Valorization of Food Waste to Produce Value-Added Products Based on Its Bioactive Compounds
Abstract
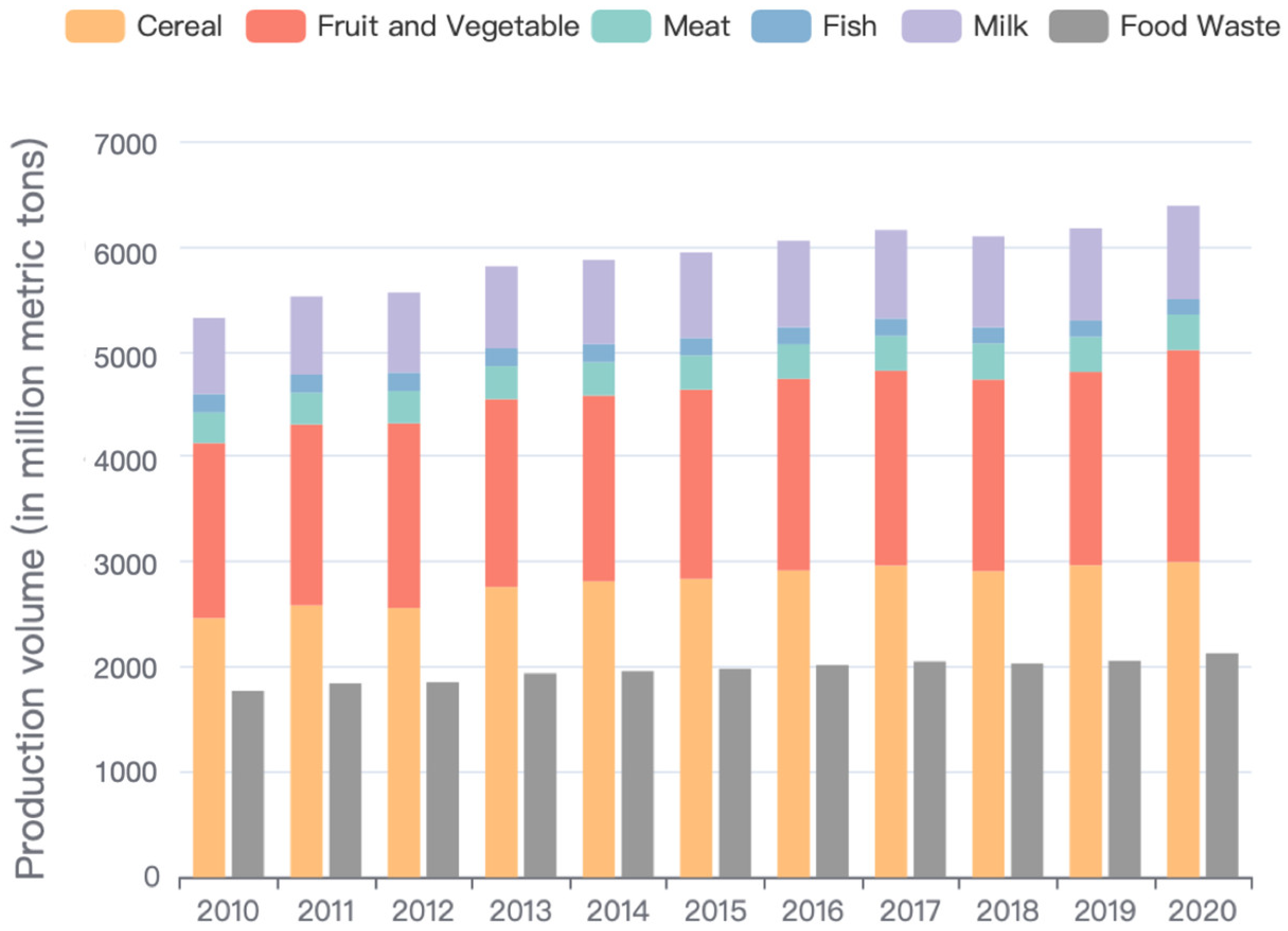
:1. Introduction
2. Food Waste Generation Sources
2.1. Cereals and Pulses
2.2. Fruits and Vegetables
2.3. Dairy
2.4. Edible Oil
2.5. Meat, Poultry, and Eggs
2.6. Seafoods and Aquatic Life
2.7. Agricultural Waste
3. Bioactive Compounds in Food Waste
3.1. Polyphenols
| Compound Name | Classification | Structure | Molecular Formula | Source | Extraction Method | References |
|---|---|---|---|---|---|---|
| Cyanidin | Anthocyanidin | 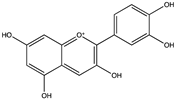 | C15H11O6+ | Grape seed, blueberry waste, cranberry pomace, carrot waste, apple peel | Chemical extraction, Supercritical CO2 extraction, pulsed electric field, UAE, SPI co-drying, Spray-drying | Agcam, Akyıldız and Balasubramaniam [77], Roopchand, Krueger, Moskal, Fridlender, Lila and Raskin [81], Lončarić, Celeiro, Jozinović, Jelinić, Kovač, Jokić, Babić, Moslavac, Zavadlav and Lores [84] |
| Catechin/Epicatechin | Catechin, flavan-3-ol |  | C15H14O6 | Citrus peel, grape skin, spent tea leaves | Chemical extraction, microwave-assisted extraction, UAE, Supercritical CO2 extraction | Vuong, et al. [89], M’hiri, et al. [90] |
| Quercetin | Flavonol | 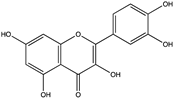 | C15H10O7 | Grape skin, apple pomace, tomato waste | Chemical extraction, deep eutectic solvents, pressurized liquid extraction | Yu and Bulone [72], da Silva, Souza, Sumere, Silva, da Cunha, Barbero, Bezerra and Rostagno [73] |
| Resveratrol | Fitoalexin stilbene | 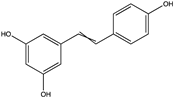 | C14H12O3 | Grape marc, peanuts waste | Chemical extraction, CO2 extraction, microwave-assisted extraction | Kammerer, Claus, Carle and Schieber [80], Sales and Resurreccion [91], Casas, et al. [92] |
| Hesperidin | Flavonoid |  | C28H34O15 | Orange waste, orange peel | Chemical extraction | Victor, et al. [93] |
| Proanthocyanidin | Proanthocyanidin |  | C30H26O12 | Cranberry pomace, grape seed | Chemical extraction, SPI co-drying, UAE | Roopchand, Krueger, Moskal, Fridlender, Lila and Raskin [81], Unusan [94] |
| Ferulic acid | Catechin | 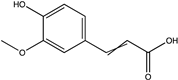 | C10H10O4 | Citrus peel, beetroot waste | Chemical extraction | Ozturk, et al. [95], Aarabi, et al. [96] |
3.2. Proteins
3.3. Dietary Fiber
3.4. Vitamins and Minerals
4. Value-Added Products Obtained by Converting Bioactive Compounds in Food Waste
4.1. Nutraceuticals
4.2. Food Additives
4.3. Biosurfactants
4.4. Single-Cell Protein
4.5. Organic Fertilizers
4.6. Bioplastics
4.7. Animal Feed
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rukikaire, K.; Loran, S. Tackling Food Loss and Waste: A Triple Win Opportunity; Food and Agriculture Organization: Roma, Italy, 2022. [Google Scholar]
- Jan, O.; Tostivint, C.; Turbé, A.; O‘Connor, C.; Lavelle, P. Food Wastage Footprint: Impacts on Natural Resources; Food and Agriculture Organization of the United Nations: Roma, Italy, 2013. [Google Scholar]
- Sharma, P.; Gaur, V.K.; Sirohi, R.; Larroche, C.; Kim, S.H.; Pandey, A. Valorization of cashew nut processing residues for industrial applications. Ind. Crops Prod. 2020, 152, 112550. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L. Natural bioactive compounds from food waste: Toxicity and safety concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef]
- Caldeira, C.; Vlysidis, A.; Fiore, G.; De Laurentiis, V.; Vignali, G.; Sala, S. Sustainability of food waste biorefinery: A review on valorisation pathways, techno-economic constraints, and environmental assessment. Bioresour. Technol. 2020, 312, 123575. [Google Scholar] [CrossRef]
- Fao.org. FAO Cereal Supply and Demand Brief I World Food Situation; Food and Agriculture Organization of the United Nations 2023: Roma, Italy, 2022. [Google Scholar]
- Statista. Pulses: Production Volume Worldwide 2021 Statista. 2021. Available online: https://www.statista.com/statistics/721945/pulses-production-volume-worldwide/ (accessed on 4 February 2023).
