In Vitro Cytotoxicity Assessment of Abutilon pannosum Chloroform Fraction and Its Phytoconstituents Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection, Crude Extracts, and Fractions Preparation
2.2. Cell Culture and Antiproliferative Assay
2.3. Flow Cytometric Analysis of DNA Content
2.4. Annexin V/Propidium Iodide Assay
2.5. RT-PCR
2.6. Western Blot Analysis
2.7. GC-MS Analysis of Phytochemical Compounds
2.8. Statistical Analysis
3. Results
3.1. Antiproliferative Activity of A. pannosum Extracts
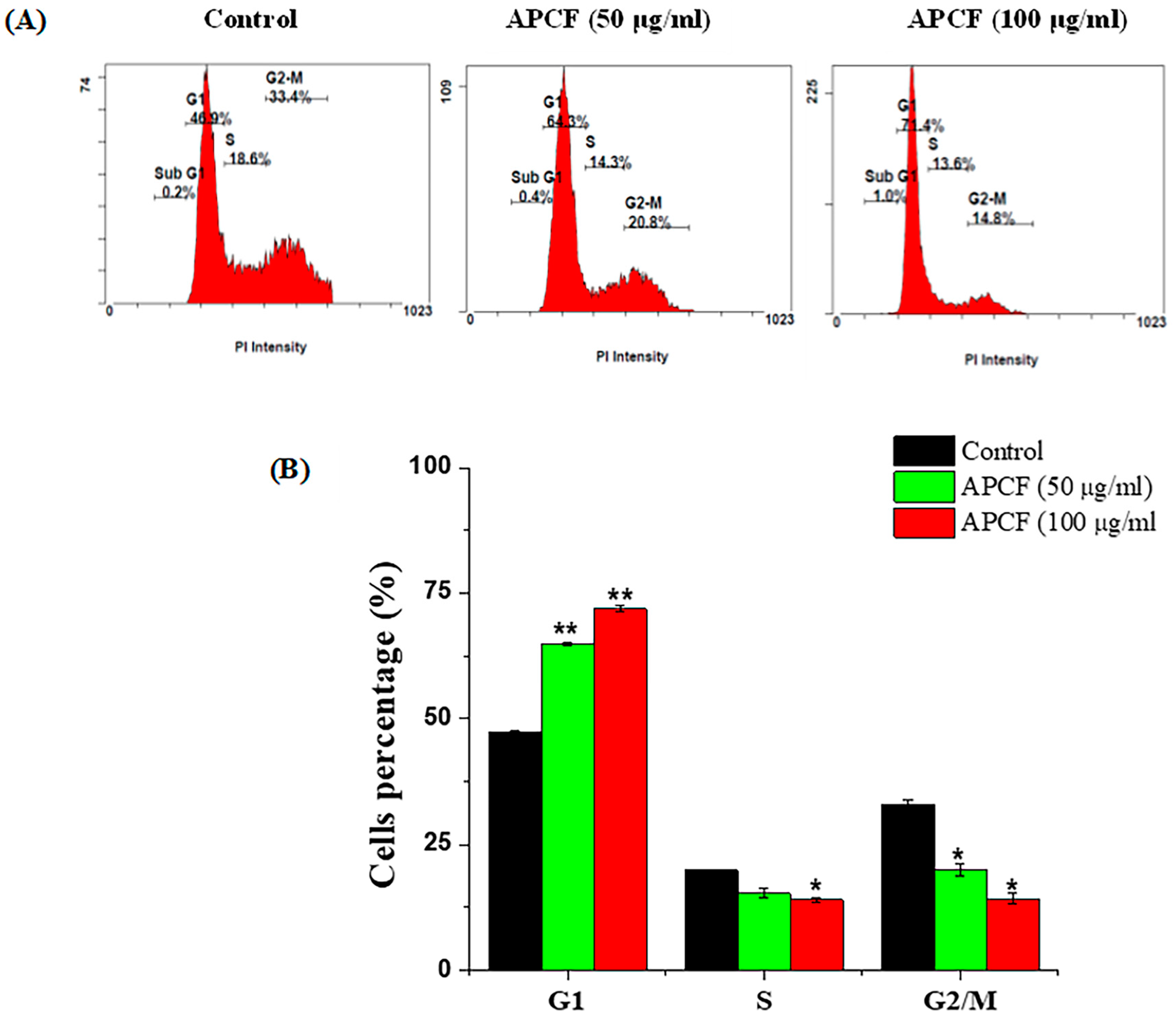
3.2. Cell Cycle Distribution after Treatment with APCF
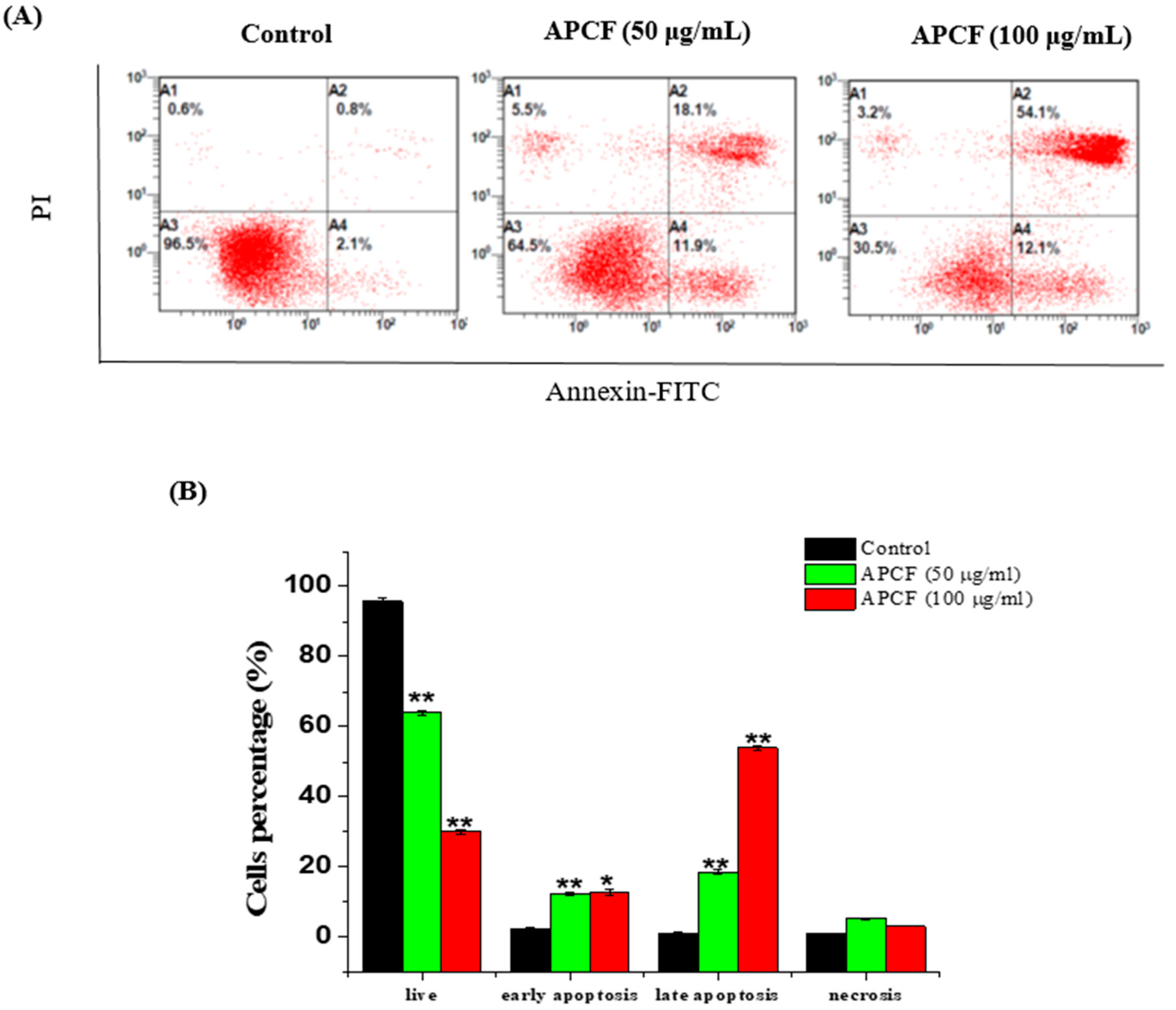
3.3. Apoptosis Evaluation of by Flow Cytometric Analysis
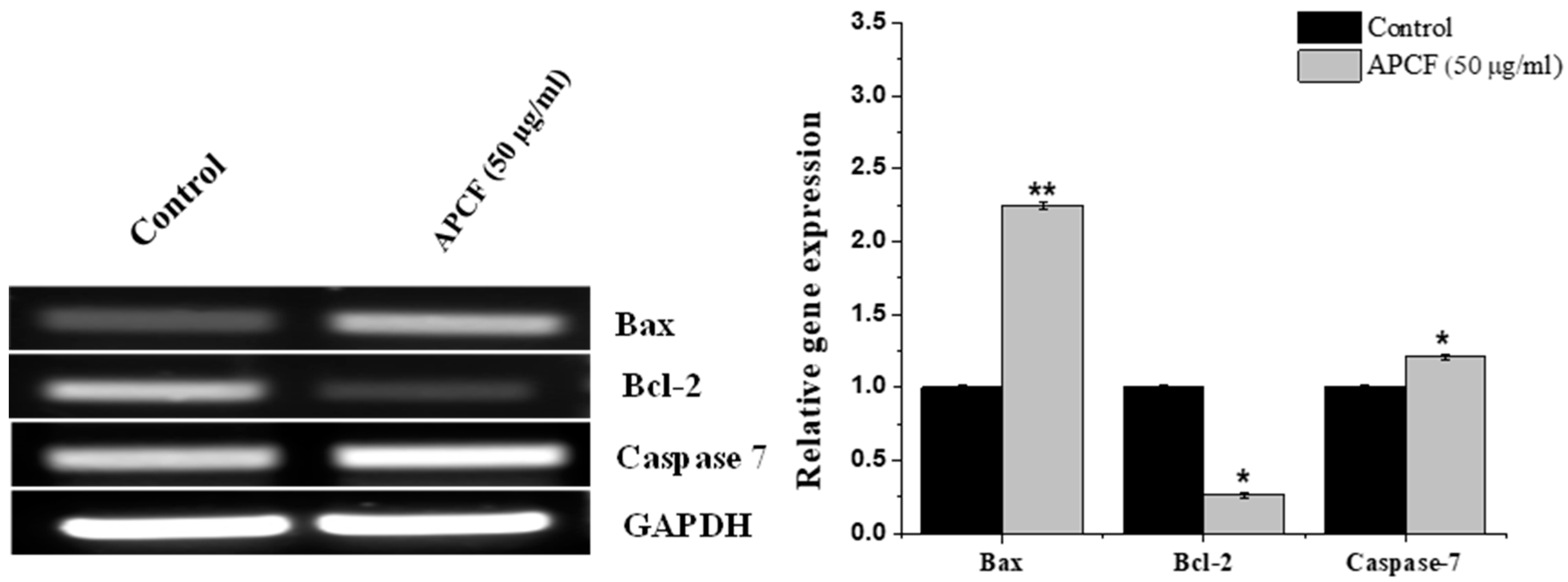
3.4. RT-PCR Analysis
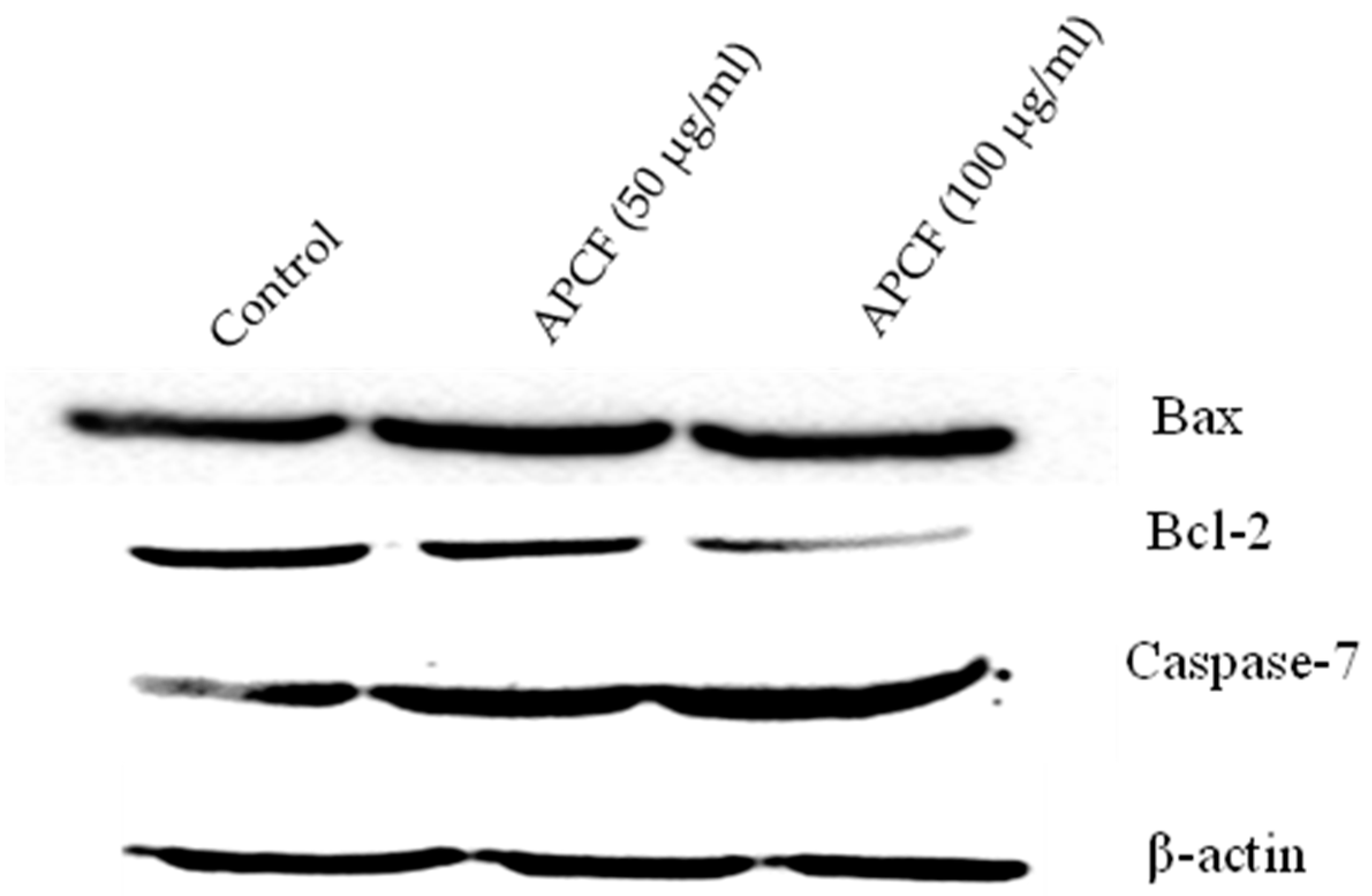
3.5. Alteration of Apoptosis Related Proteins Expression
3.6. Phytochemical Analysis of APCF by GC/MS
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: The trends projection analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- Majolo, F.; de Oliveira Becker Delwing, L.K.; Marmitt, D.J.; Bustamante-Filho, I.C.; Goettert, M.I. Medicinal plants and bioactive natural compounds for cancer treatment: Important advances for drug discovery. Phytochem. Lett. 2019, 31, 196–207. [Google Scholar] [CrossRef]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharm. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Christenhusz, M.J.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Pearman, N.; Moxon, S.; Carnachan, S.M.; Cooke, M.E.; Nep, E.I.; Sims, I.M.; Morris, G.; Smith, A. Investigating potential wound healing properties of polysaccharides extracted from Grewia mollis Juss. and Hoheria populnea A. Cunn. (Malvaceae). Bioact. Carbohydr. Diet. Fibre 2019, 20, 100201. [Google Scholar] [CrossRef]
