Effect of LiCl Electrolyte Concentration on Energy Storage of Supercapacitor with Multilayered Ti3C2Tx MXene Electrodes Synthesized by Hydrothermal Etching
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Multilayered Ti3C2Tx MXene
2.2. Materials Characterization
2.3. Electrochemical Testing
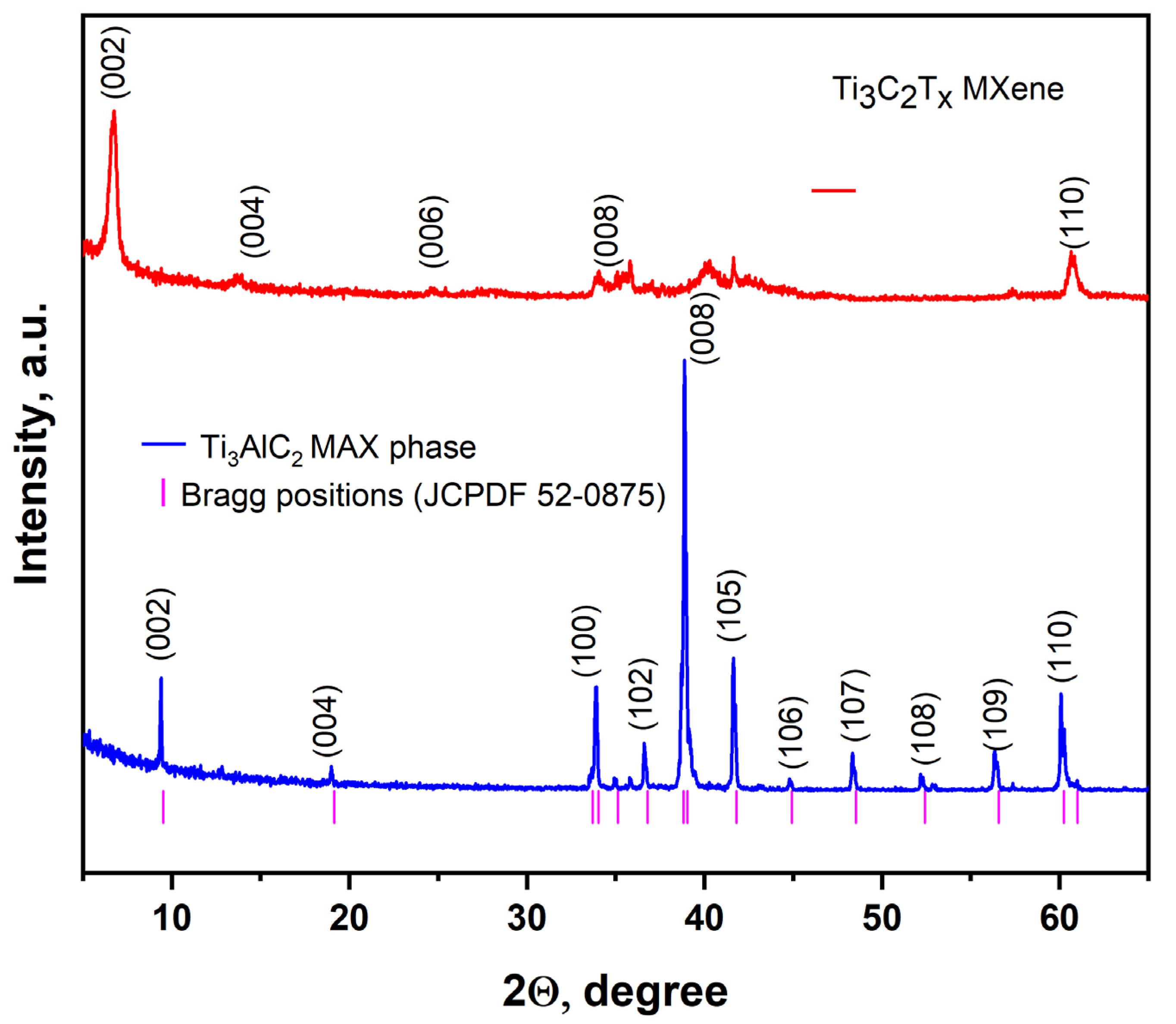
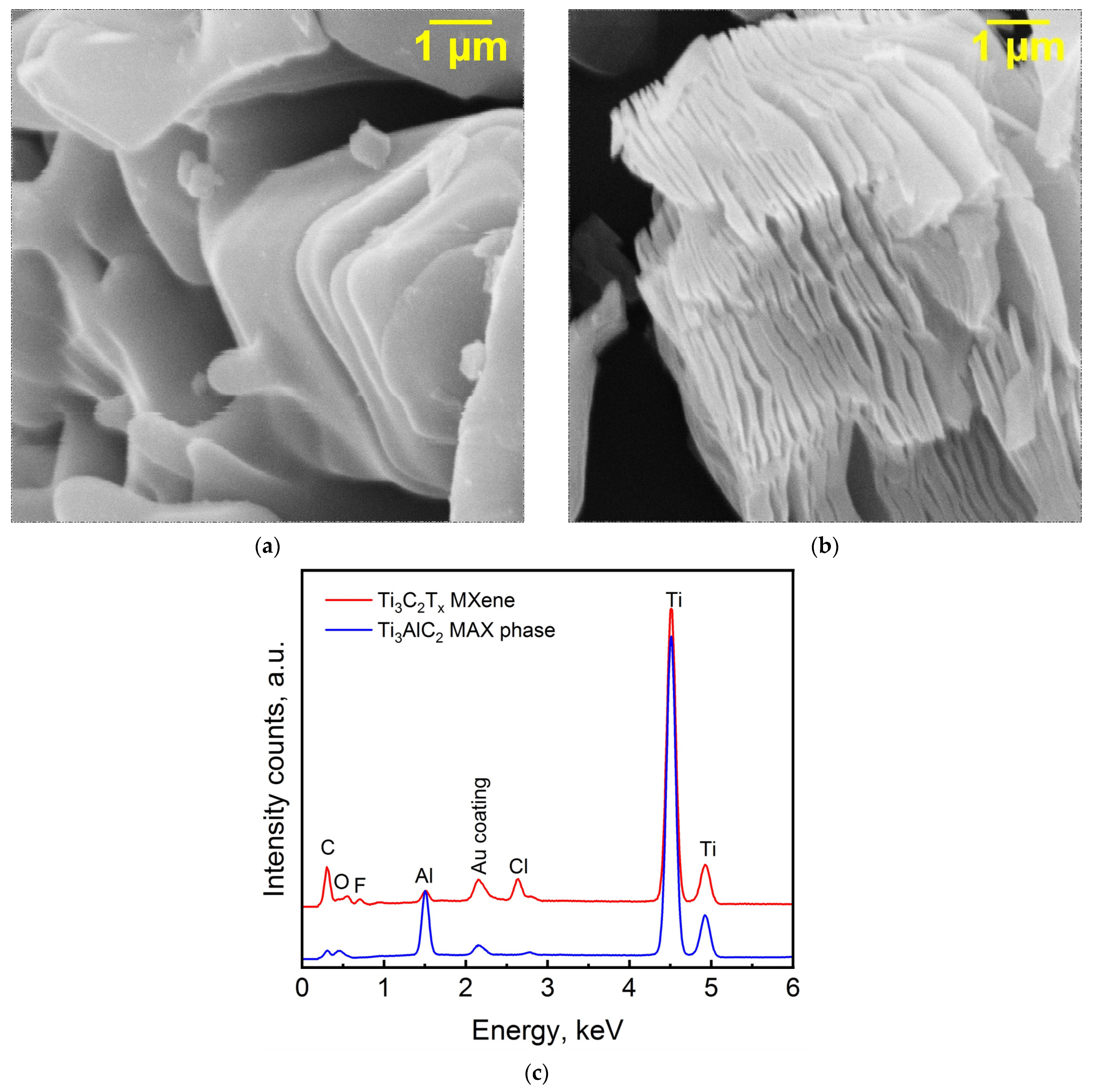
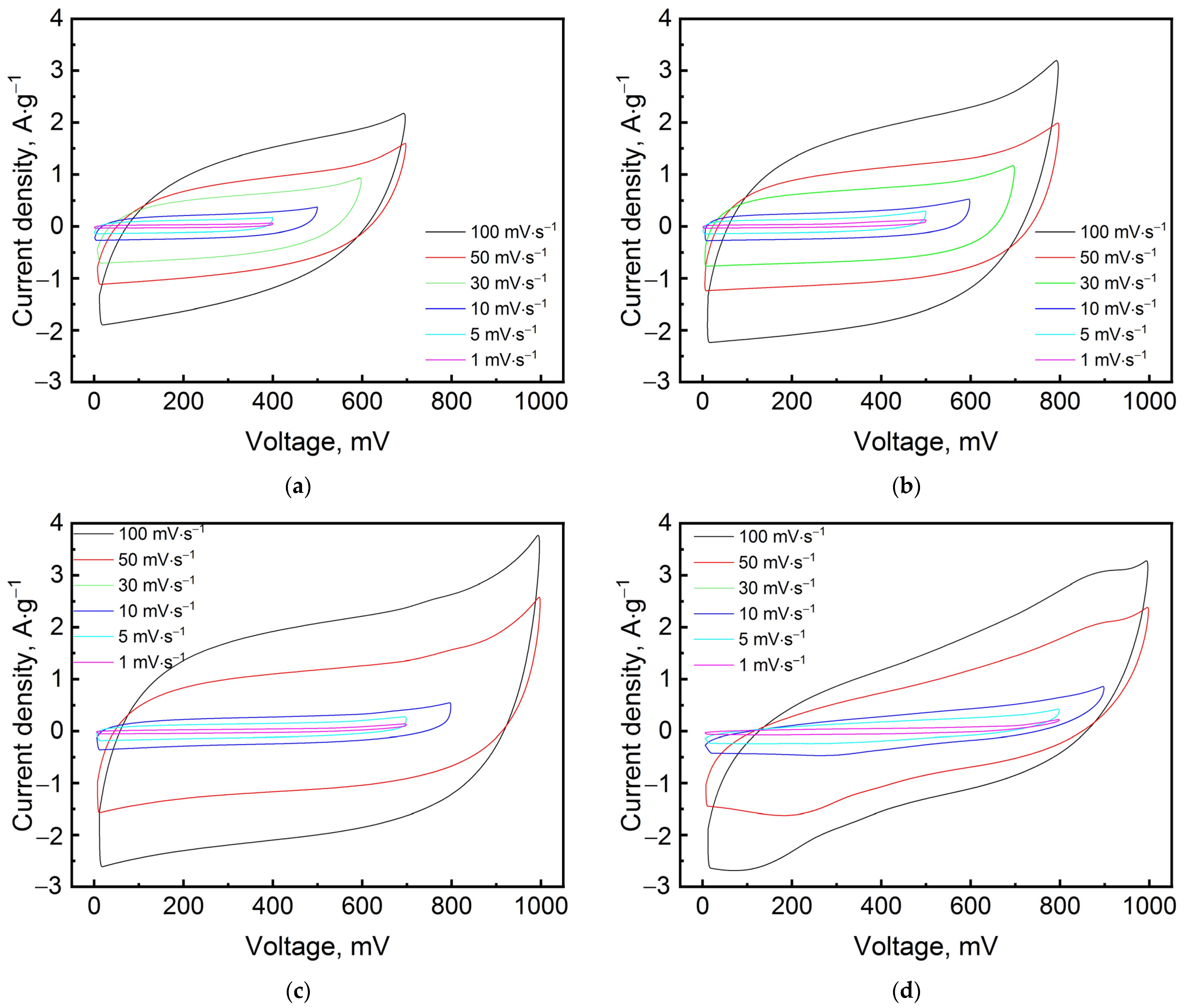
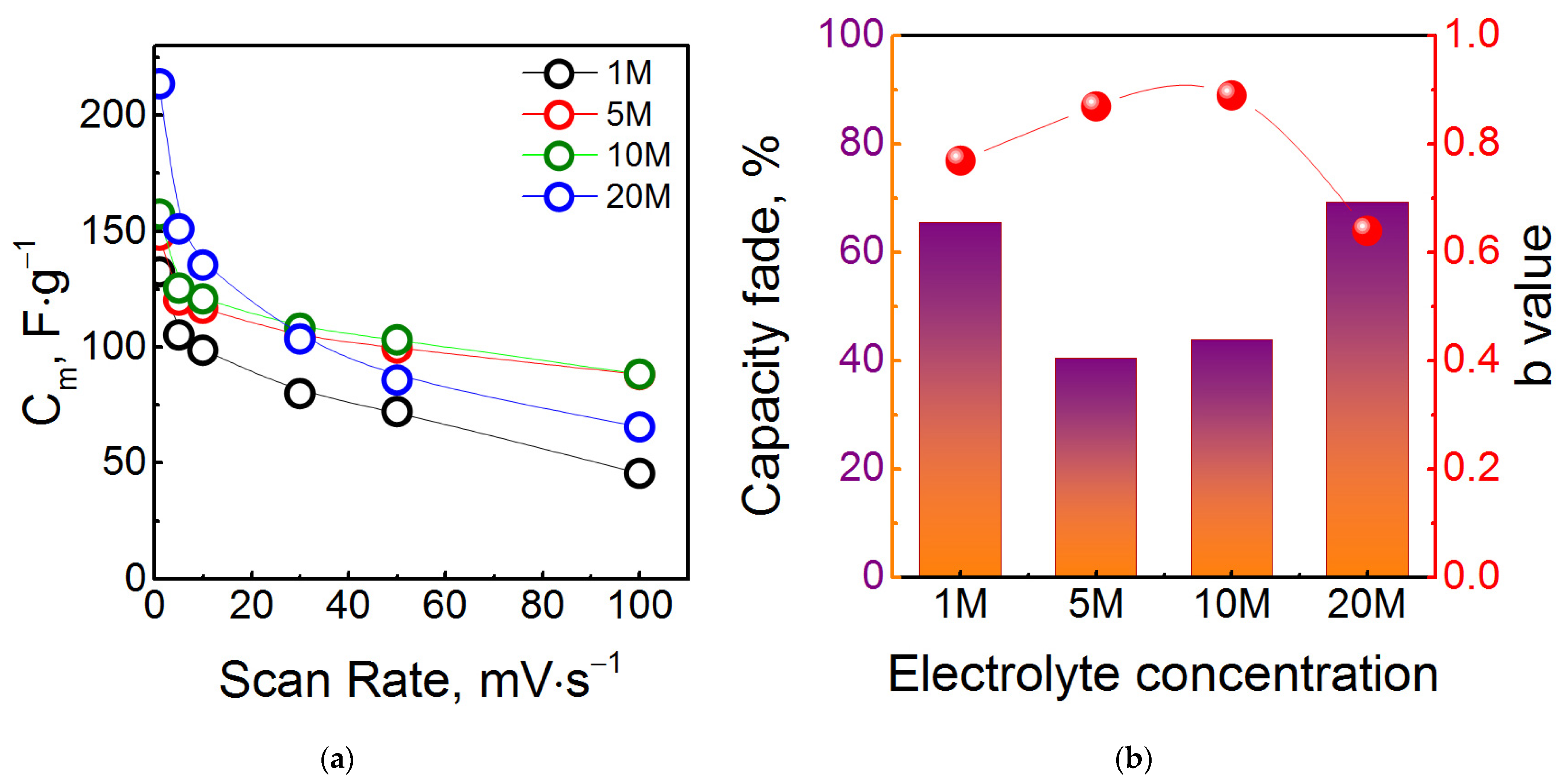
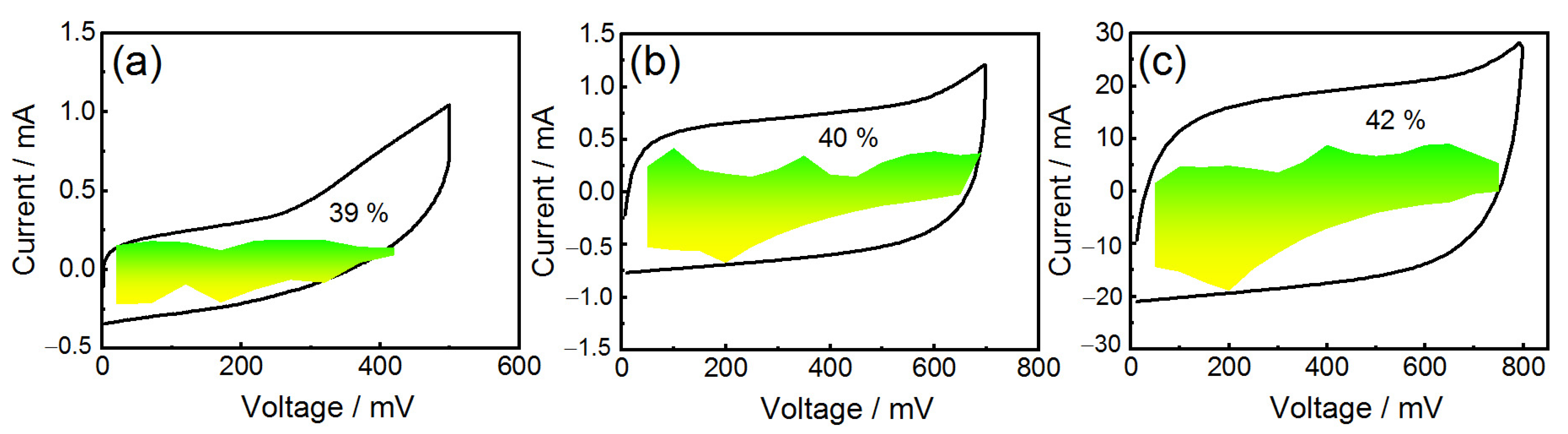
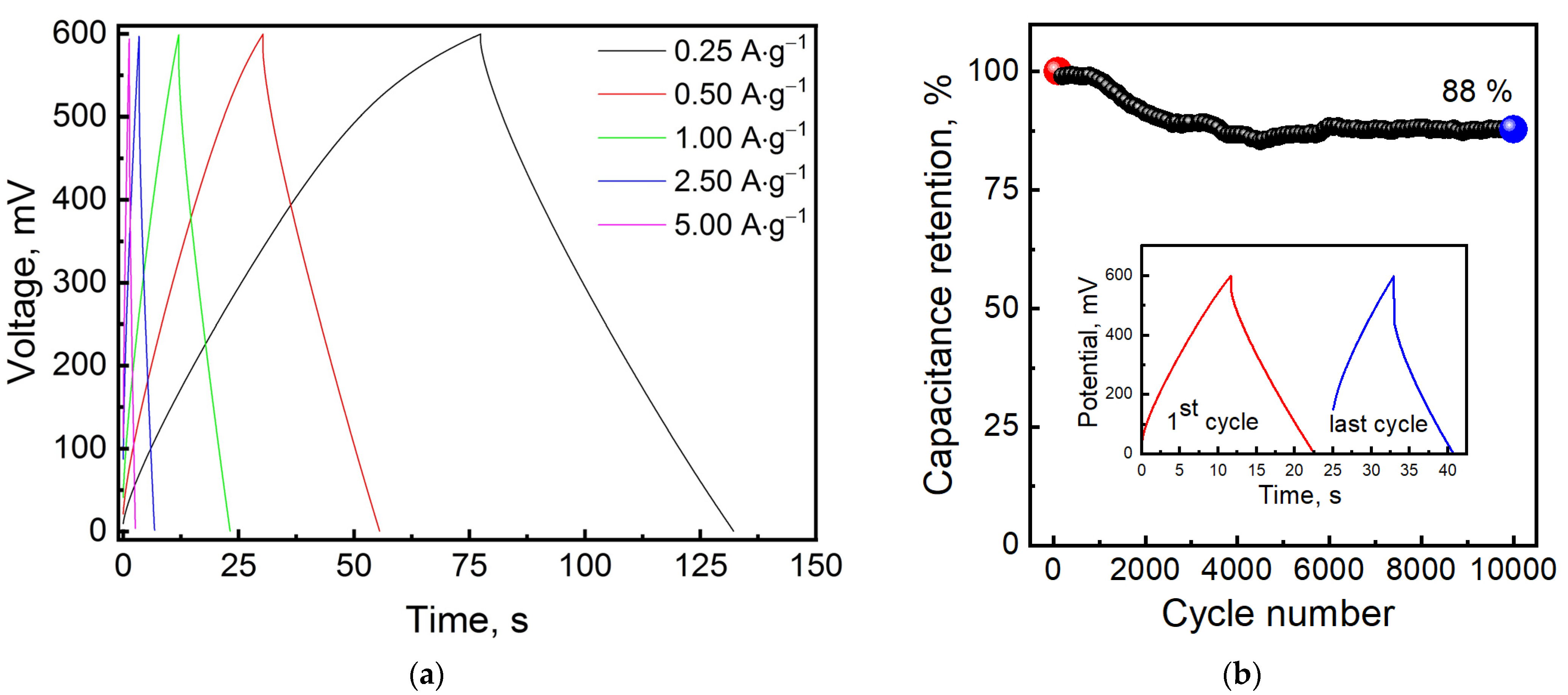
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Collath, N.; Tepe, B.; Englberger, S.; Jossen, A.; Hesse, H. Aging aware operation of lithium-ion battery energy storage systems: A review. J. Energy Storage 2022, 55, 105634. [Google Scholar] [CrossRef]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.N.; Li, Y.H.; Xu, D.F.; Zhou, W.; Xiang, K.X.; Chen, H. Three-dimensional hierarchically porous nitrogen-doped carbon from water hyacinth as selenium host for high-performance lithium–selenium batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [PubMed]
- Deng, W.; Xu, Y.; Zhang, X.; Li, C.; Liu, Y.; Xiang, K.; Chen, H. (NH4)2Co2V10O28·16H2O/(NH4)2V10O25·8H2O heterostructure as cathode for high-performance aqueous Zn-ion batteries. J. Alloys Compd. 2022, 903, 163824. [Google Scholar] [CrossRef]
- Zhou, W.; Zeng, G.; Jin, H.; Jiang, S.; Huang, M.; Zhang, C.; Chen, H. Bio-Template Synthesis of V2O3@Carbonized Dictyophora Composites for Advanced Aqueous Zinc-Ion Batteries. Molecules 2023, 28, 2147. [Google Scholar] [CrossRef]
- Wen, X.; Luo, J.; Xiang, K.; Zhou, W.; Zhang, C.; Chen, H. High-performance monoclinic WO3 nanospheres with the novel NH4+ diffusion behaviors for aqueous ammonium-ion batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar] [CrossRef]
- Xiao, J.; Li, H.; Zhang, H.; He, S.; Zhang, Q.; Liu, K.; Jiang, S.; Duan, G.; Zhang, K. Nanocellulose and its derived composite electrodes toward supercapacitors: Fabrication, properties, and challenges. J. Bioresour. Bioprod. 2022, 7, 245–269. [Google Scholar]
