Electrochemical Aptasensor Based on ZnO-Au Nanocomposites for the Determination of Ochratoxin A in Wine and Beer
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Apparatus
2.3. Fabrication of Aptasensor
2.3.1. Preparation of Flower-like ZnO-Au Composites
2.3.2. Aptasensor Development
2.4. Electrochemical Detection
2.5. Real Sample Analysis
3. Results and Discussion
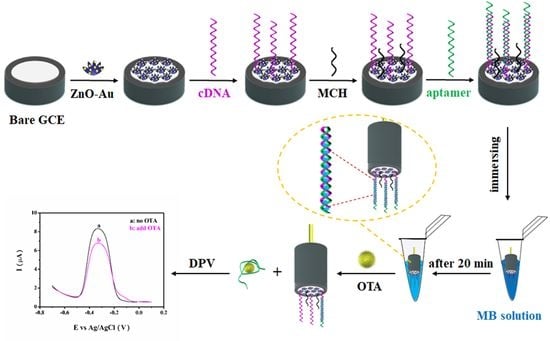
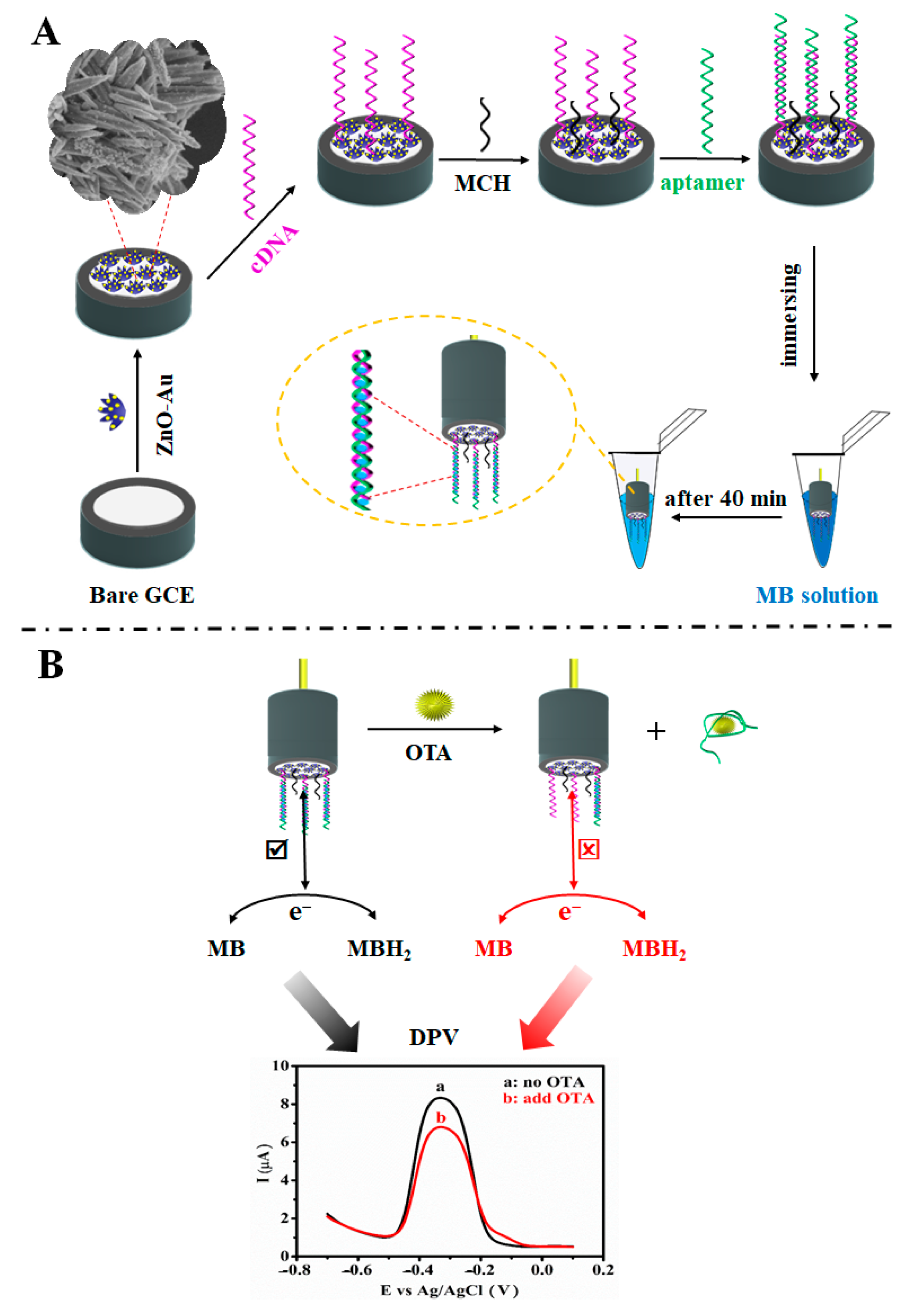
3.1. Principle of the OTA Aptasensor
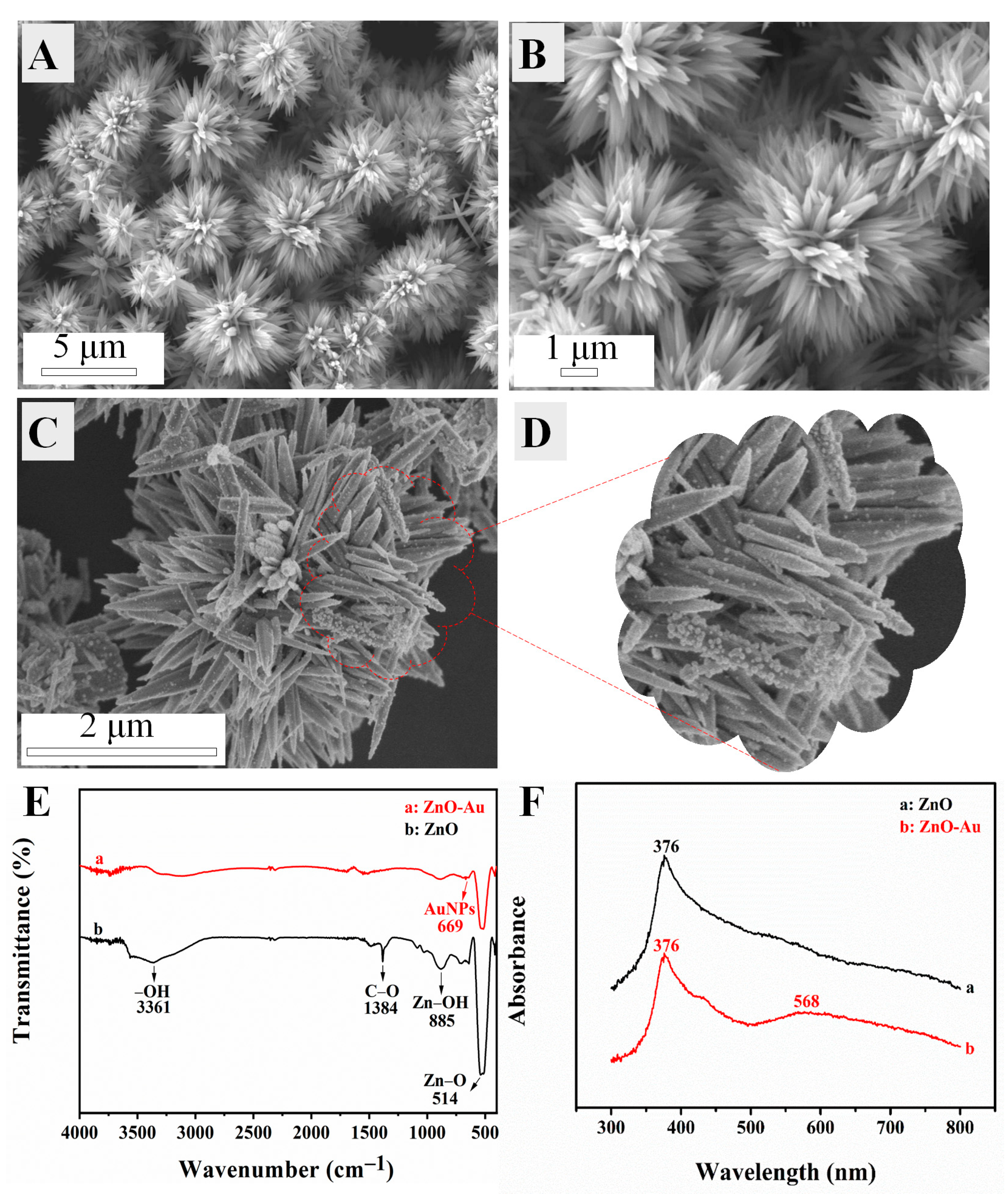
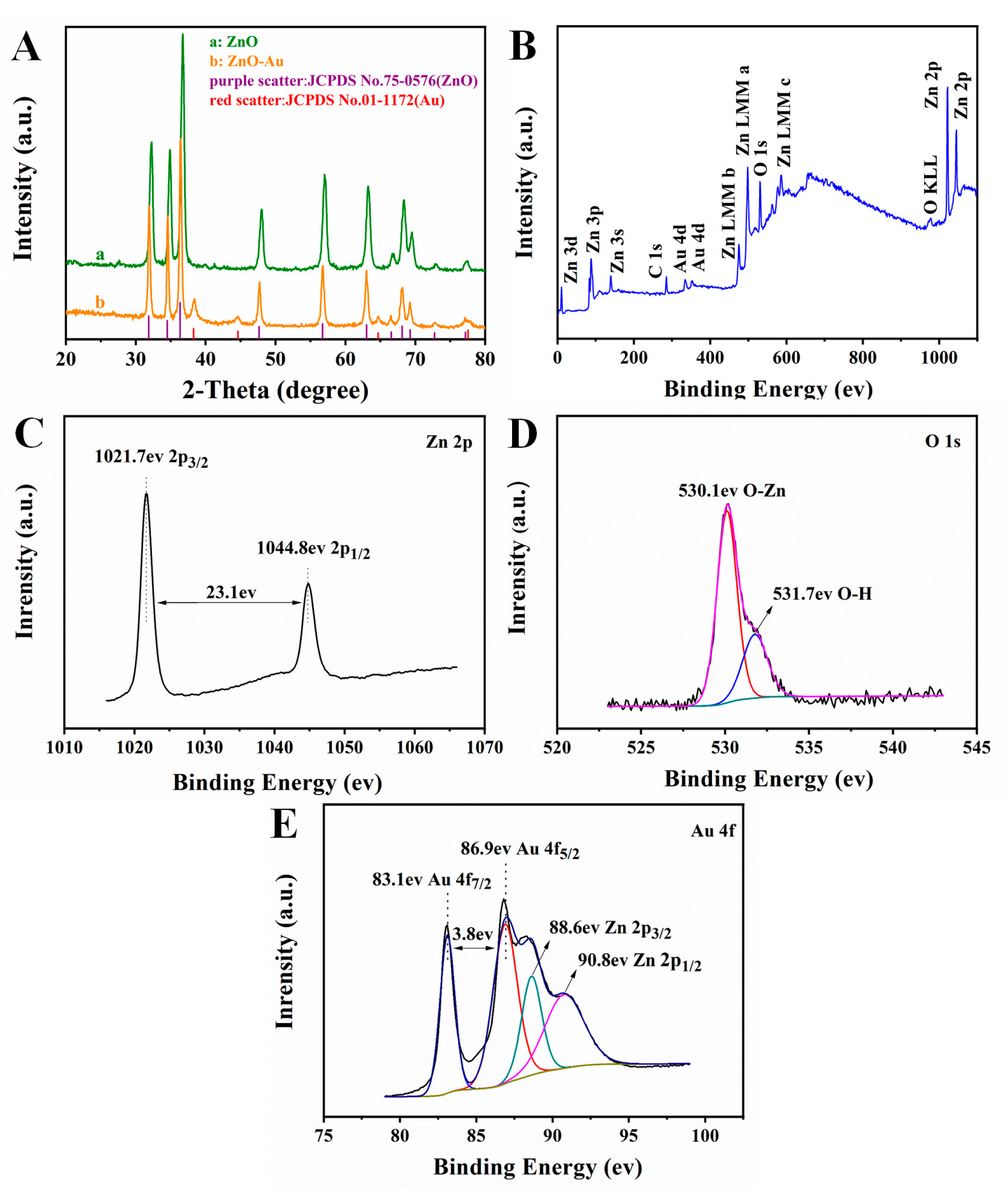
3.2. Characterization of Composites and Modified Electrode
3.3. Optimization
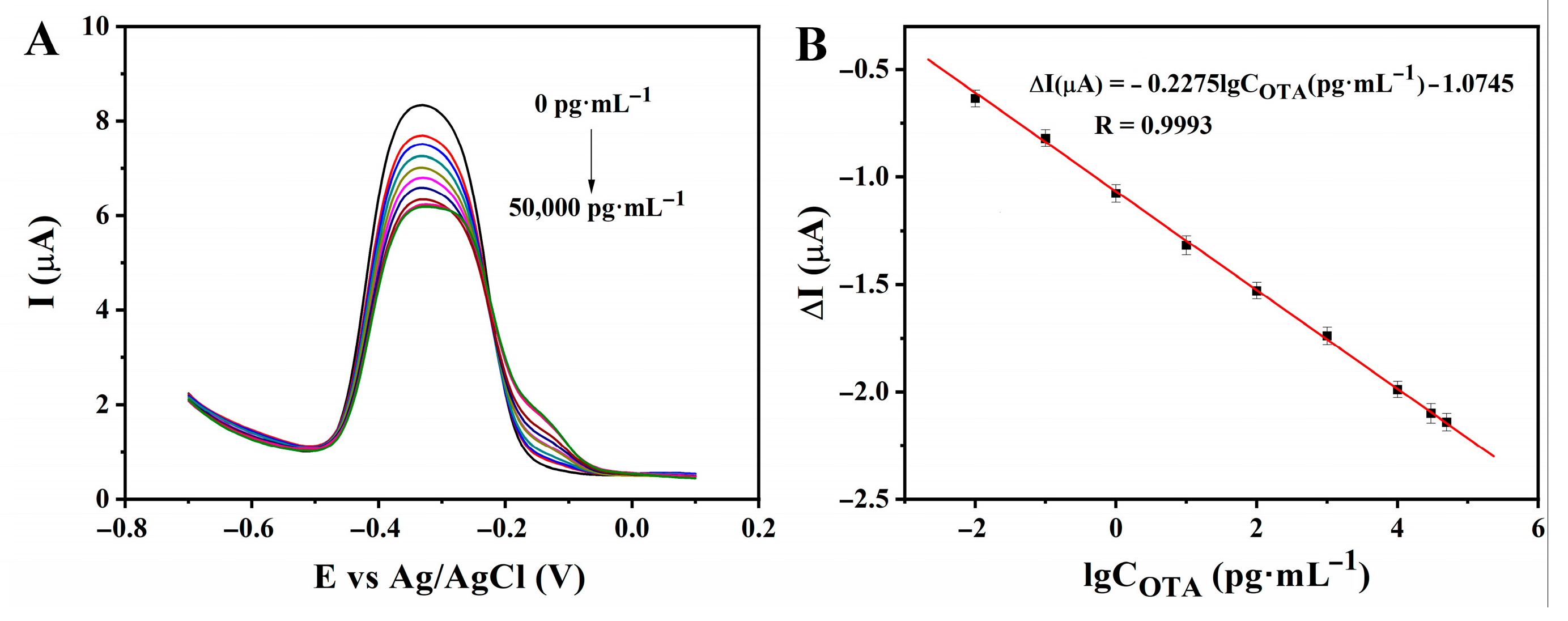
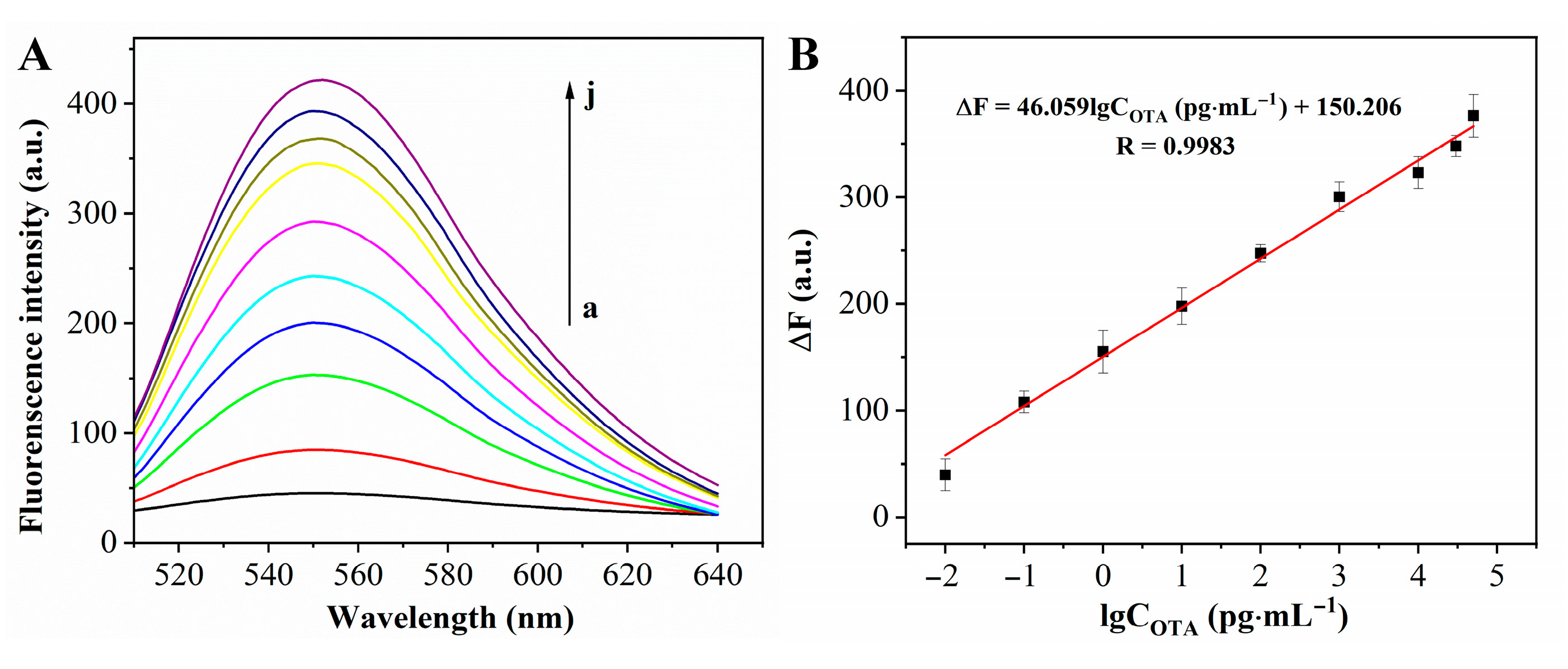
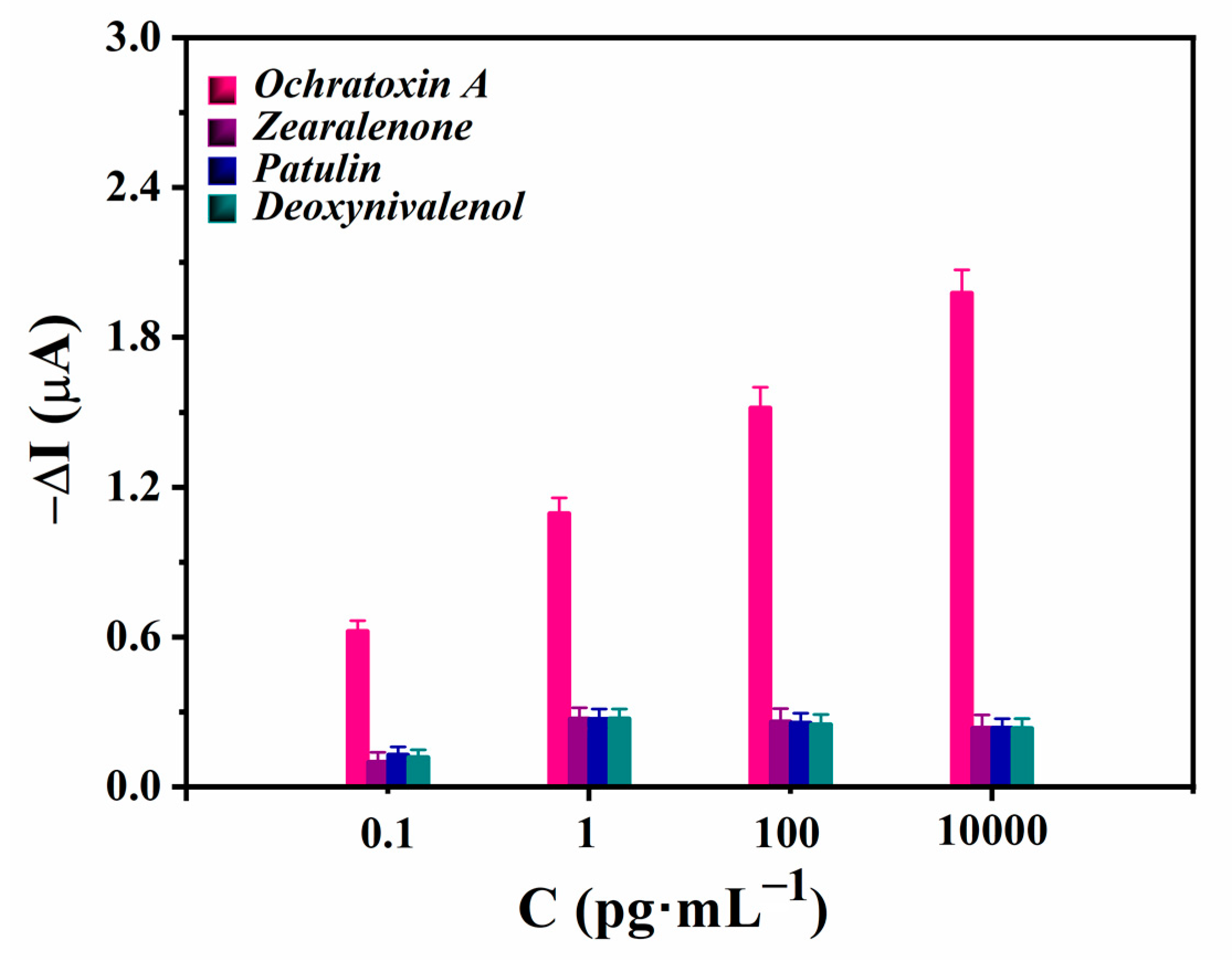
3.4. Analytical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.J.; Ma, W.; Ma, Z.Y.; Zhang, Q.H.; Li, H.M. Recent progress in determination of ochratoxin A in foods by chromatographic and mass spectrometry methods. Crit. Rev. Food Sci. Nutr. 2021, 13, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Heussner, A.H.; Bingle, L.E.H. Comparative ochratoxin toxicity: A review of the available data. Toxins 2015, 7, 4253–4282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.K.; Lakshmi, G.B.V.S.; Dhiman, T.K.; Kaushik, A.; Solanki, P.R. Bio-Active free direct optical sensing of aflatoxin B1 and ochratoxin A using a manganese oxide nano-system. Front. Nanotechnol. 2021, 2, 621681. [Google Scholar] [CrossRef]
- GB 2761-2017; National Standards of the People’s Republic of China. Mycotoxins in Food Limited. Standards Press of China: Beijing, China, 2017.
- Alizadeh, N.; Hashemi, J.; Shahdost-Fard, F. Spectrofluorimetric study of the complexation of ochratoxin A and Cu2+: Towards the hybrid fluorimetric sensor and visual detection of ochratoxin A in wheat flour samples from farm to fork. Food Chem. 2021, 350, 129204. [Google Scholar] [CrossRef] [PubMed]
- Moez, E.; Noel, D.; Brice, S.; Benjamin, G.; Pascaline, A.; Didier, M. Aptamer assisted ultrafiltration cleanup with high performance liquid chromatography-fluorescence detector for the determination of OTA in green coffee. Food Chem. 2020, 310, 125851. [Google Scholar] [CrossRef]
