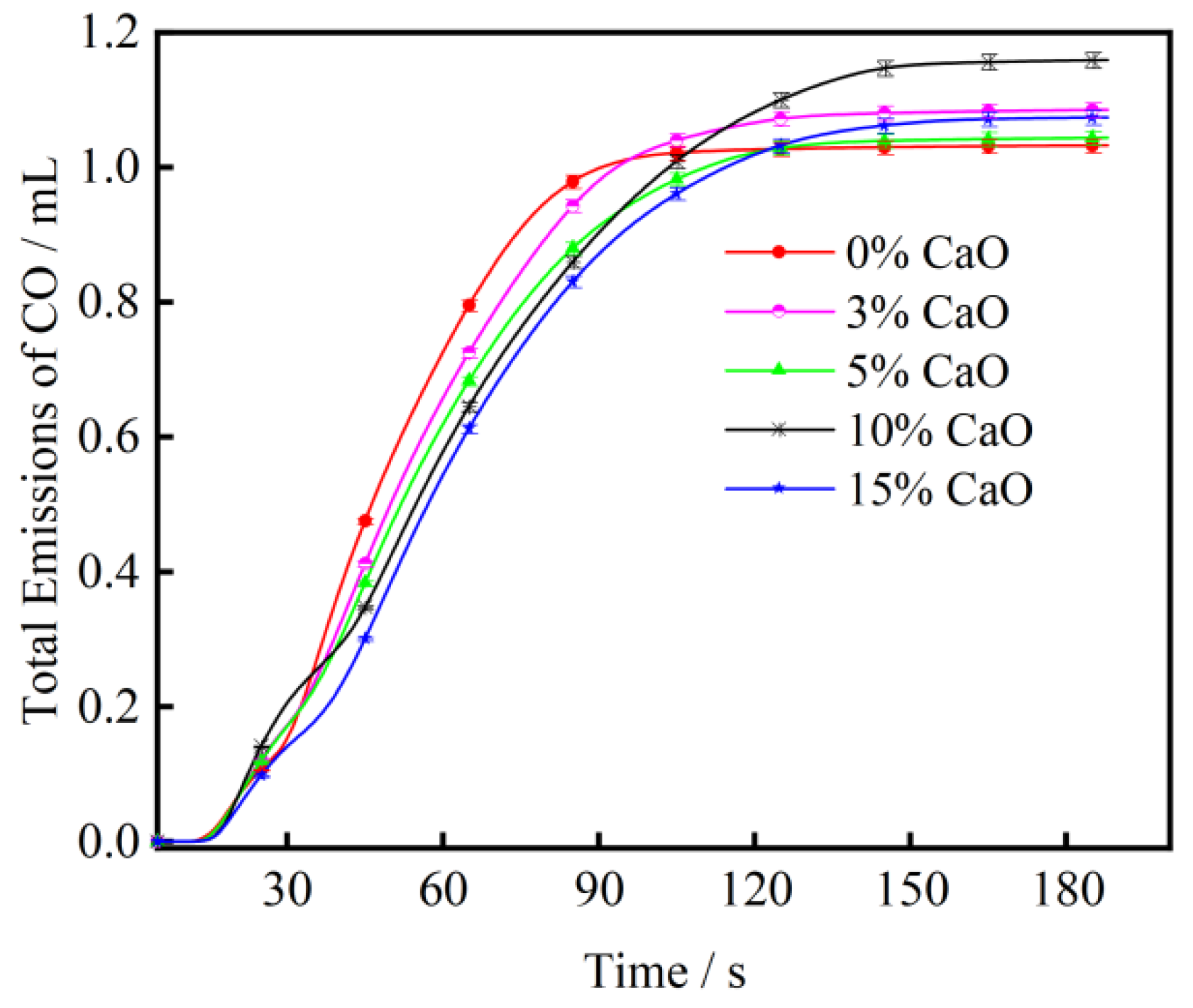
3.2. The Effect of CaO on CO Emission
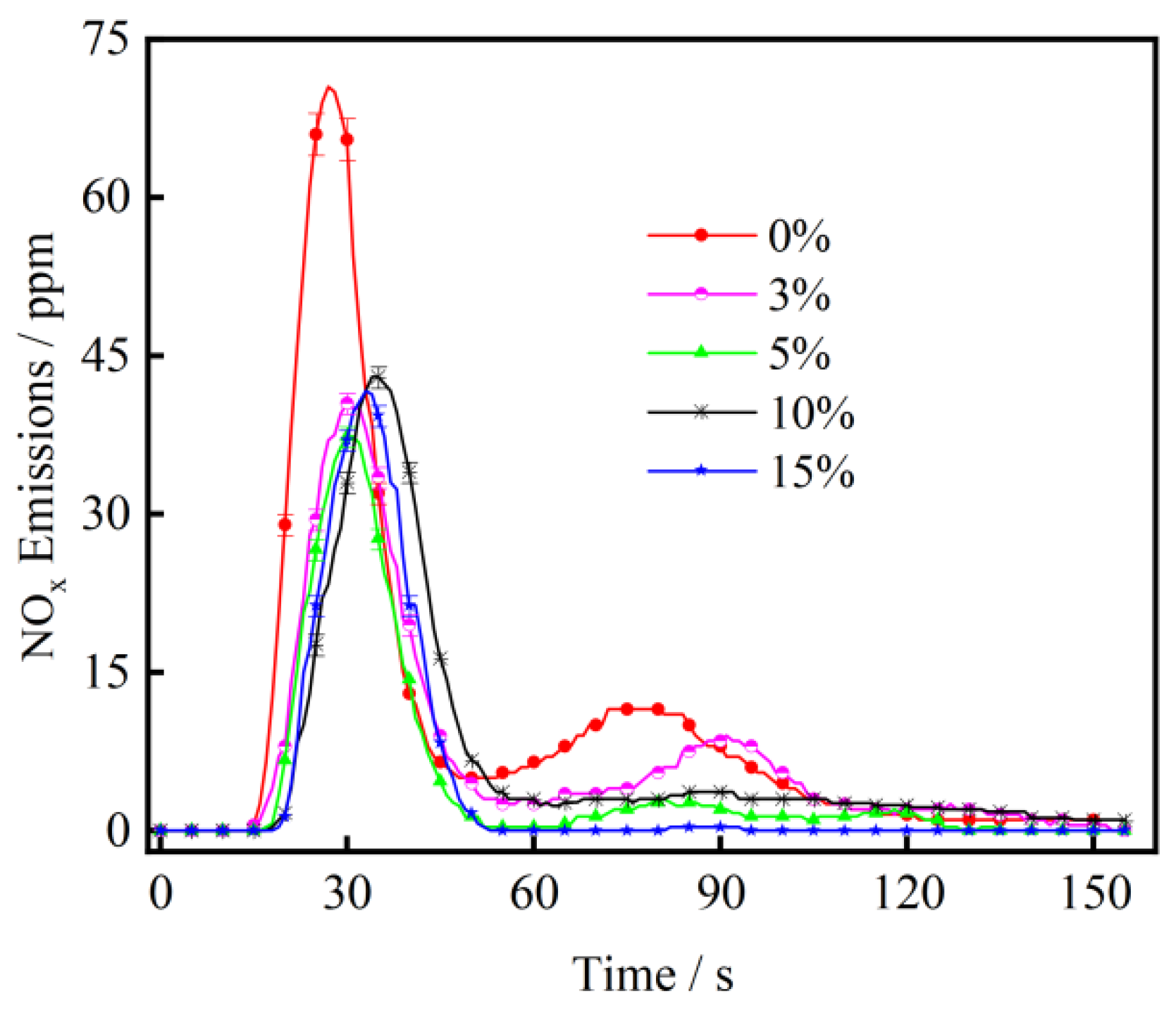
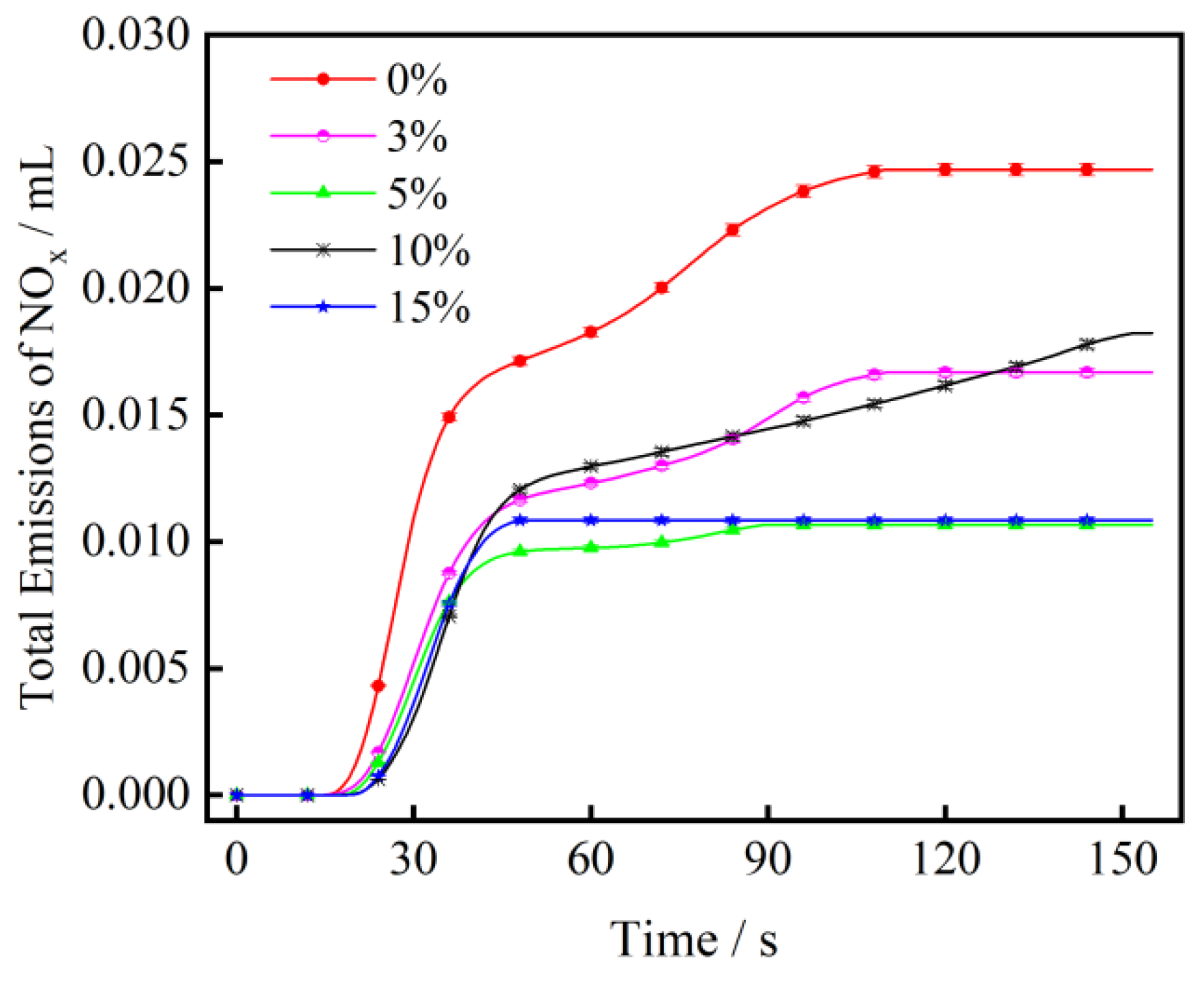
3.3. The Effect of CaO on NOx Emission
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| C | the concentration |
| V | the volume |
| M | the molar mass |
| Q | the input airflow |
| T | the temperature of the materials(K) |
| Abbreviations and acronyms | |
| AlNH4(SO4)2 | Ammonium Aluminum Sulfatehydrate |
| Ca | CalCium |
| CaCN2 | CalCium Cyanamide |
| CaO | CalCium Oxide |
| CFB | CirCulating Fluidized Bed |
| Cl | Chlorine |
| CO | Carbon Monoxide |
| CO2 | Carbon Dioxide |
| CuCl2 | CupriC Chloride |
| Cu(NO3)2 | CupriC Nitrate |
| H2 | Hydrogen |
| HCN | Hydrogen Cyanide |
| H2PtCl6 | ChloroplatiniC ACid |
| K | Potassium |
| K2CO3 | Potassium Carbonate |
| KMnO4 | Potassium Permanganate |
| MgO | Magnesium Oxide |
| MnO2 | Manganese Dioxide |
| N2 | Nitrogen |
| Na2CO3 | Sodium Carbonate |
| NH3 | Ammonia |
| NH4MgPO4 | Magnesium Ammonium Phosphate |
| NOx | Nitrogen Oxides, NO, N2O |
| PM | PartiCulate Matter |
| S | Sulfur |
| SiC | SiliCon Carbide |
| TiO2 | Titanium Dioxide |
| ZnCl2 | ZinC Chloride |
References
- Chen, C.; Fan, D.; Zhao, J.; Qi, Q.; Huang, X.; Zeng, T.; Bi, Y. Study on microwave-assisted co-pyrolysis and bio-oil of Chlorella vulgaris with high-density polyethylene under activated carbon. Energy 2022, 247, 123508. [Google Scholar] [CrossRef]
- Zhongming, Z.; Linong, L.; Xiaona, Y.; Wangqiang, Z.; Wei, L. Global Energy Review 2021; IEA: Paris, France, 2021. [Google Scholar]
- Wang, K.; Khoo, K.S.; Chew, K.W.; Selvarajoo, A.; Chen, W.; Chang, J.; Show, P.L. Microalgae: The future supply house of biohydrogen and biogas. Front. Energy Res. 2021, 9, 660399. [Google Scholar] [CrossRef]
- Recalde, M.; Woudstra, T.; Aravind, P.V. Gasifier, solid oxide fuel cell integrated systems for energy production from wet biomass. Front. Energy Res. 2019, 7, 129. [Google Scholar] [CrossRef]