- Dar, M.A.; Dinagaran, S.; Govindarajan, D.; Ahamed, S.R.; Habib, F.; Siva, C.; Moholkar, A.V.; Ahmad, Z.; Yatoo, M.A. Snx−0MnxS nanomaterial based electrodes for future-generation supercapacitor and data storage devices. J. Alloys Compd. 2023, 958, 170523. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Saikia, B.K.; Benoy, S.M.; Bora, M.; Tamuly, J.; Pandey, M.; Bhattacharya, D. A brief review on supercapacitor energy storage devices and utilization of natural carbon resources as their electrode materials. Fuel 2020, 282, 118796. [Google Scholar] [CrossRef]
- Liu, S.; Sun, S.; You, X.Z. Inorganic nanostructured materials for high performance electrochemical supercapacitors. Nanoscale 2014, 6, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Baboo, J.P.; Jakubczyk, E.; Yatoo, M.A.; Phillips, M.; Grabe, S.; Dent, M.; Hinder, S.J.; Watts, J.F.; Lekakou, C. Investigating battery-supercapacitor material hybrid configurations in energy storage device cycling at 0.1 to 10C rate. J. Power Sources 2023, 561, 232762. [Google Scholar] [CrossRef]
- Zhu, X. Recent advances of transition metal oxides and chalcogenides in pseudo-capacitors and hybrid capacitors: A review of structures, synthetic strategies, and mechanism studies. J. Energy Storage 2022, 49, 104148. [Google Scholar] [CrossRef]
- Bhojane, P. Recent advances and fundamentals of Pseudocapacitors: Materials, mechanism, and its understanding. J. Energy Storage 2022, 45, 103654. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Y.; Qiu, H.; Ma, Z.; Ruan, K.; Gu, J. A mini-review of MXene porous films: Preparation, mechanism and application. J. Mater. Sci. Technol. 2022, 103, 42–49. [Google Scholar] [CrossRef]
- Yan, J.; Li, S.; Lan, B.; Wu, Y.; Lee, P.S. Rational design of nanostructured electrode materials toward multifunctional supercapacitors. Adv. Funct. Mater. 2020, 30, 1902564. [Google Scholar] [CrossRef]