- Sikorska, M.; Matlawska, I. Polyphenolic compounds from Abutilon grandiflorum leaves. Acta Pol. Pharm. Drug Res. 2008, 65, 467–471. [Google Scholar]
- Baquar, S.R. Medicinal and Poisonous Plants of Pakistan. In Medicinal and Poisonous Plants of Pakistan; Printas: Karachi, Pakistan, 1989. [Google Scholar]
- Ahmed, Z.; Kazmi, S.N.-U.-H.; Malik, A. A new pentacyclic triterpene from Abutilon pakistanicum. J. Nat. Prod. 1990, 53, 1342–1344. [Google Scholar] [CrossRef]
- Hussain, M.; Zahra, D.N.; Hussain, S.S.; Ahmed, E.; Ahmad, I.; Malik, A.; Ahmed, Z. Structure determination of new steroids from Abutilon pakistanicum by NMR techniques. Magn. Reson. Chem. 2008, 46, 274–277. [Google Scholar] [CrossRef]
- Migahid, A. Flora of Saudi Arabia, 4th ed.; Routledge: London, UK, 1996; Volume 1, pp. 103–105. [Google Scholar]
- Abedin, S. Abutilon-muticum and Abutilon-pannosum complex. Pak. J. Bot. 1980, 12, 43–48. [Google Scholar]
- Ali, B.; Fatima, I.; Malik, A.; Ahmed, Z. New glycosidic constituents of Abutilon pakistanicum. Helv. Chim. Acta 2010, 93, 2245–2250. [Google Scholar] [CrossRef]
- Aadesariya, M.K.; Ram, V.R.; Dave, P.N. Extraction, Isolation and Identification of Useful Phyto Constituents from Dichloromethane Leave Extract of Abutilon pannosum and Grewia tenax Using Q-TOF LC/MS. Int. J. Adv. Res. Chem. Sci. 2017, 4, 1–14. [Google Scholar]
