Indolyl-Derived 4H-Imidazoles: PASE Synthesis, Molecular Docking and In Vitro Cytotoxicity Assay
Abstract
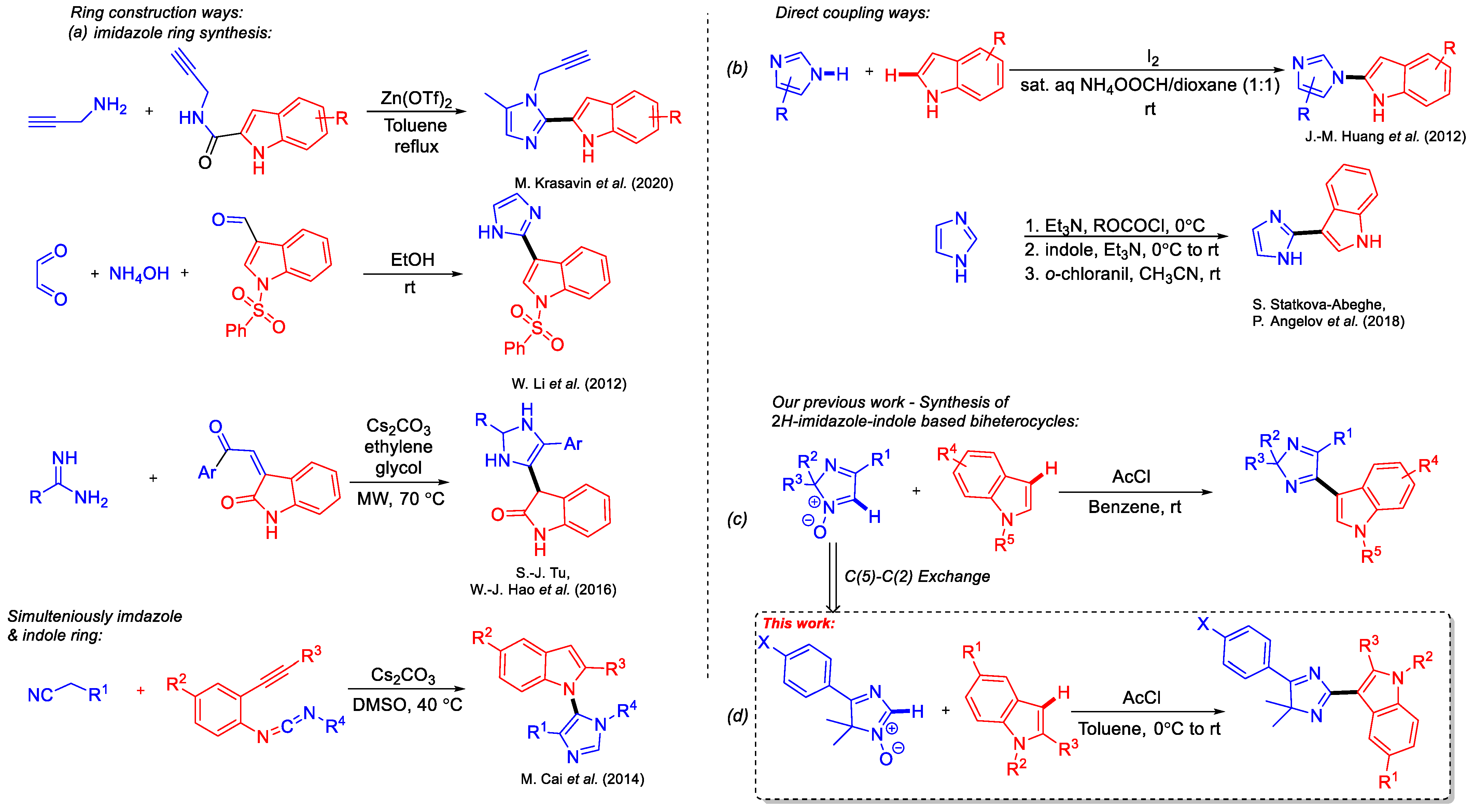
1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.1.1. Synthesis of 1-Hydroxy-2,5-Dihydroimidazoles 2a–c and 4H-Imidazole 3-Oxides 3a–c
2.1.2. General Procedure for the Synthesis of Hydrochloride Salt of Indolyl Imidazole Derivatives (5a–d)
2.1.3. General Procedure for the Synthesis of Indolyl Imidazole Derivatives (6e–h)
2.2. Molecular Docking Studies
2.3. In Vitro Studies
2.3.1. Cell Culture
2.3.2. Viability Assessment
2.3.3. Statistical Analysis
3. Results and Discussion
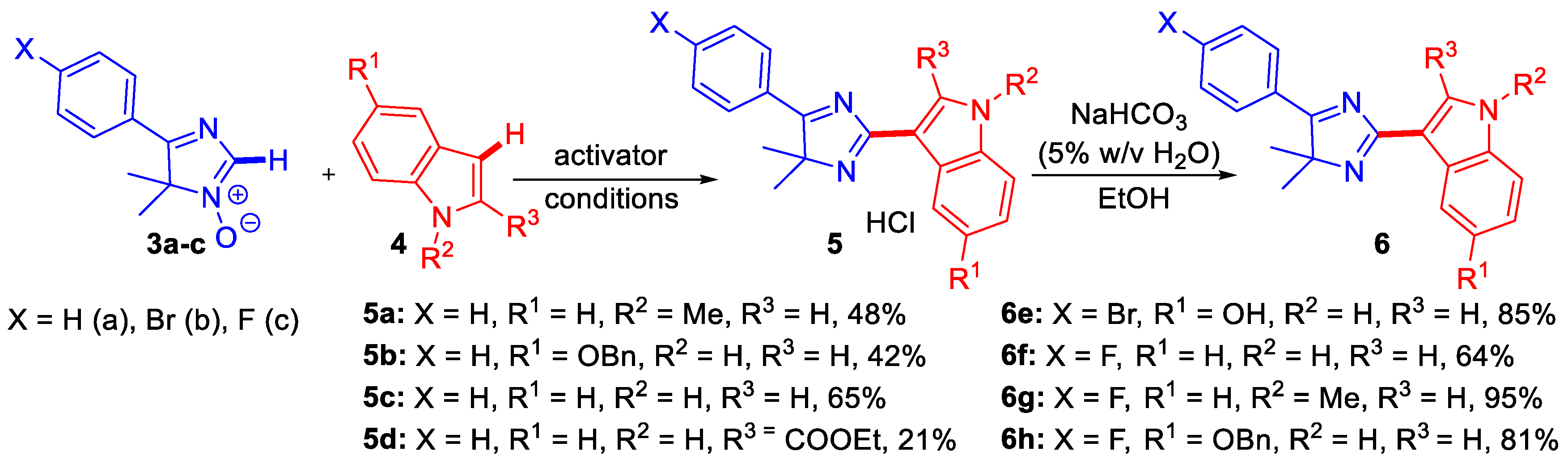
3.1. Synthesis
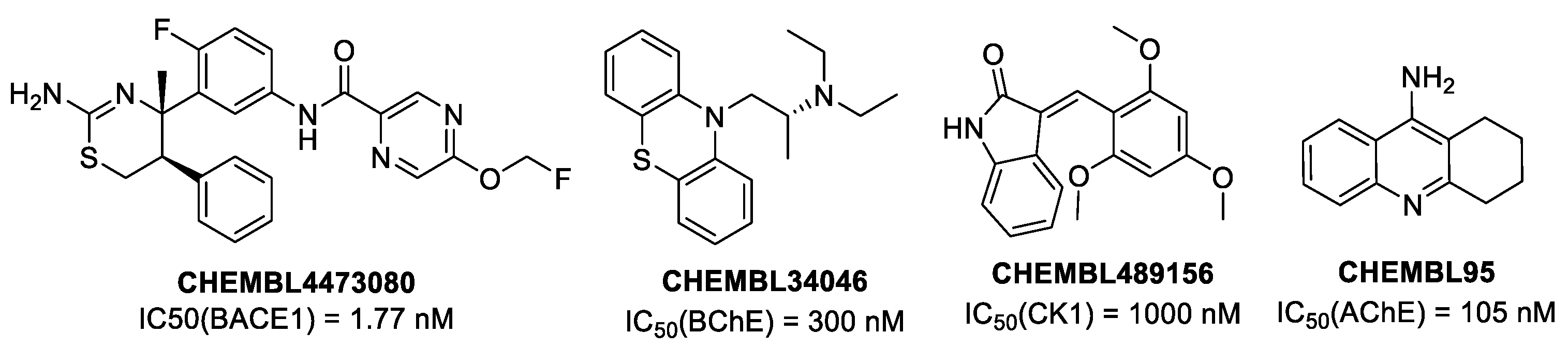
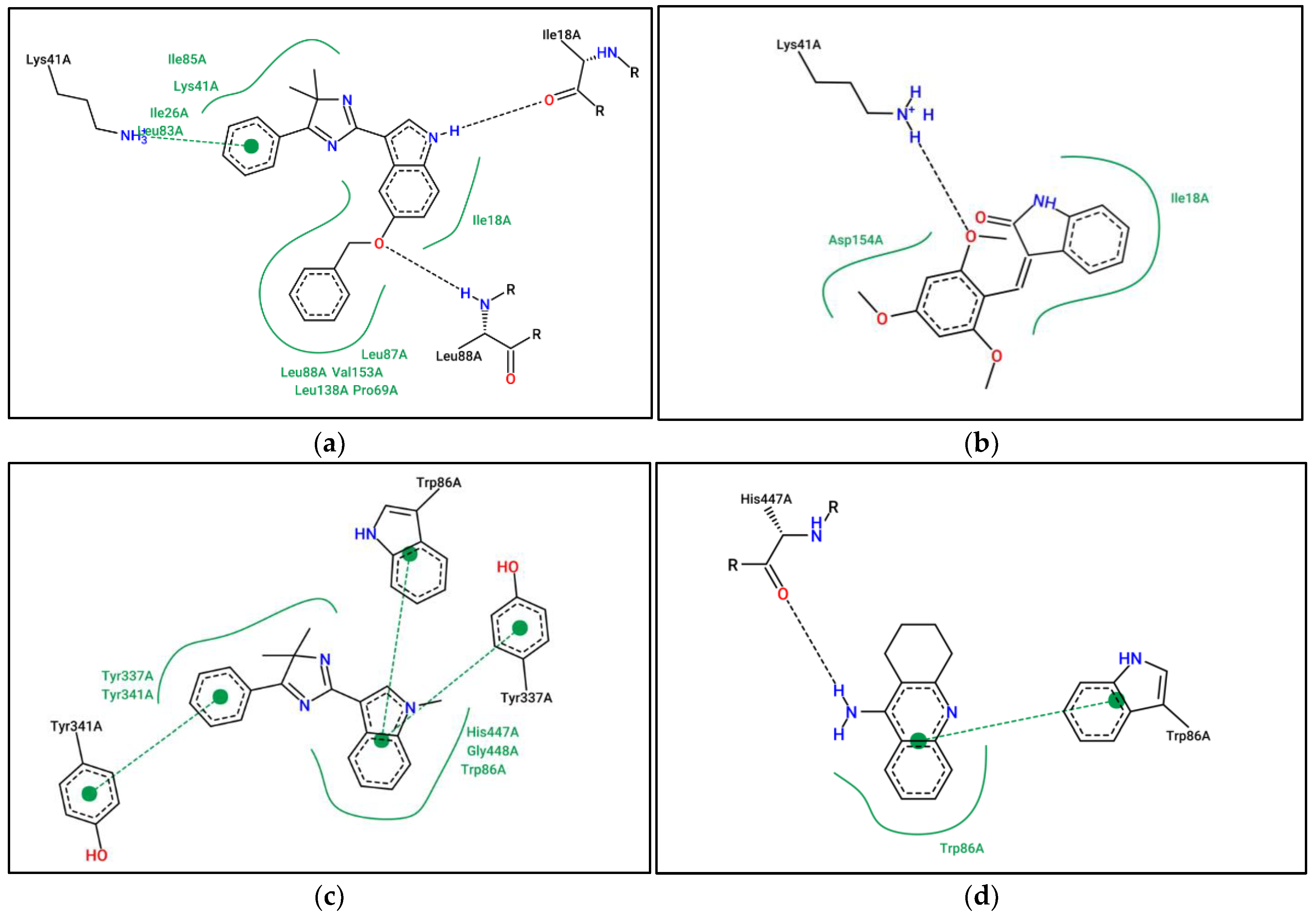
3.2. In Silico Studies
3.3. In Vitro Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Erkkinen, M.G.; Kim, M.-O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, A.; Carcaillon, L.; Kab, S.; Moisan, F. Epidemiology of Parkinson’s Disease. Rev. Neurol. 2016, 172, 14–26. [Google Scholar] [CrossRef]
- Min, Y.G.; Choi, S.-J.; Hong, Y.-H.; Kim, S.-M.; Shin, J.-Y.; Sung, J.-J. Dissociated Leg Muscle Atrophy in Amyotrophic Lateral Sclerosis/Motor Neuron Disease: The ‘Split-Leg’ Sign. Sci. Rep. 2020, 10, 15661. [Google Scholar] [CrossRef] [PubMed]
- Dayalu, P.; Albin, R.L. Huntington Disease. Neurol. Clin. 2015, 33, 101–114. [Google Scholar] [CrossRef]
- Murphy, M.P.; Hartley, R.C. Mitochondria as a Therapeutic Target for Common Pathologies. Nat. Rev. Drug Discov. 2018, 17, 865–886. [Google Scholar] [CrossRef] [PubMed]
- Ramrao, S.P.; Verma, A.; Waiker, D.K.; Tripathi, P.N.; Shrivastava, S.K. Design, Synthesis, and Evaluation of Some Novel Biphenyl Imidazole Derivatives for the Treatment of Alzheimer’s Disease. J. Mol. Struct. 2021, 1246, 131152. [Google Scholar] [CrossRef]
