Mathematical Modelling and CFD Simulation for Oxygen Removal in a Multi-Function Gas-Liquid Contactor
Abstract
:1. Introduction
- This paper presents the mathematical models and CFD models for degassing of oxygen from water in the laboratory-scale multi-function gas-liquid contactor, which can switch between GIAT, CSTR, and BC, to guide the modelling and design of various degassing systems.
- The optimum correlations of the overall are determined by the mathematical models established for specific reactor types.
- The CFD simulations are conducted to further reveal the mass transfer details in the contactor.
2. Model Development
2.1. Degassing System
2.2. Mathematical Modelling
2.2.1. Continuous Bubble Degasser
Degassing in a Horizontal Pipe
Degassing in the Stirred Tank
Degassing in the Bubble Column
2.2.2. Semi-Batch Bubble Degasser
2.3. CFD Simulation
2.3.1. Case Description
2.3.2. Model Description
2.3.3. Numerical Configurations
2.3.4. Grid Independency
3. Results and Discussion
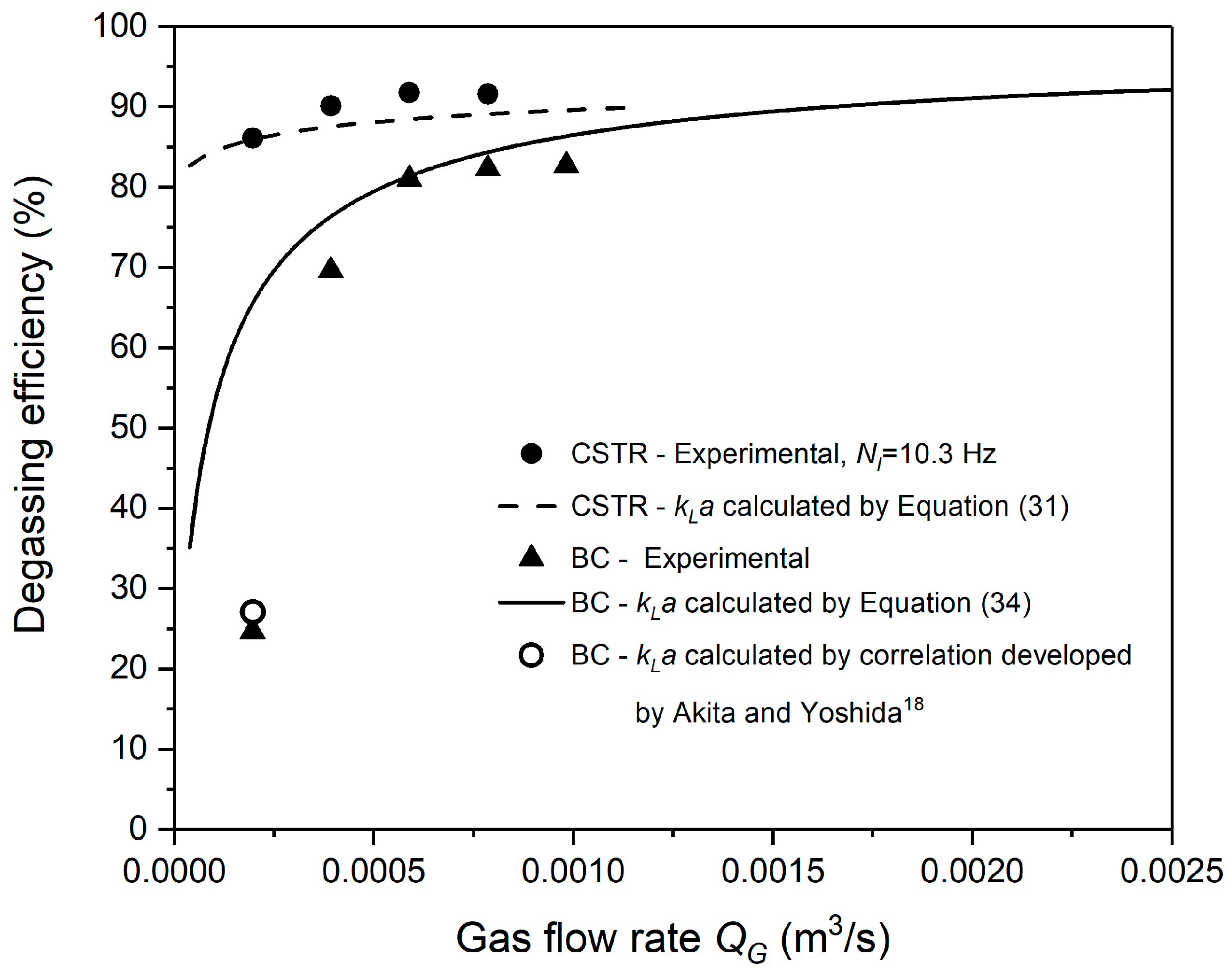
3.1. Effects of Agitation Speed on Degassing Efficiency
3.2. Effects of Flow Rate of Purge Nitrogen on Degassing Efficiency
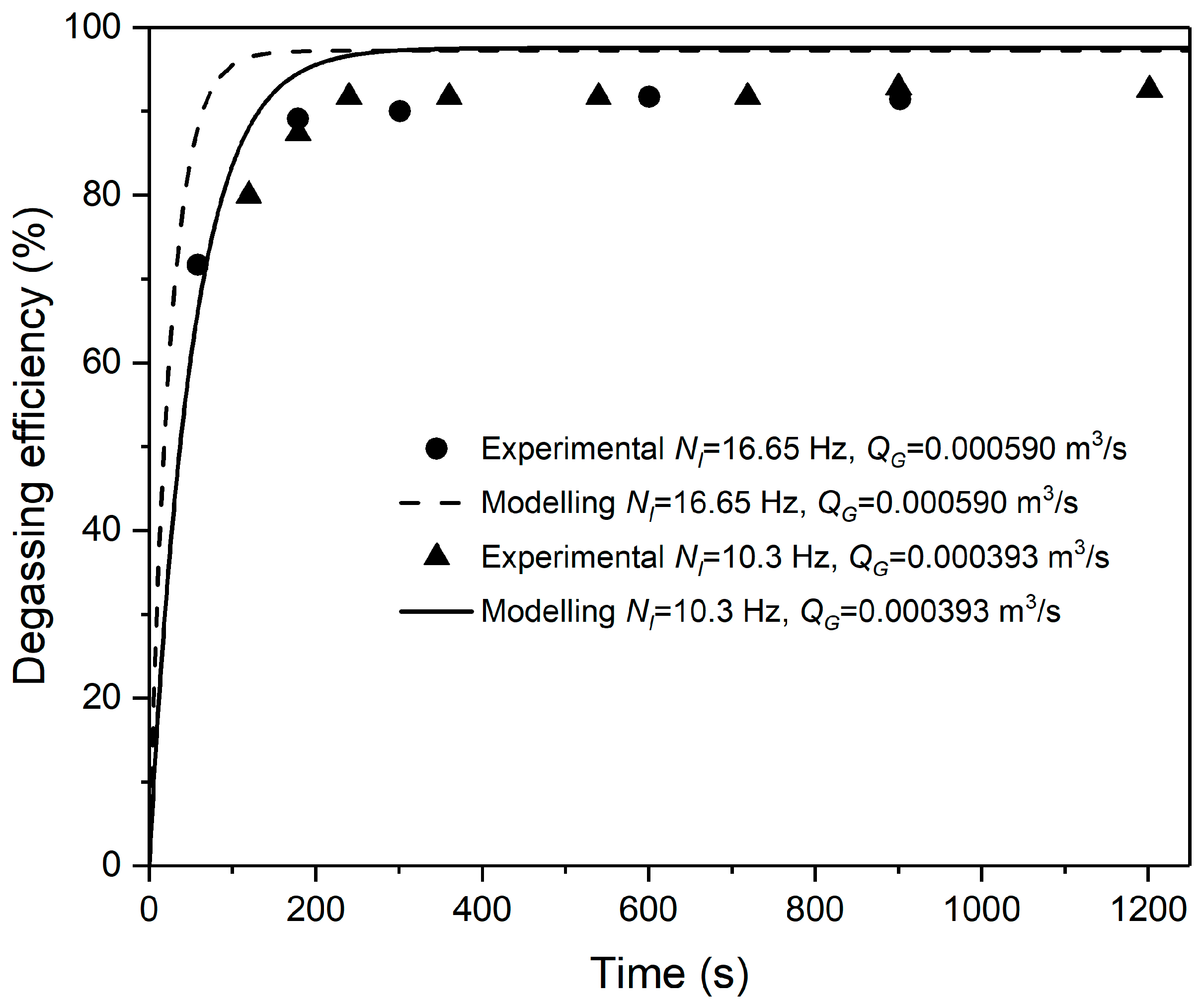
3.3. Degassing Efficiencies of a Semi-Batch Reactor
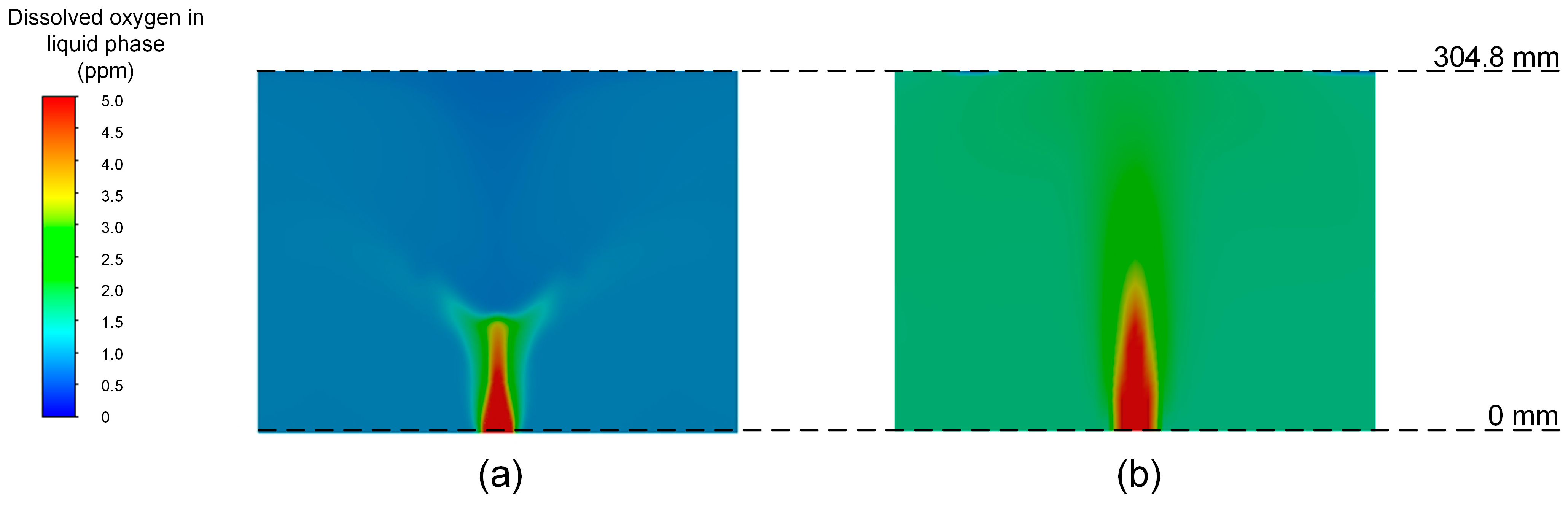
3.4. Comparison between Mathematical and CFD Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lei, H. Experimental and Modeling Studies of Bubble Degassing. Master’s Thesis, University of Calgary, Calgary, AB, Canada, 2010. [Google Scholar]