- Anal, A.K. Food Processing By-Products and Their Utilization: Introduction. In Food Processing By-Products and Their Utilization; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 1–10. [Google Scholar]
- Papageorgiou, M.; Skendi, A. Introduction to cereal processing and by-products. In Sustainable Recovery and Reutilization of Cereal Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–25. [Google Scholar]
- Kumar, S.; Sangwan, P.; Dhankhar, R.M.V.; Bidra, S. Utilization of rice husk and their ash: A review. Res. J. Chem. Env. Sci 2013, 1, 126–129. [Google Scholar]
- Wanyo, P.; Meeso, N.; Siriamornpun, S. Effects of different treatments on the antioxidant properties and phenolic compounds of rice bran and rice husk. Food Chem. 2014, 157, 457–463. [Google Scholar] [CrossRef]
- Dede, O.H.; Ozdemir, S. Development of nutrient-rich growing media with hazelnut husk and municipal sewage sludge. Environ. Technol. 2018, 39, 2223–2230. [Google Scholar] [CrossRef]
- Bolivar-Telleria, M.; Turbay, C.; Favarato, L.; Carneiro, T.; de Biasi, R.S.; Fernandes, A.A.R.; Santos, A.; Fernandes, P. Second-generation bioethanol from coconut husk. BioMed. Res. Int. 2018, 2018, 4916497. [Google Scholar] [CrossRef]
- Schieber, A. Side streams of plant food processing as a source of valuable compounds: Selected examples. Annu. Rev. Food Sci. Technol. 2017, 8, 97–112. [Google Scholar] [CrossRef]
- FAODocuments. Publication Preview Page|FAO|Food and Agriculture Organization of the United Nations, 2023, Roma, Italy. 2020. Available online: https://www.fao.org/documents/card/en/c/cb9180en (accessed on 5 February 2023).
- Wang, Y.; Pan, S.; Yin, J.; Feng, H.; Wang, M.; Chen, T. Resource potential and global warming potential of fruit and vegetable waste in China based on different treatment strategies. Waste Manag. 2022, 140, 225–232. [Google Scholar] [CrossRef]
- Ji, C.; Kong, C.-X.; Mei, Z.-L.; Li, J. A Review of the Anaerobic Digestion of Fruit and Vegetable Waste. Appl. Biochem. Biotechnol. 2017, 183, 906–922. [Google Scholar] [CrossRef]
- Dessie, W.; Zhang, W.; Xin, F.; Dong, W.; Zhang, M.; Ma, J.; Jiang, M. Succinic acid production from fruit and vegetable wastes hydrolyzed by on-site enzyme mixtures through solid state fermentation. Bioresour. Technol. 2018, 247, 1177–1180. [Google Scholar] [CrossRef]
- Matharu, A.S.; de Melo, E.M.; Houghton, J.A. Opportunity for high value-added chemicals from food supply chain wastes. Bioresour. Technol. 2016, 215, 123–130. [Google Scholar] [CrossRef]
- Díaz, A.I.; Laca, A.; Laca, A.; Díaz, M. Treatment of supermarket vegetable wastes to be used as alternative substrates in bioprocesses. Waste Manag. 2017, 67, 59–66. [Google Scholar] [CrossRef]
- Panda, S.K.; Mishra, S.S.; Kayitesi, E.; Ray, R.C. Microbial-processing of fruit and vegetable wastes for production of vital enzymes and organic acids: Biotechnology and scopes. Environ. Res. 2016, 146, 161–172. [Google Scholar] [CrossRef]
- Sabu, A.; Sarita, S.; Pandey, A.; Bogar, B.; Szakacs, G.; Soccol, C.R. Solid-state fermentation for production of phytase by Rhizopus oligosporus. Appl. Biochem. Biotechnol. 2002, 102, 251–260. [Google Scholar] [CrossRef]
- Bogar, B.; Szakacs, G.; Linden, J.; Pandey, A.; Tengerdy, R. Optimization of phytase production by solid substrate fermentation. J. Ind. Microbiol. Biotechnol. 2003, 30, 183–189. [Google Scholar] [CrossRef]
- Pandey, A.; Carlos, R. Soccol. Economic utilization of crop residues for value addition: A futuristic approach. J. Sci. Ind. Res. 2000, 59, 12–22. [Google Scholar]
- Benjamin, S.; Pandey, A. Coconut cake–a potent substrate for the production of lipase by Candida rugosa in solid-state fermentation. Acta Biotechnol. 1997, 17, 241–251. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, H.; Zheng, M.; Wang, K. Lactic acid production from acidogenic fermentation of fruit and vegetable wastes. Bioresour. Technol. 2015, 191, 53–58. [Google Scholar] [CrossRef]
- Ritchie, H.; Rosado, P.; Roser, M. Meat and Dairy Production. Our world in Data, 25 August 2017. Available online: https://ourworldindata.org/meatproduction (accessed on 4 February 2023).
- Ritchie, H.; Rosado, P.; Roser, M. Agricultural Production. Our world in Data. 2020. Available online: https://ourworldindata.org/agriculturalproduction (accessed on 4 February 2023).
- Statista. Fresh Fruit Production Worldwide 2021 Statista. 2021. Available online: https://www.statista.com/statistics/262266/global-production-of-fresh-fruit/ (accessed on 4 February 2023).
- Cappiello, J. The Dairy Industry Dumps 128 Million Tons of Milk Every Year - Mercy for Animals. Mercy For Animals, 30 November 2018. Available online: https://mercyforanimals.org/blog/the-dairy-industry-dumps-128-million-tons/ (accessed on 4 February 2023).
- Sharma, P.; Gaur, V.K.; Kim, S.-H.; Pandey, A. Microbial strategies for bio-transforming food waste into resources. Bioresour. Technol. 2020, 299, 122580. [Google Scholar] [CrossRef]
- Parashar, A.; Jin, Y.; Mason, B.; Chae, M.; Bressler, D.C. Incorporation of whey permeate, a dairy effluent, in ethanol fermentation to provide a zero waste solution for the dairy industry. J. Dairy Sci. 2016, 99, 1859–1867. [Google Scholar] [CrossRef] [Green Version]
- Kushwaha, J.P.; Srivastava, V.C.; Mall, I.D. An overview of various technologies for the treatment of dairy wastewaters. Crit. Rev. Food Sci. Nutr. 2011, 51, 442–452. [Google Scholar] [CrossRef]
- Fewtrell, L. Drinking-water nitrate, methemoglobinemia, and global burden of disease: A discussion. Environ. Health Perspect. 2004, 112, 1371–1374. [Google Scholar] [CrossRef] [Green Version]
- Dias, T.; Fragoso, R.; Duarte, E. Anaerobic co-digestion of dairy cattle manure and pear waste. Bioresour. Technol. 2014, 164, 420–423. [Google Scholar] [CrossRef]
- Mahboubi, A.; Ferreira, J.A.; Taherzadeh, M.J.; Lennartsson, P.R. Value-added products from dairy waste using edible fungi. Waste Manag. 2016, 59, 518–525. [Google Scholar] [CrossRef]
- Okino-Delgado, C.H.; Prado, D.Z.d.; Facanali, R.; Marques, M.M.O.; Nascimento, A.S.; Fernandes, C.J.d.C.; Zambuzzi, W.F.; Fleuri, L.F. Bioremediation of cooking oil waste using lipases from wastes. PLoS ONE 2017, 12, e0186246. [Google Scholar] [CrossRef] [Green Version]
- Kreps, F.; Dubaj, T.; Krepsová, Z. Accelerated oxidation method and simple kinetic model for predicting thermooxidative stability of edible oils under storage conditions. Food Packag. Shelf Life 2021, 29, 100739. [Google Scholar] [CrossRef]
- Chang, F.-C.; Tsai, M.-J.; Ko, C.-H. Agricultural waste derived fuel from oil meal and waste cooking oil. Environ. Sci. Pollut. Res. 2018, 25, 5223–5230. [Google Scholar] [CrossRef]
- Chen, C.; Sun, N.; Li, D.; Long, S.; Tang, X.; Xiao, G.; Wang, L. Optimization and characterization of biosurfactant production from kitchen waste oil using Pseudomonas aeruginosa. Environ. Sci. Pollut. Res. 2018, 25, 14934–14943. [Google Scholar] [CrossRef]
- Mulligan, C.N. Sustainable Production of Biosurfactants Using Waste Substrates. In Advancements in Biosurfactants Research; Springer: Berlin/Heidelberg, Germany, 2023; pp. 57–77. [Google Scholar]
- Diwan, B.; Parkhey, P.; Gupta, P. From agro-industrial wastes to single cell oils: A step towards prospective biorefinery. Folia Microbiol. 2018, 63, 547–568. [Google Scholar] [CrossRef]
- Sherazi, S.T.H.; Mahesar, S.A. Vegetable oil deodorizer distillate: A rich source of the natural bioactive components. J. Oleo Sci. 2016, 65, 957–966. [Google Scholar] [CrossRef] [Green Version]
- Statista. Production of Meat Worldwide by Meat Type 2022|Statista. 2022. Available online: https://www.statista.com/statistics/237632/production-of-meat-worldwide-since-1990/ (accessed on 4 February 2023).