- Bolcato, G.; Cescon, E.; Pavan, M.; Bissaro, M.; Bassani, D.; Federico, S.; Spalluto, G.; Sturlese, M.; Moro, S. A Computational Workflow for the Identification of Novel Fragments Acting as Inhibitors of the Activity of Protein Kinase CK1δ. Int. J. Mol. Sci. 2021, 22, 9741. [Google Scholar] [CrossRef]
- Kaur, G.; Goyal, B. Deciphering the Molecular Mechanism of Inhibition of Β-Secretase (BACE1) Activity by a 2-Amino-imidazol-4-one Derivative. ChemistrySelect 2022, 7, e202202561. [Google Scholar] [CrossRef]
- Gujjarappa, R.; Kabi, A.K.; Sravani, S.; Garg, A.; Vodnala, N.; Tyagi, U.; Kaldhi, D.; Velayutham, R.; Singh, V.; Gupta, S.; et al. Overview on Biological Activities of Imidazole Derivatives. In Nanostructured Biomaterials. Materials Horizons: From Nature to Nanomaterials; Swain, B., Ed.; Springer: Singapore, 2022; pp. 135–227. ISBN 978-981-16-8399-2. [Google Scholar]
- Wu, J.; Liu, Q.; Hu, Y.; Wang, W.; Gao, X. Discovery of Novel Procaine-Imidazole Derivative as Inhibitor of Monoamine Oxidase-B for Potential Benefit in Parkinson’s Disease. ChemistrySelect 2020, 5, 10928–10932. [Google Scholar] [CrossRef]
- Cornec, A.-S.; Monti, L.; Kovalevich, J.; Makani, V.; James, M.J.; Vijayendran, K.G.; Oukoloff, K.; Yao, Y.; Lee, V.M.-Y.; Trojanowski, J.Q.; et al. Multitargeted Imidazoles: Potential Therapeutic Leads for Alzheimer’s and Other Neurodegenerative Diseases. J. Med. Chem. 2017, 60, 5120–5145. [Google Scholar] [CrossRef]
- Nirwan, N.; Pareek, C.; Swami, V.K. Indolylimidazoles: Synthetic Approaches and Biological Activities. Curr. Chem. Lett. 2020, 9, 31–50. [Google Scholar] [CrossRef]
- Kawano, T.; Inokuchi, J.; Eto, M.; Murata, M.; Kang, J.-H. Activators and Inhibitors of Protein Kinase C (PKC): Their Applications in Clinical Trials. Pharmaceutics 2021, 13, 1748. [Google Scholar] [CrossRef]
