Abstract
Antibiotics are often prescribed to treat infections caused by group B Streptococcus; however, inappropriate use of antibiotics can develop resistance. Because of this, the research was carried out with the aim of evaluating the in vitro effect of the hydroalcoholic extract of Caesalpinia spinosa (Molina) Kuntze known as Taya or Tara on the viability of β-hemolytic streptococci; an experimental investigation of increasing stimulation was carried out. The hydroalcoholic extract of C. spinosa pods was worked in concentrations of 250, 500, 750, and 1000 mg/mL, which were placed on filter paper discs to perform the sensitivity test following the Kirby–Bauer method. The greatest inhibition of bacterial viability was observed in the penicillin control group (GPT-01) followed by the TCT-04 group (hydroalcoholic Tara extract 1000 mg/mL). In addition, it was found that these groups are statistically different from the rest (p < 0.05), while the lowest bacterial inhibition was obtained for the erythromycin group and the TCT-01 group (250–1000 mg/mL). It was concluded that the hydroalcoholic extract of the pods of C. spinosa (Molina) Kuntze affects the viability of β-hemolytic streptococci associated with strep pharyngitis and that this antimicrobial activity is due to the presence of tannins, steroidal flavonoid, and alkaloids. Likewise, the tested concentrations of hydroalcoholic Tara extract were found to have better antibacterial activity than erythromycin (15 µg/mL) against β-hemolytic streptococci. These results are hopeful for the traditional or herbal medicine field. However, future in vivo research is needed to determine its effectiveness in humans.
1. Introduction
The Streptococcus genus bacteria are Gram-positive, catalase-negative, and coagulase-negative cocci disposed of in pairs or chains. This bacterium can colonize the pharynx, anus, and genital mucosa. Transmission might occur through airborne droplets, hand contact with nasal discharge or surfaces contaminated with this bacterium, skin contact with contaminated lesions, or contaminated food sources. Some species belonging to group A, such as S. pyogenes, are a major human-specific bacterial pathogen, β-hemolytic, and cause a broad spectrum of acute infections such as acute pharyngitis, also known as strep throat [1]. However, presenting recurrent infections or prolonged exposure to S. pyogenes group A might lead to life-threatening conditions [2]. Approximately 1.78 million new cases associated with Streptococcus pyogenes occur each year. More than 500 thousand deaths were estimated to be caused by S. pyogenes worldwide [3].
S. pyogenes is associated with 37% of all sore throat cases in children and up to 5–10% in adults reporting millions of cases worldwide [3]. The medical treatment consists of antibiotics. It is known that Streptococcus is susceptible to β-lactam antibiotics, including penicillin, and cephalosporins [4,5,6]. However, since the 1940s, failure in treatment was reported [4]. On the other hand, there is a public health problem related to antibiotics resistances due to excessive use, which, in some cases, is associated with very high health costs [7,8]. For example, Abraham and Sistla [9] found that the erythromycin and tetracycline resistance in S. pyogenes from group A continues to be reported due to the over-prescription and use of these antibiotics. This antibiotic resistance in Streptococcus is increasing due to diverse mechanisms, such as efflux pumps and modifications of the antimicrobial target, as explained by Alves-Barroco et al. [10]
An alternative solution for antibiotic resistance is herbal medicine, which is increasing worldwide [11]. Herbal medicine is a potential source of future drugs for human health care; moreover, approximately 80% of the world population uses this alternative medicine [12,13]. The increasing herbal consumption market led to standardization and herbal-derived products modernization with present pharmacological criteria [14]. Plants were used since ancient times to treat infections and health disorders, this was possible because plants might synthesize various biological molecules known as metabolites secondary with broad-structural diversity and wide-robust pharmacological and biological activity [15,16,17]. Herbs might be used as plant extracts or as their active components [18].
The use of medicinal plants to cure infectious diseases was recognized as one of the primary health management systems. In the historical documents of ancient civilizations, evidence can be seen that Neanderthals already took advantage of the healing potential of some plants. At the end of the 1990s, a new branch of research called the complementary alternative to medication emerged, and it was found that using plants produced fewer side effects than those observed with conventional medications, high tolerability, low toxicity, and lower cost. Thus, herbal pharmaceuticals appeared to cure a wide range of diseases. Several plant secondary metabolites such as flavonoids, tannins, alkaloids, anthocyanidins, glycosides, essential oils, and terpenoids were found to have significant antimicrobial activity [19].
Some research reports the potential of plant extracts with antimicrobial activity. Hsieh’s study [20] showed that the juice of Allium tuberosum Rottler is very effective in inhibiting a wide range of microorganisms. Likewise, Hsieh et al. [21] demonstrated that extracts of Corni fructus, Cinnamon, and Chinese onion combined in a proportional volume of 8:1:1 have a complete antimicrobial spectrum against common foodborne microorganisms, including bacteria, yeasts, and molds, being very stable under heat treatment conditions and at acidic pH values. On the other hand, other research suggests that essential oils of some plants have antimicrobial effects and stimulate the immune response against some Gram-positive bacteria, such as, for example, some Staphylococcus bacteria [22,23].
Peru is one of the most biodiverse (megadiverse) countries, having 10% of the most plant species identified worldwide [24]. With at least 3000 species of medicinal plants reported, many species are located in the Amazon region and north of Peru [25,26,27,28]. Likewise, to treat respiratory infections, some species of medicinal plants were used [29]. On the other hand, it is known that in many places in the Sierra and the Jungle of Peru, access to different medicines is expensive, and, in many cases, they cannot access them. Because of this, many people choose to consume natural medicine, obtaining an improvement in their health depending on the disease or discomfort they suffer.
