Mechanism of Surface Wettability of Nanostructure Morphology Enhancing Boiling Heat Transfer: Molecular Dynamics Simulation
Abstract
:1. Introduction
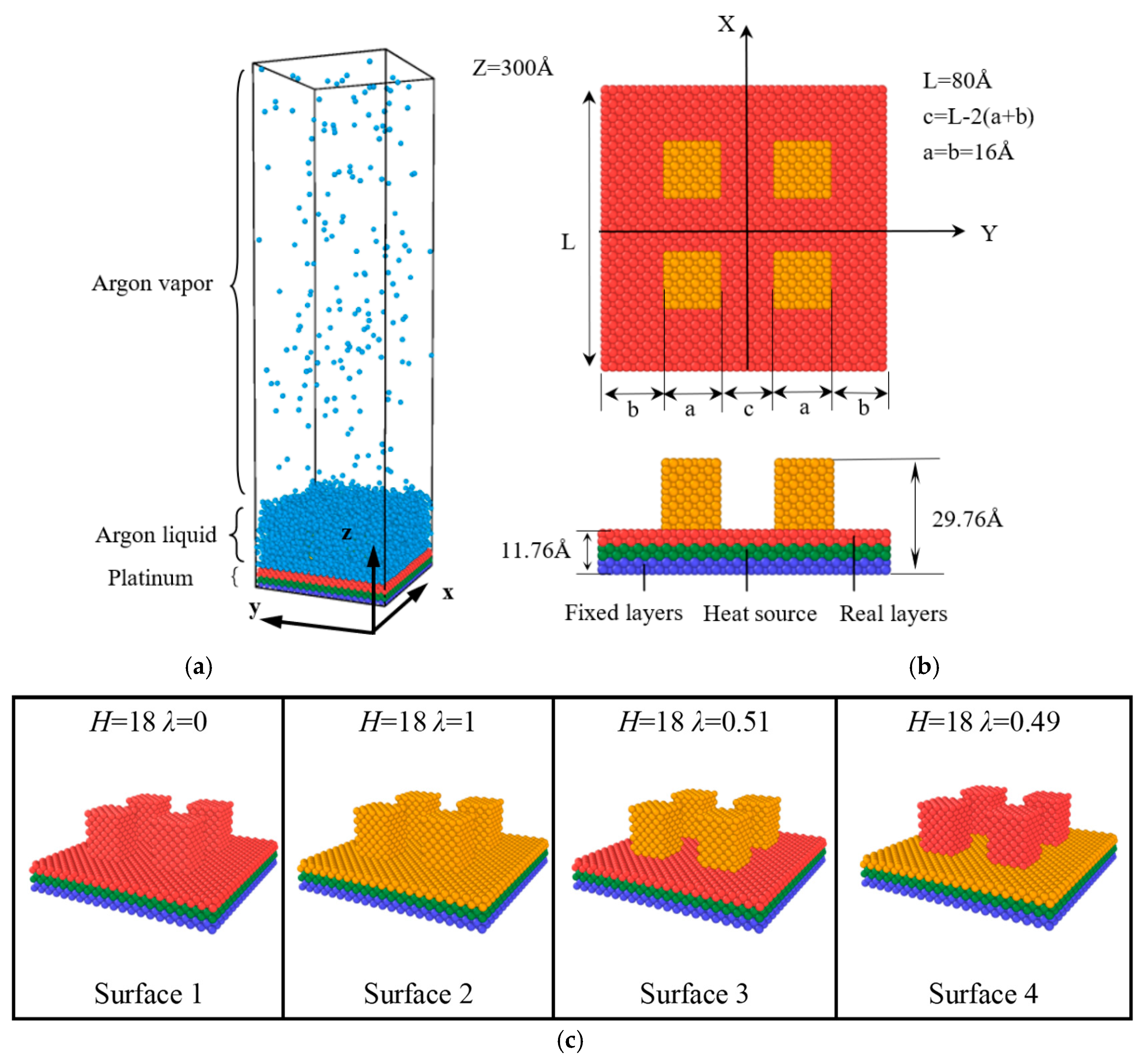
2. Models and Methods
3. Results and Discussion
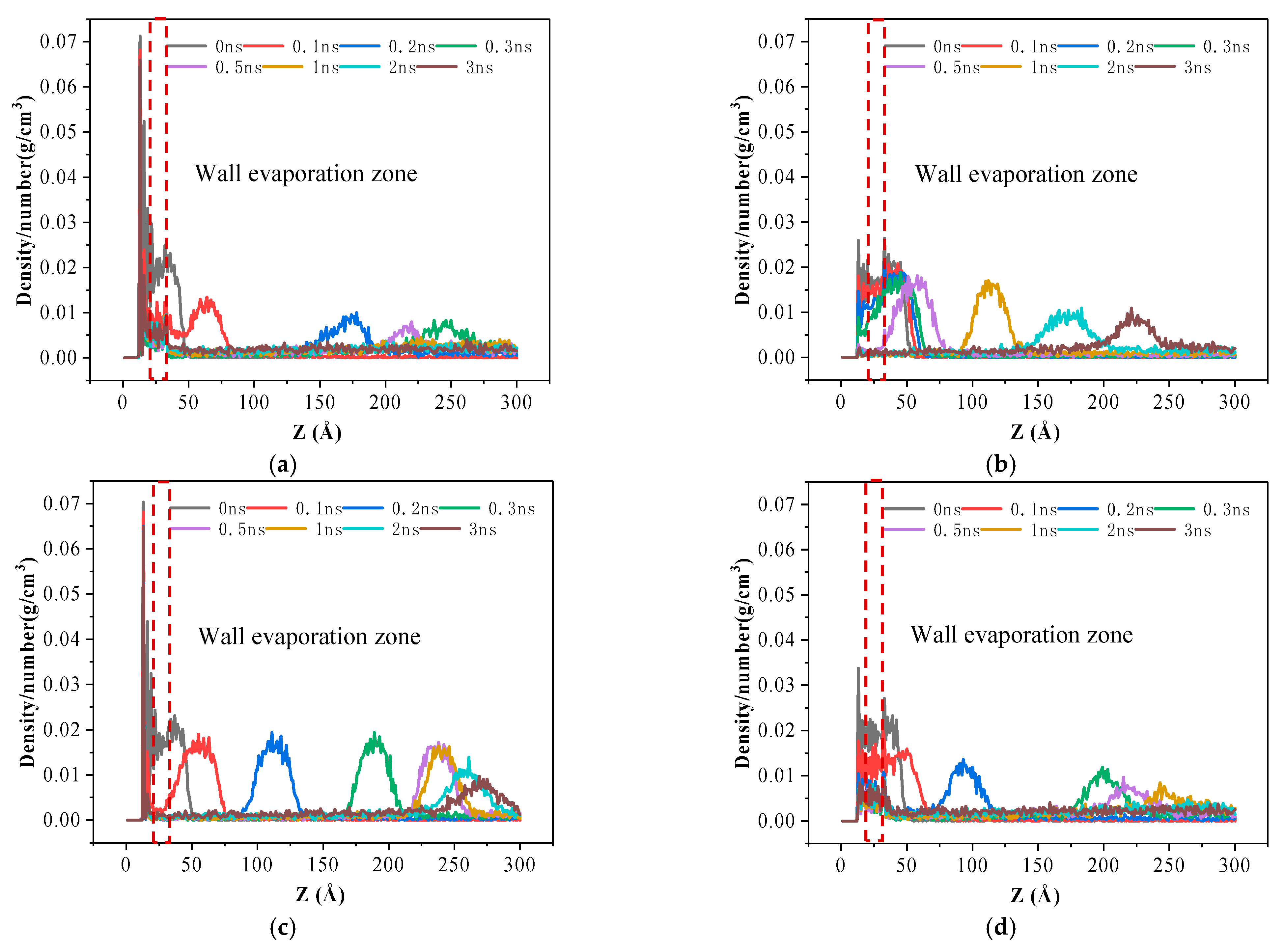
3.1. Movement and Distribution of Argon Atoms
3.2. Surface Dynamics Analysis and Nucleation of Nanostructures
3.3. Thermodynamic Analysis
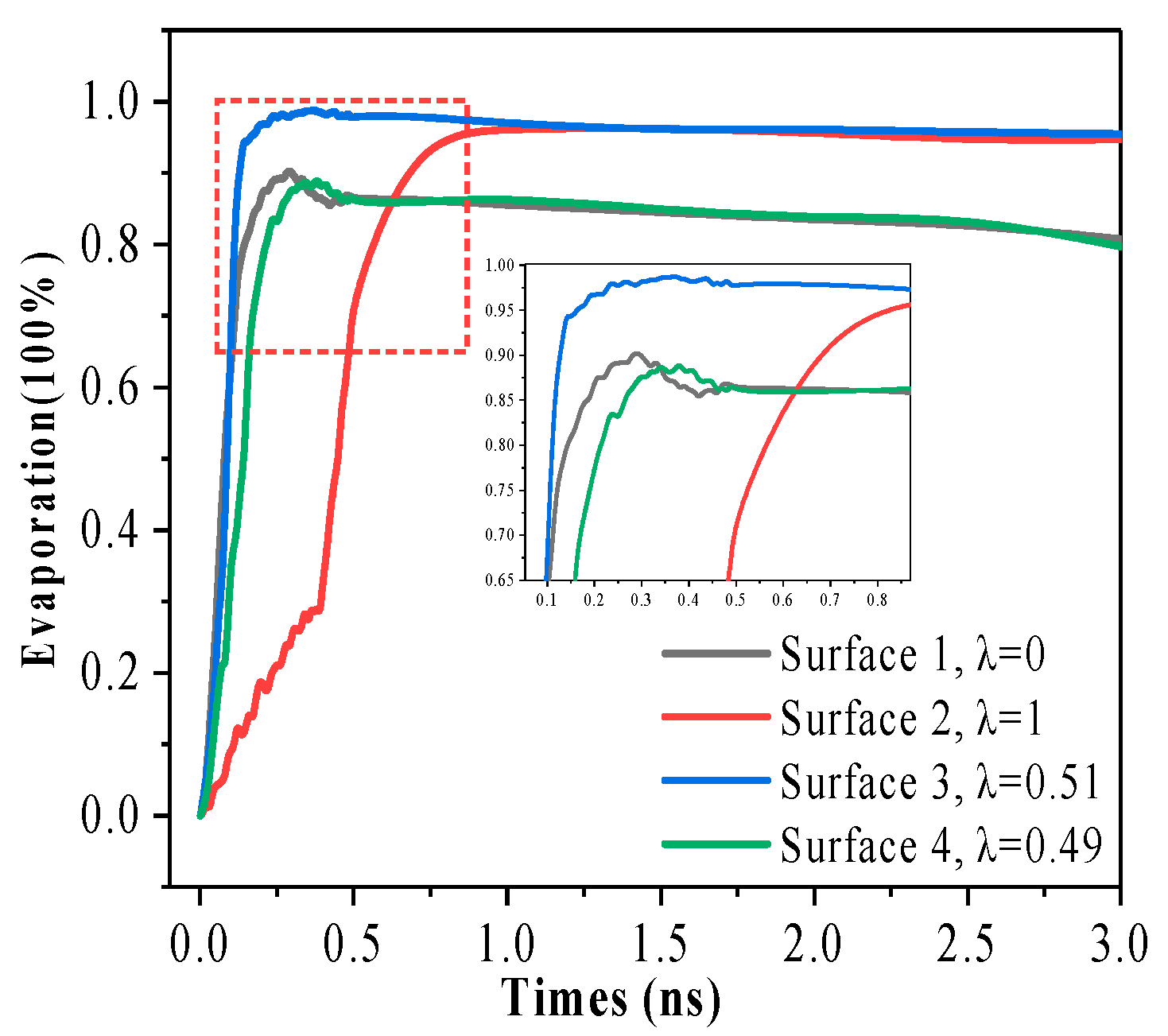
3.4. Evaporation Rate of Nanostructure Wetted Surface
4. Conclusions
- The pure hydrophilic nanostructure surface requires a significantly lower surface superheat for the gas–liquid phase transition to become evident compared to the pure hydrophobic nanostructure surface. It is worth pointing out that the hydrophilic surface responds quickly to the temperature change; the fluid temperature rises rapidly, reaches the critical point of gas–liquid phase change faster, and enhances the heat transfer.
- Due to the increase of surface hydrophobicity, the atoms staying on the solid surface are affected by kinetic energy and potential energy, showing a repulsion phenomenon. The change of atomic number density in the evaporation area of the solid surface is called the evaporation rate. The change in evaporation rate confirmed that the force of pure hydrophobic nanostructure surface on argon atoms was weak, so more argon atoms diffused from the surface, and the evaporation rate was significantly improved.