- Hogendorf, A.S.; Hogendorf, A.; Popiołek-Barczyk, K.; Ciechanowska, A.; Mika, J.; Satała, G.; Walczak, M.; Latacz, G.; Handzlik, J.; Kieć-Kononowicz, K.; et al. Fluorinated indole-imidazole conjugates: Selective orally bioavailable 5-HT7 receptor low-basicity agonists, potential neuropathic painkillers. Eur. J. Med. Chem. 2019, 170, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Bakholdina, A.; Lukin, A.; Bakulina, O.; Guranova, N.; Krasavin, M. Dual Use of Propargylamine Building Blocks in the Construction of Polyheterocyclic Scaffolds. Tetrahedron Lett. 2020, 61, 151970. [Google Scholar] [CrossRef]
- Chen, J.; Ahn, S.; Wang, J.; Lu, Y.; Dalton, J.T.; Miller, D.D.; Li, W. Discovery of Novel 2-Aryl-4-Benzoyl-Imidazole (ABI-III) Analogues Targeting Tubulin Polymerization As Antiproliferative Agents. J. Med. Chem. 2012, 55, 7285–7289. [Google Scholar] [CrossRef] [PubMed]
- Suvorov, N.N.; Smushkevich, Y.I.; Mar’yanovskaya, N.N.; Sulima, A.V. Indole Derivatives. LXI. Synthesis of 4(5)-(Indolyl-3)-Imidazole. Pharm. Chem. J. 1970, 4, 68–70. [Google Scholar] [CrossRef]
- El-Nakkady, S.S.; Hanna, M.M.; Roaiah, H.M.; Ghannam, I.A.Y. Synthesis, Molecular Docking Study and Antitumor Activity of Novel 2-Phenylindole Derivatives. Eur. J. Med. Chem. 2012, 47, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Dai, M.-L.; Pan, Y.-Q.; Zhang, C.; Tu, S.-J.; Hao, W.-J. Regioselective Synthesis of 3-(Imidazol-4-Yl) Indolin-2-Ones under Microwave Heating. J. Heterocycl. Chem. 2016, 54, 1479–1485. [Google Scholar] [CrossRef]
- Hary, U.; Roettig, U.; Paal, M. Efficient Synthesis of 3-(4,5-Dihydro-1H-Imidazole-2-Yl)-1H-Indoles. Tetrahedron Lett. 2001, 42, 5187–5189. [Google Scholar] [CrossRef]
- Hao, W.; Jiang, Y.; Cai, M. Synthesis of Indolyl Imidazole Derivatives via Base-Promoted Tandem Reaction of N-[2-(1-Alkynyl)Phenyl]Carbodiimides with Isocyanides. J. Org. Chem. 2014, 79, 3634–3640. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-B.; Huang, J.-M. Highly Regioselective C–N Bond Formation through C–H Azolation of Indoles Promoted by Iodine in Aqueous Media. Org. Lett. 2012, 14, 5832–5835. [Google Scholar] [CrossRef] [PubMed]