- Liu, X.; Tang, Z.W.; Duan, Z.H.; He, Z.Y.; Shu, M.; Wang, X.X.; Gee, S.J.; Hammock, B.D.; Xu, Y. Nanobody-based enzyme immunoassay for ochratoxin A in cereal with high resistance to matrix interference. Talanta 2017, 164, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Yuan, W.; Lu, L.X.; Lu, Y.C.; Xiong, X.; Li, Y.; Cui, X.W.; Liu, Y.J.; Xiong, X.H. Synergistic effects of DNA structure for ultrasensitive detecting OTA in grains. Food Anal. Methods 2021, 14, 2308–2316. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Beluomini, M.A.; Silva, J.L.; Sá, A.C.D.; Buffon, E.; Pereira, T.C.; Stradiotto, N.R. Electrochemical sensors based on molecularly imprinted polymer on nanostructured carbon materials: A review. J. Electroanal. Chem. 2019, 840, 343–366. [Google Scholar] [CrossRef]
- Sun, X.F.; Chen, H.H.; Wang, S.L.; Zhang, Y.P.; Tian, Y.P.; Zhou, N.D. Electrochemical detection of sequence-specific DNA based on formation of G-quadruplex-hemin through continuous hybridization chain reaction. Anal. Chim. Acta 2018, 1021, 121–128. [Google Scholar] [CrossRef]
- Zhao, K.; Quan, X. Carbon-based materials for electrochemical reduction of CO2 to C2+ oxygenates: Recent progress and remaining challenges. ACS Catal. 2021, 11, 2076–2097. [Google Scholar] [CrossRef]
- Daviddi, E.; Chen, Z.; Massani, B.B.; Lee, J.; Bentley, C.L.; Unwin, P.R.; Ratcliff, E.L. Nanoscale visualization and multiscale electrochemical analysis of conductive polymer electrodes. ACS Nano 2019, 13, 13271–13284. [Google Scholar] [CrossRef]
- Chen, S.B.; Yuan, B.Q.; Liu, G.; Zhang, D.J. Electrochemical sensors based on covalent organic frameworks: A critical review. Front. Chem. 2020, 8, 601044. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Y.X.; Wang, N.; Xu, Q.Q.; Xu, L.; Lin, M. Copper-based metal-organic framework for non-enzymatic electrochemical detection of glucose. Electroanalysis 2018, 30, 474–478. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Zhang, X.; Chi, K.N.; Yang, T.; Yang, Y.H. Bifunctional MOFs-based ratiometric electrochemical sensor for multiplex heavy metal ions. ACS Appl. Mater. Interfaces 2020, 12, 30770–30778. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Ahmed, A.; Singh, A.; Oruganti, S.K.; Khosla, A.; Arya, S. Review-recent advances in tin oxide nanomaterials as electrochemical/chemiresistive sensors. J. Electrochem. Soc. 2021, 168, 027505. [Google Scholar] [CrossRef]
- Prathap, M.U.A.; Kaur, B.; Srivastava, R. Electrochemical sensor platforms based on nanostructured metal oxides, and zeolite-based materials. Chem. Rec. 2019, 19, 883–907. [Google Scholar] [CrossRef]
- Ortiz-Casas, B.; Galdámez-Martínez, A.; Gutiérrez-Flores, J.; Ibañez, A.B.; Panda, P.K.; Santana, G.; Vega, H.A.D.L.; Suar, M.; Rodelo, C.G.; Kaushik, A.; et al. Bio-acceptable 0D and 1D ZnO nanostructures for cancer diagnostics and treatment. Mater. Today 2021, 50, 533–569. [Google Scholar] [CrossRef]
- Das, S.; Mukhopadhyay, S.; Chatterjee, S.; Devi, P.S.; Kumar, G.S. Fluorescent ZnO-Au nanocomposite as a probe for elucidating specificity in DNA interaction. ACS Omega 2018, 3, 7494–7507. [Google Scholar] [CrossRef]
- Mahajan, H.; Bae, J.; Yun, K. Facile synthesis of ZnO-Au nanocomposites for high-performance supercapacitors. J. Alloys Compd. 2018, 758, 131–139. [Google Scholar] [CrossRef]
- Yao, C.J.; Lin, J.M.; Wu, L.; Li, L.Q.; Xu, N.; Sun, J.; Wu, J.D. High visible light photocatalytic activity of ZnO-Au nanocomposites synthesized by a controlled hydrothermal method. Phys. Status Solidi (A) Appl. Mater. Sci. 2021, 218, 2100150. [Google Scholar] [CrossRef]
- Ilgu, M.; Nilsen-Hamilton, M. Aptamers in analytics. Analyst 2016, 141, 1551–1568. [Google Scholar] [CrossRef] [Green Version]
- Röthlisberger, P.; Hollenstein, M. Aptamer chemistry. Adv. Drug Deliv. Rev. 2018, 134, 3–21. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Qiao, X.J.; Zhang, X.L.; Niu, C.; Yue, T.L.; Sheng, Q.L. Simultaneous electrochemical aptasensing of patulin and ochratoxin A in apple juice based on gold nanoparticles decorated black phosphorus nanomaterial. Anal. Bioanal. Chem. 2021, 413, 3131–3140. [Google Scholar] [CrossRef]