- Ning, Z.; Zhang, H.; Li, W.; Zhang, R.; Liu, G.; Chen, C. Anaerobic digestion of lipid-rich swine slaughterhouse waste: Methane production performance, long-chain fatty acids profile and predominant microorganisms. Bioresour. Technol. 2018, 269, 426–433. [Google Scholar] [CrossRef]
- Mozhiarasi, V.; Natarajan, T.S. Slaughterhouse and poultry wastes: Management practices, feedstocks for renewable energy production, and recovery of value added products. Biomass Convers. Biorefinery 2022, 1–24. [Google Scholar] [CrossRef]
- Yaakob, M.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Tiey, A.; Kassim, A.H.M. Optimising of Scenedesmus sp. biomass production in chicken slaughterhouse wastewater using response surface methodology and potential utilisation as fish feeds. Environ. Sci. Pollut. Res. 2019, 26, 12089–12108. [Google Scholar] [CrossRef]
- Ashayerizadeh, O.; Dastar, B.; Samadi, F.; Khomeiri, M.; Yamchi, A.; Zerehdaran, S. Study on the chemical and microbial composition and probiotic characteristics of dominant lactic acid bacteria in fermented poultry slaughterhouse waste. Waste Manag. 2017, 65, 178–185. [Google Scholar] [CrossRef]
- Adhikari, B.B.; Chae, M.; Bressler, D.C. Utilization of slaughterhouse waste in value-added applications: Recent advances in the development of wood adhesives. Polymers 2018, 10, 176. [Google Scholar] [CrossRef] [Green Version]
- Marques, R.V.; Paz, M.F.d.; Duval, E.H.; Corrêa, L.B.; Corrêa, É.K. Staphylococcus xylosus fermentation of pork fatty waste: Raw material for biodiesel production. Braz. J. Microbiol. 2016, 47, 675–679. [Google Scholar] [CrossRef] [Green Version]
- Statista. World Fish Production 2022|Statista. 2022. Available online: https://www.statista.com/statistics/264577/total-world-fish-production-since-2002/ (accessed on 5 February 2023).
- Kumar, A.; Kumar, D.; George, N.; Sharma, P.; Gupta, N. A process for complete biodegradation of shrimp waste by a novel marine isolate Paenibacillus sp. AD with simultaneous production of chitinase and chitin oligosaccharides. Int. J. Biol. Macromol. 2018, 109, 263–272. [Google Scholar] [CrossRef]
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef] [Green Version]
- Prameela, K.; Venkatesh, K.; Immandi, S.B.; Kasturi, A.P.K.; Krishna, C.R.; Mohan, C.M. Next generation nutraceutical from shrimp waste: The convergence of applications with extraction methods. Food Chem. 2017, 237, 121–132. [Google Scholar] [CrossRef]
- Hussein, M.H.; El-Hady, M.F.; Shehata, H.A.; Hegazy, M.A.; Hefni, H.H. Preparation of some eco-friendly corrosion inhibitors having antibacterial activity from sea food waste. J. Surfactants Deterg. 2013, 16, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Valcarcel, J.; Novoa-Carballal, R.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A. Glycosaminoglycans from marine sources as therapeutic agents. Biotechnol. Adv. 2017, 35, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Directive, E. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on waste and repealing certain Directives. Off. J. Eur. Union L 2008, 312, 22. [Google Scholar]
- Dai, Y.; Sun, Q.; Wang, W.; Lu, L.; Liu, M.; Li, J.; Yang, S.; Sun, Y.; Zhang, K.; Xu, J. Utilizations of agricultural waste as adsorbent for the removal of contaminants: A review. Chemosphere 2018, 211, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Footprint, F.W. Food Wastage Footprint: Impacts on Natural Resources: Summary Report; Food & Agriculture Org: Roma, Italy, 2013. [Google Scholar]
- Heredia-Guerrero, J.A.; Heredia, A.; Domínguez, E.; Cingolani, R.; Bayer, I.S.; Athanassiou, A.; Benítez, J.J. Cutin from agro-waste as a raw material for the production of bioplastics. J. Exp. Bot. 2017, 68, 5401–5410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madurwar, M.V.; Ralegaonkar, R.V.; Mandavgane, S.A. Application of agro-waste for sustainable construction materials: A review. Constr. Build. Mater. 2013, 38, 872–878. [Google Scholar] [CrossRef]
- Cheng, H.; Hu, Y. Municipal solid waste (MSW) as a renewable source of energy: Current and future practices in China. Bioresour. Technol. 2010, 101, 3816–3824. [Google Scholar] [CrossRef]
- Bogar, B.; Szakacs, G.; Pandey, A.; Abdulhameed, S.; Linden, J.C.; Tengerdy, R.P. Production of Phytase by Mucorracemosus in Solid-State Fermentation. Biotechnol. Prog. 2003, 19, 312–319. [Google Scholar] [CrossRef]
- Singh, J.S.; Strong, P. Biologically derived fertilizer: A multifaceted bio-tool in methane mitigation. Ecotoxicol. Environ. Saf. 2016, 124, 267–276. [Google Scholar] [CrossRef]
- Selvakumar, P.; Ashakumary, L.; Pandey, A. Biosynthesis of glucoamylase from Aspergillus niger by solid-state fermentation using tea waste as the basis of a solid substrate. Bioresour. Technol. 1998, 65, 83–85. [Google Scholar] [CrossRef]
- Philippoussis, A.N. Production of mushrooms using agro-industrial residues as substrates. In Biotechnology for Agro-Industrial Residues Utilisation; Springer: Berlin/Heidelberg, Germany, 2009; pp. 163–196. [Google Scholar]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Shi, X.; He, F.; Wu, T.; Jiang, L.; Normakhamatov, N.; Sharipov, A.; Wang, T.; Wen, M.; Aisa, H.A. Valorization of Food Processing Waste to Produce Valuable Polyphenolics. J. Agric. Food Chem. 2022, 70, 8855–8870. [Google Scholar] [CrossRef] [PubMed]
- Martău, G.A.; Teleky, B.-E.; Ranga, F.; Pop, I.D.; Vodnar, D.C. Apple pomace as a sustainable substrate in sourdough fermentation. Front. Microbiol. 2021, 12, 3850. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.U.; Shabbir, M.A.; Mustafa, S.; Hina, S.; Quddoos, M.Y.; Mahmood, S.; Maryam, Y.; Faisal, F.; Rafique, A. Effect of Apple peel as an antioxidant on the quality characteristics and oxidative stability of mayonnaise. Appl. Food Res. 2021, 1, 100023. [Google Scholar] [CrossRef]
- Hobbi, P.; Okoro, O.V.; Hajiabbas, M.; Hamidi, M.; Nie, L.; Megalizzi, V.; Musonge, P.; Dodi, G.; Shavandi, A. Chemical Composition, Antioxidant Activity and Cytocompatibility of Polyphenolic Compounds Extracted from Food Industry Apple Waste: Potential in Biomedical Application. Molecules 2023, 28, 675. [Google Scholar] [CrossRef]
- Yu, L.; Bulone, V. De-glycosylation and enhanced bioactivity of flavonoids from apple pomace during extraction with deep eutectic solvents. Green Chem. 2021, 23, 7199–7209. [Google Scholar] [CrossRef]
- da Silva, L.C.; Souza, M.C.; Sumere, B.R.; Silva, L.G.; da Cunha, D.T.; Barbero, G.F.; Bezerra, R.M.; Rostagno, M.A. Simultaneous extraction and separation of bioactive compounds from apple pomace using pressurized liquids coupled on-line with solid-phase extraction. Food Chem. 2020, 318, 126450. [Google Scholar] [CrossRef]
- Iorizzo, M.; Curaba, J.; Pottorff, M.; Ferruzzi, M.G.; Simon, P.; Cavagnaro, P.F. Carrot anthocyanins genetics and genomics: Status and perspectives to improve its application for the food colorant industry. Genes 2020, 11, 906. [Google Scholar] [CrossRef]
- Tian, L.; Tan, Y.; Chen, G.; Wang, G.; Sun, J.; Ou, S.; Chen, W.; Bai, W. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 982–991. [Google Scholar] [CrossRef]