Tara (Caesalpinia spinosa) (Molina) Kuntze (Fabaceae), known as Taya or Tara, is a leguminous tree indigenous to South America with economic and cultural importance [30,31]. Tara has 8–10 cm long red or yellow pods and can be found in Venezuela, Colombia, Ecuador, Peru, Bolivia, and up to the north of Chile [31]. The leaves and fruits contain a high concentration of tannins (26.4–60.0%). On the other hand, C. spinosa extract contains active compounds for the in vitro control of Gram-positive and Gram-negative bacteria [32]. Likewise, Aguilar-Galvez et al. [33] reported that gallotannins have the potential to inhibit pathogenic bacteria and their antimicrobial activity can enhance by acid hydrolysis.
These bacterial growth inhibition properties were demonstrated when working with the hydroalcoholic extract of Caesalpinia spinosa, where its inhibitory effect on the growth of great negative bacteria such as Salmonella typhi and Escherichia coli was observed; and it is postulated that this effect would be due to the active compounds found in the pods of this plant, among which are tannins, quinones, phenols, and flavonoids, which act by combining with the proteins of the cell membrane of the bacteria and inhibit enzyme activity, resulting in protein denaturation. These properties make this plant a good candidate for testing bacteria that cause upper respiratory tract infections, such as pharyngitis caused by β-hemolytic streptococci [34].
In this sense, the research aims to evaluate in vitro effect of the Tara pod hydroalcoholic extract (C. spinosa (Molina) Kuntze) against β-hemolytic streptococci viability. The purpose is to contribute scientific knowledge about herbal medicine topics and validate the use of Tara in complementary and alternative medicine against Streptococcus associated with strep throat. The findings are useful for complementary medicine, and future research with Tara-based preparations is needed to validate their use as a first alternative to antibiotics, thus preventing antibiotic resistance due to improper use of these.
2. Materials and Methods
2.1. Samples
Tara pod samples (C. spinosa (Molina) Kuntze) come from cultivates located in Otuzco province (La Libertad, Peru) at an altitude of 2645 m.s.n.m. The collection of the samples was carried out using the conventional method of herborization. Botanical identification was performed in the Herbarium truxillense at Universidad Nacional de Trujillo (Peru) where it was deposited with the Code N° 63835.
The β-hemolytic streptococci strains were isolated by microbiologic conventional techniques from pharyngeal secretions in the Bacteriology Laboratory of the Universidad Nacional de Trujillo (Peru). Identification of the genus, macroscopic (Blood Agar and BHI broth), and microscopic (Gram stain) characteristics were analyzed. On blood Agar, typical β-hemolytic streptococci colonies were observed (beta (β)-hemolysis). The typical growth of the genus Streptococcus was shown in the BHI medium, where it grows forming lumps due to its arrangement in chains. Gram-positive cocci disposed of in chains stained by Gram staining was observed (1000×).
2.2. Hydroalcoholic Extract Obtaining
The samples were transferred to the bacteriology laboratory of the School of Microbiology and Parasitology (Universidad Nacional de Trujillo) to eliminate any foreign compounds present in the plant material. Then, the samples were washed with distilled water and disinfected with 0.5% sodium hypochlorite, and finally, they were rinsed with plenty of distilled water to remove hypochlorite residues. The samples were taken out at 40 °C in an oven. The seeds were separated from the pods and smashed in a mortar. The powder (50 g) was then sieved to homogenize the particle size and kept in an amber-colored container until utilization. The powder was macerated with 100 mL 80% hydroalcoholic solution and refluxed at 2 h. In order to obtain a hydroalcoholic extract, a process of pre-evaporation, filtration, and concentration was performed. Finally, to dry the hydroalcoholic extract, it was placed in an oven at 40 °C.
2.3. Detection of Phytoconstituents
The methods in order to separate and identify the phytoconstituents were carried out according to Lock Sing de Ugaz (2022) [35] and Carvajal Rojas [36], where solvents with different polarities were used.
2.3.1. Tannin Detection (Fraction A)
To detection of tannins, 10% ferric chloride had to be added to 2 mL of the hydroalcoholic extract, resulting in a bluish-green to black coloration.
2.3.2. Flavonoid Detection (Fraction A, D, E)
The detection of flavonoids was carried out by the Shinoda reaction. First, to 2 mL of hydroalcoholic extract, 0.5 g of magnesium powder was added. After this, drops of concentrated HCl had to be added, resulting in a yellow coloration due to the presence of isoflavones. However, if the color varied from yellow to red, it was because of the presence of the flavones and flavonols. On the other hand, if the color changed from red to magenta, it was due to flavanols. Finally, if the color varied from red-magenta to violet-blue, it was due to flavonoids.
2.3.3. Steroid Detection (Fraction B, C, D)
Preliminary steroid analysis was necessary by the Liebermann–Burchard test. Acetic acid (drops) was transferred to a 0.2 mL sample of fraction B, and then, 3 mL of a solution of acetic anhydride/sulfuric acid (50:1) was added, resulting in the formation of a blue-green coloration due to steroidal compounds.
2.3.4. Detection of Cardenolides (Fraction C)
The cardenolides were detected using the Kedde reaction. According to the procedure, one drop of fraction C must be applied to a paper filter, followed by 0.1 mL of Kedde’s reagent, and the appearance of a persistent purple or violet coloration shows the presence of cardenolides.
