Activation Energy of Ion Diffusion in an Electrode Material: Theoretical Calculation and Experimental Estimation with LiCoVO4 as an Example
Abstract
:1. Introduction
2. Materials and Methods
2.1. Computational Details
2.2. Experimental Section
2.2.1. Material Synthesis Procedure
2.2.2. Making a Working Electrode and Electrochemical Measurements
3. Results
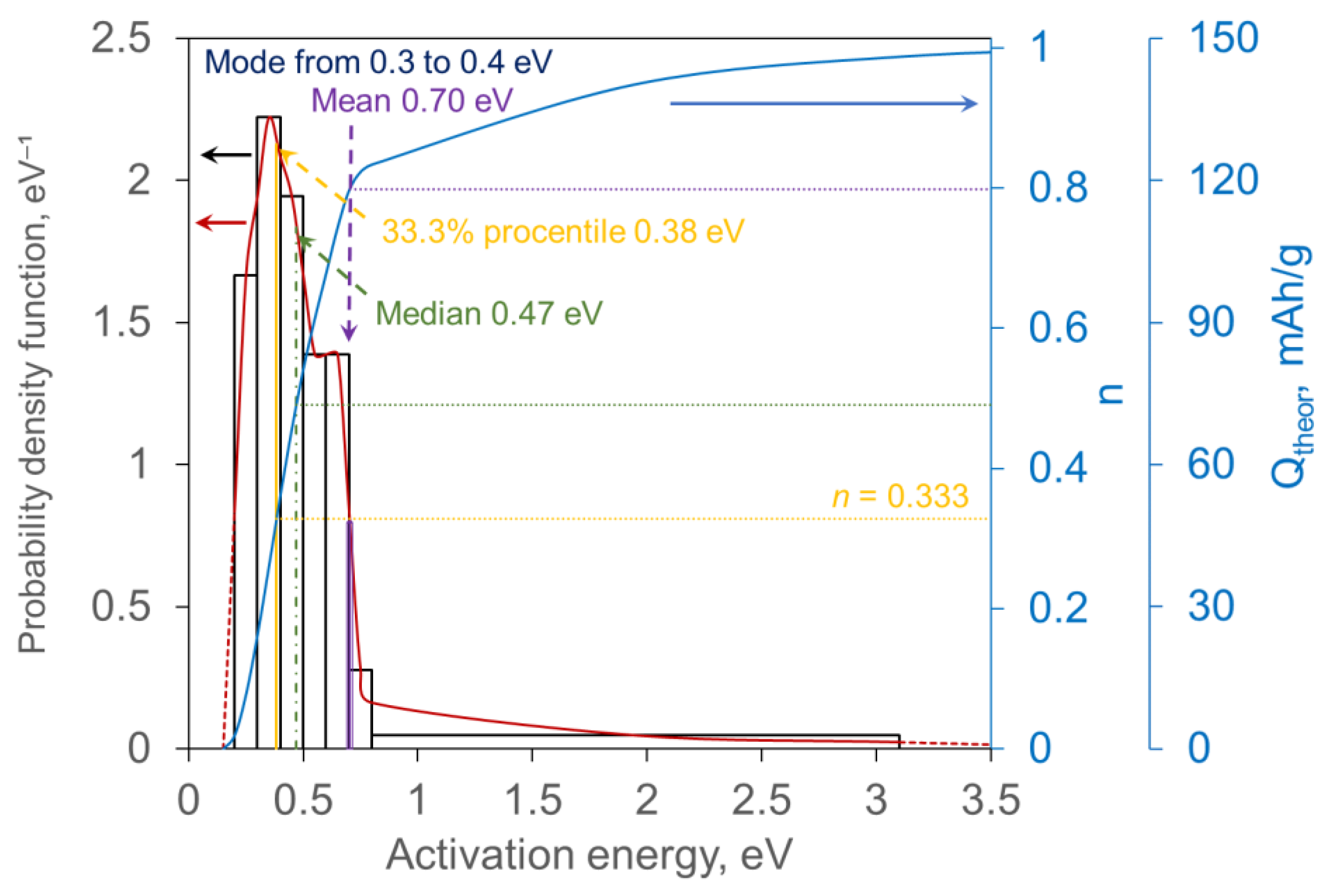
3.1. Theoretical Study
3.2. Experimental Study
3.2.1. Cyclic Voltammetry
3.2.2. Galvanostatic and Potentiostatic Impulses
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Kharbachi, A.; Zavorotynska, O.; Latroche, M.; Cuevas, F.; Yartys, V.; Fichtner, M. Exploits, advances and challenges benefiting beyond Li-ion battery technologies. J. Alloy Compd. 2020, 817, 153261. [Google Scholar] [CrossRef]
- Daniel, C. Materials and processing for lithium-ion batteries. JOM 2008, 60, 43–48. [Google Scholar] [CrossRef]
- Harks, P.; Mulder, F.; Notten, P. In situ methods for Li-ion battery research: A review of recent developments. J. Power Sources 2015, 288, 92–105. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, W.; Trottier, J.; Gagnon, C.; Barray, F.; Guerfi, A.; Mauger, A.; Groult, H.; Julien, C.M.; Goodenough, J.B.; et al. Spinel materials for high-voltage cathodes in Li-ion batteries. RSC Adv. 2014, 4, 154–167. [Google Scholar] [CrossRef]
- Darjazi, H.; Madinabeitia, I.; Zarrabeitia, M.; Gonzalo, E.; Acebedo, B.; Javad Rezvani, S.; Muñoz-Márquez, M.Á. LiNi0.5Mn1.5O4 Thin Films Grown by Magnetron Sputtering under Inert Gas Flow Mixtures as High-Voltage Cathode Materials for Lithium-Ion Batteries. ChemElectroChem 2023, 10, e202201004. [Google Scholar] [CrossRef]
- Wong-Ng, W.; McMurdie, H.F.; Paretzkin, B.; Zhang, Y.; Davis, K.L.; Hubbard, C.R.; Dragoo, A.L.; Stewart, J.M. Reference X-Ray Diffraction Powder Patterns of Fifteen Ceramic Phases. Powder Diffr. 1987, 2, 257–265. [Google Scholar] [CrossRef]
- Bernier, J.C.; Poix, P.; Michel, A. Etude cristallographique et magnetique de deux vanadates mixtes spinelles [Magnetic and crystallographic study of two mixed spinel vanadates]. Bull. Soc. Chim. Fr. 1963, 1963, 445–446. [Google Scholar]
- Leonidova, O.N.; Voronin, V.; Leonidov, I.A.; Samigullina, R.F.; Slobodin, B.V. Crystal Structures of Double Vanadates LiCoVO4 and Li0.5Co1.25VO4. J. Struct. Chem. 2004, 44, 277–283. [Google Scholar] [CrossRef]
- Rybakov, K.S.; Ushakov, A.V. High-voltage Cathode Material Based on LiCoVO4 for Lithium-Ion Battery: Development and Research. Electrochem. Energetics 2019, 19, 90–104. Available online: https://energetica.sgu.ru/en/articles/high-voltage-cathode-material-based-on-licovo-for-lithium-ion-battery-development-and (accessed on 6 April 2023). [CrossRef]
