Expansive Soil Stabilization Using Alkali-Activated Fly Ash
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Experimental Work
3. Results and Discussion
3.1. Unloaded Swelling Rate Test
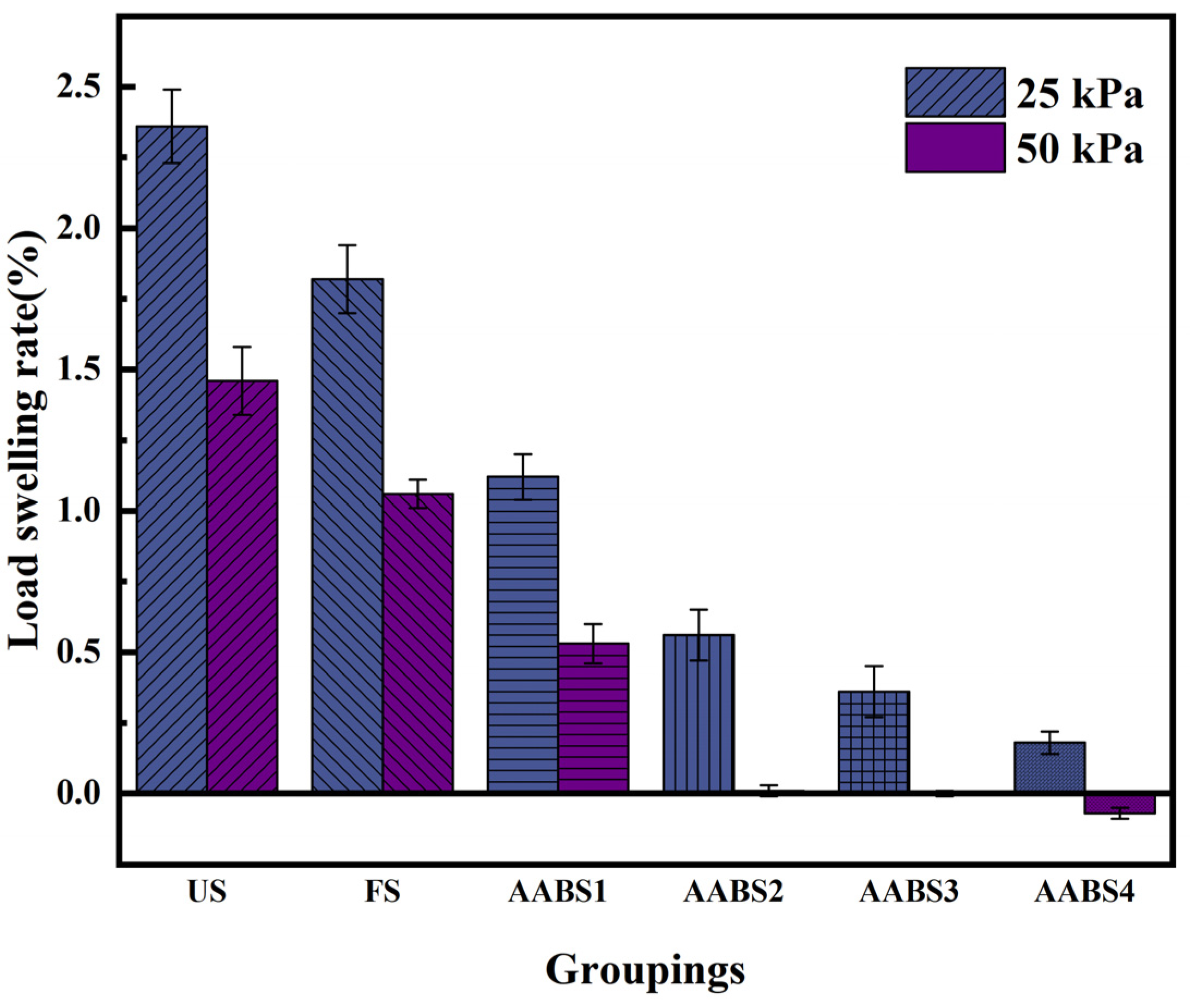
3.2. Load Swelling Rate Test
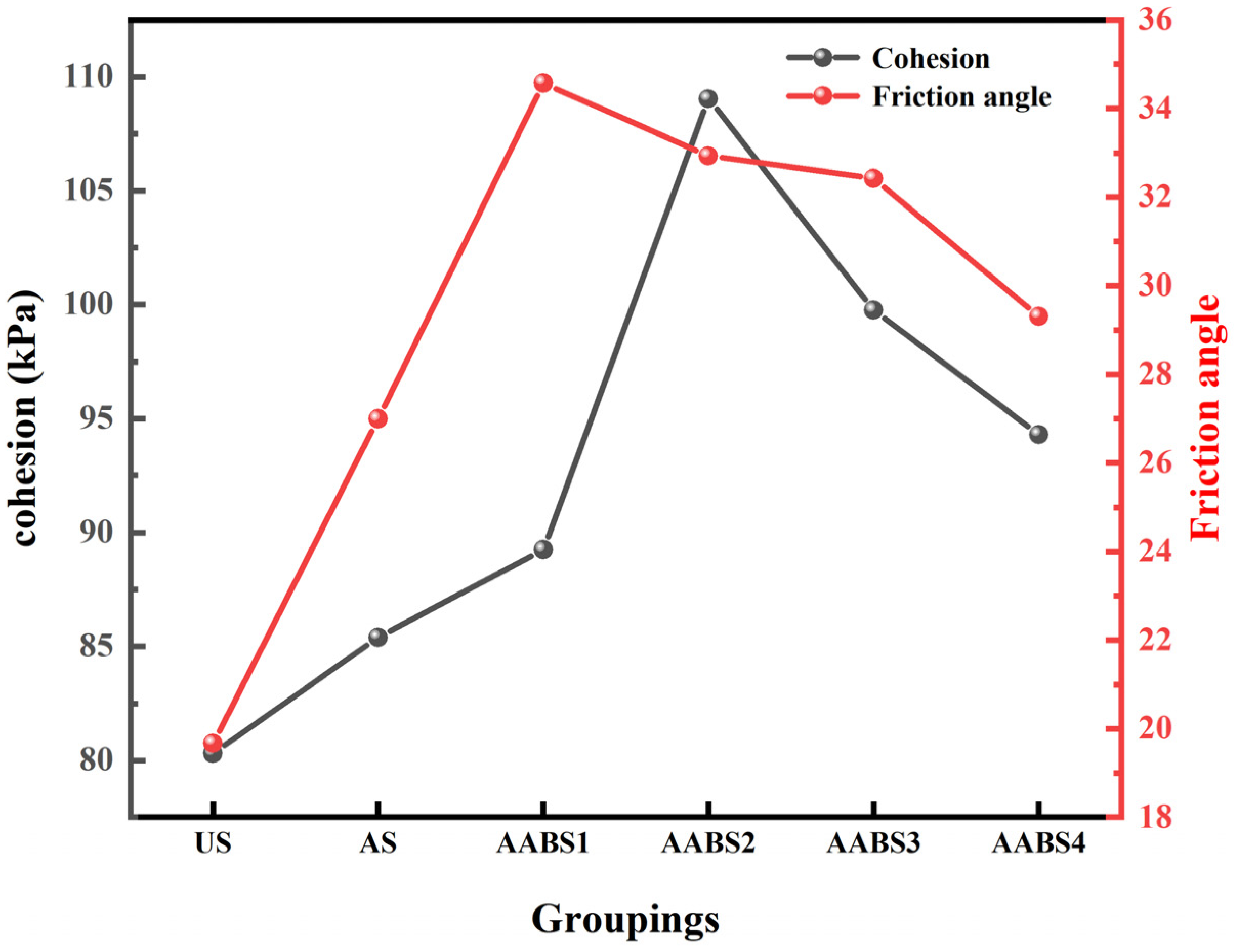
3.3. Direct Shear Test
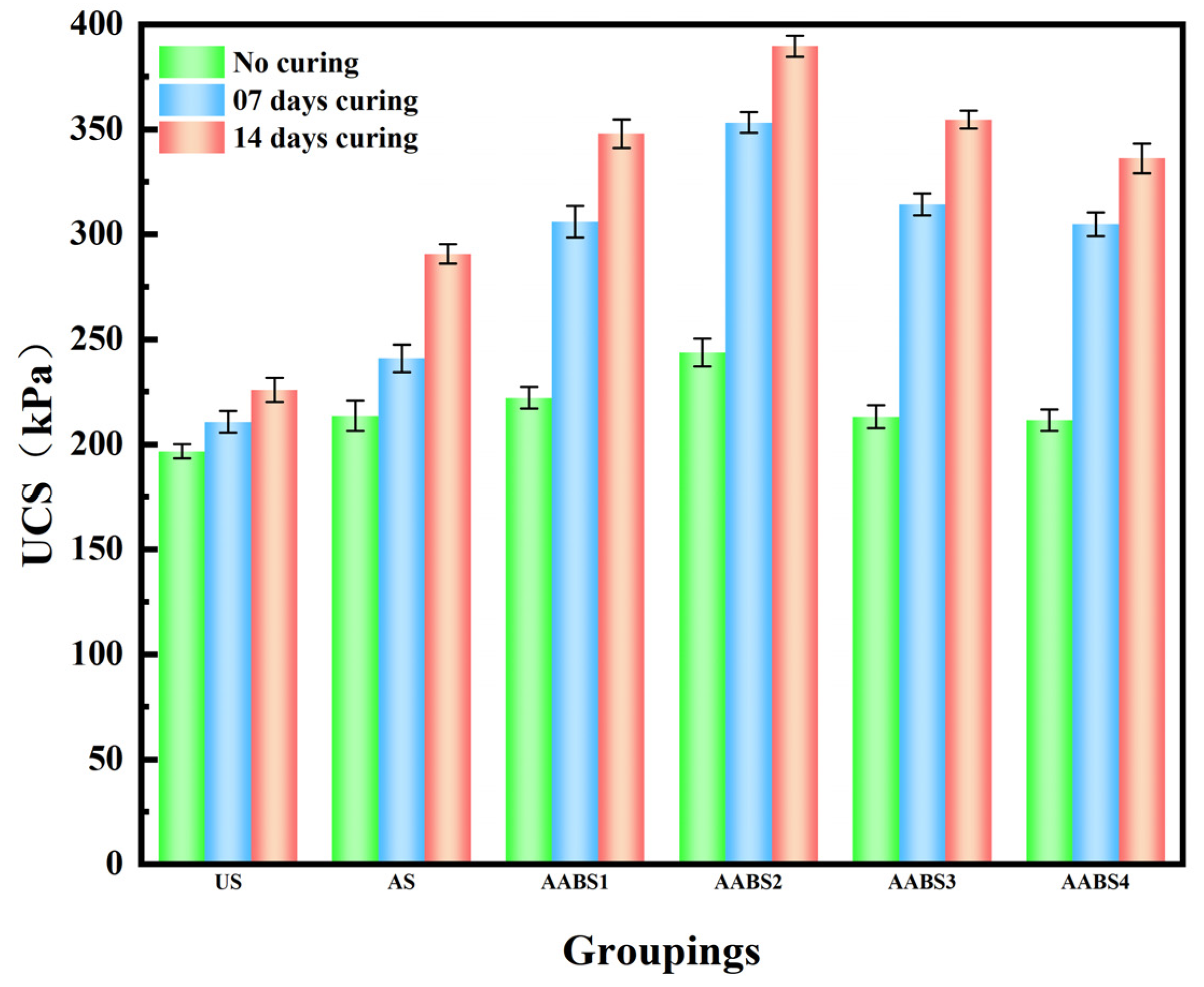