- Amin, S.; Jung, S.; Kang, I.; Duval, A. Valorization of baby carrot processing waste. J. Culin. Sci. Technol. 2021, 21, 1–17. [Google Scholar] [CrossRef]
- Agcam, E.; Akyıldız, A.; Balasubramaniam, V. Optimization of anthocyanins extraction from black carrot pomace with thermosonication. Food Chem. 2017, 237, 461–470. [Google Scholar] [CrossRef]
- Xiao, L.; Ye, F.; Zhou, Y.; Zhao, G. Utilization of pomelo peels to manufacture value-added products: A review. Food Chem. 2021, 351, 129247. [Google Scholar] [CrossRef]
- Pastor, R.F.; Restani, P.; Di Lorenzo, C.; Orgiu, F.; Teissedre, P.-L.; Stockley, C.; Ruf, J.C.; Quini, C.I.; Garcìa Tejedor, N.; Gargantini, R. Resveratrol, human health and winemaking perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 1237–1255. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Krueger, C.G.; Moskal, K.; Fridlender, B.; Lila, M.A.; Raskin, I. Food-compatible method for the efficient extraction and stabilization of cranberry pomace polyphenols. Food Chem. 2013, 141, 3664–3669. [Google Scholar] [CrossRef] [Green Version]
- Waterhouse, G.I.; Sun-Waterhouse, D.; Su, G.; Zhao, H.; Zhao, M. Spray-drying of antioxidant-rich blueberry waste extracts; interplay between waste pretreatments and spray-drying process. Food Bioprocess Technol. 2017, 10, 1074–1092. [Google Scholar] [CrossRef]
- Bener, M.; Shen, Y.; Apak, R.; Finley, J.W.; Xu, Z. Release and degradation of anthocyanins and phenolics from blueberry pomace during thermal acid hydrolysis and dry heating. J. Agric. Food Chem. 2013, 61, 6643–6649. [Google Scholar] [CrossRef]
- Lončarić, A.; Celeiro, M.; Jozinović, A.; Jelinić, J.; Kovač, T.; Jokić, S.; Babić, J.; Moslavac, T.; Zavadlav, S.; Lores, M. Green extraction methods for extraction of polyphenolic compounds from blueberry pomace. Foods 2020, 9, 1521. [Google Scholar] [CrossRef]
- Pujol, D.; Liu, C.; Gominho, J.; Olivella, M.; Fiol, N.; Villaescusa, I.; Pereira, H. The chemical composition of exhausted coffee waste. Ind. Crops Prod. 2013, 50, 423–429. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R. Spent coffee grounds as a valuable source of phenolic compounds and bioenergy. J. Clean. Prod. 2012, 34, 49–56. [Google Scholar] [CrossRef]
- Leung, L.K.; Su, Y.; Chen, R.; Zhang, Z.; Huang, Y.; Chen, Z.-Y. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. J. Nutr. 2001, 131, 2248–2251. [Google Scholar] [CrossRef] [Green Version]
- Abdeltaif, S.A.; SirElkhatim, K.A.; Hassan, A.B. Estimation of phenolic and flavonoid compounds and antioxidant activity of spent coffee and black tea (processing) waste for potential recovery and reuse in Sudan. Recycling 2018, 3, 27. [Google Scholar] [CrossRef] [Green Version]
- Vuong, Q.V.; Golding, J.B.; Nguyen, M.; Roach, P.D. Extraction and isolation of catechins from tea. J. Sep. Sci. 2010, 33, 3415–3428. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Ghoul, M.; Boudhrioua, N.M. Extraction methods of citrus peel phenolic compounds. Food Rev. Int. 2014, 30, 265–290. [Google Scholar] [CrossRef]
- Sales, J.M.; Resurreccion, A.V. Resveratrol in peanuts. Crit. Rev. Food Sci. Nutr. 2014, 54, 734–770. [Google Scholar] [CrossRef]
- Casas, L.; Mantell, C.; Rodríguez, M.; de la Ossa, E.M.; Roldán, A.; De Ory, I.; Caro, I.; Blandino, A. Extraction of resveratrol from the pomace of Palomino fino grapes by supercritical carbon dioxide. J. Food Eng. 2010, 96, 304–308. [Google Scholar] [CrossRef]
- Victor, M.M.; David, J.M.; Cortez, M.V.; Leite, J.L.; da Silva, G.S. A high-yield process for extraction of hesperidin from orange (Citrus sinensis L. osbeck) peels waste, and its transformation to diosmetin, A valuable and bioactive flavonoid. Waste Biomass Valoriz. 2021, 12, 313–320. [Google Scholar] [CrossRef]
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Aarabi, A.; Mizani, M.; Honarvar, M.; Faghihian, H.; Gerami, A. Extraction of ferulic acid from sugar beet pulp by alkaline hydrolysis and organic solvent methods. J. Food Meas. Charact. 2016, 10, 42–47. [Google Scholar] [CrossRef]
- Kamal, H.; Le, C.F.; Salter, A.M.; Ali, A. Extraction of protein from food waste: An overview of current status and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2455–2475. [Google Scholar] [CrossRef] [PubMed]
- Apprich, S.; Tirpanalan, Ö.; Hell, J.; Reisinger, M.; Böhmdorfer, S.; Siebenhandl-Ehn, S.; Novalin, S.; Kneifel, W. Wheat bran-based biorefinery 2: Valorization of products. LWT-Food Sci. Technol. 2014, 56, 222–231. [Google Scholar] [CrossRef]
- Stevenson, L.; Phillips, F.; O’sullivan, K.; Walton, J. Wheat bran: Its composition and benefits to health, a European perspective. Int. J. Food Sci. Nutr. 2012, 63, 1001–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil cakes and their biotechnological applications–A review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, D.; Li, K.; Yang, Y.; Lei, Z.; Zhang, Z. Soybean curd residue: Composition, utilization, and related limiting factors. Int. Sch. Res. Not. 2013, 2013, 423590. [Google Scholar] [CrossRef]
- Prandi, B.; Faccini, A.; Lambertini, F.; Bencivenni, M.; Jorba, M.; Van Droogenbroek, B.; Bruggeman, G.; Schöber, J.; Petrusan, J.; Elst, K. Food wastes from agrifood industry as possible sources of proteins: A detailed molecular view on the composition of the nitrogen fraction, amino acid profile and racemisation degree of 39 food waste streams. Food Chem. 2019, 286, 567–575. [Google Scholar] [CrossRef]
- Negi, T.; Kumar, Y.; Sirohi, R.; Singh, S.; Tarafdar, A.; Pareek, S.; Awasthi, M.K.; Sagar, N.A. Advances in bioconversion of spent tea leaves to value-added products. Bioresour. Technol. 2021, 346, 126409. [Google Scholar] [CrossRef]
- Perera, G.; Amarakoon, A.; Illeperuma, D.; Muthukumarana, P. Application of Membrane Filtration Technique in Preparation of Protein-Rich Feed from Spent Tea. J. Food Agric. 2019, 12, 13–22. [Google Scholar] [CrossRef]
- Khiari, Z. Sustainable Upcycling of Fisheries and Aquaculture Wastes Using Fish-Derived Cold-Adapted Proteases. Front. Nutr. 2022, 9, 875697. [Google Scholar] [CrossRef]
- Mo, W.Y.; Man, Y.B.; Wong, M.H. Use of food waste, fish waste and food processing waste for China’s aquaculture industry: Needs and challenge. Sci. Total Environ. 2018, 613, 635–643. [Google Scholar] [CrossRef]
- Ramakrishnan, V.; Ghaly, A.; Brooks, M.; Budge, S. Extraction of proteins from mackerel fish processing waste using alcalase enzyme. Bioprocess Biotech 2013, 3, 2–10. [Google Scholar]
- Simon, C.; Salini, M.; Irvin, S.; Blyth, D.; Bourne, N.; Smullen, R. The effect of poultry protein concentrate and phosphorus supplementation on growth, digestibility and nutrient retention efficiency in barramundi Lates calcarifer. Aquaculture 2019, 498, 305–314. [Google Scholar] [CrossRef]
- Teshnizi, Z.M.; Robatjazi, S.M.; Mosaabadi, J.M. Optimization of the enzymatic hydrolysis of poultry slaughterhouse wastes using alcalase enzyme for the preparation of protein hydrolysates. Appl. Food Biotechnol. 2020, 7, 153–160. [Google Scholar]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Wang, M.; Yang, F.; Yan, X.; Chao, X.; Zhang, W.; Yuan, C.; Zeng, Q. Anti-diabetic effect of banana peel dietary fibers on type 2 diabetic mellitus mice induced by streptozotocin and high-sugar and high-fat diet. J. Food Biochem. 2022, 46, e14275. [Google Scholar] [CrossRef]