- Stremski, Y.; Statkova-Abeghe, S.; Angelov, P.; Ivanov, I. Synthesis of Camalexin and Related Analogues. J. Heterocycl. Chem. 2018, 55, 1589–1595. [Google Scholar] [CrossRef]
- Liu, X.; He, K.; Gao, N.; Jiang, P.; Lin, J.; Jin, Y. A Radical-Mediated Multicomponent Cascade Reaction for the Synthesis of Azide-Biindole Derivatives. Chem. Commun. 2021, 57, 9696–9699. [Google Scholar] [CrossRef] [PubMed]
- Bergman, J.; Renström, L.; Sjöberg, B. Synthesis of Aromatic Aldehydes via 2-Aryl-n,n’-Diacyl-4-Imidazolines. Tetrahedron 1980, 36, 2505–2511. [Google Scholar] [CrossRef]
- Varaksin, M.V.; Utepova, I.A.; Chupakhin, O.N.; Charushin, V.N. Palladium(II)-Catalyzed Oxidative C–H/C–H Coupling and Eliminative SNH Reactions in Direct Functionalization of Imidazole Oxides with Indoles. J. Org. Chem. 2012, 77, 9087–9093. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, L. Green Shades in Organic Synthesis. Eur. J. Org. Chem. 2020, 2020, 4273–4283. [Google Scholar] [CrossRef]
- de Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of Green Chemistry and Its Multidimensional Impacts: A Review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef]
- Gujral, S.S.; Sheela, M.A.; Khatri, S.; Singla, R.K. A Focus & Review on the Advancement of Green Chemistry. Indo Glob. J. Pharm. Sci. 2012, 02, 397–408. [Google Scholar] [CrossRef]
- Charushin, V.N.; Chupakhin, O.N. Nucleophilic C—H Functionalization of Arenes: A Contribution to Green Chemistry. Russ. Chem. Bull. 2019, 68, 453–471. [Google Scholar] [CrossRef]
- Chupakhin, O.N.; Charushin, V.N. Recent Advances in the Field of Nucleophilic Aromatic Substitution of Hydrogen. Tetrahedron Lett. 2016, 57, 2665–2672. [Google Scholar] [CrossRef]
- Akulov, A.A.; Varaksin, M.V.; Charushin, V.N.; Chupakhin, O.N. C(sp2)–H Functionalization of Aldimines and Related Compounds: Advances and Prospects. Russ. Chem. Rev. 2021, 90, 374–394. [Google Scholar] [CrossRef]