- Yu, D. Simulation Studies on Water Deoxygenation Process in A Rotating Packed Bed. Master’s Thesis, Dalian University of Technology, Dalian, China, 2014. [Google Scholar]
- Zhao, Z.; Liu, Z.; Xiang, Y.; Arowo, M.; Shao, L. Removal of Dissolved Oxygen from Water by Nitrogen Stripping Coupled with Vacuum Degassing in a Rotor-Stator Reactor. Processes 2021, 9, 1354. [Google Scholar] [CrossRef]
- Yu, S. Numerical Modeling of Dehydrogenation and Denitrogenation in Industrial Vacuum Tank Degassers. Ph.D. Thesis, Aalto University, Helsinki, Finland, 2014. [Google Scholar]
- Riedel, E.; Köhler, P.; Ahmed, M.; Hellmann, B.; Horn, I.; Scharf, S. Industrial suitable and digitally recordable application of ultrasound for the enviromentally friendly degassing of aluminium melts before tilt casting. Procedia CIRP 2021, 98, 589–594. [Google Scholar] [CrossRef]
- Xie, D.; Wu, P.; Wu, D. The Design of a Degassing Pump Based on Numerical and Experimental Research. In Proceedings of the ASME/JSME/KSME 2015 Joint Fluids Engineering Division Summer Meeting, Seoul, Republic of Korea, 26–31 July 2015. [Google Scholar]
- Che, D.; Yao, g.; Kang, W.; Zhang, X. Effect of Vacuum Degassing on Composites preparation. In Proceedings of the TMS 2010 Annual Meeting Supplemental Proceedings on Materials Processing and Properties, Seattle, WA, USA, 14–18 February 2010; pp. 301–306. [Google Scholar]
- Oosterom, S.v.; Schreier, A.; Battley, M.; Bickerton, S.; Allen, T. Influence of dissolved gasses in epoxy resin on resin infusion part quality. Compos. Part A Appl. Sci. Manuf. 2020, 132, 105818. [Google Scholar] [CrossRef]