3.4. Unconfined Compressive Strength Test
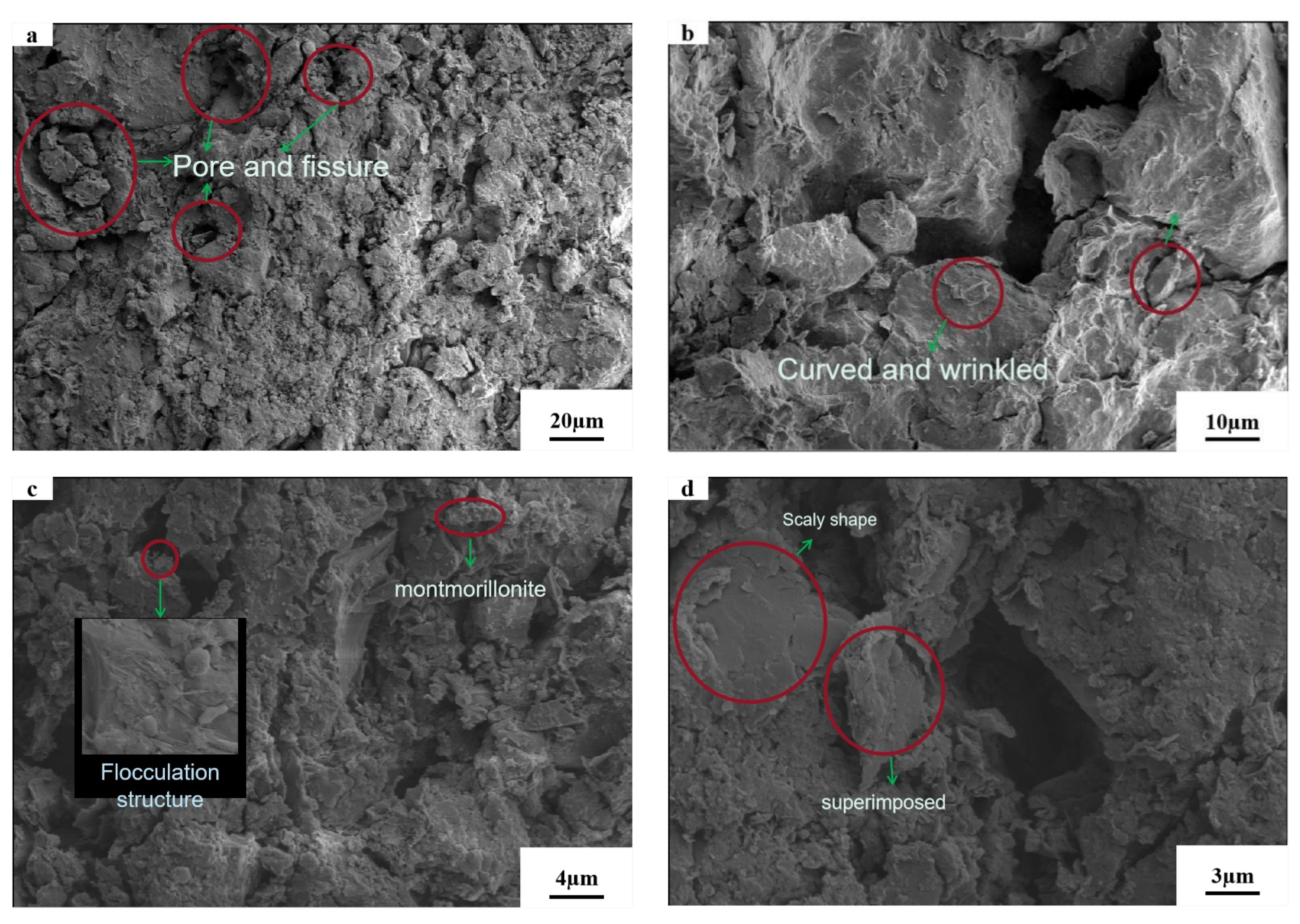
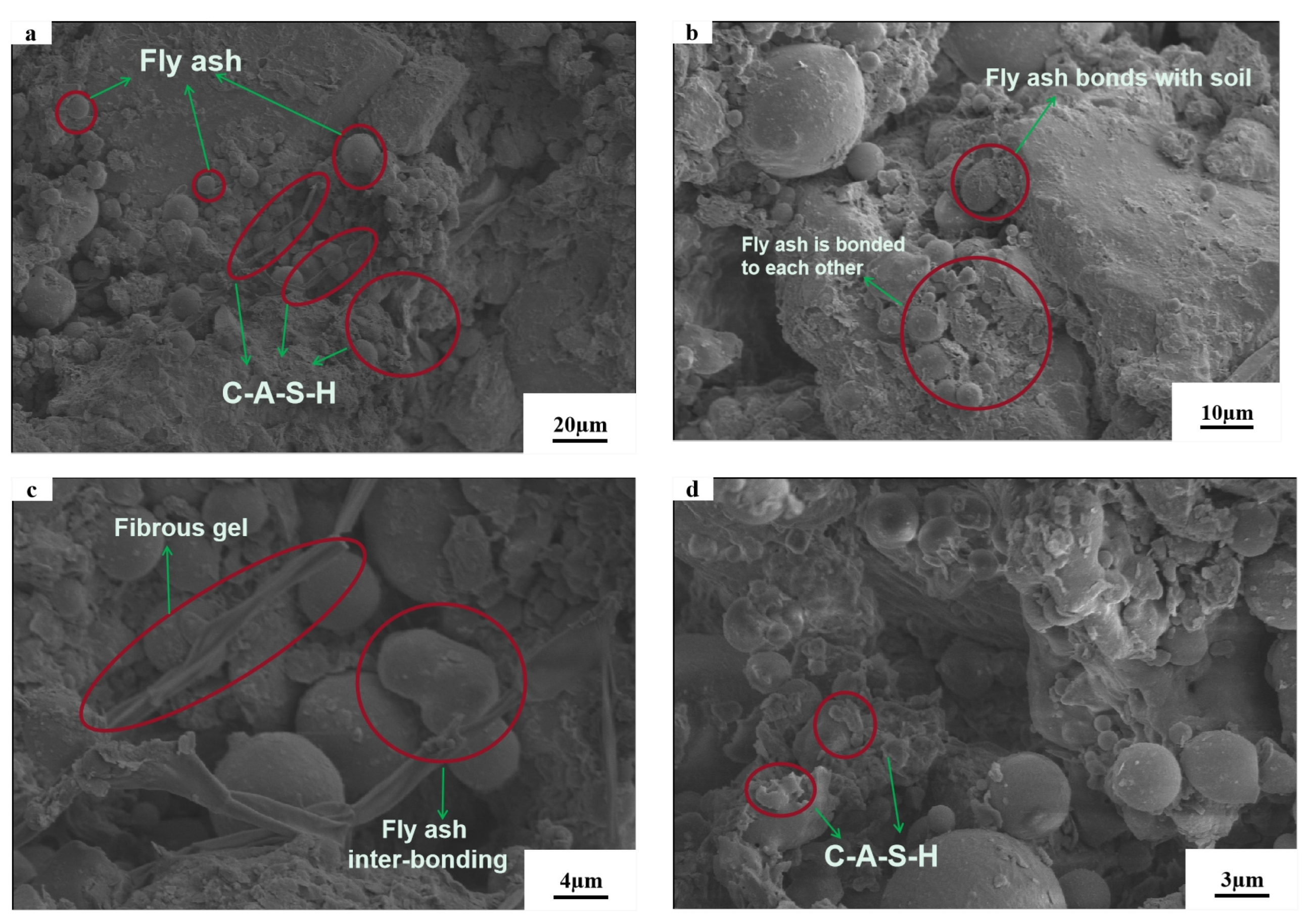
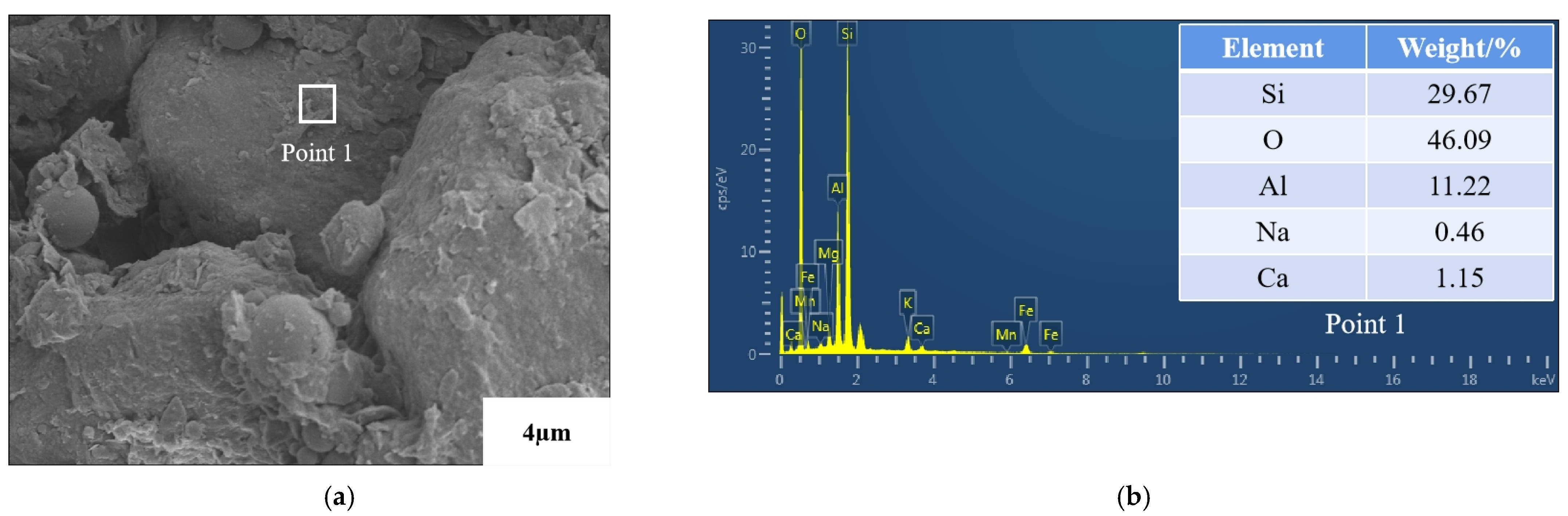