- Arbi, H.M.; Vijayalakshmi, L.; Anil Kumar, Y.; Alzahmi, S.; Gopi, C.V.V.M.; Rusydi, A.; Obaidat, I.M. A Facile Two-Step Hydrothermal Synthesis of Co(OH)2@NiCo2O4 Nanosheet Nanocomposites for Supercapacitor Electrodes. Nanomaterials 2023, 13, 1981. [Google Scholar] [CrossRef]
- Kumar, S.; Ahmed, F.; Shaalan, N.M.; Arshi, N.; Dalela, S.; Chae, K.H. Investigations of Structural, Magnetic, and Electrochemical Properties of NiFe2O4 Nanoparticles as Electrode Materials for Supercapacitor Applications. Materials 2023, 16, 4328. [Google Scholar] [CrossRef]
- Senthil, R.A.; Min, A.; Theerthagiri, J.; Kim, G.A.; Choi, H.C.; Choi, M.Y. Insights on Ni-based layered double hydroxides for electrochemical supercapacitors: Underlying aspects in rational design and structural evolution. J. Energy Storage 2023, 72, 108305. [Google Scholar] [CrossRef]
- Kandasamy, M.; Sahoo, S.; Nayak, S.K.; Chakraborty, B.; Rout, C.S. Recent advances in engineered metal oxide nanostructures for supercapacitor applications: Experimental and theoretical aspects. J. Mater. Chem. A 2021, 9, 17643–17700. [Google Scholar] [CrossRef]
- Bokhari, S.W.; Siddique, A.H.; Sherrell, P.C.; Yue, X.; Karumbaiah, K.M.; Wei, S.; Ellis, A.V.; Gao, W. Advances in graphene-based supercapacitor electrodes. Energy Rep. 2020, 6, 2768–2784. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Zeng, W. Preparation and Application of 2D MXene-Based Gas Sensors: A Review. Chemosensors 2021, 9, 225. [Google Scholar] [CrossRef]
- Perera, A.A.P.R.; Madhushani, K.A.U.; Punchihewa, B.T.; Kumar, A.; Gupta, R.K. MXene-Based Nanomaterials for Multifunctional Applications. Materials 2023, 16, 1138. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; He, J.; Huang, R.; Tao, L.; Zhao, Y.; Yang, Y. Ti3C2Tx MXene Quantum Dots with Surface-Terminated Groups (–F, –OH, =O, –Cl) for Ultrafast Photonics. Nanomaterials 2022, 12, 2043. [Google Scholar] [CrossRef]