- Juan, J.; Silva, A.; Tornero, J.A.; Gámez, J.; Salán, N. Void Content Minimization in Vacuum Infusion (VI) via Effective Degassing. Polymers 2021, 13, 2876. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; The, J.; Tan, Z.; Feng, X. Modeling SO2 absorption into water accompanied with reversible reaction in a hollow fiber membrane contactor. Chem. Eng. Sci. 2016, 156, 136–146. [Google Scholar] [CrossRef]
- Astarita, G. Mass transfer with chemical reaction. Ind. Eng. Chem. 1967, 58, 379–383. [Google Scholar]
- Ma, Q.; Shang, C.; Zhang, G.; Fang, H. Numerical Investigation of Degassing Behavior of Highly Viscous Non-Newtonian Fluids under Stirring. Chem. Eng. Technol. 2020, 43, 157–167. [Google Scholar] [CrossRef]
- Jaszczur, M.; Mlynarczykowska, A. A General Review of the Current Development of Mechanically Agitated Vessels. Processes 2020, 8, 982. [Google Scholar] [CrossRef]
- Tari, F.; Zarrinpashne, S.; Shekarriz, M.; Ruzbehani, A. Modeling of mass transfer and hydrodynamic investigation of H2S removal from molten sulfur using porous Sparger. Heat Mass Transf. 2020, 56, 1641–1648. [Google Scholar] [CrossRef]
- Yu, H.S.; Tan, Z.C. New Correlations of Volumetric Liquid-Phase Mass Transfer Coefficients in Gas-Inducing Agitated Tank Reactors. Int. J. Chem. React. Eng. 2012, 10, 1585–1611. [Google Scholar] [CrossRef]
- Yawalkar, A.A.; Heesink, A.B.M.; Versteeg, G.F.; Pangarkar, V.G. Gas-liquid mass transfer coefficient in stirred tank reactors. Can. J. Chem. Eng. 2002, 80, 840–848. [Google Scholar] [CrossRef] [Green Version]