- Further research shows that the capillary transmission is enhanced on the mixed wettability nanostructure surface, and the wettability is limited by the surface pits. The efficiency of three-phase heat transmission between solid, liquid, and gas is improved when compared to pure hydrophobic surfaces, and when compared to pure hydrophilic surfaces, the rate of evaporation and the formation of nanobubbles are encouraged.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verlag, S. Springer Handbook of Nanotechnology; Harbin Institute of Technology Press: Harbin, China, 2013. [Google Scholar]
- Carey, V.P. Molecular dynamics simulations and liquid-vapor phase-change phenomena. Microscale Thermophys. Eng. 2002, 6, 1–2. [Google Scholar] [CrossRef]
- Ceccio, S.L. Friction Drag Reduction of External Flows with Bubble and Gas Injection. Annu. Rev. Fluid Mech. 2010, 42, 183–203. [Google Scholar] [CrossRef] [Green Version]
- Rothstein, J.P. Slip on Superhydrophobic Surfaces. Annu. Rev. Fluid Mech. 2010, 42, 89–109. [Google Scholar] [CrossRef]
- Elbing, B.R.; Winkel, E.S.; Lay, K.A.; Ceccio, S.L.; Dowling, D.R.; Perlin, M. Bubble-induced skin-friction drag reduction and the abrupt transition to air-layer drag reduction. J. Fluid Mech. 2008, 612, 201–236. [Google Scholar] [CrossRef]
- Elbing, B.R.; Mkiharju, S.; Wiggins, A.; Perlin, M.; Dowling, D.R.; Ceccio, S.L. On the scaling of air layer drag reduction. J. Fluid Mech. 2013, 717, 484–513. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef] [Green Version]
- Nosonovsky, M. Materials science: Slippery when wetted. Nature 2011, 477, 412–413. [Google Scholar] [CrossRef]
- Betz, A.R.; Jenkins, J.; Kim, C.-J.; Attinger, D. Boiling heat transfer on superhydrophilic, superhydrophobic, and superbiphilic surfaces. Int. J. Heat Mass Transf. 2013, 57, 733–741. [Google Scholar] [CrossRef] [Green Version]
- Liang, G.; Mudawar, I. Review of pool boiling enhancement by surface modification. Int. J. Heat Mass Transf. 2019, 128, 892–933. [Google Scholar] [CrossRef]
- Kim, J. Review of nucleate pool boiling bubble heat transfer mechanisms. Int. J. Multiph. Flow 2009, 35, 1067–1076. [Google Scholar] [CrossRef]
- Lu, Y. Molecular-continuum simulation of elevated temperature drag reduction by nanostructure-induced vapor layer. Int. J. Heat Mass Transf. 2019, 148, 119100. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, S.Y.; Lu, G.; Wang, X.-D. Effects of wettability on explosive boiling of nanoscale liquid films: Whether the classical nucleation theory fails or not? Int. J. Heat Mass Transf. 2019, 132, 1277–1283. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Li, M.; Wei, J.; Tao, W. Bubble nucleation over patterned surfaces with different wettabilities: Molecular dynamics investigation. Int. J. Heat Mass Transf. 2019, 136, 1–9. [Google Scholar] [CrossRef]
- Diaz, R.; Guo, Z. Molecular dynamics study of wettability and pitch effects on maximum critical heat flux in evaporation and pool boiling heat transfer. Numer. Heat Transf. Part A Appl. 2017, 72, 891–903. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, J.; Liu, G.; Lei, J. Nucleate boiling on nanostructured surfaces using molecular dynamics simulations. Int. J. Therm. Sci. 2020, 152, 106325. [Google Scholar] [CrossRef]
- Seyf, H.R.; Zhang, Y. Effect of nanotextured array of conical features on explosive boiling over a flat substrate: A nonequilibrium molecular dynamics study. Int. J. Heat Mass Transf. 2013, 66, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Fu, T.; Mao, Y.; Tang, Y.; Zhang, Y.; Yuan, W. Effect of nanostructure on rapid boiling of water on a hot copper plate: A molecular dynamics study. Heat Mass Transf. 2016, 52, 1469–1478. [Google Scholar] [CrossRef]