- Mu, L.; Li, T.; Wang, Z.; Shang, Y.; Yin, H. Influence of water/acid washing pretreatment of aquatic biomass on ash transformation and slagging behavior during co-firing with bituminous coal. Energy 2021, 234, 121286. [Google Scholar] [CrossRef]
- Kobyłecki, R.; Zarzycki, R.; Bis, Z.; Panowski, M.; Wiński, M. Numerical analysis of the combustion of straw and wood in a stoker boiler with vibrating grate. Energy 2021, 222, 119948. [Google Scholar] [CrossRef]
- Lei, X. Carbon sink grass and its carbon sink mechanism. Agric. Eng. 2015, 5, 38–43. [Google Scholar]
- Lei, X. Comprehensively carry out general secretary Xi Jinping’s important strategic thought on ecological civilization construction. Energy China 2017, 668, 101–107. [Google Scholar]
- Liu, H.; Hong, Q.; Liu, H.; Huang, Z.; Zhang, X.; Chen, W.; Zeng, X.; Pan, S. Effects of temperature and additives on NOx emission from combustion of Fast-Growing grass. Front. Energy Res. 2021, 9, 772755. [Google Scholar] [CrossRef]
- Hu, B. Strength and Thermal Properties of Pennisetum—Gypsum Wall Material. Master’s Thesis, Hunan University, Changsha, China, 2018. [Google Scholar]
- Nie, S.; Li, Q.; Li, P.; Wu, Q.; Liu, K. Pavement performance of fast-growing grass fiber asphalt mixture. Highw. Eng. 2021, 46, 183–187. [Google Scholar]
- Wang, Q. The Preparation and Characterization of Fast-Growing Wood/Plastic Composites. Master’s Thesis, Central South University of Forestry & Technology, Changsha, China, 2018. [Google Scholar]
- Szatyłowicz, E.; Skoczko, I. Evaluation of the PAH content in soot from solid fuels combustion in low power boilers. Energies 2019, 12, 4254. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, S.; Jiang, J.; Bhattarai, N.; Li, X.; Chang, X.; Qiu, X.; Zheng, M.; Hua, Y.; Hao, J. Nitrate dominates the chemical composition of PM2.5 during haze event in Beijing, China. Sci. Total Environ. 