2.3.5. Alkaloid Detection (Fraction C and D)
Most alkaloids create soluble salts in water when 5% hydrochloric acid is added to them (extraction procedure), and these salts are subsequently precipitated utilizing heavy metal salts such as those of Mayer’s reagent (potassium and mercury iodide). The occurrence of a white or light yellow, amorphous, or crystalline precipitate is owing to the presence of alkaloids.
2.4. Discs with Tara Hydroalcoholic Extract and Inoculum of Streptococcus Preparation
Dilutions of the dry hydroalcoholic extract of 25, 50, 75, and 100% were made. Up to 0.5 McFarland turbidity (1.5 × 108 bacteria/mL) suspensions in sterile water distilled from a 24 h culture of β-hemolytic streptococci were generated using a nephelometer.
Whatman Grade 2 cellulose filter papers, on the other hand, were utilized for disc preparation. Discs (5–6 mm diameter) were sterilized at 120 °C for 20 min before drying for 12 h on a stove at 37 °C. Finally, 50 L of each dilution’s hydroalcoholic extract (25, 50, 75, and 100%) was transferred to the discs.
2.5. Antibiotic Sensitivity of β-Hemolytic Streptococci by Diffusion Agar Test
The Bauer method (1996) [37] and the CLSI update (2013) [38] were employed. Muller Hilton Agar was used. With a Drigalski spatula, Streptococcus cultures were seeded on the medium’s surface. Each bacteria-containing medium received five discs of each dilution, which were then incubated for 24 h at 37 °C. Each dilution was tested three times, with three negative controls. After incubation time, the inhibition halo was measured using a Bernier.
2.6. Statistics
SPPS v.22 software was used to perform the statistical analysis. An ANOVA test was utilized to test the assumption of normality. Following that, Dunnet’s T3 multiple comparisons test was used, which allowed comparison in pairs based on the maximum studentized modulus, which is appropriate when the variances are unequal. In addition, to determine the size of the effect, the means of each experimental group were first compared with the control group (erythromycin) using Student’s t-test with a 5% significance level. The effect size was then evaluated using the Social Science Statistics online calculator by applying Cohen’s D formula to groups with equal standard deviations and sample sizes (15 observations). Finally, a bar graph generated with InfoStat (version 1.1) was used to perform the graphical analysis.
3. Results
Different phytoconstituents were determined in the hydroalcoholic extract of Tara. In the five fractions analyzed, the presence of tannins and flavonoids in fraction A; steroids in fraction B; cardenolics, steroids, alkaloids, and flavonoids in fraction C; cardenolics steroids and alkaloids in fraction D; and flavonoids in fraction E was observed (Table 1).

Table 1.
Phytoconstituents in Tara pods (C. spinosa) according to the photochemical march of Lock Sing by Ugaz (2022) [35].
Figure 1 compares the results of mean inhibition taken as a measure of the diameter of the halo when applying four increasing concentrations of hydroalcoholic extract of tare from (250 to 1000 mg/mL) and two antibiotics (penicillin and erythromycin) as positive control groups. It is shown that the greatest bacterial inhibition was observed in the penicillin control group (GPT-01), followed by the TCT-04 group (hydroalcoholic extract of Tara 1000 mg/mL). In turn, these groups were statistically different from the rest (p < 0.05), while the lowest bacterial inhibition was obtained for the erythromycin group and the TCT-01 group (250 mg/mL), not being statistically different (the whiskers did not exceed the edge of the bar). Whiskers were generated based on DMS value (1.13).
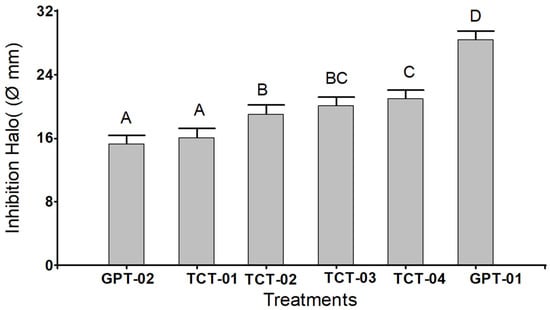
Figure 1.
Bar chart for each group of treatments with hydroalcoholic extract of Tara in four different concentrations and two positive control groups on the inhibition of β-hemolytic streptococci. Positive control group (GPT-01): Penicillin, positive control group (GPT-02): Erythromycin, Tare concentration (TCT-01): 250 mg/mL, Tare concentration (TCT-02): 500 mg/mL, Tare concentration (TCT-03): 750 mg/mL, Tara concentration (TCT-04): 1000 mg/mL.
The analysis of variance showed highly significant differences (p < 0.0001); between at least two of the means of the treatments and the control groups; so, it was decided to apply Dunnett’s T3 multiple comparison test to find pairs of different means. This test found four significance ranges (A, B, C, and D), where the equal letters were not significantly different (p > 0.05) (Figure 1).
According to Cohen’s D (Table 2) coefficient, it was found that the magnitude of the difference between the means was significant (p < 0.05) for three of the four doses of Tara extract used (500, 750, and 1000 mg/L) versus GPT-02 and that the effect size was 2.87, 4.46, and 4.97, respectively, considering these values in the category of a very large magnitude ≥ 1.00.

Table 2.
Pairwise comparisons between the control group and each treatment by Student t-test and estimation of the effect size by Cohen’s D.
4. Discussion
Medicinal plants played an important role in ancestral cultures. These plants were used in the treatment of various diseases, thus finding a great diversity of plants; so, their use presents a promising future within the field of natural or traditional medicine [39]. For example, the study performed by Orlando (2005), which aimed at determining the antimicrobial activity of the raw hydroalcoholic extract of the leaves of Stryphnodendron adstringenss (Martus) Coville (Barbatimau), a type of legume, against bacteria and yeasts, using the disc technique, demonstrated the antibacterial in vitro activity of the plant against Staphylococcus aureus [40].