- Pereira, B.S.; de Freitas, C.; Contiero, J.; Brienzo, M. Enzymatic Production of Xylooligosaccharides from Xylan Solubilized from Food and Agroindustrial Waste. BioEnergy Res. 2022, 15, 1195–1203. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, L.; Li, J.; Wang, L.; Song, X. Extraction and Characterization of Cellulose from Jerusalem Artichoke Residue and Its Application in Blueberry Preservation. Foods 2022, 11, 1065. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, W.; Wu, B.; Wu, P.; Duan, Y.; Yang, Q.; Ma, H. Modification of garlic skin dietary fiber with twin-screw extrusion process and in vivo evaluation of Pb binding. Food Chem. 2018, 268, 550–557. [Google Scholar] [CrossRef]
- Rahim, M.A.; Saeed, F.; Khalid, W.; Hussain, M.; Anjum, F.M. Functional and nutraceutical properties of fructo-oligosaccharides derivatives: A review. Int. J. Food Prop. 2021, 24, 1588–1602. [Google Scholar] [CrossRef]
- Chelliah, R.; Park, S.J.; Oh, S.; Lee, E.; Daliri, E.B.-M.; Elahi, F.; Park, C.R.; Sultan, G.; Madar, I.H.; Oh, D.H. An effective universal protocol for the Extraction of Fructooligosaccharide from the different agricultural byproducts. MethodsX 2023, 10, 102096. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Nezafat, Z.; Shafiei, N. Polysaccharide biopolymer chemistry. In Biopolymer Based Metal Nanoparticle Chemistry for Sustainable Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 45–105. [Google Scholar]
- Müller-Maatsch, J.; Bencivenni, M.; Caligiani, A.; Tedeschi, T.; Bruggeman, G.; Bosch, M.; Petrusan, J.; Van Droogenbroeck, B.; Elst, K.; Sforza, S. Pectin content and composition from different food waste streams. Food Chem. 2016, 201, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Bioactive potential of fruit and vegetable wastes. Adv. Food Nutr. Res. 2020, 91, 157–225. [Google Scholar]
- Konrade, D.; Kļava, D.; Grāmatiņa, I.; Kampuse, S.; Kince, T. Crispbreads with carrot and pumpkin processing by-products. Proc. Latv. Acad. Sciences. Sect. B Nat. Exact Appl. Sci. 2018, 72, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Kultys, E.; Kurek, M.A. Green extraction of carotenoids from fruit and vegetable byproducts: A review. Molecules 2022, 27, 518. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Cassano, R.; Procopio, D.; Di Gioia, M.L.; Barone, E. Valorization of tomato waste as a source of carotenoids. Molecules 2021, 26, 5062. [Google Scholar] [CrossRef]
- Saini, R.K.; Moon, S.H.; Keum, Y.-S. An updated review on use of tomato pomace and crustacean processing waste to recover commercially vital carotenoids. Food Res. Int. 2018, 108, 516–529. [Google Scholar] [CrossRef]
- Quan, C.; Turner, C. Extraction of astaxanthin from shrimp waste using pressurized hot ethanol. Chromatographia 2009, 70, 247–251. [Google Scholar] [CrossRef]
- Ambati, R.R.; Siew Moi, P.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Czech, A.; Zarycka, E.; Yanovych, D.; Zasadna, Z.; Grzegorczyk, I.; Kłys, S. Mineral content of the pulp and peel of various citrus fruit cultivars. Biol. Trace Elem. Res. 2020, 193, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Khattak, K.; Rahman, T. Analysis of vegetable’s peels as a natural source of vitamins and minerals. Int. Food Res. J. 2017, 24, 292–297. [Google Scholar]
- Hussain, A.; Kausar, T.; Din, A.; Murtaza, M.A.; Jamil, M.A.; Noreen, S.; Rehman, H.U.; Shabbir, H.; Ramzan, M.A. Determination of total phenolic, flavonoid, carotenoid, and mineral contents in peel, flesh, and seeds of pumpkin (Cucurbita maxima). J. Food Process. Preserv. 2021, 45, e15542. [Google Scholar] [CrossRef]
- Yatnatti, S.; Vijayalakshmi, D.; Chandru, R. Processing and nutritive value of mango seed kernel flour. Curr. Res. Nutr. Food Sci. J. 2014, 2, 170–175. [Google Scholar] [CrossRef]
- Lucey, J.; Fox, P. Importance of calcium and phosphate in cheese manufacture: A review. J. Dairy Sci. 1993, 76, 1714–1724. [Google Scholar] [CrossRef]
- Souza, S.O.; Santos, V.S.; Santos, E.S.; Ávila, D.V.L.; Nascimento, C.C.; Costa, S.S.L.; Garcia, C.A.B.; Araujo, R.G.O. Evaluation of the mineral content in milk and yogurt types using chemometric tools. Microchem. J. 2018, 143, 1–8. [Google Scholar] [CrossRef]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef] [Green Version]
- Balandrán-Quintana, R.R.; Mercado-Ruiz, J.N.; Mendoza-Wilson, A.M. Wheat bran proteins: A review of their uses and potential. Food Rev. Int. 2015, 31, 279–293. [Google Scholar] [CrossRef]
- Kiran, E.U.; Trzcinski, A.P.; Ng, W.J.; Liu, Y. Bioconversion of food waste to energy: A review. Fuel 2014, 134, 389–399. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; Domínguez-Avila, J.A.; Yahia, E.M.; Belmonte-Herrera, B.H.; Wall-Medrano, A.; Montalvo-González, E.; González-Aguilar, G. Avocado fruit and by-products as potential sources of bioactive compounds. Food Res. Int. 2020, 138, 109774. [Google Scholar] [CrossRef]
- Nobre, B.P.; Palavra, A.F.; Pessoa, F.L.; Mendes, R.L. Supercritical CO2 extraction of trans-lycopene from Portuguese tomato industrial waste. Food Chem. 2009, 116, 680–685. [Google Scholar] [CrossRef]
- Lappalainen, K.; Kärkkäinen, J.; Joensuu, P.; Lajunen, M. Modification of potato peel waste with base hydrolysis and subsequent cationization. Carbohydr. Polym. 2015, 132, 97–103. [Google Scholar] [CrossRef]
- Okuno, S.; Yoshinaga, M.; Nakatani, M.; Ishiguro, K.; Yoshimoto, M.; Morishita, T.; Uehara, T.; Kawano, M. Extraction of antioxidants in sweetpotato waste powder with supercritical carbon dioxide. Food Sci. Technol. Res. 2002, 8, 154–157. [Google Scholar] [CrossRef] [Green Version]
- Kühn, S.; Wollseifen, H.; Galensa, R.; Schulze-Kaysers, N.; Kunz, B. Adsorption of flavonols from onion (Allium cepa L.) processing residues on a macroporous acrylic resin. Food Res. Int. 2014, 65, 103–108. [Google Scholar] [CrossRef]
- Kiassos, E.; Mylonaki, S.; Makris, D.P.; Kefalas, P. Implementation of response surface methodology to optimise extraction of onion (Allium cepa) solid waste phenolics. Innov. Food Sci. Emerg. Technol. 2009, 10, 246–252. [Google Scholar] [CrossRef]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; López-Andréu, F.J.; Terry, L.A.; Esteban, R.M. The impact of pasteurisation and sterilisation on bioactive compounds of onion by-products. Food Bioprocess Technol. 2013, 6, 1979–1989. [Google Scholar] [CrossRef]
- Hua, X.; Yang, R.; Zhang, W.; Fei, Y.; Jin, Z.; Jiang, B. Dual-enzymatic synthesis of lactulose in organic-aqueous two-phase media. Food Res. Int. 2010, 43, 716–722. [Google Scholar] [CrossRef]
- Lappa, I.K.; Papadaki, A.; Kachrimanidou, V.; Terpou, A.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Cheese whey processing: Integrated biorefinery concepts and emerging food applications. Foods 2019, 8, 347. [Google Scholar] [CrossRef] [Green Version]
- Henkel, M.; Müller, M.M.; Kügler, J.H.; Lovaglio, R.B.; Contiero, J.; Syldatk, C.; Hausmann, R. Rhamnolipids as biosurfactants from renewable resources: Concepts for next-generation rhamnolipid production. Process Biochem. 2012, 47, 1207–1219. [Google Scholar] [CrossRef]