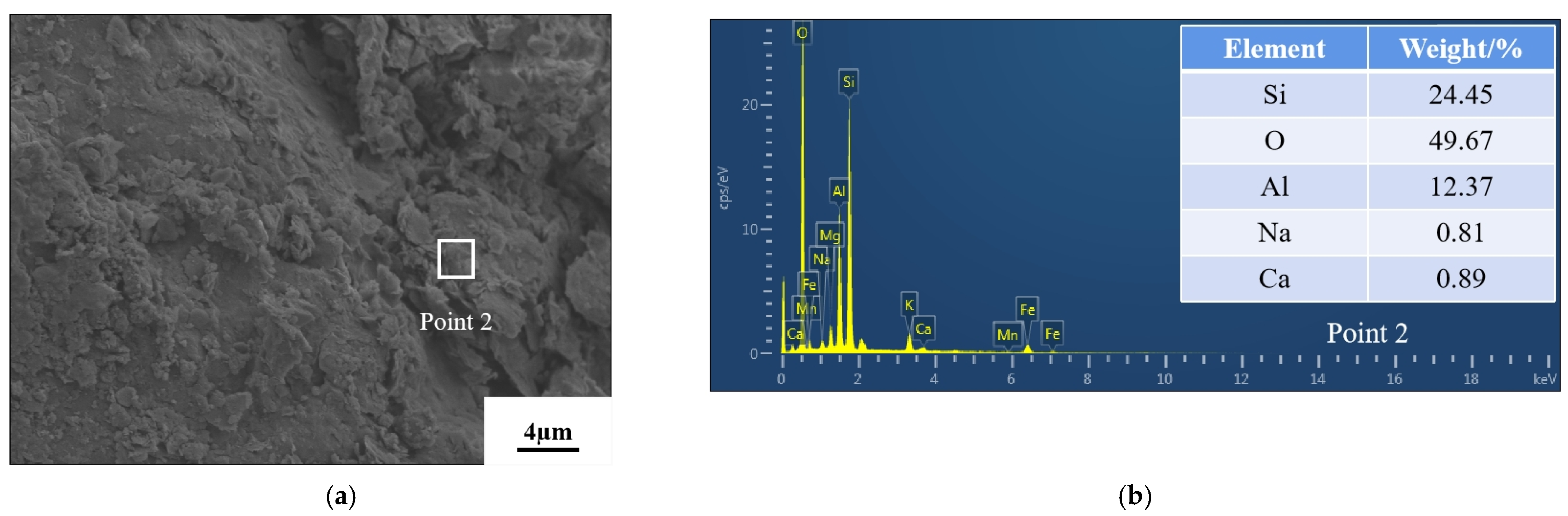
3.5. Microstructure Analysis
3.6. X-ray Diffraction XRD
4. Conclusions
- According to the unloaded swelling test and the loaded swelling test, it was found that AAB can further reduce the swelling property of the soil, but the swelling property does not change significantly when the NaOH content is greater than 8%.