- Prakash, D.; Masuda, Y.; Sanjeeviraja, C. Structural, electrical and electrochemical studies of LiCoVO4 cathode material for lithium rechargeable batteries. Powder Technol. 2013, 235, 454–459. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Xu, H.; Wang, L.; Lu, X.; He, X. Li4Ti5O12 spinel anode: Fundamentals and advances in rechargeable batteries. Infomat 2022, 4, e12228. [Google Scholar] [CrossRef]
- Okhotnikov, K.; Charpentier, T.; Cadars, S. Supercell program: A combinatorial structure-generation approach for the local-level modeling of atomic substitutions and partial occupancies in crystals. J. Chemin. 2016, 8, 17. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Henkelman, G.; Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]
- BatteryMaterials. Available online: https://batterymaterials.info/downloads (accessed on 6 April 2023).
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Aksyonov, D.; Fedotov, S.; Stevenson, K.; Zhugayevych, A. Understanding migration barriers for monovalent ion insertion in transition metal oxide and phosphate based cathode materials: A DFT study. Comput. Mater. Sci. 2018, 154, 449–458. [Google Scholar] [CrossRef]
- Xiao, Y.; Miara, L.J.; Wang, Y.; Ceder, G. Computational Screening of Cathode Coatings for Solid-State Batteries. Joule 2019, 3, 1252–1275. [Google Scholar] [CrossRef]
- Xu, S.; Jacobs, R.M.; Nguyen, H.M.; Hao, S.; Mahanthappa, M.; Wolverton, C.; Morgan, D. Lithium transport through lithium-ion battery cathode coatings. J. Mater. Chem. A 2015, 3, 17248–17272. [Google Scholar] [CrossRef]
- Deng, Z.; Mo, Y.; Ong, S.P. Computational studies of solid-state alkali conduction in rechargeable alkali-ion batteries. NPG Asia Mater. 2016, 8, e254. [Google Scholar] [CrossRef]
- Aksyonov, D.; Boev, A.; Fedotov, S.; Abakumov, A. Computational insights into ionic conductivity of transition metal electrode materials for metal-ion batteries—A review. Solid State Ion. 2023, 393, 116170. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, X. Factors that affect activation energy for Li diffusion in LiFePO4: A first-principles investigation. Solid State Ion. 2010, 181, 907–913. [Google Scholar] [CrossRef]
- Bagotsky, V.S. Fundamentals of Electrochemistry, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Churikov, A.V. Mathematics of Diffusion in Application to Lithium Electrochemical Systems (Transliteration of Russian into English: Matematika Diffuzii v Prilozhenii k Litiyevym Elektrokhimicheskim Sistemam); Nauka: Moscow, Russia, 2015. [Google Scholar]









| No | ID | a (Å) | b (Å) | c (Å) | α (°) | Β (°) | Γ (°) | V (Å3) | E (eV) |