- Edeleva, M.V.; Parkhomenko, D.A.; Morozov, D.A.; Dobrynin, S.A.; Trofimov, D.G.; Kanagatov, B.; Kirilyuk, I.A.; Bagryanskaya, E.G. Controlled/Living Polymerization of Methyl Methacrylate Using New Sterically Hindered Imidazoline Nitroxides Prepared via Intramolecular 1,3-Dipolar Cycloaddition Reaction. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 929–943. [Google Scholar] [CrossRef]
- Volodarskii, L.B.; Sevast’yanova, T.K. Synthesis and properties of α-hydroxylamino ketones. Zh. Org. Khim. 1971, 7, 1687–1692. [Google Scholar]
- Amitina, S.A.; Zaytseva, E.V.; Dmitrieva, N.A.; Lomanovich, A.V.; Kandalintseva, N.V.; Ten, Y.A.; Artamonov, I.A.; Markov, A.F.; Mazhukin, D.G. 5-Aryl-2-(3,5-Dialkyl-4-Hydroxyphenyl)-4,4-Dimethyl-4H-Imidazole 3-Oxides and Their Redox Species: How Antioxidant Activity of 1-Hydroxy-2,5-Dihydro-1H-Imidazoles Correlates with the Stability of Hybrid Phenoxyl–Nitroxides. Molecules 2020, 25, 3118. [Google Scholar] [CrossRef]
- Kirilyuk, I.A.; Grigor’ev, I.A.; Volodarskii, L.B. Synthesis of 3-imidazolines and 3-imidazoline 3-oxides containing a hydrogen atom at C-2. Izv. SO AN SSSR Ser. Khim. 1989, 2, 99–106. [Google Scholar]
- Grigor’ev, I.A.; Kirilyuk, I.A.; Volodarskii, L.B. NMR Spectra of Cyclic Nitrones. 4. Synthesis and 13C NMR Spectra of 4H-Imidazole N-Oxides and N,N-Dioxides. Chem. Heterocycl. Compd. 1988, 24, 1355–1362. [Google Scholar] [CrossRef]
- Russell, W.C.; Graham, F.L.; Smiley, J.; Nairn, R. Characteristics of a Human Cell Line Transformed by DNA from Human Adenovirus Type 5. J. Gen. Virol. 1977, 36, 59–72. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Voinov, M.A.; Volodarsky, L.B. Synthesis and Properties Of N-[1-Hydroxyimino-2-Methyl-1-(2-Pyridyl)Prop-2-Yl]Hydroxylamine and Heterocyclic Derivatives Based on It. Russ. Chem. Bull. 1997, 46, 126–132. [Google Scholar] [CrossRef]