- According to the straight shear test and unconfined compressive strength test, AAB can improve the mechanical strength of soil, AABS2 can reach the maximum strength, and more NaOH will make the soil sample brittle and cause strength to decline. Excess NaOH decreases the cohesion and friction angle.
- Microscopic tests demonstrated that the reactions in AAB produced C–S–H and (C,N)–A–S–H, and that the new substances generated changed the internal structural arrangement of the soil, filled the pores and created adhesion with the clay. After the gel was hydrated and hardened, the high strength of the soil was ensured and the newly generated gel wraps around the clay particles, thus suppressing the swelling properties.
- This study proposed an eco-friendly sustainable binder, which can be used to improve the performance of expansive soil.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, F.H. Foundations on Expansive Soils; Elsevier: Amsterdam, The Netherlands, 1975. [Google Scholar]
- Mazhar, S.; Anasua, G.; Divyam, G. Strength characterisation of fiber reinforced expansive subgrade soil stabilized with alkali activated binder. Road Mater. Pavement. 2021, 23, 1037–1060. [Google Scholar] [CrossRef]
- Yi, Y.; Gu, L.; Liu, S. Microstructural and mechanical properties of marine soft clay stabilized by lime-activated ground granulated blast furnace slag. Appl. Clay Sci. 2015, 103, 71–76. [Google Scholar] [CrossRef]
- Puppala, A.J.; Pedarla, A. Innovative ground improvement techniques for expansive soils. Innov. Infrastruct. Solut. 2017, 2, 24. [Google Scholar] [CrossRef]
- Woldesenbet, T.T. Experimental Study on Stabilized Expansive Soil by Blending Parts of the Soil Kilned and Powdered Glass Wastes. Adv. Civ. Eng. 2022, 12, 96455589. [Google Scholar] [CrossRef]
- Zha, F.; Liu, S.; Du, Y.; Cui, K. Behavior of expansive soils stabilized with fly ash. Nat. Hazards 2008, 47, 509–523. [Google Scholar] [CrossRef]
- Jones, L.D.; Jefferson, J. ICE Manual of Geotechnical Engineering Volume 1: Geotechnical Engineering Principles, Problematic Soils and Site Investigation; The National Academies of Sciences, Engineering, and Medicine: Washington, DC, USA, 2012. [Google Scholar]
- Kamei, T.; Ahmed, A.; Ugai, K. Durability of soft clay soil stabilized with recycled Bassanite and furnace cement mixtures. Soils Found. 2013, 53, 155–163. [Google Scholar] [CrossRef]
- Higgins, D. Briefing: GGBS and sustainability. Const. Mater. 2007, 160, 99–101. [Google Scholar] [CrossRef]
- Shihab, A.M.; Abbas, J.M.; Ibrahim, A.M. Effects of temperature in different initial duration time for soft clay stabilized by fly ash based geopolymer. Civ. Eng. J. 2018, 4, 2082–2096. [Google Scholar] [CrossRef]
- Suhendro, B. Toward green concrete for better sustainable environment. Procedia Eng. 2014, 95, 305–320. [Google Scholar] [CrossRef]
- Krivenko, P. Why alkaline activation—60 years of the theory and practice of alkali-activated materials. J. Ceram. Sci. Technol. 2017, 8, 323–333. [Google Scholar] [CrossRef]
- Davidovits, J. False values on CO2 emission for geopolymer cement/concrete published in Scientific Papers. Geopolym. Inst. Libr. Tech. Pap. 2015, 24, 1–9. [Google Scholar]
- Palomo, A.; Krivenko, P.; Garcia-Lodeiro, I.; Kavalerova, E.; Maltseva, O.; Fernández-Jiménez, A. A review on alkaline activation: New analytical perspectives. Mater. Construcc. 2014, 64, 315. [Google Scholar] [CrossRef]