- Tsyganov, A.; Vikulova, M.; Artyukhov, D.; Zheleznov, D.; Gorokhovsky, A.; Gorshkov, N. Intercalation Effects on the Dielectric Properties of PVDF/Ti3C2Tx MXene Nanocomposites. Nanomaterials 2023, 13, 1337. [Google Scholar] [CrossRef]
- Tseluikin, V.; Dzhumieva, A.; Tribis, A.; Tikhonov, D.; Tsyganov, A.; Gorshkov, N.; Lopukhova, M. Study of Electrodeposition and Properties of Composite Nickel Coatings Modified with Ti3C2TX MXene. Coatings 2023, 13, 1042. [Google Scholar] [CrossRef]
- Raja Sulaiman, R.R.; Hanan, A.; Wong, W.Y.; Mohamad Yunus, R.; Shyuan Loh, K.; Walvekar, R.; Chaudhary, V.; Khalid, M. Structurally Modified MXenes-Based Catalysts for Application in Hydrogen Evolution Reaction: A Review. Catalysts 2022, 12, 1576. [Google Scholar] [CrossRef]
- Murali, G.; Reddy Modigunta, J.K.; Park, Y.H.; Lee, J.H.; Rawal, J.; Lee, S.Y.; In, I.; Park, S.J. A review on MXene synthesis, stability, and photocatalytic applications. ACS Nano 2022, 16, 13370–13429. [Google Scholar] [CrossRef]
- Arumugam, B.; Mayakrishnan, G.; Subburayan Manickavasagam, S.K.; Kim, S.C.; Vanaraj, R. An Overview of Active Electrode Materials for the Efficient High-Performance Supercapacitor Application. Crystals 2023, 13, 1118. [Google Scholar] [CrossRef]
- Liang, W.; Xu, R.; Nawwar, M.; Zhitomirsky, I. Multifunctional MXene–Fe3O4–Carbon Nanotube Composite Electrodes for High Active Mass Asymmetric Supercapacitors. Batteries 2023, 9, 327. [Google Scholar] [CrossRef]
- Guan, G.; Guo, F. A Review of Nb2CTx MXene: Synthesis, Properties and Applications. Batteries 2023, 9, 235. [Google Scholar] [CrossRef]
- Forouzandeh, P.; Pillai, S.C. MXenes-based nanocomposites for supercapacitor applications. Curr. Opin. Chem. Eng. 2021, 33, 100710. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, R.; Tian, J.; Huang, W.; Liu, J. MXene-based nanocomposites for energy conversion and storage applications. Chem. Eur. J. 2020, 26, 6342–6359. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, Q.; Wu, D.; Jiang, Y.; Liu, Z.; Chen, B.; Zhu, M.; Schmidt, O.G. Flexible MXene films for batteries and beyond. Carbon Energy 2022, 4, 598–620. [Google Scholar] [CrossRef]