Several substances called active ingredients are produced as part of plant metabolism. Some of these active ingredients belong to the category of secondary plant metabolites, which include phenolics, alkaloids, terpenes, and carbohydrates that were incorporated into many secondary metabolites through glycosylation linkages. These compounds are responsible for the pharmacological action of medicinal plants, and so, they have a beneficial potential [41,42]. Both roots and stems were reported to be the main organs responsible for the accumulation of these substances [43]. In this way, in the phytochemical study of the hydroalcoholic extract of Tara, tannins, flavonoids in greater proportion, and alkaloids, steroids in smaller proportion, were identified as active ingredients, as shown in Table 1. It is also worth mentioning that a study carried out by Ballesteros-Ramírez [44] confirmed that at concentrations of 1000 mg of extract of Caesalpina spinosa, there was no evidence of mutagenic effect or aberrations in vivo tests through the micronucleus test, which guaranteed the use of this plant as an alternative treatment to combat microorganisms.
Tannins are phenolic compounds with antiviral, antibacterial, antiparasitic, astringent, and antioxidant properties. There were reports of gallotannins being extracted from Galla chinensis that have antimicrobial activity. Likewise, compounds were identified as 5–7 galloylglucopyranoses (GG) inhibited Salmonella typhimurium, 6–7 GG inhibited Bacillus cereus, while 2 GG produced a very low antimicrobial activity against both mentioned strains [45]. Likewise, Aguilar et al. [46] obtained gallotannins from extracts and products of acid hydrolysis during 4 and 9 h of the tare; having as the objective of study its antioxidant, antimicrobial activity, and the minimum inhibitory concentration. It is worth mentioning that the gallotannins in the Tara extract showed greater inhibitory activity against Saphylococcus aeureus, followed by Pseudomonas fluorescens, while the tare extract after hydrolysis enhanced antibacterial activity against S. aureus. It should be noted that Kim et al. [47] showed that hydrolysable tannins and their derivatives possess bacteriostatic and bactericidal activities against Aeromonas hydrophila, Enterobacter sakazakii, Escherichia coli, Klebsiella pneumoniae, Listeria monocytogenes, Staphylococcus aureus, Salmonella Typhi, and Salmonella typhimurium.
On the other hand, the results of this study showed that the active ingredients of C. spinosa (Molina) Kuntze have bactericidal activity against β-hemolytic streptococci due to the presence of the high concentration of tannins. Tannins are complex substances that occur as a mixture of difficult-to-separate polyphenols, to which antimicrobial properties are attributed [46,47]. In this context, it should be noted that there are three hypotheses regarding its mechanism of action: (i) the inhibition of enzymes of microorganisms by binding to the substrates of these enzymes; (ii) action on the cell membrane; (iii) modifying its metabolism, and (iv) by the complementation of tannins with metal ions, decreasing these ions essential for the metabolism of microorganisms [47,48,49,50,51].
Flavonoids, a kind of secondary plant metabolite, were also identified. This active component is discovered in high concentrations and is known to have in vitro action as an enzyme inhibitor and also has antibacterial, antiallergic, and antiviral activity. Alkaloids and steroids, which have antibacterial action, were also discovered in smaller proportions. A steroid derivative such as fusidic acid is known to have antibacterial effects, as well as pharmacologically exert effects that counteract the anti-inflammatory activity of tissues [51,52,53,54,55,56,57,58,59,60,61,62,63,64].
Several reports corroborate the antimicrobial effect of Tara, for example Acho [65], evaluated the antimicrobial effect of Caesalpinia spinosa (Tara) against mixed salivary flora, finding that concentrations of 50 and 75 mg/mL inhibited the growth of mixed salivary flora. Likewise, Araujo et al. [66] and Liu et al. [67] demonstrated that the in vitro antibacterial effect of Caesalpinia spinosa extract (Molina) Kuntze (Tara) on the growth of bacteria such as: Staphylococcus aureus, Escherichia coli, Pseudomona aeruginosa, Klebsiella sp., and Shiguella flexnerii, using the disc technique.
It was shown in vitro that the extract of C. spinosa (Molina) Kuntze has antimicrobial activity against Staphylococcus aureus, Escherichia coli, Pseudomona aeruginosa, Klebsiella sp., and Shigella flexnerii when the Agar diffusion method is used by the disc technique. Some plants belonging to the Leguminosae family have potential use as antimicrobials due to their chemical constituents mainly tannins and flavonoids [68]. In other studies, the effect of the hydroalcoholic extract of Caesalpinia spinosa (Fabaceae) on the growth of Salmonella typhi and Escherichia coli was evaluated and the results showed a directly proportional relationship between the increase in the concentration of the extract and the inhibition of growth of both bacteria, being attributed mainly to the antibacterial action of compounds such as tannins, quinones, phenols, and flavonoids [34].
Figure 1 shows that the hydroalcoholic extract of Tara in the concentration of 1000 mg/mL presented the highest halo of inhibition concerning the control of erythromycin. However, it was observed that the positive control of penicillin had a much higher halo. This is because excellent in vitro efficacy of penicillin was reported [69]. In addition, penicillin contains pure active ingredients, while the hydroalcoholic extract of Tara contains antibacterial active ingredients and various alcohol-soluble substances [70]. In this sense, it should be considered that the size of the growth inhibition zone by the diffusion method is quite influenced by the diffusion speed of the agar substances [71].