- Kapic, A.; Heindel, T.J. Correlating gas-liquid mass transfer in a stirred-tank reactor. Chem. Eng. Res. Des. 2006, 84, 239–245. [Google Scholar] [CrossRef]
- Akita, K.; Yoshida, F. Gas Holdup and Volumetric Mass Transfer Coefficient in Bubble Columns. Effects of Liquid Properties. Ind. Eng. Chem. Process Des. Dev. 1973, 12, 76–80. [Google Scholar] [CrossRef]
- Manjrekar, O.N. Hydrodynamics and Mass Transfer in Bubble Columns. Ph.D. Thesis, Washington University, St. Louis, MO, USA, 2016. [Google Scholar]
- Deckwer, W.D.; Burckhart, R.; Zoll, G. Mixing and mass transfer in tall bubble columns. Chem. Eng. Sci. 1974, 29, 2177–2188. [Google Scholar] [CrossRef]
- Guo, K.Y.; Wang, T.F.; Liu, Y.F.; Wang, J.F. CFD-PBM simulations of a bubble column with different liquid properties. Chem. Eng. J. 2017, 329, 116–127. [Google Scholar] [CrossRef]
- Zhang, H.H.; Sayyar, A.; Wang, Y.L.; Wang, T.F. Generality of the CFD-PBM coupled model for bubble column simulation. Chem. Eng. Sci. 2020, 219, 115514. [Google Scholar] [CrossRef]
- Sideman, S.; Hortasu, N.; Fulton, J.W. Mass transfer in gas-liquid contacting systems. Ind. Eng. Chem. 2002, 58, 32–47. [Google Scholar] [CrossRef]
- Ramkrishna, D. Population Balances Theory and Applications to Particulate Systems in Engineering; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Li, L.; Hao, R.; Jin, X.; Hao, Y.; Fu, C.; Zhang, C.; Gu, X. A Turbulent Mass Diffusivity Model for Predicting Species Concentration Distribution in the Biodegradation of Phenol Wastewater in an Airlift Reactor. Processes 2023, 11, 484. [Google Scholar] [CrossRef]