- Jia, Z.; Yang, Y.; Yang, L.; Zhang, Y.; Sun, Z. Hydration products, internal relative humidity and drying shrinkage of alkali activated slag mortar with expansion agents. Constr. Build. Mater. 2018, 158, 198–207. [Google Scholar] [CrossRef]
- Yi, Y.; Li, C.; Liu, S. Alkali-activated ground-granulated blast furnace slag for stabilization of marine soft clay. J. Mater. Civ. Eng. 2015, 27, 4. [Google Scholar] [CrossRef]
- Rivera, J.F.; Orobio, A.; Mejía, R.; Cristelo, N. Clayey soil stabilization using alkali-activated cementitious materials. Mater. Constr. 2020, 70, 211. [Google Scholar] [CrossRef]
- Miraki, H.; Shariatmadari, N.; Ghadir, P.; Jahandari, S.; Tao, Z.; Siddique, R. Clayey soil stabilization using alkali-activated volcanic ash and slag. J. Rock Mech. Geotech. Eng. 2022, 14, 576–591. [Google Scholar] [CrossRef]
- Syed, M.; GuhaRay, A.; Kar, A. Stabilization of expansive clayey soil with alkali activated binders. Geotech. Geol. Eng. 2020, 38, 6657–6677. [Google Scholar] [CrossRef]
- Adeyanju, E.; Okeke, C.A.; Akinwumi, I.; Busari, A. Subgrade stabilization using rice husk ash-based geopolymer (GRHA) and cement kiln dust (CKD). Case Stud. Constr. Mater. 2020, 13, 00388. [Google Scholar] [CrossRef]
- Bruschi, G.J.; dos Santos, C.P.; de Araújo, M.T.; Ferrazzo, S.T.; Marques, S.; Consoli, N.C. Green stabilization of bauxite tailings: A mechanical study on alkaliactivated materials. J. Mater. Civ. Eng. 2021, 31, 11. [Google Scholar] [CrossRef]
- Bruschi, J.; dos Santos, C.P.; Ferrazzo, S.T.; de Araújo, M.T.; Consoli, N.C. Parameters controlling loss of mass and stiffness degradation of green stabilized bauxite tailings. Proc. Inst. Civ. Eng.-Geotech. Eng. 2021, 62, 177–183. [Google Scholar] [CrossRef]
- Pannirselvam, N.; Chandramouli, K.; Anitha, V. Pulse Velocity Test on BananaFibre Concrete with Nano Silica. Int. J. Civ. Eng. Technol. 2018, 9, 2853–2858. [Google Scholar]
- Kar, A.; Halabe, U.B.; Ray, I.; Unnikrishnan, A. Nondestructive characterizations of alkali activated fly ash and/orslag concrete. Eur. Sci. J. ESJ 2013, 9, 52–74. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Phetchuay, C.; Chinkulkijniwat, A. Soil stabilization by calcium carbide residue and fly ash. J. Mater. Civ. Eng. 2012, 24, 184–193. [Google Scholar] [CrossRef]
- Miao, S.; Shi, J.; Sun, Y.; Zhang, P.; Shen, Z.; Nian, H. Mineral abundances quantification to reveal the swelling property of the black cotton soil in Kenya. Appl. Clay Sci. 2018, 161, 524–532. [Google Scholar] [CrossRef]
- Gupta, S.; GuhaRay, A.; Kar, A.; Komaravolu, V.P. Performance of alkali-activated binder-treated jute geotextile as reinforcement for subgrade stabilization. Int. J. Geotech. Eng. 2018, 15, 298–312. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Udriştioiu, E.G.; Aboul-Enein, H.Y. X-ray Diffraction: Instrumentation and Applications. Crit. Rev. Anal. Chem. 2015, 45, 289–299. [Google Scholar] [CrossRef]
- Das, R.; Eaqub, A.; Hamid, A.; Bee, S. Current applications of x-ray powder diffraction—A review. Rev. Adv. Mater. Sci. 2014, 38, 95–109. [Google Scholar]
- Mohammed, A.; Abdullah, A. Scanning Electron Microscopy (SEM): A Review. In Proceedings of the 2018 International Conference on Hydraulics and Pneumatics, Băile Govora, Romania, 7–9 November 2018. [Google Scholar]
- Mypati, V.; Sireesh, S. Feasibility of Alkali-Activated Low-Calcium Fly Ash as a Binder for Deep Soil Mixing. J. Mater. Civil. Eng. 2021, 34, 04021410. [Google Scholar] [CrossRef]
- Alsafi, S.; Farzadnia, N.; Asadi, A.; Huat, B.K. Collapsibility potential of gypseous soil stabilized with fly ash geopolymer; characterization and assessment. Constr. Build. Mater. 2017, 137, 390–409. [Google Scholar] [CrossRef]