- Wang, J.; He, J.; Kan, D.; Chen, K.; Song, M.; Huo, W. MXene Film Prepared by Vacuum-Assisted Filtration: Properties and Applications. Crystals 2022, 12, 1034. [Google Scholar] [CrossRef]
- Xi, W.; Zhang, Y.; Zhang, J.; Wang, R.; Gong, Y.; He, B.; Wang, H.; Jin, J. Constructing MXene hydrogels and aerogels for rechargeable supercapacitors and batteries. J. Mater. Chem. C 2023, 11, 2414–2429. [Google Scholar] [CrossRef]
- Li, K.; Li, J.; Zhu, Q.; Xu, B. Three-dimensional MXenes for supercapacitors: A review. Small Methods 2022, 6, 2101537. [Google Scholar] [CrossRef]
- Hu, M.; Li, Z.; Hu, T.; Zhu, S.; Zhang, C.; Wang, X. High-capacitance mechanism for Ti3C2Tx MXene by in situ electrochemical raman spectroscopy investigation. ACS Nano 2016, 10, 11344–11350. [Google Scholar] [CrossRef]
- Fu, J.; Li, L.; Lee, D.; Yun, J.M.; Ryu, B.K.; Kim, K.H. Enhanced electrochemical performance of Ti3C2Tx MXene film based supercapacitors in H2SO4/KI redox additive electrolyte. Appl. Surf. Sci. 2020, 504, 144250. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.; Lian, W.; Hu, Q.; Liu, X.; Zhou, A. The preparation of V2CTx by facile hydrothermal-assisted etching processing and its performance in lithium-ion battery. J. Mater. Res. Technol. 2020, 9, 984–993. [Google Scholar] [CrossRef]
- Ghosh, A.; Pal, H.; Das, T.; Chatterjee, S.; Das, A. Synthesis and Characterization of MXene from MAX phase. Mater. Today Proc. 2022, 58, 714–716. [Google Scholar] [CrossRef]
- Jiang, Q.; Kurra, N.; Alhabeb, M.; Gogotsi, Y.; Alshareef, H.N. All pseudocapacitive MXene-RuO2 asymmetric supercapacitors. Adv. Energy Mater. 2018, 8, 1703043. [Google Scholar] [CrossRef]
- Tao, Q.; Dahlqvist, M.; Lu, J.; Kota, S.; Meshkian, R.; Halim, J.; Palisaitis, J.; Hultman, L.; Barsoum, M.W.; Persson, P.O.A.; et al. Two-dimensional Mo1.33C MXene with divacancy ordering prepared from parent 3D laminate with in-plane chemical ordering. Nat. Commun. 2017, 8, 14949. [Google Scholar] [CrossRef]