- Pica, A.; Krauss, I.R.; Parente, V.; Tateishi-Karimata, H.; Nagatoishi, S.; Tsumoto, K.; Sugimoto, N.; Sica, F. Through-bond effects in the ternary complexes of thrombin sandwiched by two DNA aptamers. Nucleic Acids Res. 2017, 45, 461–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.J.; Wang, W.; Liu, Y.L. Enhanced acetone sensing performance of Au nanoparticles functionalized flower-like ZnO. Sens. Actuators B Chem. 2012, 168, 39–45. [Google Scholar] [CrossRef]
- Tabrizi, M.A.; Ferré-Borrull, J.; Marsal, L.F. Remote sensing of Salmonella-specific DNA fragment by using nanoporous alumina modified with the single-strand DNA probe. Sens. Actuators B 2020, 304, 127302. [Google Scholar] [CrossRef]
- Mudasir; Wahyuni, E.T.; Tjahjono, D.H.; Yoshioka, N.; Inoue, H. Spectroscopic studies on the thermodynamic and thermal denaturation of the ct-DNA binding of methylene blue. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 528–534. [Google Scholar] [CrossRef]
- Palinska, A.; Grodzka, A.; Elzanowska, H.; Kepska, B.; Zwierkowska, E.; Achmatowicz, S.; Maj-Zurawska, M. Methylene blue interactions with chromosomal and plasmid DNA on screen-printed carbon electrodes. Electroanalysis 2010, 22, 1306–1313. [Google Scholar] [CrossRef]
- Han, N.S.; Kim, D.H.; Lee, J.W.; Kim, J.; Shim, H.S.; Lee, Y.J.; Lee, D.; Song, J.K. Unexpected size effect observed in ZnO-Au composite photocatalysts. ACS Appl. Mater. Interfaces 2016, 8, 1067–1072. [Google Scholar] [CrossRef]
- Wang, M.K.; Shen, Y.; Liu, Y.; Wang, T.; Zhao, F.; Liu, B.F.; Dong, S.J. Direct electrochemistry of microperoxidase 11 using carbon nanotube modified electrodes. J. Electroanal. Chem. 2005, 578, 121–127. [Google Scholar] [CrossRef]
- Pillai, R.; Preetha, S.; Narasimhamurthy, B.; Lekshmi, I.C. Biosensing of catechol via amperometry using laccase immobilized nickel oxide/graphite modified screen-printed electrodes. Mater. Today Proc. 2022, 62, 5434–5438. [Google Scholar] [CrossRef]