- Vescovi, V.; Rojas, M.J.; Baraldo, A.; Botta, D.C.; Santana, F.A.M.; Costa, J.P.; Machado, M.S.; Honda, V.K.; de Lima Camargo Giordano, R.; Tardioli, P.W. Lipase-catalyzed production of biodiesel by hydrolysis of waste cooking oil followed by esterification of free fatty acids. J. Am. Oil Chem. Soc. 2016, 93, 1615–1624. [Google Scholar] [CrossRef]
- Pattara, C.; Cappelletti, G.; Cichelli, A. Recovery and use of olive stones: Commodity, environmental and economic assessment. Renew. Sustain. Energy Rev. 2010, 14, 1484–1489. [Google Scholar] [CrossRef]
- Rodríguez, G.; Lama, A.; Rodríguez, R.; Jiménez, A.; Guillén, R.; Fernández-Bolanos, J. Olive stone an attractive source of bioactive and valuable compounds. Bioresour. Technol. 2008, 99, 5261–5269. [Google Scholar] [CrossRef]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487. [Google Scholar]
- Ullah, N.; Nadhman, A.; Siddiq, S.; Mehwish, S.; Islam, A.; Jafri, L.; Hamayun, M. Plants as antileishmanial agents: Current scenario. Phytother. Res. 2016, 30, 1905–1925. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.M.M.; Campos, M.R.S. Bioactive compounds as therapeutic alternatives. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–264. [Google Scholar]
- Ayala-Zavala, J.; Rosas-Domínguez, C.; Vega-Vega, V.; González-Aguilar, G. Antioxidant enrichment and antimicrobial protection of fresh-cut fruits using their own byproducts: Looking for integral exploitation. J. Food Sci. 2010, 75, R175–R181. [Google Scholar] [CrossRef] [PubMed]
- Rebecca, L.J.; Seshiah, C.; Tissopi, T. Extraction of caffeine from used tea leaves. Ann. Valahia Univ. Târgovişte 2014, 19–22. [Google Scholar]
- Sharma, P.; Gaur, V.K.; Sirohi, R.; Varjani, S.; Kim, S.H.; Wong, J.W. Sustainable processing of food waste for production of bio-based products for circular bioeconomy. Bioresour. Technol. 2021, 325, 124684. [Google Scholar] [CrossRef] [PubMed]
- Pratap, K.; Taki, A.C.; Johnston, E.B.; Lopata, A.L.; Kamath, S.D. A comprehensive review on natural bioactive compounds and probiotics as potential therapeutics in food allergy treatment. Front. Immunol. 2020, 11, 996. [Google Scholar] [CrossRef]
- Palaniappan, A.; Antony, U.; Emmambux, M.N. Current status of xylooligosaccharides: Production, characterization, health benefits and food application. Trends Food Sci. Technol. 2021, 111, 506–519. [Google Scholar] [CrossRef]
- Fischer, C.; Kleinschmidt, T. Synthesis of galactooligosaccharides in milk and whey: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 678–697. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.; Tomar, S.; Singh, R.; Singh, A.; Ali, B. Galactooligosaccharides: Novel components of designer foods. J. Food Sci. 2011, 76, R103–R111. [Google Scholar] [CrossRef]
- Liu, F.; Li, P.; Chen, M.; Luo, Y.; Prabhakar, M.; Zheng, H.; He, Y.; Qi, Q.; Long, H.; Zhang, Y. Fructooligosaccharide (FOS) and galactooligosaccharide (GOS) increase Bifidobacterium but reduce butyrate producing bacteria with adverse glycemic metabolism in healthy young population. Sci. Rep. 2017, 7, 11789. [Google Scholar] [CrossRef] [Green Version]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic compounds in the potato and its byproducts: An overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Huang, V.; Quiambao, Q.; Noritake, S.; Liu, J.; Kwon, O.; Chintalapati, S.; Young, J.; Levin, C.E.; Tam, C. Potato peels and their bioactive glycoalkaloids and phenolic compounds inhibit the growth of pathogenic trichomonads. J. Agric. Food Chem. 2018, 66, 7942–7947. [Google Scholar] [CrossRef]
- Muíño, I.; Díaz, M.T.; Apeleo, E.; Pérez-Santaescolástica, C.; Rivas-Cañedo, A.; Pérez, C.; Cañeque, V.; Lauzurica, S.; de la Fuente, J. Valorisation of an extract from olive oil waste as a natural antioxidant for reducing meat waste resulting from oxidative processes. J. Clean. Prod. 2017, 140, 924–932. [Google Scholar] [CrossRef]
- Bitalebi, S.; Nikoo, M.; Rahmanifarah, K.; Noori, F.; Ahmadi Gavlighi, H. Effect of apple peel extract as natural antioxidant on lipid and protein oxidation of rainbow trout (Oncorhynchus mykiss) mince. Int. Aquat. Res. 2019, 11, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.L.; Patel, J.; Jaroni, D.; Friedman, M.; Ravishankar, S. Antimicrobial activity of apple, hibiscus, olive, and hydrogen peroxide formulations against Salmonella enterica on organic leafy greens. J. Food Prot. 2011, 74, 1676–1683. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.M.; Rabii, N.S.; Garbaj, A.M.; Abolghait, S.K. Antibacterial effect of olive (Olea europaea L.) leaves extract in raw peeled undeveined shrimp (Penaeus semisulcatus). Int. J. Vet. Sci. Med. 2014, 2, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.; Stepanyan, V.; Allen, P.; O’grady, M.; Kerry, J. Effect of lutein, sesamol, ellagic acid and olive leaf extract on the quality and shelf-life stability of packaged raw minced beef patties. Meat Sci. 2010, 84, 613–620. [Google Scholar] [CrossRef]
- Martínez, L.; Castillo, J.; Ros, G.; Nieto, G. Antioxidant and antimicrobial activity of rosemary, pomegranate and olive extracts in fish patties. Antioxidants 2019, 8, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanatt, S.R.; Chander, R.; Sharma, A. Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. Int. J. Food Sci. Technol. 2010, 45, 216–222. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-food byproducts as a new source of natural food additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Rezende, C.A.; Rodrigues, R.A.; Barbero, G.F.; Rosa, P.; Martínez, J. Encapsulation of anthocyanin-rich extract from blackberry residues by spray-drying, freeze-drying and supercritical antisolvent. Powder Technol. 2018, 340, 553–562. [Google Scholar] [CrossRef]
- Ledesma-Escobar, C.A.; de Castro, M.D.L. Towards a comprehensive exploitation of citrus. Trends Food Sci. Technol. 2014, 39, 63–75. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Carocho, M.; Morales, P.; Ferreira, I.C. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-S.; Wang, A.-B.; Zang, X.-P.; Tan, L.; Xu, B.-Y.; Chen, H.-H.; Jin, Z.-Q.; Ma, W.-H. Physicochemical, functional and emulsion properties of edible protein from avocado (Persea americana Mill.) oil processing by-products. Food Chem. 2019, 288, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Chel-Guerrero, L.; Barbosa-Martín, E.; Martínez-Antonio, A.; González-Mondragón, E.; Betancur-Ancona, D. Some physicochemical and rheological properties of starch isolated from avocado seeds. Int. J. Biol. Macromol. 2016, 86, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Gaur, V.K.; Bajaj, A.; Regar, R.K.; Kamthan, M.; Jha, R.R.; Srivastava, J.K.; Manickam, N. Rhamnolipid from a Lysinibacillus sphaericus strain IITR51 and its potential application for dissolution of hydrophobic pesticides. Bioresour. Technol. 2019, 272, 19–25. [Google Scholar] [CrossRef]