- MacArthur, D.M. The hydrated nickel hydroxide electrode potential sweep experiments. J. Electrochem. Soc. 1970, 117, 422–426. [Google Scholar] [CrossRef]
- Kim, H.S.; Cook, J.B.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3−x. Nat. Mater. 2017, 16, 454–460. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xu, C.; Jiang, H.; Li, C.; Zhang, L.; Lin, J.; Shen, Z.X. Advanced energy storage devices: Basic principles, analytical methods, and rational materials design. Adv. Sci. 2018, 5, 1700322. [Google Scholar] [CrossRef]
- Chao, D.; Liang, P.; Chen, Z.; Bai, L.; Shen, H.; Liu, X.; Xia, X.; Zhao, Y.; Savilov, S.V.; Lin, J.; et al. Pseudocapacitive Na-ion storage boosts high rate and areal capacity of self-branched 2D layered metal chalcogenide nanoarrays. ACS Nano 2016, 10, 10211–10219. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Zhu, C.; Yang, P.; Xia, X.; Liu, J.; Wang, J.; Fan, X.; Savilov, S.V.; Lin, J.; Fan, H.J.; et al. Array of nanosheets render ultrafast and high-capacity Na-ion storage by tunable pseudocapacitance. Nat. Commun. 2016, 7, 12122. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, T.; Vedarajan, R.; Rajalakshmi, N.; Reddy, L.R. Dynamic electrochemical impedance spectroscopy as a rapid screening tool for supercapacitor electrode materials. J. Mater. Sci. Mater. Electron. 2020, 31, 1681–1690. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical impedance spectroscopy. Nat. Rev. Methods Primers 2021, 1, 41. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, W.; Yuan, H.; Jin, C.; Zhang, L.; Huang, H.; Liang, C.; Xia, Y.; Zhang, J.; Gan, Y.; et al. Pillared Structure Design of MXene with Ultralarge Interlayer Spacing for High-Performance Lithium-Ion Capacitors. ACS Nano 2017, 11, 2459–2469. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Dong, L.; Dong, M. Co(OH)2/MXene composites for tunable pseudo-capacitance energy storage. Electrochim. Acta 2020, 353, 136607. [Google Scholar] [CrossRef]