- Khalil, I.; Ghani, M.; Khan, M.R.; Akbar, F. Evaluation of biological activities and in vivo amelioration of CCl4 induced toxicity in lung and kidney with Abutilon pannosum (G. Forst.) Schltdl. in rat. J. Ethnopharmacol. 2020, 249, 112395. [Google Scholar] [CrossRef]
- Bano, I.; Deora, G. Studies on micro morphological taxonomic variations in Abutilon species of Indian Thar Desert. IOSR J. Pharm. Biol. Sci. 2017, 12, 60–68. [Google Scholar]
- Khan, R.S.; Senthi, M.; Rao, P.C.; Basha, A.; Alvala, M.; Tummuri, D.; Masubuti, H.; Fujimoto, Y.; Begum, A.S. Cytotoxic constituents of Abutilon indicum leaves against U87MG human glioblastoma cells. Nat. Prod. Res. 2015, 29, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Gouda, H.M.; Morsy, A.A.; Youssef, A.K.; Tolba, I.A.E.-M.; Selim, A.A.M.A. The ethyl acetate extract from Abutilon fruticosum Guill and Perr. as a potential diabetes–cancer prophylactic: A cytotoxic, α-glucosidase, and in-silico study. S. Afr. J. Bot. 2023, 156, 110–114. [Google Scholar] [CrossRef]
- Alqahtani, A.S.; Ghorab, M.M.; Nasr, F.A.; Ahmed, M.Z.; Al Mishari, A.A.; Attia, S.M.; Farooq Khan, M. Cytotoxicity of Newly Synthesized Quinazoline–Sulfonamide Derivatives in Human Leukemia Cell Lines and Their Effect on Hematopoiesis in Zebrafish Embryos. Int. J. Mol. Sci. 2022, 23, 4720. [Google Scholar] [CrossRef]
- Nasr, F.A.; Noman, O.M.; Alqahtani, A.S.; Qamar, W.; Ahamad, S.R.; Al-Mishari, A.A.; Alyhya, N.; Farooq, M. Phytochemical constituents and anticancer activities of Tarchonanthus camphoratus essential oils grown in Saudi Arabia. Saudi Pharm. J. 2020, 28, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- McLafferty, F.W.; Stauffer, D.B. The Wiley/NBS Registry of Mass Spectral Data; Wiley: New York, NY, USA, 1989; Volume 1. [Google Scholar]