2019, 689, 1293–1303. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, F.; Yi, H.; Yang, C.; Zhang, R.; Zhou, Y.; Tang, X. Recent advances in selective catalytic oxidation of nitric oxide (NO-SCO) in emissions with excess oxygen: A review on catalysts and mechanisms. Environ. Sci. Pollut. Res. 2021, 28, 2549–2571. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Fan, X.; Gan, M.; Chen, X. Effect of Ca-Fe oxides additives on NOx reduction in iron ore sintering. J. Iron Steel Res. Int. 2017, 24, 1184–1189. [Google Scholar] [CrossRef]
- Zamansky, V.M.; Lissianski, V.V.; Maly, P.M.; Ho, L.; Rusli, D.; Gardiner, W.C., Jr. Reactions of sodium species in the promoted SNCR process. Combust. Flame 1999, 117, 821–831. [Google Scholar] [CrossRef]
- Bae, S.W.; Roh, S.A.; Kim, S.D. NO removal by reducing agents and additives in the selective non-catalytic reduction (SNCR) process. Chemosphere 2006, 65, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Han, K.; Lu, C. Experimental study on the effect of urea and additive injection for controlling nitrogen oxides emissions. Environ. Eng. Sci. 2010, 27, 47–53. [Google Scholar] [CrossRef]
- Gasnot, L.; Dao, D.Q.; Pauwels, J.F. Experimental and kinetic study of the effect of additives on the ammonia based SNCR process in low temperature conditions. Energy Fuels 2012, 26, 2837–2849. [Google Scholar] [CrossRef]
- Wang, F.; Chen, T.; Jin, M.; Lu, P. Simultaneous Desulfurization and Denitrification from Flue Gas Using Urea/Piperazine Solution. Adv. Mater. Res. 2014, 881–883, 641–644. [Google Scholar] [CrossRef]
- Chen, C.; Chen, F.; Cheng, Z.; Chan, Q.; Kook, S.; Yeoh, G. Emissions characteristics of NOx and SO2 in the combustion of microalgae biomass using a tube furnace. J. Energy Inst. 2017, 90, 806–812. [Google Scholar] [CrossRef]
- Liu, C.; Liu, J.; Evrendilek, F.; Xie, W.; Kuo, J.; Buyukada, M. Bioenergy and emission characterizations of catalytic combustion and pyrolysis of litchi peels via TG-FTIR-MS and Py-GC/MS. Renew. Energy 2020, 148, 1074–1093. [Google Scholar] [CrossRef]