- Kurra, N.; Alhabeb, M.; Maleski, K.; Wang, C.H.; Alshareef, H.N.; Gogotsi, Y. Bistacked titanium carbide (MXene) anodes for hybrid sodium-ion capacitors. ACS Energy Lett. 2018, 3, 2094–2100. [Google Scholar] [CrossRef]
- Syamsai, R.; Grace, A.N. Ta4C3 MXene as supercapacitor electrodes. J. Alloys Compd. 2019, 792, 1230–1238. [Google Scholar] [CrossRef]
- Xu, P.; Xiao, H.; Liang, X.; Zhang, T.; Zhang, F.; Liu, C.; Lang, B.; Gao, Q. A MXene-based EDA-Ti3C2Tx intercalation compound with expanded interlayer spacing as high performance supercapacitor electrode material. Carbon 2021, 173, 135–144. [Google Scholar] [CrossRef]
- Habib, I.; Ferrer, P.; Ray, S.C.; Ozoemena, K.I. Interrogating the impact of onion-like carbons on the supercapacitive properties of MXene (Ti2CTX). J. Appl. Phys. 2019, 126, 134301. [Google Scholar] [CrossRef]
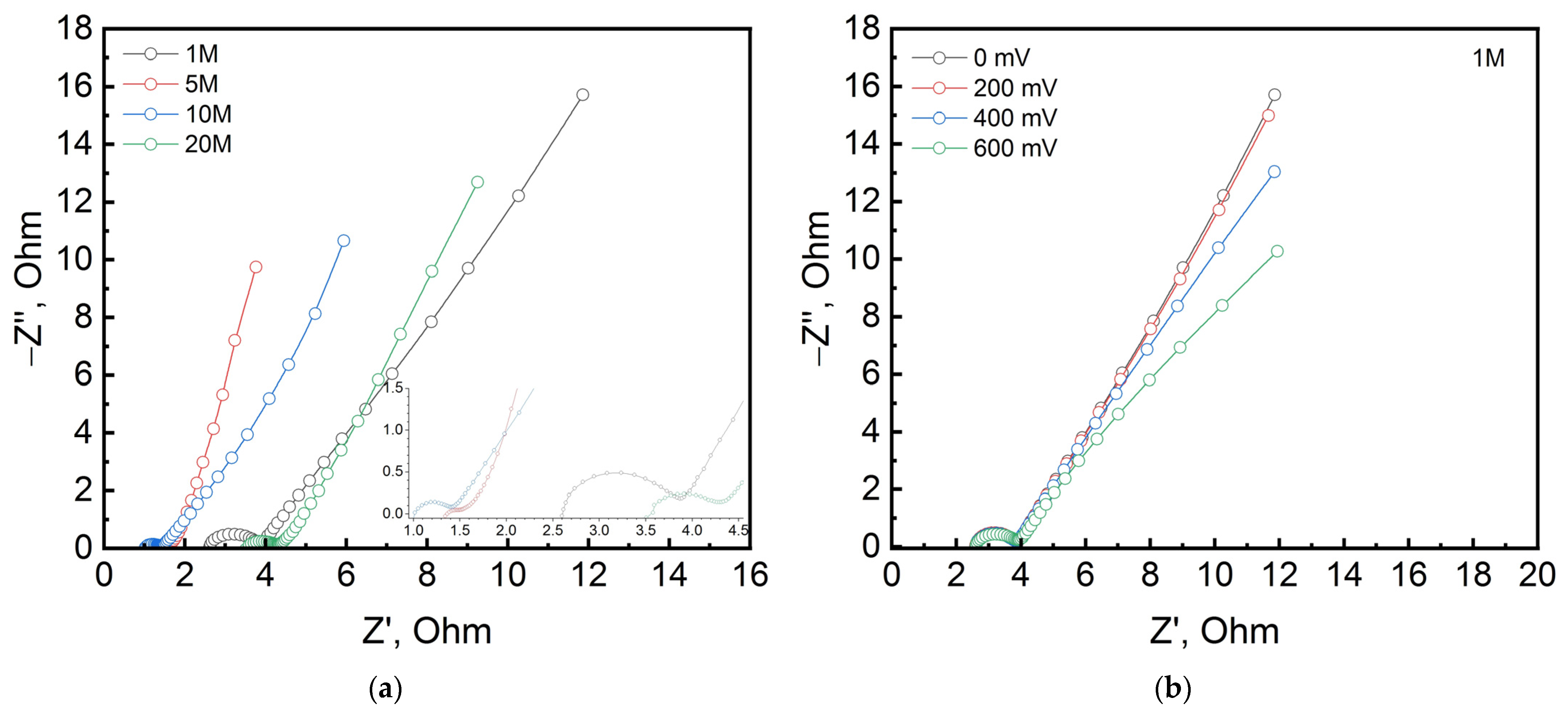
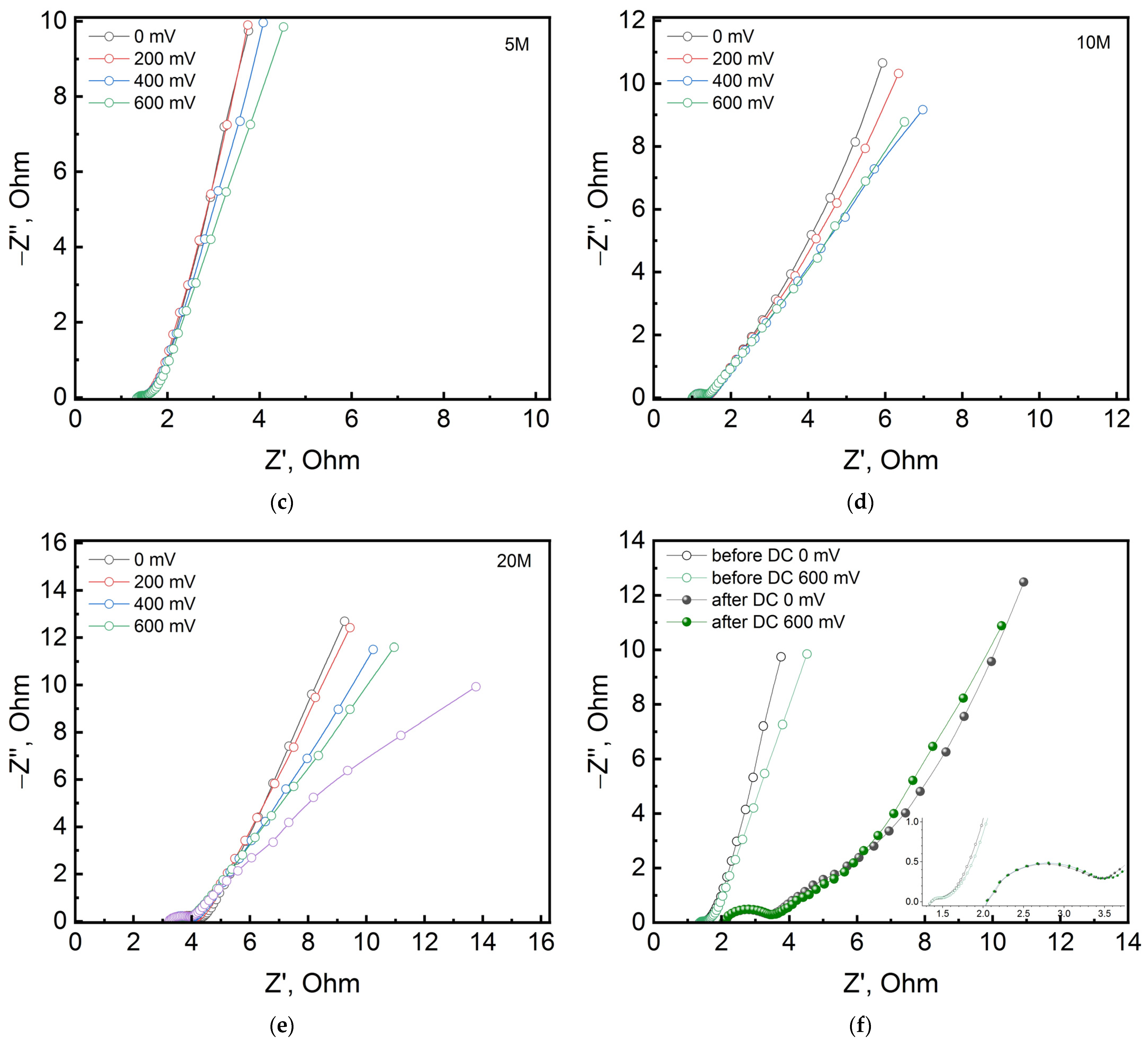
| Electrolyte Concentration | 1 M | 5 M | 5 M after Cycling | 10 M | 20 M |
|---|---|---|---|---|---|
| Rs, Ohm | 2.594 | 1.172 | 1.918 | 0.940 | 3.503 |
| CPEdl, mF∙s(a−1) | 0.1 | 7.228 | 191 | 0.286 | 0.96 |
| ndl | 0.888 | 0.486 | 0.750 | 0.817 | 0.688 |
| Rct, Ohm | 1.19 | 0.358 | 1.384 | 0.423 | 0.789 |
| W, Ohm∙s−1/2 | 1.372 | 1.530 | 9.560 | 2.453 | 1.964 |
| CPEel, F∙s(a−1) | 0.087 | 0.191 | 0.148 | 0.179 | 0.136 |
| nel | 0.718 | 1 | 0.982 | 0.837 | 0.851 |
| Applied Voltage, mV | 0 | 200 | 400 | 600 | 600 after Cycling |
|---|---|---|---|---|---|
| Rs, Ohm | 1.172 | 1.228 | 1.325 | 1.208 | 1.898 |
| CPEdl, mF∙s(a−1) | 7.228 | 8.697 | 2.323 | 93 | 0.237 |
| ndl | 0.486 | 0.510 | 0.730 | 0.304 | 0.731 |
| Rct, Ohm | 0.358 | 0.297 | 0.206 | 0.481 | 1.475 |
| W, Ohm∙s−1/2 | 1.530 | 1.480 | 1.434 | 0.798 | 8.245 |
| CPEel, F∙s(a−1) | 0.191 | 0.186 | 0.184 | 0.164 | 0.128 |
| nel | 1 | 1 | 0.960 | 0.883 | 0.777 |