Finally, the results showed that there is a significant difference between the control and experimental groups. Tara, on the other hand, was more effective than erythromycin but less efficient than penicillin at all concentrations except 250 mg/mL. Concentrations of 500, 750, and 1000 mg/mL exhibited considerable antimicrobial effects against β-hemolytic streptococci. In this regard, macrolides such as erythromycin and lincosamides were recommended as second-line antibiotics for the treatment of infections in individuals allergic to penicillin, as well as in patients who had failed previous treatment with penicillin or other oral lactic acids [72,73]. Thus, resistance to macrolides represented by erythromycin, azithromycin, and roxithromycin was reported in a variety of settings.
The findings contribute to complementary medicine, which is on the rise in many developed countries such as Japan, China, South Korea, etc., because many people feel confident with the healing properties of plants. Likewise, in some places, complementary medicine is the only source of alternative medicine to conventional. On the other hand, one study showed that many people opt for this type of alternative medicine in the face of dissatisfaction with conventional medicine [74,75,76]. Similarly, a study by Santiváñez-Acosta [77] found that 19.5% of residents (out of 179 respondents) in Peru’s Ucayali region made use of herbal medicine throughout the year. Another study demonstrated the potential of Tara to combat other diseases as a promise for the topical treatment of cutaneous leishmaniasis [78]. These investigations support the use of Tara as an alternative medication; however, they still require more research where the effects in patients with bacterial diseases such as Streptococcus associated with pharyngitis are evaluated.
5. Conclusions
The hydroalcoholic extract obtained from the fruits of C. spinosa (Molina) Kuntze has an in vitro effect on the viability of β-hemolytic Streptococci associated with strep throat. In addition, the antimicrobial activity was due to tannins, steroidal flavonoids, and alkaloids. Additionally, the concentrations tested (250, 500, 75, and 1000 mg/mL) of hydroalcoholic extract of Tara showed better antibacterial activity than erythromycin (15 µg/mL) for β-hemolytic streptococci. Because the findings represent a different treatment option that does not cause resistance to develop over time as pharmacological antibiotics, they may be hopeful in natural or complementary medicine. A restriction of the study was that it was not possible to ascertain the MIC and MBC, as well as the purification of the active compounds, the stability of the compounds, and the molecular identification of the strain of β-hemolytic Streptococci. So, it is suggested that future research includes these elements, which will be valuable in other applications.
Author Contributions
Conceptualization, M.D.L.C.-N. and I.M.R.-H.; methodology, S.M.B. and L.K.B.-G.; software, I.M.R.-H. and L.K.B.-G.; validation, L.C.-C. and M.L.S.-C.; formal analysis, M.D.L.C.-N. and M.L.S.-C.; investigation, M.D.L.C.-N. and N.M.O.; resources, L.C.-C. and K.M.-V.; data curation, S.M.B.; writing—original draft preparation, W.R.-V., S.R.-F. and N.M.O.; writing—review and editing, W.R.-V., S.R.-F. and S.M.B.; visualization, K.M.-V.; supervision, S.R.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kanwal, S.; Vaitla, P. Streptococcus Pyogenes. In StatPearls [Internet]; StatPearls Publishing: Rockville, MD, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554528/ (accessed on 8 May 2023).
- Castro, S.A.; Dorfmueller, H.C. A Brief Review on Group A Streptococcus Pathogenesis and Vaccine Development. R. Soc. Open Sci. 2021, 8, 201991. [Google Scholar] [CrossRef] [PubMed]
- Wijesundara, N.M.; Lee, S.F.; Cheng, Z.; Davidson, R.; Rupasinghe, H.P.V. Carvacrol Exhibits Rapid Bactericidal Activity against Streptococcus Pyogenes through Cell Membrane Damage. Sci. Rep. 2021, 11, 1487. [Google Scholar] [CrossRef] [PubMed]
- Cattoir, V. Mechanisms of Antibiotic Resistance. In Streptococcus Pyogenes: Basic Biology to Clinical Manifestations [Internet]; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 10 February 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK333414/ (accessed on 8 May 2023).
- Yu, D.; Zheng, Y.; Yang, Y. Is There Emergence of β-Lactam Antibiotic-Resistant Streptococcus Pyogenes in China? Infect. Drug Resist. 2020, 13, 2323–2327. [Google Scholar] [CrossRef] [PubMed]
- Kebede, D.; Admas, A.; Mekonnen, D. Prevalence and Antibiotics Susceptibility Profiles of Streptococcus Pyogenes among Pediatric Patients with Acute Pharyngitis at Felege Hiwot Comprehensive Specialized Hospital, Northwest Ethiopia. BMC Microbiol. 2021, 21, 135. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4378521/ (accessed on 8 May 2023).