- Ulusoy, B.; Anicic, B.; Zhao, L.; Lin, W.; Lu, B.; Wang, W.; Dam-Johansen, K.; Wu, H. Multifunctional additives for NOx abatement in fluidized bed biomass combustion. Energy Fuels 2021, 35, 12367–12379. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X.; Xie, G.; Yu, Y. NO emission characteristic during fluidized combustion of biomass with limestone addition. Fuel 2021, 291, 120264. [Google Scholar]
- Gaze, B.; Knutel, B.; Jajczyk, M.; Wacławek, A.; Bukowski, P.; Dębowski, M. Analysis of the use of catalytic additives for combustion with wood pellets in a low-power boiler. Rynek Energii 2021, 4, 93–98. [Google Scholar]
- Gaze, B.; Romanski, L.; Kulazynski, M. The concept of using urea to reduce NO (x) emissions from low power biomass boilers. Przem. Chem. 2020, 99, 569–573. [Google Scholar]
- Gaze, B.; Hrywna, D.; Romanski, L.; Kulazynski, M. Effect of fuel type and active substance addition on exhaust gas emissions from vehicles powered by a spark ignition engine. Przem. Chem. 2021, 100, 73–78. [Google Scholar]
- Meng, J.; Zhang, X.; Ai, Y.; Yue, H.; Li, X.; Chen, R. Deslagging of Eichhornia crassipers and Pistia stratiotes biomass pellets. Energy Rep. 2021, 7, 822–829. [Google Scholar] [CrossRef]
- Lindström, E.; Sandström, M.; Boström, D.; Öhman, M. Slagging characteristics during combustion of cereal grains rich in phosphorus. Energy Fuels 2007, 21, 710–717. [Google Scholar] [CrossRef]
- Najser, T.; Gaze, B.; Knutel, B.; Verner, A.; Najser, J.; Mikeska, M.; Chojnacki, J.; Němček, O. Analysis of the effect of catalytic additives in the agricultural waste combustion process. Materials 2022, 15, 3526. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, Z.; Liu, L.; Wang, Z.; Li, X. Study on the migration characteristics of sulfur and nitrogen during combustion of oil sludge with CaO additive. Energy Fuels 2020, 34, 6124–6135. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Hu, H.; Liu, P.; Hu, X.; Li, A.; Yao, H. Catalytic role of conditioner CaO in nitrogen transformation during sewage sludge pyrolysis. Proc. Combust. Inst. 2015, 35, 2759–2766. [Google Scholar] [CrossRef]