- Fu, T.; Mao, Y.; Tang, Y.; Zhang, Y.; Yuan, Q. Molecular Dynamics Simulation on Rapid Boiling of Thin Water Films on Cone-Shaped Nanostructure Surfaces. Nanoscale Microscale Thermophys. Eng. 2015, 19, 17–30. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, L.; Du, X. Bubble nucleation on grooved surfaces with hybrid wettability: Molecular dynamics study under a transient temperature boundary condition. Int. J. Heat Mass Transf. 2021, 166, 120752. [Google Scholar] [CrossRef]
- Chen, Y.J.; Yu, B.; Zou, Y.; Chen, B.-N.; Tao, W.-Q. Molecular dynamics studies of bubble nucleation on a grooved substrate. Int. J. Heat Mass Transf. 2020, 158, 119850. [Google Scholar] [CrossRef]
- Jo, H.J.; Ahn, H.S.; Kang, S.H.; Kim, M.H. A study of nucleate boiling heat transfer on hydrophilic, hydrophobic and heterogeneous wetting surfaces. Int. J. Heat Mass Transf. 2011, 54, 5643–5652. [Google Scholar] [CrossRef]
- Bourdon, B.; Marco, P.D.; Rioboo, R.; Marengo, M.; De Coninck, J. Enhancing the onset of pool boiling by wettability modification on nanometrically smooth surfaces. Int. Commun. Heat Mass Transf. 2013, 45, 11–15. [Google Scholar] [CrossRef]
- Wang, W.; Shenghong, H.; Xisheng, L. MD simulation on nano-scale heat transfer mechanism of sub-cooled boiling on nano-structured surface. Int. J. Heat Mass Transf. 2016, 100, 276–286. [Google Scholar] [CrossRef]
- Li, X.; Cole, I.; Tu, J. A review of nucleate boiling on nanoengineered surfaces—The nanostructures, phenomena and mechanisms. Int. J. Heat Mass Transf. 2019, 141, 20–33. [Google Scholar] [CrossRef]
- Wu, N.; Zeng, L.; Fu, T.; Wang, Z.; Lu, C. Molecular dynamics study of rapid boiling of thin liquid water film on smooth copper surface under different wettability conditions. Int. J. Heat Mass Transf. 2020, 147, 118905.1–118905.5. [Google Scholar] [CrossRef]
- Cao, Q.; Shao, W.; Ren, X.; Ma, X.; Shao, K.; Cui, Z.; Liu, Y. Molecular dynamics simulations of the liquid film evaporation heat transfer on different wettability hybrid surfaces at the nanoscale. J. Mol. Liq. 2020, 314, 113610. [Google Scholar] [CrossRef]
- Liang, Z.; Chandra, A.; Bird, E.; Keblinski, P. A molecular dynamics study of transient evaporation and condensation. Int. J. Heat Mass Transf. 2020, 149, 119152. [Google Scholar] [CrossRef]
- Carey, V.P.; Wemhoff, A.P. Thermodynamic analysis of near-wall effects on phase stability and homogeneous nucleation during rapid surface heating. Int. J. Heat Mass Transf. 2005, 48, 5431–5445. [Google Scholar] [CrossRef]
- Shen, B.; Yamada, M.; Mine, T.; Hidaka, S.; Kohno, M.; Takahashi, K.; Takata, Y. Depinning of bubble contact line on a biphilic surface in subatmospheric boiling: Revisiting the theories of bubble departure. Int. J. Heat Mass Transf. 2018, 126 Pt B, 715–720. [Google Scholar] [CrossRef]
- Meloni, S.; Giacomello, A.; Casciola, C.M. Focus Article: Theoretical aspects of vapor/gas nucleation at structured surfaces. J. Chem. Phys. 2016, 145, 211802. [Google Scholar] [CrossRef] [Green Version]
- Verlet, L. Computer “Experiments” on Classical Fluids. I. Thermodynamical Properties of Lennard-Jones Molecules. Phys. Rev. 1967, 159, 98–103. [Google Scholar] [CrossRef] [Green Version]



| Molecules | ε (eV) | σ (Å) |
|---|---|---|
| Argon–Argon (Ar–Ar) | 0.010438 | 3.405 |
| Argon–Platinum (hydrophilic, c = 1) (Ar–Pt) | 0.060486 | 2.990 |
| Argon–Platinum (hydrophobic, c = 0.66) (Ar–Pt) | 0.004175 | 2.990 |
| Platinum–Platinum (Pt–Pt) | 0.351 | 2.574 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Zeng, L.; Liu, Z. Mechanism of Surface Wettability of Nanostructure Morphology Enhancing Boiling Heat Transfer: Molecular Dynamics Simulation. Processes 2023, 11, 857. https://doi.org/10.3390/pr11030857
Guo W, Zeng L, Liu Z. Mechanism of Surface Wettability of Nanostructure Morphology Enhancing Boiling Heat Transfer: Molecular Dynamics Simulation. Processes. 2023; 11(3):857. https://doi.org/10.3390/pr11030857
Chicago/Turabian StyleGuo, Wenting, Liangcai Zeng, and Zhuoyuan Liu. 2023. "Mechanism of Surface Wettability of Nanostructure Morphology Enhancing Boiling Heat Transfer: Molecular Dynamics Simulation" Processes 11, no. 3: 857. https://doi.org/10.3390/pr11030857