- Abraham, T.; Sistla, S. Trends in Antimicrobial Resistance Patterns of Group A Streptococci, Molecular Basis and Implications. Indian J. Med. Microbiol. 2018, 36, 186–191. [Google Scholar] [CrossRef]
- Alves-Barroco, C.; Rivas-García, L.; Fernandes, A.R.; Baptista, P.V. Tackling Multidrug Resistance in Streptococci—From Novel Biotherapeutic Strategies to Nanomedicines. Front. Microbiol. 2020, 11, 579916. [Google Scholar] [CrossRef]
- Welz, A.N.; Emberger-Klein, A.; Menrad, K. Why People Use Herbal Medicine: Insights from a Focus-Group Study in Germany. BMC Complement. Altern. Med. 2018, 18, 92. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, M.; Guo, H.; Zheng, G.; Yang, J.; Lu, A. Chinese Herbal Medicines for Rheumatoid Arthritis. In Advances in Botanical Research; Shyur, L.-F., Lau, A.S.Y., Eds.; Elsevier: San Diego, CA, USA, 2012; Volume 62, pp. 273–313. ISBN 9780123945914. [Google Scholar]
- Woo, C.S.J.; Lau, J.S.H.; El-Nezami, H. Herbal Medicine. In Advances in Botanical Research; Shyur, L.-F., Lau, A.S.Y., Eds.; Elsevier: San Diego, CA, USA, 2012; Volume 62, pp. 365–384. ISBN 9780123945914. [Google Scholar]
- Nguyen-Vo, T.-H.; Nguyen, L.; Do, N.; Nguyen, T.-N.; Trinh, K.; Cao, H.; Le, L. Plant Metabolite Databases: From Herbal Medicines to Modern Drug Discovery. J. Chem. Inf. Model. 2020, 60, 1101–1110. [Google Scholar] [CrossRef]
- Shafi, A.; Zahoor, I. Metabolomics of Medicinal and Aromatic Plants: Goldmines of Secondary Metabolites for Herbal Medicine Research. In Medicinal and Aromatic Plants; Aftab, T., Hakeem, K.R., Eds.; Elsevier: San Diego, CA, USA, 2021; pp. 261–287. ISBN 9780128195901. [Google Scholar]
- Wijesundara, N.M.; Rupasinghe, H.P.V. Herbal Tea for the Management of Pharyngitis: Inhibition of Streptococcus Pyogenes Growth and Biofilm Formation by Herbal Infusions. Biomedicines 2019, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef] [PubMed]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Nandy, S.; Mundhra, A.; Das, N.; Pandey, D.K.; Dey, A. A Review on Antimicrobial Botanicals, Phytochemicals and Natural Resistance Modifying Agents from Apocynaceae Family: Possible Therapeutic Approaches against Multidrug Resistance in Pathogenic Microorganisms. Drug Resist. Updates 2020, 51, 100695. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.C. Antimicrobial E¡ect of Cinnamon Extract. Taiwan. J. Agric. Chem. Food Sci. 2000, 38, 184–193. [Google Scholar]
- Hsieh, P.-C.; Mau, J.-L.; Huang, S.-H. Antimicrobial Effect of Various Combinations of Plant Extracts. Food Microbiol. 2001, 18, 35–43. [Google Scholar] [CrossRef]
- Gismondi, A.; Di Marco, G.; Redi, E.L.; Ferrucci, L.; Cantonetti, M.; Canini, A. The Antimicrobial Activity of Lavandula Angustifolia Mill. Essential Oil against Staphylococcus Species in a Hospital Environment. J. Herb. Med. 2021, 26, 100426. [Google Scholar] [CrossRef]
- Giovannini, D.; Gismondi, A.; Basso, A.; Canuti, L.; Braglia, R.; Canini, A.; Mariani, F.; Cappelli, G. Lavandula Angustifolia Mill. Essential Oil Exerts Antibacterial and Anti-Inflammatory Effect in Macrophage Mediated Immune Response to Staphylococcus Aureus. Immunol. Investig. 2016, 45, 11–28. [Google Scholar] [CrossRef]
- Friso, F.; Mendive, F.; Soffiato, M.; Bombardelli, V.; Hesketh, A.; Heinrich, M.; Menghini, L.; Politi, M. Implementation of Nagoya Protocol on Access and Benefit-Sharing in Peru: Implications for Researchers. J. Ethnopharmacol. 2020, 259, 112885. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Sharon, D. Traditional Medicinal Plant Use in Northern Peru: Tracking Two Thousand Years of Healing Culture. J. Ethnobiol. Ethnomed. 2006, 2, 47. [Google Scholar] [CrossRef]
- Cierto, L.E.O.; Quintana, E.D.; Escalante, C.A.; Dumont, J.R.D.; Curo, G.G. Diversity, Abundance and Ecological Importance of Plant Species for Medical Use in Tropical Forest of Tingo Maria, Peru. Bol. Malariol. Salud Ambient. 2022, 62, 1055–1066. [Google Scholar] [CrossRef]
- Corroto, F.; Gamarra Torres, O.A.; Macía, M.J. Different Patterns in Medicinal Plant Use along an Elevational Gradient in Northern Peruvian Andes. J. Ethnopharmacol. 2019, 239, 111924. [Google Scholar] [CrossRef] [PubMed]
- de Pascoa Júnior, J.G.; Souza, C.L.L. de Medicinal Plants Used in the Amazon Region: A Systematic Review. Res. Soc. Dev. 2021, 10, e163101419965. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Glenn, A. Medicinal Plants Used in Peru for the Treatment of Respiratory Disorders. Rev. Peru. Biol. 2010, 17, 331–346. [Google Scholar]
- Villena Velásquez, J.J.; Seminario Cunya, J.F.; Valderrama Cabrera, M.A. Variabilidad Morfológica de La ‘Tara’ Caesalpinia spinosa (Molina.) Kuntze (Fabaceae), En Poblaciones Naturales de Cajamarca: Descriptores de Fruto y Semilla. Arnaldoa 2019, 26, 555–574. [Google Scholar]
- Skowyra, M.; Janiewicz, U.; Salejda, A.M.; Krasnowska, G.; Almajano, M.P. Effect of Tara (Caesalpinia spinosa) Pod Powder on the Oxidation and Colour Stability of Pork Meat Batter during Chilled Storage. Food Technol. Biotechnol. 2015, 53, 419–427. [Google Scholar] [CrossRef]
- Salirrosas, D.; Reategui-Pinedo, N.; Crespo, J.P.; Sánchez-Tuesta, L.; Arqueros, M.; Cabrera, A.; Martinez, R.M.; Ayala, C.; Baby, A.R.; Prieto, Z.A. Safety Profile of Caesalpinia spinosa Aqueous Extract Tested in Oreochromis Niloticus Toward Its Application in Dermocosmetics. Front. Sustain. 2021, 2, 696289. [Google Scholar] [CrossRef]
- Ramesh, C.H.; Vinithkumar, N.V.; Kirubagaran, R. Marine pigmented bacteria: A prospective source of antibacterial compounds. J. Nat. Sci. Biol. Med. 2019, 10, 104–113. [Google Scholar] [CrossRef]
- Cholán Pacheco, K.; Zavaleta Espejo, G.; Saldaña Jiménez, J.; Blas Cerdán, W. Efecto Del Extracto Hidroalcohólico de Caesalpinia spinosa (Fabaceae) Sobre El Crecimiento de Salmonella Typhi y Escherichia Coli. Arnaldoa 2019, 26, 699–712. [Google Scholar]
- Lock Sing de Ugaz, O. Investigación Fitoquímica: Métodos En El Estudio de Productos Naturales; Pontificia Universidad Católica del Perú: Lima, Peru, 2022; ISBN 9786124664779. [Google Scholar]
- Carvajal Rojas, L.; Uribe, Y.H.; Sierra Martínez, N.; Rueda Niño, D. Análisis fitoquímico preliminar de hojas, tallos y semillas de cupatá (Strychnos schultesiana Krukoff). Colomb. For. 2008, 12, 161. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Clsi Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2013; ISBN 9781562388669.