- Kochuveedu, S.T.; Oh, J.H.; Do, Y.R.; Kim, D.H. Surface-plasmon-enhanced band emission of ZnO nanoflowers decorated with Au nanoparticles. Chem. Eur. J. 2012, 18, 7467–7472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.H.; Wang, L.W.; Yang, T.L.; Guo, X.Z.; Wu, S.H.; Wang, S.R.; Zhang, S.M. Au-functionalized hematite hybrid nanospindles: General synthesis, gas sensing and catalytic properties. J. Phys. Chem. C 2011, 115, 5352–5357. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhang, J.; Wang, L.W.; Yang, T.L.; Guo, X.Z.; Wu, S.H.; Wang, S.R. 3D hierarchically porous ZnO structures and their functionalization by Au nanoparticles for gas sensors. J. Mater. Chem. 2011, 21, 349–356. [Google Scholar] [CrossRef]
- Fang, L.X.; Huang, K.J.; Zhang, B.L.; Liu, Y.J.; Zhang, Q.Y. A label-free electrochemistry biosensor based flower-like 3-dimensional ZnO superstructures for detection of DNA arrays. New J. Chem. 2014, 38, 5918–5924. [Google Scholar] [CrossRef]
- Liu, X.W. Selective growth of Au nanoparticles on (111) facets of Cu2O microcrystals with an enhanced electrocatalytic property. Langmuir 2011, 27, 9100–9104. [Google Scholar] [CrossRef]
- Rivas, L.; Mayorga-Martinez, C.C.; Quesada-Gonzalez, D.; Zamora-Gálvez, A.; Escosura-Muñiz, A.D.L.; Merkoçi, A. Label-free impedimetric aptasensor for ochratoxin-A detection using iridium oxide nanoparticles. Anal. Chem. 2015, 87, 5167–5172. [Google Scholar] [CrossRef]
- Cao, J.L.; Kong, W.J.; Zhou, S.J.; Yin, L.H.; Wan, L.; Yang, M.H. Molecularly imprinted polymer-based solid phase clean-up for analysis of ochratoxin A in beer, red wine, and grape juice. J. Sep. Sci. 2013, 36, 1291–1297. [Google Scholar] [CrossRef]
- Cina, M.; Ponce, M.D.; Martinez, L.D.; Cerutti, S. Development of a novel UHPLC-MS/MS method for the determination of ochratoxin A in tea. Heliyon 2021, 7, e06663. [Google Scholar] [CrossRef]
- Cina, M.; Ponce, M.D.V.; Fernandez, L.; Cerutti, S. A green approach for Ochratoxin A determination in coffee infusions. J. Food Comp. Anal. 2022, 114, 104777. [Google Scholar] [CrossRef]
- Streit, B.; Czabany, T.; Weingart, G.; Marchetti-Deschmann, M.; Prasad, S. Toolbox for the Extraction and Quantification of Ochratoxin A and Ochratoxin Alpha Applicable for Different Pig and Poultry Matrices. Toxins 2022, 14, 432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, W.T.; Liu, Z.J.; Fu, X.L.; Du, D.L. Establishment of a Chemiluminescence Immunoassay Combined with Immunomagnetic Beads for Rapid Analysis of Ochratoxin A. J. Aoac. Int. 2022, 105, 346–351. [Google Scholar] [CrossRef]