- Higbie, R. The rate of absorption of a pure gas into a still liquid during short periods of exposure. Trans. AIChE 1935, 31, 365–377. [Google Scholar]
- Lamont, J.C.; Scott, D.S. An eddy cell model of mass transfer into the surface of a turbulent liquid. AIChE J. 1970, 16, 513–519. [Google Scholar] [CrossRef]
- Wang, T.F.; Wang, J.F. Numerical simulations of gas-liquid mass transfer in bubble columns with a CFD-PBM coupled model. Chem. Eng. Sci. 2007, 62, 7107–7118. [Google Scholar] [CrossRef]
- Buffo, A.; Vanni, M.; Marchisio, D.L. Multidimensional population balance model for the simulation of turbulent gas-liquid systems in stirred tank reactors. Chem. Eng. Sci. 2012, 70, 31–44. [Google Scholar] [CrossRef]
- Petitti, M.; Vanni, M.; Marchisio, D.L.; Buffo, A.; Podenzani, F. Simulation of coalescence, break-up and mass transfer in a gas-liquid stirred tank with CQMOM. Chem. Eng. J. 2013, 228, 1182–1194. [Google Scholar] [CrossRef]
- Moreno-Casas, P.A.; Scott, F.; Delpiano, J.; Abell, J.A.; Caicedo, F.; Munoz, R.; Vergara-Fernandez, A. Mechanistic Description of Convective Gas-Liquid Mass Transfer in Biotrickling Filters Using CFD Modeling. Environ. Sci. Technol. 2020, 54, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Enersul. CUSTOM SULPHUR DEGASSING-HYSPEC™ Degassing Systems. Available online: https://www.enersul.com/degassing (accessed on 30 May 2023).