- Talib, W.H.; Daoud, S.; Mahmod, A.I.; Hamed, R.A.; Awajan, D.; Abuarab, S.F.; Odeh, L.H.; Khater, S.; Al Kury, L.T. Plants as a Source of Anticancer Agents: From Bench to Bedside. Molecules 2022, 27, 4818. [Google Scholar] [CrossRef] [PubMed]
- Willis, K. State of the World’s Plants 2017; Royal Botanics Gardens Kew: Richmond, UK, 2017. [Google Scholar]
- Agrawal, T. Abutilon a vulnerable genus: A review. Res. Rev. J. Pharmacogn. Phytochem. 2017, 5, 44–46. [Google Scholar]
- Elshikh, A.A.; ElNour, M.E.; Elkamali, H.H.; Kabbashi, A.S. In-vitro Testing of Antioxidant, Anti-Parasite Activities, Cytotoxicity, and Chemical Evaluation of Abutilon pannosum and Cassia occidentalis Ethanolic Extracts. Annu. Res. Rev. Biol. 2021, 36, 112–121. [Google Scholar] [CrossRef]
- Suski, J.M.; Braun, M.; Strmiska, V.; Sicinski, P. Targeting cell-cycle machinery in cancer. Cancer Cell 2021, 39, 759–778. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Dhanalakshmi, S.; Agarwal, R. Phytochemicals as cell cycle modulators—A less toxic approach in halting human cancers. Cell Cycle 2002, 1, 156–161. [Google Scholar] [CrossRef]
- Mata, R.; Nakkala, J.R.; Sadras, S.R. Polyphenol stabilized colloidal gold nanoparticles from Abutilon indicum leaf extract induce apoptosis in HT-29 colon cancer cells. Colloids Surf. B Biointerfaces 2016, 143, 499–510. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- van Engeland, M.; Nieland, L.J.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 1998, 31, 1–9. [Google Scholar] [CrossRef]