- Darius, Z.; Danute, V.; Stelmokaitis, G.; Viktoras, D. Clayey Soil Strength Improvement by Using Alkali Activated Slag Reinforcing. Minerals 2020, 10, 1076. [Google Scholar] [CrossRef]
- Yu, S.; Xuejue, C.; Jie, A. Experimental study on the deformation characteristics and microstructure of clay polluted by alkaline solution. China Sci. Pap. 2015, 10, 1578–1582. [Google Scholar]
- Parhi, P.S.; Garanayak, L.; Mahamaya, M. Stabilization of an Expansive Soil Using Alkali Activated Fly Ash Based Geopolymer. In Sustainable Civil Infrastructures; Springer: Cham, Switzerland, 2018; pp. 36–50. [Google Scholar] [CrossRef]
- Santhikala, R.; Chandramouli, K.; Pannirselvam, N. Stabilization of expansive soil using fly ash based geopolymer. Mater. Proc. 2022, 68, 110–114. [Google Scholar] [CrossRef]
- Alemshet, D.; Fayissa, B.; Geremew, A. Amelioration Effect of Fly Ash and Powdered Ground Steel Slag for Improving Expansive Subgrade Soil. Oxid. Med. Cell. Longev. 2023, 10, 1700857. [Google Scholar] [CrossRef]
- Zailani, W.W.A.; Bouaissi, A.; Fansuri, H. Bonding Strength characteristics of FA-based geopolymer paste as a repair material when applied on OPC substrate. Appl. Sci. 2020, 10, 3321. [Google Scholar] [CrossRef]
- Kar, A.; Ray, I.; Halabe, U.B.; Unnikrishnan, A.; Dawson-Andoh, B. Characterizations and quantitative estimation of alkali-activated binder paste from microstructures. Int. J. Concr. Struct. Mater. 2014, 8, 213–228. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A. Hydration kinetics in hybrid binders: Early reaction stages. Cem. Concr. Compos. 2013, 39, 82–92. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, H.; Yi, C.; Fan, J.; Chen, H.; Xu, X.; Wang, T. Preparation and reaction mechanism characterization of alkali-activated coal gangue-slag materials. Materials 2019, 12, 2250. [Google Scholar] [CrossRef]




| Free Swelling Rate | Liquid Limit/% | Plastic Limit/% | Plasticity Index | Maximum Dry Density/(g/cm3) | Optimum Moisture Content/% | Specific Gravity | Classification of Soil |
|---|---|---|---|---|---|---|---|
| 55 | 44.52 | 17.39 | 27.13 | 1.61 | 20 | 2.76 | CH |
| Composition | Wight/% |
|---|---|
| SiO2 | 56.01 |
| Al2O3 | 30.27 |
| Fe2O3 | 4.36 |
| CaO | 2.36 |
| K2O | 1.71 |
| Notation | Expansive Soil, wt.% | Fly Ash, wt.% | NaOH, wt.% | W/s |
|---|---|---|---|---|
| Untreated expansive soil (US) | 100 | 0 | 0 | 0.2 |
| Fly ash stabilized expansive soil (FS) | 91 | 9 | 0 | 0.2 |
| AAB stabilized expansive soil (AABS1) | 90.64 | 9 | 0.36 | 0.2 |
| AAB stabilized expansive soil (AABS2) | 90.46 | 9 | 0.54 | 0.2 |
| AAB stabilized expansive soil (AAB3) | 90.28 | 9 | 0.72 | 0.2 |
| AAB stabilized expansive soil (AAB4) | 90.1 | 9 | 0.9 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, T.; Yan, C.; Wang, J. Expansive Soil Stabilization Using Alkali-Activated Fly Ash. Processes 2023, 11, 1550. https://doi.org/10.3390/pr11051550
Wang H, Liu T, Yan C, Wang J. Expansive Soil Stabilization Using Alkali-Activated Fly Ash. Processes. 2023; 11(5):1550. https://doi.org/10.3390/pr11051550
Chicago/Turabian StyleWang, Huan, Tengjiao Liu, Chao Yan, and Jianqi Wang. 2023. "Expansive Soil Stabilization Using Alkali-Activated Fly Ash" Processes 11, no. 5: 1550. https://doi.org/10.3390/pr11051550
APA StyleWang, H., Liu, T., Yan, C., & Wang, J. (2023). Expansive Soil Stabilization Using Alkali-Activated Fly Ash. Processes, 11(5), 1550. https://doi.org/10.3390/pr11051550