- Li, D.Y.; Xia, H.Y.; Sun, Y.Y.; Liu, W.J.; Liu, W.; Yu, J.N.; Jing, G.X.; Zhang, J.; Li, W.S. Colorimetric aptasensor for the sensitive detection of ochratoxin A based on a triple cascade amplification strategy. Anal. Chim. Acta 2022, 1237, 340616. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, L.; Li, M.; She, Y.X.; Zhu, C.; Yan, M.M. An Alkyne-Mediated SERS Aptasensor for Anti-Interference Ochratoxin A Detection in Real Samples. Foods 2022, 11, 3407. [Google Scholar] [CrossRef]
- Wu, S.; Liu, L.; Duan, N.; Wang, W.; Yu, Q.; Wang, Z. A test strip for ochratoxin A based on the use of aptamer-modified fluorescence upconversion nanoparticles. Microchim. Acta 2018, 185, 497. [Google Scholar] [CrossRef]
- Liu, M.; Liu, S.; Ma, Y.; Li, B. Construction of a fluorescence biosensor for ochratoxin A based on magnetic beads and exonuclease III-assisted DNA cycling signal amplification. Anal. Methods 2022, 14, 734–740. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, X.P.; Ma, L.Y.; Fang, H.J.; Yang, H.L.; Zhou, Y. Fluorescence and absorbance dual-mode immunoassay for detecting Ochratoxin A. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 279, 121440. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; He, S.; Cao, H.; Su, B.; Huang, T.; Chen, Q.; Liu, M.; Yang, D.P. Electrochemiluminescence Immunosensor Based on Nanobody and Au/CaCO3 Synthesized Using Waste Eggshells for Ultrasensitive Detection of Ochratoxin A. ACS Omega 2021, 6, 30148–30156. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guo, Y.; Luo, F.; Rao, P.; Fu, C.; Wang, S. Homogeneous Electrochemical Method for Ochratoxin A Determination Based on Target Triggered Aptamer Hairpin Switch and Exonuclease III-Assisted Recycling Amplification. Food Anal. Methods 2017, 10, 1982–1990. [Google Scholar] [CrossRef]