- Dar, R.A.; Shahnawaz, M.; Qazi, P.H. General overview of medicinal plants: A review. J. Phytopharm. 2017, 6, 349–351. [Google Scholar] [CrossRef]
- Orlando, S.C. Evaluación de la Actividad Antimicrobiana del Extracto Hidroalcohólico Crudo Obtenido de Hojas de Stryhorodendrona Dstringes (Martius) Coville (Barbatimao); Universidad de Francia: Lima, Peru, 2005. [Google Scholar]
- Carrillo-Tomalá, C.; Díaz-Torres, R. Actividad Antimicrobiana de Extractos Hidroalcohólicos de Hojas de Dos Variedades de Mangifera indica L. Revista Ciencia Unemi 2020, 13, 69–77. [Google Scholar] [CrossRef]
- Verdecía, D.M.; Herrera-Herrera, R.d.C.; Torres, E.; Sánchez, A.R.; Hernández-Montiel, L.G.; Herrera, R.S.; Ramírez, J.L.; Bodas, R.; Giráldez, F.J.; Guillaume, J.; et al. Metabolitos primarios y secundario de seis especies de árboles, arbustos y leguminosas herbáceas. Cuban J. Agric. Sci. 2021, 55, 77–93. [Google Scholar]
- Hussein, R.A.; El-Anssary, A.A. Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants. In Herbal Medicine; IntechOpen: London, UK, 2019; ISBN 9781789847826. [Google Scholar] [CrossRef]
- Ballesteros-Ramírez, R.; Durán, M.I.; Fiorentino, S. Genotoxicity and Mutagenicity Assessment of a Standardized Extract (P2Et) Obtained from Caesalpinia spinosa. Toxicol. Rep. 2021, 8, 258–263. [Google Scholar] [CrossRef]
- Tian, F.; Li, B.; Ji, B.; Zhang, G.; Luo, Y. Identification and Structure–Activity Relationship of Gallotannins Separated from Galla Chinensis. Lebenson. Wiss. Technol. 2009, 42, 1289–1295. [Google Scholar] [CrossRef]
- Aguilar-Galvez, A.; Noratto, G.; Chambi, F.; Debaste, F.; Campos, D. Potential of Tara (Caesalpinia spinosa) Gallotannins and Hydrolysates as Natural Antibacterial Compounds. Food Chem. 2014, 156, 301–304. [Google Scholar] [CrossRef]
- Kim, T.J.; Silva, J.L.; Kim, M.K.; Jung, Y.S. Enhanced Antioxidant Capacity and Antimicrobial Activity of Tannic Acid by Thermal Processing. Food Chem. 2010, 118, 740–746. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Anil Kumar, N.V.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Tsouh Fokou, P.V.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Singh, A.P.; Kumar, S. Applications of Tannins in Industry. In Tannins—Structural Properties, Biological Properties and Current Knowledge; IntechOpen: London, UK, 2020; ISBN 9781789847963. [Google Scholar] [CrossRef]
- Baldwin, A.; Booth, B.W. Biomedical Applications of Tannic Acid. J. Biomater. Appl. 2022, 36, 1503–1523. [Google Scholar] [CrossRef]
- Reygaert, W.C. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Egorov, A.M.; Ulyashova, M.M.; Rubtsova, M.Y. Bacterial Enzymes and Antibiotic Resistance. Acta Nat. 2018, 10, 33–48. [Google Scholar] [CrossRef]
- Noma, S.A.A.; Ulu, A.; Acet, Ö.; Sanz, R.; Sanz-Pérez, E.S.; Odabaşı, M.; Ateş, B. Comparative Study of ASNase Immobilization on Tannic Acid-Modified Magnetic Fe3O4/SBA-15 Nanoparticles to Enhance Stability and Reusability. New J. Chem. 2020, 44, 4440–4451. [Google Scholar] [CrossRef]
- Deng, L.; Qi, Y.; Liu, Z.; Xi, Y.; Xue, W. Effect of Tannic Acid on Blood Components and Functions. Colloids Surf. B Biointerfaces 2019, 184, 110505. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on Tannins: Extraction Processes, Applications and Possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Prospectives and Actual Industrial Applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef]
- D’Amelia, V.; Aversano, R.; Chiaiese, P.; Carputo, D. The Antioxidant Properties of Plant Flavonoids: Their Exploitation by Molecular Plant Breeding. Phytochem. Rev. 2018, 17, 611–625. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Rakha, A.; Umar, N.; Rabail, R.; Butt, M.S.; Kieliszek, M.; Hassoun, A.; Aadil, R.M. Anti-Inflammatory and Anti-Allergic Potential of Dietary Flavonoids: A Review. Biomed. Pharmacother. 2022, 156, 113945. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral Activities of Flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef] [PubMed]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Doğan, A.; Otlu, S.; Çelebï, Ö.; Aksu Kiliçle, P.; Gülmez Sağlam, A.; Doğan, A.N.C.; Mutlu, N. An investigation of antibacterial effects of steroids. Turk. J. Vet. Anim. Sci. 2017, 41, 302–305. [Google Scholar] [CrossRef]