- Yu, H. Absorption of Nitric Oxide from Flue Gas Using Ammoniacal Cobalt(II) Solutions. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2012. [Google Scholar]
- Mandhane, J.M.; Gregory, G.A.; Aziz, K. A flow pattern map for gas-liquid flow in horizontal pipes. Int. J. Multiph. Flow 1974, 1, 537–553. [Google Scholar] [CrossRef]
- Jepsen, J.C. Mass transfer in two-phase flow in horizontal pipelines. AIChE J. 1970, 16, 705–711. [Google Scholar] [CrossRef]
- Taitel, Y.; Dukler, A.E. A theoretical approach to the Lockhart-Martinelli correlation for stratified flow. Int. J. Multiph. Flow 1976, 2, 591–595. [Google Scholar] [CrossRef]
- Chen, J.J.J.; Spedding, P.L. An analysis of holdup in horizontal two-phase gas-liquid flow. Int. J. Multiph. Flow 1983, 9, 147–159. [Google Scholar] [CrossRef]
- Spedding, P.L.; Hand, N.P. Prediction in stratified gas-liquid co-current flow in horizontal pipelines. Int. J. Heat Mass Transf. 1997, 40, 1923–1935. [Google Scholar] [CrossRef]
- Forrester, S.E.; Rielly, C.D.; Carpenter, K.J. Gas-inducing impeller design and performance characteristics. Chem. Eng. Sci. 1998, 53, 603–615. [Google Scholar] [CrossRef]
- Akita, K.; Yoshida, F. Bubble Size, Interfacial Area, and Liquid-Phase Mass Transfer Coefficient in Bubble Columns. Ind. Eng. Chem. Process Des. Dev. 1974, 13, 84–91. [Google Scholar] [CrossRef]
- Kresta, S.M.; Mao, D.M.; Roussinova, V. Batch blend time in square stirred tanks. Chem. Eng. Sci. 2006, 61, 2823–2825. [Google Scholar] [CrossRef]
- Deckwer, W.D.; Nguyen-Tien, K.; Kelkar, B.G.; Shah, Y.T. Applicability of axial dispersion model to analyze mass transfer measurements in bubble columns. AIChE J. 1983, 29, 915–922. [Google Scholar] [CrossRef]
- Yu, H.S.; Tan, Z.C. On the Kinetics of the Absorption of Nitric Oxide into Ammoniacal Cobalt(II) Solutions. Environ. Sci. Technol. 2014, 48, 2453–2463. [Google Scholar] [CrossRef]
- Yu, H.S.; Tan, Z.C.; The, J.; Feng, X.S.; Croiset, E.; Anderson, W.A. Kinetics of the Absorption of Carbon Dioxide into Aqueous Ammonia Solutions. AIChE J. 2016, 62, 3673–3684. [Google Scholar] [CrossRef]
- Linek, V.; Vacek, V.; Bene, P. A critical review and experimental verification of the correct use of the dynamic method for the determination of oxygen transfer in aerated agitated vessels to water, electrolyte solutions and viscous liquids. Chem. Eng. J. 1987, 34, 11–34. [Google Scholar] [CrossRef]
- Robinson, C.W.; Wilke, C.R. Oxygen absorption in stirred tanks: A correlation for ionic strength effects. Biotechnol. Bioeng. 1973, 15, 755–782. [Google Scholar] [CrossRef]