- Sun, A.L.; Zhang, Y.F.; Sun, G.P.; Wang, X.N.; Tang, D. Homogeneous electrochemical detection of ochratoxin A in foodstuff using aptamer-graphene oxide nanosheets and DNase I-based target recycling reaction. Biosens. Bioelectron. 2017, 89, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Huang, Y.; Cheng, Y.; Huang, L.; Zhuang, J.; Tang, D. Two-dimensional MoS2 as a nano-binder for ssDNA: Ultrasensitive aptamer based amperometric detection of Ochratoxin A. Microchim. Acta 2018, 185, 162. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Shan, Y.Q.; Gong, M.; Jin, X.; Lv, L.; Jiang, M.; Xu, J. A novel electrochemical sensor for ochratoxin A based on the hairpin aptamer and double report DNA via multiple signal amplification strategy. Sens. Actuators B 2019, 281, 595–601. [Google Scholar] [CrossRef]
- Kunene, K.; Weber, M.; Sabela, M.; Voiry, D.; Kanchi, S.; Bisetty, K.; Bechelany, M. Highly-efficient electrochemical label-free immunosensor for the detection of ochratoxin A in coffee samples. Sens. Actuators B 2020, 305, 127438. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, H.Y.; Hu, J.Y.; Yang, D.T. Disposable electrochemical aptasensor based on graphene oxide-DNA complex as signal amplifier towards ultrasensitive detection of ochratoxin A. Micromachines 2022, 13, 834. [Google Scholar] [CrossRef]
| Detection Method | Material | Real Sample | Linear Range (pg·mL−1) | LOD (pg·mL−1) | Reference |
|---|---|---|---|---|---|
| LC | NA | Beer, wine, and juice | 1000–50,000 | 25 | [40] |
| UHPLC-MS/MS | NA | Tea | 500–70,000,000 | 500 | [41] |
| UHPLC-(ESI+)-MS/MS | NA | Coffee beverages | 300–70,000 | 300 | [42] |
| LC-MS/MS | NA | Pig and poultry matrices | 250–250,000 | 1000 | [43] |
| Chemiluminescence method | Anti-OTA antibodies-magnetic beads | Grains | 5–405 | 2.05 | [44] |
| Colorimetric method | G-quadruplex/hemin DNAzyme | Rice | 0.404–40.4 | 3.5 | [45] |
| Surface-enhanced Raman spectroscopy | Fe3O4 NPs | Soybean, grape, and milk | 40.4–16,160 | 12 | [46] |
| Fluorescence method | UCNPs | Beer and wheat | 5000–100,000 | 1860 | [47] |
| Fluorescence method | Magnetic beads- exonuclease | Wheat and corn | 500–75,000 | 28 | [48] |
| Fluorescence and absorbance dual-mode immunoassay | G-quadruplex-TMB | Corn, oats, and rice | 49–25,000 | 28 | [49] |