- Gaur, V.K.; Regar, R.K.; Dhiman, N.; Gautam, K.; Srivastava, J.K.; Patnaik, S.; Kamthan, M.; Manickam, N. Biosynthesis and characterization of sophorolipid biosurfactant by Candida spp.: Application as food emulsifier and antibacterial agent. Bioresour. Technol. 2019, 285, 121314. [Google Scholar] [CrossRef]
- Singh, P.; Patil, Y.; Rale, V. Biosurfactant production: Emerging trends and promising strategies. J. Appl. Microbiol. 2019, 126, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Kopsahelis, A.; Kourmentza, C.; Zafiri, C.; Kornaros, M. Gate-to-gate life cycle assessment of biosurfactants and bioplasticizers production via biotechnological exploitation of fats and waste oils. J. Chem. Technol. Biotechnol. 2018, 93, 2833–2841. [Google Scholar] [CrossRef]
- Patowary, R.; Patowary, K.; Kalita, M.C.; Deka, S. Utilization of paneer whey waste for cost-effective production of rhamnolipid biosurfactant. Appl. Biochem. Biotechnol. 2016, 180, 383–399. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhao, Y. Chemical composition and functional properties of three soy processing by-products (soy hull, okara and molasses). Qual. Assur. Saf. Crops Foods 2015, 7, 651–660. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Moreira, F.S.; Cardoso, V.L.; de Resende, M.M. Soy molasses as a fermentation substrate for the production of biosurfactant using Pseudomonas aeruginosa ATCC 10145. Environ. Sci. Pollut. Res. 2017, 24, 18699–18709. [Google Scholar] [CrossRef]
- Ramírez, M.V.; Escobar, A.H.; Garces, A. Hydrothermal coordination considering wind and pumping storage unit in the Colombian smart grid. In Proceedings of the 2015 IEEE PES Innovative Smart Grid Technologies Latin America (ISGT LATAM), Montevideo, Uruguay, 5–7 October 2015; pp. 231–236. [Google Scholar]
- Olasanmi, I.O.; Thring, R.W. The role of biosurfactants in the continued drive for environmental sustainability. Sustainability 2018, 10, 4817. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, Y.; Rasoul-Amini, S.; Morowvat, M. Algae for the production of SCP. In Bioprocess Sciences and Technology; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 163–184. [Google Scholar]
- Bertasini, D.; Binati, R.L.; Bolzonella, D.; Battista, F. Single Cell Proteins production from food processing effluents and digestate. Chemosphere 2022, 296, 134076. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Z.; Hu, P.; Zhang, S.; Luo, G. Two-stage fermentation enhanced single-cell protein production by Yarrowia lipolytica from food waste. Bioresour. Technol. 2022, 361, 127677. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, Y.; Hu, W.; Zheng, X.; Chen, Y. Valorization of food waste fermentation liquid into single cell protein by photosynthetic bacteria via stimulating carbon metabolic pathway and environmental behaviour. Bioresour. Technol. 2022, 361, 127704. [Google Scholar] [CrossRef] [PubMed]
- Pillaca-Pullo, O.S.; Lopes, A.M.; Rodriguez-Portilla, L.M.; Estela-Escalante, W. Optimizing medium composition with wastewater from Coffea arabica processing to produce single-cell protein using Candida sorboxylosa. J. Chem. Technol. Biotechnol. 2022, 4, 18. [Google Scholar] [CrossRef]
- Khan, M.K.I.; Asif, M.; Razzaq, Z.U.; Nazir, A.; Maan, A.A. Sustainable food industrial waste management through single cell protein production and characterization of protein enriched bread. Food Biosci. 2022, 46, 101406. [Google Scholar] [CrossRef]
- Zhu, N.-m.; Luo, T.; Guo, X.-j.; Zhang, H.; Deng, Y. Nutrition potential of biogas residues as organic fertilizer regarding the speciation and leachability of inorganic metal elements. Environ. Technol. 2015, 36, 992–1000. [Google Scholar] [CrossRef]
- Tampio, E.; Salo, T.; Rintala, J. Agronomic characteristics of five different urban waste digestates. J. Environ. Manag. 2016, 169, 293–302. [Google Scholar] [CrossRef]
- Cheong, J.C.; Lee, J.T.; Lim, J.W.; Song, S.; Tan, J.K.; Chiam, Z.Y.; Yap, K.Y.; Lim, E.Y.; Zhang, J.; Tan, H.T. Closing the food waste loop: Food waste anaerobic digestate as fertilizer for the cultivation of the leafy vegetable, xiao bai cai (Brassica rapa). Sci. Total Environ. 2020, 715, 136789. [Google Scholar] [CrossRef]
- Zou, Q.; Xiang, H.; Jiang, J.; Li, D.; Aihemaiti, A.; Yan, F.; Liu, N. Vanadium and chromium-contaminated soil remediation using VFAs derived from food waste as soil washing agents: A case study. J. Environ. Manag. 2019, 232, 895–901. [Google Scholar] [CrossRef]
- Beiyuan, J.; Tsang, D.C.; Bolan, N.S.; Baek, K.; Ok, Y.S.; Li, X.-D. Interactions of food waste compost with metals and metal-chelant complexes during soil remediation. J. Clean. Prod. 2018, 192, 199–206. [Google Scholar] [CrossRef]
- Du, C.; Abdullah, J.J.; Greetham, D.; Fu, D.; Yu, M.; Ren, L.; Li, S.; Lu, D. Valorization of food waste into biofertiliser and its field application. J. Clean. Prod. 2018, 187, 273–284. [Google Scholar] [CrossRef] [Green Version]
- More, A.; Srinivasan, A.; Liao, P.H.; Lo, K.V. Microwave enhanced oxidation treatment of organic fertilizers. J. Sci. Food Agric. 2017, 97, 3233–3239. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Potential of using organic fertilizer to cultivate Chlorella vulgaris for biodiesel production. Appl. Energy 2012, 94, 303–308. [Google Scholar] [CrossRef]
- Gironi, F.; Piemonte, V. Bioplastics and petroleum-based plastics: Strengths and weaknesses. Energy Sources Part A Recovery Util. Environ. Eff. 2011, 33, 1949–1959. [Google Scholar] [CrossRef]
- Tsang, Y.F.; Kumar, V.; Samadar, P.; Yang, Y.; Lee, J.; Ok, Y.S.; Song, H.; Kim, K.-H.; Kwon, E.E.; Jeon, Y.J. Production of bioplastic through food waste valorization. Environ. Int. 2019, 127, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Prabisha, T.P.; Sindhu, R.; Binod, P.; Sankar, V.; Raghu, K.G.; Pandey, A. Production and characterization of PHB from a novel isolate Comamonas sp. from a dairy effluent sample and its application in cell culture. Biochem. Eng. J. 2015, 101, 150–159. [Google Scholar] [CrossRef]
- Tripathi, A.; Narayanan, S. Potassium doped graphitic carbon nitride with extended optical absorbance for solar light driven photocatalysis. Appl. Surf. Sci. 2019, 479, 1–11. [Google Scholar] [CrossRef]
- Pandian, S.R.; Deepak, V.; Kalishwaralal, K.; Rameshkumar, N.; Jeyaraj, M.; Gurunathan, S. Optimization and fed-batch production of PHB utilizing dairy waste and sea water as nutrient sources by Bacillus megaterium SRKP-3. Bioresour. Technol. 2010, 101, 705–711. [Google Scholar] [CrossRef]
- Boneberg, B.S.; Machado, G.D.; Santos, D.F.; Gomes, F.; Faria, D.J.; Gomes, L.A.; Santos, F.A. Biorefinery of lignocellulosic biopolymers. Rev. Eletrônica Científica Da UERGS 2016, 2, 79–100. [Google Scholar] [CrossRef] [Green Version]
- Ashter, S.A. Introduction to Bioplastics Engineering; William Andrew, B., Ed.; Braun Medical Inc.: Bethlehem, PA, USA, 2016. [Google Scholar]
- Westendorf, M.L. Food waste as animal feed: An introduction. In Food Waste to Animal Feed; Iowa State University Press: Ames, IA, USA, 2000; pp. 3–16. [Google Scholar]
- San Martin, D.; Ramos, S.; Zufía, J. Valorisation of food waste to produce new raw materials for animal feed. Food Chem. 2016, 198, 68–74. [Google Scholar] [CrossRef]