- Tayu, M.; Nomura, K.; Kawachi, K.; Higuchi, K.; Saito, N.; Kawasaki, T. Direct C2-Functionalization of Indoles Triggered by the Generation of Iminium Species from Indole and Sulfonium Salt. Chem.—A Eur. J. 2017, 23, 10925–10930. [Google Scholar] [CrossRef]
- Moseev, T.D.; Nikiforov, E.A.; Varaksin, M.V.; Charushin, V.N.; Chupakhin, O.N. Metal-Free C–H/C–H Coupling of 2 H -Imidazole 1-Oxides with Polyphenols toward Imidazole-Linked Polyphenolic Compounds. J. Org. Chem. 2021, 86, 13702–13710. [Google Scholar] [CrossRef] [PubMed]
- Varaksin, M.; Moseev, T.; Chupakhin, O.; Charushin, V.; Trofimov, B. Metal-Free C–H Functionalization of 2H-Imidazole 1-Oxides with Pyrrolyl Fragments in the Design of Novel Azaheterocyclic Ensembles. Org. Biomol. Chem. 2017, 15, 8280–8284. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.A. Molecular docking using ArgusLab, an efficient shape-based search algorithm and AScore scoring function. In Proceedings of the ACS Meeting, Philadelphia, PA, USA, 22–26 August 2004; p. 172. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Lee, H.J.; Barden, C.J.; Weaver, D.F. The Blood–Brain Barrier (BBB) Score. J. Med. Chem. 2019, 62, 9824–9836. [Google Scholar] [CrossRef] [PubMed]







| Docking Score (kcal/mol) | ||||
|---|---|---|---|---|
| Structure | BACE1 6jse | BChE 6eqp | CK1δ 1eh4 | AChE 7e3i |
| 5a | −11.60 | −10.96 | −9.78 | −13.57 |
| 5b | −12.57 * | −11.58 | −13.09 | −13.33 |
| 5d | −10.04 | −12.89 | −11.53 | −12.42 |
| 6g | −10.77 | −10.66 | −10.21 | −11.59 |
| 6h | −11.27 | −12.22 | −10.99 | −12.69 |
| CHEMBL4473080 | −11.27 | - | - | - |
| SCHEMBL34046 | - | −8.93 | - | - |
| CHEMBL489156 | - | - | −9.45 | - |
| CHEMBL95 | - | - | - | −8.50 |
| Entry a | Solvent | Activating Agent (Equiv) | Temperature (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| 1 | Toluene | AcCl (1.0) | 0 °C to rt | 4 | 25 b |
| 2 | Toluene | AcCl (1.0) | 0 °C to rt | 0.5 | 48 b |
| 3 | Toluene | AcCl (1.0) | 0 °C to rt | 2 | 35 b |