- Xing, X.; Xing, Y.; Yu, J.; Li, Y.; Ma, P.; Xu, B. Characteristics of CO and NO emission from municipal solid waste combustion. Chin. J. Process Eng. 2017, 17, 866–872. [Google Scholar]
- Hayhurst, A.N.; Lawrence, A.D. The effect of solid CaO on the production of NOx and N2O in fluidized bed combustors: Studies using pyridine as a prototypical nitrogenous fuel. Combust. Flame 1996, 105, 511–527. [Google Scholar] [CrossRef]
- Leckner, B.; Karlsson, M.; Mjornell, M.; Hagman, U. Emissions from a 165 MWth circulating fluidised-bed boiler. J. Inst. Energy 1992, 65, 122–130. [Google Scholar]
- Liu, X.; Luo, Z.; Yu, C. Effect of limestone on the emission of NO during petroleum coke combustion. Fuel 2018, 224, 1–9. [Google Scholar] [CrossRef]
- Xu, G.; Ou, J.; Fang, B.; Wei, H.; Hu, T.; Wang, H. NOX emission from the combustion of mixed fuel pellets of Fenton/CaO-conditioned municipal sludge and rice husk. Environ. Pollut. 2021, 281, 117018. [Google Scholar] [CrossRef]
- Vermeulen, I.; Block, C.; Vandecasteele, C. Estimation of fuel-nitrogen oxide emissions from the element composition of the solid or waste fuel. Fuel 2012, 94, 75–80. [Google Scholar] [CrossRef]
- Shah, I.A.; Gou, X.; Wu, J. Simulation study of an Oxy-Biomass-Based boiler for nearly zero emission using aspen plus. Energies 2019, 12, 1949. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Peng, H.; Li, Y.; Jiang, Z. Characteristics of flue gas emissions during large wood pellet combustion. Trans. Chin. Soc. Agric. Eng. 2015, 31, 215–220. [Google Scholar]