- ANSYS Inc. ANSYS Fluent Theory Guide; ANSYS Inc.: Canonsburg, PA, USA, 2020. [Google Scholar]
- Sato, Y.; Sekoguchi, K. Liquid velocity distribution in two-phase bubble flow. Int. J. Multiph. Flow 1975, 2, 79–95. [Google Scholar] [CrossRef]
- Seidel, S.; Eibl, D. Influence of Interfacial Force Models and Population Balance Models on the k(L)a Value in Stirred Bioreactors. Processes 2021, 9, 1185. [Google Scholar] [CrossRef]
- Wang, M.; Ni, C.; Bu, X.; Peng, Y.; Xie, G.; Tan, Z.; Yu, H. CFD-PBM Simulation of the Column Flotation Unit of FCMC: Importance of Gas-Liquid Interphase Forces Models. Can. J. Chem. Eng. 2023, 1, 1–16. [Google Scholar] [CrossRef]
- ANSYS Inc. ANSYS Fluent User’s Guide; ANSYS Inc.: Canonsburg, PA, USA, 2020. [Google Scholar]
- Prince, M.J.; Blanch, H.W. Bubble coalescence and break-up in air-sparged bubble columns. AIChE J. 1990, 36, 1485–1499. [Google Scholar] [CrossRef]
- Luo, H.; Svendsen, H.F. Theoretical model for drop and bubble breakup in turbulent dispersions. AIChE J. 2010, 42, 1225–1233. [Google Scholar] [CrossRef]
- Maluta, F.; Paglianti, A.; Montante, G. A PBM-Based Procedure for the CFD Simulation of Gas-Liquid Mixing with Compact Inline Static Mixers in Pipelines. Processes 2023, 11, 198. [Google Scholar] [CrossRef]
- Liu, M. Age distribution and the degree of mixing in continuous flow stirred tank reactors. Chem. Eng. Sci. 2012, 69, 382–393. [Google Scholar] [CrossRef]
- Lane, G.L. Improving the accuracy of CFD predictions of turbulence in a tank stirred by a hydrofoil impeller. Chem. Eng. Sci. 2017, 169, 188–211. [Google Scholar] [CrossRef]
- Aubin, J.; Fletcher, D.; Xuereb, C. Modeling turbulent flow in stirred tanks with CFD: The influence of the modeling approach, turbulence model and numerical scheme. Exp. Therm. Fluid Sci. 2004, 28, 431–445. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Jurtz, N.; Walle, A.; Kraume, M. Evaluation and application of efficient CFD-based methods for the multi-objective optimization of stirred tanks. Chem. Eng. Sci. 2022, 263, 118109. [Google Scholar] [CrossRef]
- Guan, X.; Li, X.; Yang, N.; Liu, M. CFD simulation of gas-liquid flow in stirred tanks: Effect of drag models. Chem. Eng. J. 2020, 386, 121554. [Google Scholar] [CrossRef]
- Law, D.; Battaglia, F.; Heindel, T.J. Model validation for low and high superficial gas velocity bubble column flows. Chem. Eng. Sci. 2008, 63, 4605–4616. [Google Scholar] [CrossRef]
- Monahan, S.M.; Vitankar, V.S.; Fox, R.O. CFD predictions for flow-regime transitions in bubble columns. AIChE J. 2005, 51, 1897–1923. [Google Scholar] [CrossRef]