| 4 | Toluene | Ethyl chloroformate (1.0) | 0 °C to rt | 0.5 | 20 b |
| 5 | Toluene | Oxalyl chloride (1.0) | 0 °C to rt | 0.5 | 0 c |
| 6 | PEG-400 | AcCl (1.0) | 0 °C to rt | 4 | 10 b |
| 7 | Hexane/Toluene (1/1) | AcCl (1.0) | 0 °C to rt | 4 | 15 b |
| 8 | 2-Me-THF | AcCl (1.0) | 0 °C to rt | 4 | 0 c |
| 9 | Anisole | AcCl (1.0) | 0 °C to rt | 4 | 0 c |
| Entry | Compound | IC50 ± SE |
|---|---|---|
| 1 | 5a | 306.85 ± 37.65 |
| 2 | 5b | 59.58 ± 3.63 |
| 3 | 5d | 66.60 ± 4.98 |
| 4 | 6g | 181.68 ± 20.69 |
| 5 | 6h | >256 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikiforov, E.A.; Vaskina, N.F.; Moseev, T.D.; Varaksin, M.V.; Butorin, I.I.; Melekhin, V.V.; Tokhtueva, M.D.; Mazhukin, D.G.; Tikhonov, A.Y.; Charushin, V.N.; et al. Indolyl-Derived 4H-Imidazoles: PASE Synthesis, Molecular Docking and In Vitro Cytotoxicity Assay. Processes 2023, 11, 846. https://doi.org/10.3390/pr11030846
Nikiforov EA, Vaskina NF, Moseev TD, Varaksin MV, Butorin II, Melekhin VV, Tokhtueva MD, Mazhukin DG, Tikhonov AY, Charushin VN, et al. Indolyl-Derived 4H-Imidazoles: PASE Synthesis, Molecular Docking and In Vitro Cytotoxicity Assay. Processes. 2023; 11(3):846. https://doi.org/10.3390/pr11030846
Chicago/Turabian StyleNikiforov, Egor A., Nailya F. Vaskina, Timofey D. Moseev, Mikhail V. Varaksin, Ilya I. Butorin, Vsevolod V. Melekhin, Maria D. Tokhtueva, Dmitrii G. Mazhukin, Alexsei Y. Tikhonov, Valery N. Charushin, and et al. 2023. "Indolyl-Derived 4H-Imidazoles: PASE Synthesis, Molecular Docking and In Vitro Cytotoxicity Assay" Processes 11, no. 3: 846. https://doi.org/10.3390/pr11030846
APA StyleNikiforov, E. A., Vaskina, N. F., Moseev, T. D., Varaksin, M. V., Butorin, I. I., Melekhin, V. V., Tokhtueva, M. D., Mazhukin, D. G., Tikhonov, A. Y., Charushin, V. N., & Chupakhin, O. N. (2023). Indolyl-Derived 4H-Imidazoles: PASE Synthesis, Molecular Docking and In Vitro Cytotoxicity Assay. Processes, 11(3), 846. https://doi.org/10.3390/pr11030846