- Xu, Q.; Peng, W.; Ling, C. An experimental analysis of soybean straw combustion on both CO and NOx emission characteristics in a tubular furnace. Energies 2020, 13, 1587. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, Y.; Shu, H.; Zhang, L.; Lu, X.; Bai, F.; Zhao, Q.; Tian, D. The effect of basicity of modified ground granulated blast furnace slag on its denitration performance. J. Clean. Prod. 2021, 305, 126800. [Google Scholar] [CrossRef]
- Tan, H.; Wang, X.; Wang, C.; Xu, T. Characteristics of HCN removal using CaO at high temperatures. Energy Fuels 2009, 23, 1545–1550. [Google Scholar] [CrossRef]
- Fu, S.; Song, Q.; Tang, J.; Yao, Q. Effect of CaO on the selective non-catalytic reduction deNOx process: Experimental and kinetic study. Chem. Eng. J. 2014, 249, 252–259. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.; Lu, G.; Yi, L.; Hu, H.; Chi, H.; Yao, H. Mechanism of conditioner CaO on NOx precursors evolution during sludge steam gasification. P. Combust. Inst. 2017, 36, 4003–4010. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, B.; Su, Y. Advanced control of NO emission from algal biomass combustion using loaded iron-based additives. Energy 2019, 185, 229–238. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Hong, Q.; Liu, H.; Liu, H. The Effect of CaO on the CO and NOx Emission Characteristics of Fast-Growing Grass Combustion. Processes 2023, 11, 760. https://doi.org/10.3390/pr11030760
Li Y, Hong Q, Liu H, Liu H. The Effect of CaO on the CO and NOx Emission Characteristics of Fast-Growing Grass Combustion. Processes. 2023; 11(3):760. https://doi.org/10.3390/pr11030760
Chicago/Turabian StyleLi, Yan, Qingchao Hong, Haili Liu, and Heyun Liu. 2023. "The Effect of CaO on the CO and NOx Emission Characteristics of Fast-Growing Grass Combustion" Processes 11, no. 3: 760. https://doi.org/10.3390/pr11030760
APA StyleLi, Y., Hong, Q., Liu, H., & Liu, H. (2023). The Effect of CaO on the CO and NOx Emission Characteristics of Fast-Growing Grass Combustion. Processes, 11(3), 760. https://doi.org/10.3390/pr11030760