| Electrode Material | Electrolyte | Capacitance, F g−1 | Capacitance Retention | Ref. |
|---|---|---|---|---|
| CTAB-Sn(IV)@Ti3C2//AC | 1 M LiPF6 in EC:DEC:EMC (1:1:1 v:v:v) + 1 wt.% FEC | 51 | 71.1% (4000 cycles) | [56] |
| Co(OH)2/Ti3C2Tx | 5 M LiCl | 153 | 99.0% (1000 cycles) | [57] |
| Bistacked 2D titanium carbide | Non-aqueous 1 M NaClO4 | 104 | 84.2% (4000 cycles) | [58] |
| Ta4C3 | 0.1 M H2SO4 | 120 | 89.0% (2000 cycles) | [59] |
| EDA-Ti3C2Tx | 3 M H2SO4 | 249.4 | 89.7% (10,000 cycles) | [60] |
| Ti2CTx/OLS(5%) | 1 M H2SO4 | 102.03 | 100% (10,000 cycles) | [61] |
| Multilayered Ti3C2Tx | 5 M LiCl | 120 | 88% (10,000 cycles) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsyganov, A.; Shindrov, A.; Vikulova, M.; Zheleznov, D.; Gorokhovsky, A.; Gorshkov, N. Effect of LiCl Electrolyte Concentration on Energy Storage of Supercapacitor with Multilayered Ti3C2Tx MXene Electrodes Synthesized by Hydrothermal Etching. Processes 2023, 11, 2528. https://doi.org/10.3390/pr11092528
Tsyganov A, Shindrov A, Vikulova M, Zheleznov D, Gorokhovsky A, Gorshkov N. Effect of LiCl Electrolyte Concentration on Energy Storage of Supercapacitor with Multilayered Ti3C2Tx MXene Electrodes Synthesized by Hydrothermal Etching. Processes. 2023; 11(9):2528. https://doi.org/10.3390/pr11092528
Chicago/Turabian StyleTsyganov, Alexey, Alexander Shindrov, Maria Vikulova, Denis Zheleznov, Alexander Gorokhovsky, and Nikolay Gorshkov. 2023. "Effect of LiCl Electrolyte Concentration on Energy Storage of Supercapacitor with Multilayered Ti3C2Tx MXene Electrodes Synthesized by Hydrothermal Etching" Processes 11, no. 9: 2528. https://doi.org/10.3390/pr11092528
APA StyleTsyganov, A., Shindrov, A., Vikulova, M., Zheleznov, D., Gorokhovsky, A., & Gorshkov, N. (2023). Effect of LiCl Electrolyte Concentration on Energy Storage of Supercapacitor with Multilayered Ti3C2Tx MXene Electrodes Synthesized by Hydrothermal Etching. Processes, 11(9), 2528. https://doi.org/10.3390/pr11092528