- Sahoo, A.; Sarkar, S.; Lal, B.; Kumawat, P.; Sharma, S.; De, K. Utilization of fruit and vegetable waste as an alternative feed resource for sustainable and eco-friendly sheep farming. Waste Manag. 2021, 128, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, M.; Wadhwa, M.; Makkar, H.P. Waste to worth: Vegetable wastes as animal feed. CABI Rev. 2016, 11, 1–26. [Google Scholar] [CrossRef]
- Dou, Z.; Toth, J.D.; Westendorf, M.L. Food waste for livestock feeding: Feasibility, safety, and sustainability implications. Glob. Food Secur. 2018, 17, 154–161. [Google Scholar] [CrossRef]
- Westendorf, M.; Schuler, T.; Zirkle, E.; Hays, V.; Wilson, L. Nutritional Quality of Recycled Food Plate Waste in Diets Fed to Swine12. Prof. Anim. Sci. 1999, 15, 106–111. [Google Scholar] [CrossRef]
- Cheraghi Saray, S.; Hosseinkhani, A.; Janmohammadi, H.; Zare, P.; Daghighkia, H. Thermal and probiotic treatment effects on restaurant waste for incorporation into poultry diet. Int. J. Recycl. Org. Waste Agric. 2014, 3, 7. [Google Scholar] [CrossRef]
- Myer, R.; Brendemuhl, J.; Johnson, D. Dehydrated restaurant food waste as swine feed. In Food Waste to Animal Feed; Iowa State University Press: Ames, IA, USA, 2000; pp. 113–114. [Google Scholar]
- Chen, T.; Jin, Y.; Shen, D. A safety analysis of food waste-derived animal feeds from three typical conversion techniques in China. Waste Manag. 2015, 45, 42–50. [Google Scholar] [CrossRef]
- Mo, W.; Choi, W.; Man, K.; Wong, M.H. Food waste-based pellets for feeding grass carp (Ctenopharyngodon idellus): Adding baker’s yeast and enzymes to enhance growth and immunity. Sci. Total Environ. 2020, 707, 134954. [Google Scholar] [CrossRef]


| Food Waste Category | Main Source | Processing Method | Value-Added Products and Bioactive Compounds | References |
|---|---|---|---|---|
| Barley by-product | Cereal and pulses industry | Chemical extraction | Vitamin E, phytates, insoluble dietary fiber, and phenolic compounds | Papageorgiou and Skendi [9] |
| Rice bran and husk | Cereal and pulses industry | Enzymolysis | Phenolic compounds, γ-Oryzanol, and tocopherols | Wanyo, Meeso and Siriamornpun [11] |
| Wheat | Cereal and pulses industry | Chemical extraction, fermentation | Carbohydrates, lipid soluble vitamins, folic acid, phytosterols, amino acids, oligosaccharides, phenolic compounds, and peptides | Balandrán-Quintana, et al. [134] |
| Legumes | Cereal and pulses industry | Chemical extraction, fermentation | Activated carbon, proteins, lipids, fatty acids, vitamins, minerals, and phenolic compounds | Kiran, et al. [135], Ben-Othman, et al. [136] |
| Grape by-products | Fruits processing industry | Chemical extraction, fermentation, anaerobic digestion, gasification, pyrolysis distillation, producing single cell protein | Ethanol, flavanols, anthocyanins, procyanidins, tartaric acid, dietary fibre, grape seed oil, pomace oil, oleanolic acid, malates, citric acid, single cell protein. | Muhlack, et al. [137], Schieber [14] |
| Apple juice by-product | Fruits processing industry | Enzymolysis, chemical extraction | Pectin, lactic acid, citric acid, aroma compounds, butanol, fructooligosaccharides, and pectinases | Schieber [14], Kiran, Trzcinski, Ng and Liu [135] |
| Citrus processing industry | Fruits processing industry | Chemical extraction, distillation | Phenolics, pectin, essential oil (limonene), antioxidants, ethanol, and organic acids, | Matharu, de Melo, and Houghton [19], Schieber [14] |
| Avocado by-products | Fruits processing industry | Chemical extraction, homogenized, grinding | Phenolic compounds, acetogenins, phytosterols, carotenoids, alkaloids, starch, edible protein, and animal feed | Salazar-López, et al. [138] |
| Tomato waste | Vegetable processing industry | Supercritical fluid extraction using CO2, chemical extraction | Trans-lycopene, lycopene, and pectin | Nobre, et al. [139] |
| Potato and sweet potato | Vegetable processing industry | Hydrolysis, chemical extraction, pulsed electric fields, fermentation | Lysine, protein, adsorption dyes, starch, steroidal alkaloids, β-carotene, α-tocopherol cellulolytic enzymes, and biopolymer films | Lappalainen, et al. [140], OKUNO, et al. [141], Matharu, de Melo and Houghton [19] |
| Onions | Vegetable processing industry | Chemical extraction, macroporous resin adsorption | Dietary fiber, fructans, phenolic compounds | Kühn, et al. [142], Kiassos, et al. [143], Benítez, et al. [144] |
| Milk | Dairy industry | Transglycosidation, enzymolysis, fermentation, fractionation | Prebiotics, biodiesel, ethanol, whey protein, lactose, galactooligosaccharides, baker’s yeast, and minerals | Hua, et al. [145], Lappa, et al. [146] |
| Cheese, casein, yogurt | Dairy industry | Fractionation, producing single cell protein, fermentation, enzymolysis | Ethanol, single cell protein, animal feed, whey protein, lactose | Hua, Yang, Zhang, Fei, Jin and Jiang [145], Lappa, Papadaki, Kachrimanidou, Terpou, Koulougliotis, Eriotou and Kopsahelis [146] |
| Waste cooking oil | Family kitchen or commercial kitchen | Hydrolysis, esterification, producing single cell protein, fermentation | Biosurfactants and glycolipids, biodiesel, sterols, squalene, tocopherols and single cell protein | Henkel, et al. [147], Vescovi, et al. [148] |
| Olives | Edible oil industry | Combustion, chemical extraction | Phenolic compounds, carotenoids, squalene, dietary fiber and phytosterols | Pattara, et al. [149], Rodríguez, et al. [150] |
| Meat and poultry | Meat processing industry | Fermentation, anaerobic digestion, transesterification | Fertilizer, feather meal, lactic acid, animal feed, blood meal, meat and bone meal and probiotics | Yaakob, Mohamed, Al-Gheethi, Tiey and Kassim [47], Marques, Paz, Duval, Corrêa and Corrêa [50], Ashayerizadeh, Dastar, Samadi, Khomeiri, Yamchi and Zerehdaran [48] |
| Crabs, lobster and shrimps | Seafood processing industry | Grinding, destructive, fermentation | Chitin, calcium carbonate, protein, astaxanthin, and chitinase | Yan and Chen [53], Kumar, Kumar, George, Sharma and Gupta [52], Prameela, Venkatesh, Immandi, Kasturi, Krishna and Mohan [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; de Souza, T.S.P.; Holland, B.; Dunshea, F.; Barrow, C.; Suleria, H.A.R. Valorization of Food Waste to Produce Value-Added Products Based on Its Bioactive Compounds. Processes 2023, 11, 840. https://doi.org/10.3390/pr11030840
Liu Z, de Souza TSP, Holland B, Dunshea F, Barrow C, Suleria HAR. Valorization of Food Waste to Produce Value-Added Products Based on Its Bioactive Compounds. Processes. 2023; 11(3):840. https://doi.org/10.3390/pr11030840
Chicago/Turabian StyleLiu, Ziyao, Thaiza S. P. de Souza, Brendan Holland, Frank Dunshea, Colin Barrow, and Hafiz A. R. Suleria. 2023. "Valorization of Food Waste to Produce Value-Added Products Based on Its Bioactive Compounds" Processes 11, no. 3: 840. https://doi.org/10.3390/pr11030840
APA StyleLiu, Z., de Souza, T. S. P., Holland, B., Dunshea, F., Barrow, C., & Suleria, H. A. R. (2023). Valorization of Food Waste to Produce Value-Added Products Based on Its Bioactive Compounds. Processes, 11(3), 840. https://doi.org/10.3390/pr11030840