- Levine, B.; Sinha, S.C.; Kroemer, G. Bcl-2 family members: Dual regulators of apoptosis and autophagy. Autophagy 2008, 4, 600–606. [Google Scholar] [CrossRef]
- McComb, S.; Chan, P.K.; Guinot, A.; Hartmannsdottir, H.; Jenni, S.; Dobay, M.P.; Bourquin, J.P.; Bornhauser, B.C. Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or -7. Sci. Adv. 2019, 5, eaau9433. [Google Scholar] [CrossRef]
- Konappa, N.; Udayashankar, A.C.; Krishnamurthy, S.; Pradeep, C.K.; Chowdappa, S.; Jogaiah, S. GC-MS analysis of phytoconstituents from Amomum nilgiricum and molecular docking interactions of bioactive serverogenin acetate with target proteins. Sci. Rep. 2020, 10, 16438. [Google Scholar] [CrossRef]
- Bano, I.; Deora, G. Preliminary phytochemical screening and GC-MS analysis of methanolic leaf extract of Abutilon pannosum (Forst. F.) Schlect. from Indian Thar desert. J. Pharmacogn. Phytochem. 2019, 8, 894–899. [Google Scholar]
- Shams, M.; Esfahan, S.Z.; Esfahan, E.Z.; Dashtaki, H.N.; Dursun, A.; Yildirim, E. Effects of climatic factors on the quantity of essential oil and dry matter yield of coriander (Coriandrum sativum L.). Indian J. Sci. Technol. 2016, 9, 1–4. [Google Scholar] [CrossRef]
- Harada, H.; Yamashita, U.; Kurihara, H.; Fukushi, E.; Kawabata, J.; Kamei, Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002, 22, 2587–2590. [Google Scholar] [PubMed]