|---|---|---|---|---|---|---|---|---|---|
| 1 |  | 8.2161 | 8.1431 | 8.1202 | 89.974 | 89.131 | 89.920 | 543.218 | −403.737 |
| 2 |  | 8.1552 | 8.2049 | 8.1404 | 90.249 | 89.615 | 89.777 | 544.672 | −403.816 |
| 3 |  | 8.1088 | 8.1437 | 8.2027 | 90.584 | 90.154 | 89.652 | 541.626 | −403.881 |
| No. | Structure 1 | Structure 2 | Structure 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ID | Path Length (Å) | Ea (eV) | ID | Path Length (Å) | Ea (eV) | ID | Path Length (Å) | Ea (eV) | |
| 1 |  | 2.609 | 0.35 |  | 2.795 | 0.27 |  | 2.680 | 0.25 |
| 2 |  | 3.141 | 0.65 |  | 2.762 | 0.39 |  | 2.829 | 0.28 |
| 3 |  | 2.795 | 0.37 |  | 2.903 | 0.36 |  | 2.811 | 0.40 |
| 4 |  | 2.447 | 0.20 |  | 3.050 | 0.51 |  | 2.840 | 0.39 |
| 5 |  | 2.962 | 0.77 |  | 2.750 | 0.46 |  | 3.055 | 0.48 |
| 6 |  | 2.984 | 0.34 |  | 3.051 | 0.47 |  | 2.959 | 0.69 |
| 7 |  | 2.942 | 0.62 |  | 2.969 | 0.26 |  | 5.355 | 2.90 |
| 8 |  | 3.348 | 0.51 |  | 3.046 | 0.43 |  | 5.251 | 2.42 |
| 9 |  | 3.043 | 0.62 |  | 5.248 | 2.84 |  | 2.719 | 0.32 |
| 10 |  | 3.080 | 0.59 |  | 2.884 | 0.50 |  | 2.825 | 0.24 |
| 11 |  | 5.465 | 3.05 |  | 2.851 | 0.34 |  | 2.785 | 0.47 |
| 12 |  | 3.006 | 0.50 | ||||||
| 13 |  | 3.053 | 0.48 | ||||||
| 14 |  | 2.994 | 0.61 | ||||||
| Temperature (°C) | Dan.·1013 (m2·s−1) | Dcat.·1013 (m2·s−1) | Ean. (eV 1) | Ecat., (eV 1) |
|---|---|---|---|---|
| 10 | 9.43 | 0.629 | 0.51 ± 0.34 | 0.78 ± 0.38 |
| 25 | 17.5 | 2.13 | ||
| 30 | 26.7 | 3.76 | ||
| 40 | 77.6 | 15.4 |
| Temperature (°C) | GI | PI | |||
|---|---|---|---|---|---|
| h2D (s−1) | Ea (eV) | h2D | ΔE·RF−1 (A·m−2) | Ea (eV) | |
| 5 | 24.25 | 0.25 ± 0.06 | 3.15 | 0.0017 | 0.37 ± 0.07 |
| 10 | 27.43 | 4.48 | 0.0019 | ||
| 15 | 30.31 | 6.85 | 0.0021 | ||
| 20 | 36.74 | 7.45 | 0.0023 | ||
| 25 | 41.05 | 9.96 | 0.0025 | ||
| 30 | 54.29 | 11.98 | 0.0027 | ||
| 35 | 69.53 | 14.85 | 0.0030 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybakov, K.S.; Ushakov, A.V.; Kabanov, A.A. Activation Energy of Ion Diffusion in an Electrode Material: Theoretical Calculation and Experimental Estimation with LiCoVO4 as an Example. Processes 2023, 11, 1427. https://doi.org/10.3390/pr11051427
Rybakov KS, Ushakov AV, Kabanov AA. Activation Energy of Ion Diffusion in an Electrode Material: Theoretical Calculation and Experimental Estimation with LiCoVO4 as an Example. Processes. 2023; 11(5):1427. https://doi.org/10.3390/pr11051427
Chicago/Turabian StyleRybakov, Kirill S., Arseni V. Ushakov, and Artem A. Kabanov. 2023. "Activation Energy of Ion Diffusion in an Electrode Material: Theoretical Calculation and Experimental Estimation with LiCoVO4 as an Example" Processes 11, no. 5: 1427. https://doi.org/10.3390/pr11051427