- Tarkowská, D. Plants Are Capable of Synthesizing Animal Steroid Hormones. Molecules 2019, 24, 2585. [Google Scholar] [CrossRef]
- Acho, M.H.; Perfecto, D.R. Efecto Antibacteriano de Caesalpinia spinosa (Tara) Sobre Flora Salival Mixta. Odontol. Sanmarquina 2012, 15, 27–30. [Google Scholar] [CrossRef]
- Díaz, J.A.; Asencios, R.S. Actividad antimicrobiana del extracto crudo de la vaina de Caesalpinia spinosa" tara" frente a Staphylococcus aureus. Científica 2009, 6, 142–155. [Google Scholar]
- Liu, H.; Lengua, L.A.; León, G.; Carla La Torre, D.; Huapaya, J.; Chauca, J. Evaluación de la Actividad Antibacteriana in vitro de los Extractos de Caesalpinia spinosa “tara” y Eucalyptus sp.“eucalipto”. Horiz. Médico 2002, 2. [Google Scholar]
- Das, S.; Sharangi, A.B.; Egbuna, C.; Jeevanandam, J.; Ezzat, S.M.; Adetunji, C.O.; Tijjani, H.; Olisah, M.C.; Patrick-Iwuanyanwu, K.C.; Adetunji, J.B.; et al. Health benefits of isoflavones found exclusively of plants of the Fabaceae family. In Functional Foods and Nutraceuticals; Springer International Publishing: Cham, Switzerland, 2020; pp. 473–508. ISBN 9783030423186. [Google Scholar] [CrossRef]
- Brook, I. Treatment Challenges of Group A Beta-Hemolytic Streptococcal Pharyngo-Tonsillitis. Int. Arch. Otorhinolaryngol. 2017, 21, 286–296. [Google Scholar] [CrossRef]
- Santos, M.B.; Garcia-Rojas, E.E. Recent Advances in the Encapsulation of Bioactive Ingredients Using Galactomannans-Based as Delivery Systems. Food Hydrocoll. 2021, 118, 106815. [Google Scholar] [CrossRef]
- Murray, P.R. The Clinician and the Microbiology Laboratory. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2015; pp. 191–223. ISBN 9781455748013. [Google Scholar]
- Gomes, C.; Martínez-Puchol, S.; Palma, N.; Horna, G.; Ruiz-Roldán, L.; Pons, M.J.; Ruiz, J. Macrolide Resistance Mechanisms in Enterobacteriaceae: Focus on Azithromycin. Crit. Rev. Microbiol. 2017, 43, 1–30. [Google Scholar] [CrossRef]
- Wei, B.; Kang, M. Molecular Basis of Macrolide Resistance In Campylobacter Strains Isolated from Poultry in South Korea. Biomed. Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Ghaedi, F.; Dehghan, M.; Salari, M.; Sheikhrabori, A. Complementary and Alternative Medicines: Usage and Its Determinant Factors among Outpatients in Southeast of Iran: Usage and Its Determinant Factors among Outpatients in Southeast of Iran. J. Evid. Based Complement. Altern. Med. 2017, 22, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.L.; Richards, N.; Harrison, J.; Barnes, J. Prevalence of Use of Traditional, Complementary and Alternative Medicine by the General Population: A Systematic Review of National Studies Published from 2010 to 2019. Drug Saf. 2022, 45, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Tangkiatkumjai, M.; Boardman, H.; Walker, D.-M. Potential Factors That Influence Usage of Complementary and Alternative Medicine Worldwide: A Systematic Review. BMC Complement. Med. Ther. 2020, 20, 363. [Google Scholar] [CrossRef]
- Santiváñez-Acosta, R.; Valenzuela-Oré, F.; Angulo-Bazán, Y. Uso de terapias de medicina alternativa y complementaria en la provincia de Coronel Portillo, Ucayali, Perú. Rev. Peru. Med. Exp. Salud Publica 2020, 37, 510–515. [Google Scholar] [CrossRef]
- Robledo Restrepo, S.M.; Quintero, J.; Higuita, J.; Fernández, M.; Murillo, J.; Restrepo, A.; Arbeláez, N.; Montoya, A.; Ospina, V.; Pineda, T.; et al. Caesalpinia spinosa (Molina) Kuntze: Una Nueva Promesa Para El Tratamiento Tópico de La Leishmaniasis Cutánea. Rev. Acad. Colomb. Cienc. Exactas Fis. Nat. 2020, 44, 915–936. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).