- Zhu, S.; Jiao, W.; Xu, Y.; Hou, L.; Li, H.; Shao, J.; Zhang, X.; Wang, R.; Kong, D. Palmitic acid inhibits prostate cancer cell proliferation and metastasis by suppressing the PI3K/Akt pathway. Life Sci. 2021, 286, 120046. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Correia-da-Silva, G.; Valentão, P.; Teixeira, N.; Andrade, P.B. Palmitic acid and ergosta-7, 22-dien-3-ol contribute to the apoptotic effect and cell cycle arrest of an extract from Marthasterias glacialis L. in neuroblastoma cells. Mar. Drugs 2013, 12, 54–68. [Google Scholar] [CrossRef]
- Zafaryab, M.; Fakhri, K.U.; Khan, M.A.; Hajela, K.; Rizvi, M.M.A. In vitro assessment of cytotoxic and apoptotic potential of palmitic acid for breast cancer treatment. Int. J. Life Sci. Res. 2019, 7, 166–174. [Google Scholar]
- Liang, H.; Zhong, Y.; Zhou, S.; Li, Q.Q. Palmitic acid-induced apoptosis in pancreatic β-cells is increased by liver X receptor agonist and attenuated by eicosapentaenoate. In Vivo 2011, 25, 711–718. [Google Scholar] [PubMed]
- Yu, G.; Luo, H.; Zhang, N.; Wang, Y.; Li, Y.; Huang, H.; Liu, Y.; Hu, Y.; Liu, H.; Zhang, J.; et al. Loss of p53 Sensitizes Cells to Palmitic Acid-Induced Apoptosis by Reactive Oxygen Species Accumulation. Int. J. Mol. Sci. 2019, 20, 6268. [Google Scholar] [CrossRef] [PubMed]
- Samimi, S.; Ardestani, M.S.; Dorkoosh, F.A. Preparation of carbon quantum dots-quinic acid for drug delivery of gemcitabine to breast cancer cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102287. [Google Scholar] [CrossRef]
- Karam, L.; Abou Staiteieh, S.; Chaaban, R.; Hayar, B.; Ismail, B.; Neipel, F.; Darwiche, N.; Abou Merhi, R. Anticancer activities of parthenolide in primary effusion lymphoma preclinical models. Mol. Carcinog. 2021, 60, 567–581. [Google Scholar] [CrossRef]