| Electrochemiluminescence method | Au-CaCO3 nanocomposites | Beer and wheat | 10–100,000 | 5.7 | [50] |
| Electrochemical method | Hairpin Aptamer | Wheat | 1000–500,000 | 0.58 | [51] |
| Electrochemical method | Aptamer-graphene oxide nanosheets | Wheat | 10–50,000 | 5.6 | [52] |
| Electrochemical method | Aptamer-MoS2 | Wine | 0.5–1000 | 0.23 | [53] |
| Electrochemical method | Aptamer-Au nanoparticles | NA | 1–1000 | 0.5 | [54] |
| Electrochemical method | OTA Antibody-palladium nanoparticles | Coffee | 500–2000 | 96 | [55] |
| Electrochemical method | GO-DNA complex | Oat and rice | 0.01–1000 | 0.005 | [56] |
| Electrochemical method | ZnO-Au composites | Wine & Beer | 0.1–30,000 | 0.05 | This work |
| Spiked OTA (pg·mL−1) | Real Sample (pg·mL−1) | RSD (n = 5) | Recovery (n = 5) | |||
|---|---|---|---|---|---|---|
| Beverage 1 | Beverage 2 | Beverage 1 | Beverage 2 | Beverage 1 | Beverage 2 | |
| 0.1 | 0.11 | 0.106 | 3.8% | 2.7% | 110.0% | 106.0% |
| 1 | 1.095 | 1.05 | 3.6% | 3.5% | 109.1% | 104.5% |
| 100 | 105.2 | 106.0 | 3.4% | 4.2% | 105.2% | 106.0% |
| 1000 | 1022.0 | 1057.0 | 3.28 | 4.3% | 102.2% | 105.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wang, Y.; Sheng, Q.; Yue, T. Electrochemical Aptasensor Based on ZnO-Au Nanocomposites for the Determination of Ochratoxin A in Wine and Beer. Processes 2023, 11, 864. https://doi.org/10.3390/pr11030864
Zhang S, Wang Y, Sheng Q, Yue T. Electrochemical Aptasensor Based on ZnO-Au Nanocomposites for the Determination of Ochratoxin A in Wine and Beer. Processes. 2023; 11(3):864. https://doi.org/10.3390/pr11030864
Chicago/Turabian StyleZhang, Sai, Yahui Wang, Qinglin Sheng, and Tianli Yue. 2023. "Electrochemical Aptasensor Based on ZnO-Au Nanocomposites for the Determination of Ochratoxin A in Wine and Beer" Processes 11, no. 3: 864. https://doi.org/10.3390/pr11030864
APA StyleZhang, S., Wang, Y., Sheng, Q., & Yue, T. (2023). Electrochemical Aptasensor Based on ZnO-Au Nanocomposites for the Determination of Ochratoxin A in Wine and Beer. Processes, 11(3), 864. https://doi.org/10.3390/pr11030864