- Alencar, M.; Islam, M.T.; Ali, E.S.; Santos, J.V.O.; Paz, M.; Sousa, J.M.C.; Dantas, S.; Mishra, S.K.; Cavalcante, A. Association of Phytol with Toxic and Cytotoxic Activities in an Antitumoral Perspective: A Meta-Analysis and Systemic Review. Anticancer Agents Med. Chem. 2018, 18, 1828–1837. [Google Scholar] [CrossRef]
- Pejin, B.; Kojic, V.; Bogdanovic, G. An insight into the cytotoxic activity of phytol at in vitro conditions. Nat. Prod. Res. 2014, 28, 2053–2056. [Google Scholar] [CrossRef]
- Pezzani, R.; Salehi, B.; Vitalini, S.; Iriti, M.; Zuñiga, F.A.; Sharifi-Rad, J.; Martorell, M.; Martins, N. Synergistic effects of plant derivatives and conventional chemotherapeutic agents: An update on the cancer perspective. Medicina 2019, 55, 110. [Google Scholar] [CrossRef] [PubMed]


| Cell Type | IC50 Values (μg/mL) | ||||
|---|---|---|---|---|---|
| Crude | CHCl3 | BuOH | MeOH | Doxorubicin | |
| Lung (A549) | 174.2 ± 5.6 | 86.2 ± 2.9 | 169.1 ± 2.6 | 167.9 ± 2.9 | 0.98 ± 0.02 |
| liver (HepG2) | 147.9 ± 3.2 | 78.5 ± 2.1 | 112.5 ± 3.5 | 174.7 ± 4.5 | 1.3 ± 0.4 |
| Breast (MCF-7) | 99.5 ± 1.5 | 50.1 ± 1.2 | 147.6 ± 4.2 | 181.5 ± 4.9 | 1.1 ± 0.3 |
| Compound Name | Chemical Formula | Molecular Weight (g/mol) | RT (min) | %Area |
|---|---|---|---|---|
| p-Menthan-8-yl acetate | C12H22O2 | 198.30 | 13.35 | 2.690 |
| Alpha-d-glucopyranoside | C6H11O6 | 179.15 | 16.16 | 11.150 |
| Quinic acid | C7H12O6 | 192.17 | 19.05 | 11.840 |
| D-Glycero-d-ido-heptose | C7H14O7 | 210.18 | 19.68 | 1.780 |
| Dihydro-alpha-ionone | C13H22O | 194.31 | 21.77 | 0.280 |
| Methyl palmitate | C17H34O2 | 270.5 | 21.84 | 0.700 |
| Ethyl palmitate | C18H36O2 | 284.5 | 22.53 | 0.490 |
| Stearic acid | C18H36O2 | 284.5 | 23.38 | 1.740 |
| alpha-Linolenic acid | C18H30O2 | 278.4 | 23.62 | 0.610 |
| Phytol | C20H40O | 296.5 | 23.74 | 6.650 |
| Caryophyllene epoxide | C15H24O | 220.35 | 23.98 | 0.420 |
| Parthenolide | C15H20O3 | 248.32 | 24.48 | 9.650 |
| gamma-elemene | C15H24 | 204.35 | 24.64 | 0.960 |
| palmitic acid | C16H32O2 | 256.42 | 25.77 | 50.460 |
| 14-Pentadecenoic acid | C15H28O2 | 240.38 | 28.01 | 0.320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-zharani, M.; Nasr, F.A.; Barnawi, I.O.; Noman, O.M.; Herqash, R.N.; Alsufyani, S.A.; Qurtam, A.A.; Rudayni, H.A.; Aleissa, M.S.; Alqahtani, A.S. In Vitro Cytotoxicity Assessment of Abutilon pannosum Chloroform Fraction and Its Phytoconstituents Analysis. Processes 2023, 11, 1306. https://doi.org/10.3390/pr11051306
Al-zharani M, Nasr FA, Barnawi IO, Noman OM, Herqash RN, Alsufyani SA, Qurtam AA, Rudayni HA, Aleissa MS, Alqahtani AS. In Vitro Cytotoxicity Assessment of Abutilon pannosum Chloroform Fraction and Its Phytoconstituents Analysis. Processes. 2023; 11(5):1306. https://doi.org/10.3390/pr11051306
Chicago/Turabian StyleAl-zharani, Mohammed, Fahd A. Nasr, Ibrahim O. Barnawi, Omar M. Noman, Rashed N. Herqash, Sami A. Alsufyani, Ashraf Ahmed Qurtam, Hassan A. Rudayni, Mohammed S. Aleissa, and Ali S. Alqahtani. 2023. "In Vitro Cytotoxicity Assessment of Abutilon pannosum Chloroform Fraction and Its Phytoconstituents Analysis" Processes 11, no. 5: 1306. https://doi.org/10.3390/pr11051306