- Picardi, R.; Zhao, L.; Battaglia, F. On the Ideal Grid Resolution for Two-Dimensional Eulerian Modeling of Gas–Liquid Flows. J. Fluids Eng. 2016, 138, 114503. [Google Scholar] [CrossRef]
- Roache, P.J. Perspective: A Method for Uniform Reporting of Grid Refinement Studies. J. Fluids Eng. 1994, 116, 405–413. [Google Scholar] [CrossRef]
- Sosnowski, M. Evaluation of Heat Transfer Performance of a Multi-Disc Sorption Bed Dedicated for Adsorption Cooling Technology. Energies 2019, 12, 4660. [Google Scholar] [CrossRef] [Green Version]
- Maluta, F.; Buffo, A.; Marchisio, D.L.; Montante, G.; Paglianti, A.; Vanni, M. Effect of turbulent kinetic energy dissipation rate on the prediction of droplet size distribution in stirred tanks. Int. J. Multiph. Flow 2021, 136, 103547. [Google Scholar] [CrossRef]
- Li, D.; Chen, W. Effects of impeller types on gas-liquid mixing and oxygen mass transfer in aerated stirred reactors. Process Saf. Environ. Prot. 2022, 158, 360–373. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Castro, E.G. Estimation of oxygen mass transfer coefficient in stirred tank reactors using artificial neural networks. Enzym. Microb. Tech. 2001, 28, 560–569. [Google Scholar] [CrossRef]
- Miller, D.N. Scale-up of agitated vessels gas-liquid mass transfer. AIChE J. 1974, 20, 445–453. [Google Scholar] [CrossRef]
- Calderbank, P.H. Physical rate process in industrial fermentation. Part II. Mass transfer coefficients in gas–liquid contacting with and without mechanical agitation. Trans. Inst. Chem. Eng. 1959, 37, 173–185. [Google Scholar]
- Sawant, S.B.; Joshi, J.B.; Pangarkar, V.G.; Mhaskar, R.D. Mass transfer and hydrodynamic characteristics of the Denver type of flotation cells. Chem. Eng. J. 1981, 21, 11–19. [Google Scholar] [CrossRef]
- Basavarajappa, M.; Miskovic, S. Investigation of gas dispersion characteristics in stirred tank and flotation cell using a corrected CFD-PBM quadrature-based moment method approach. Miner. Eng. 2016, 95, 161–184. [Google Scholar] [CrossRef]
- Chen, M.N.; Wang, J.J.; Zhao, S.W.; Xu, C.Z.; Feng, L.F. Optimization of Dual-Impeller Configurations in a Gas-Liquid Stirred Tank Based on Computational Fluid Dynamics and Multiobjective Evolutionary Algorithm. Ind. Eng. Chem. Res. 2016, 55, 9054–9063. [Google Scholar] [CrossRef]
- Ranganathan, P.; Sivaraman, S. Investigations on hydrodynamics and mass transfer in gasliquid stirred reactor using computational fluid dynamics. Chem. Eng. Sci. 2011, 66, 3108–3124. [Google Scholar] [CrossRef]




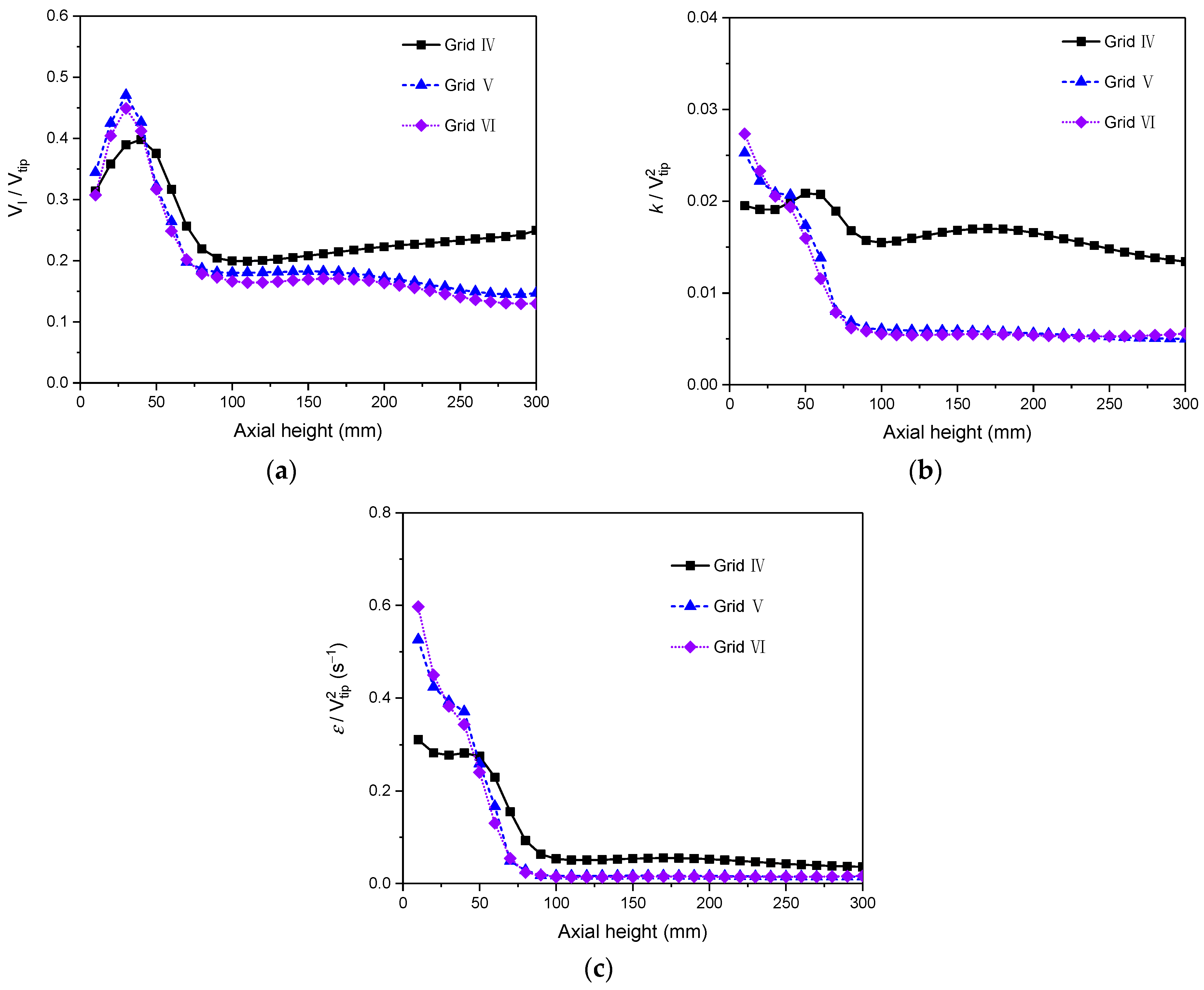

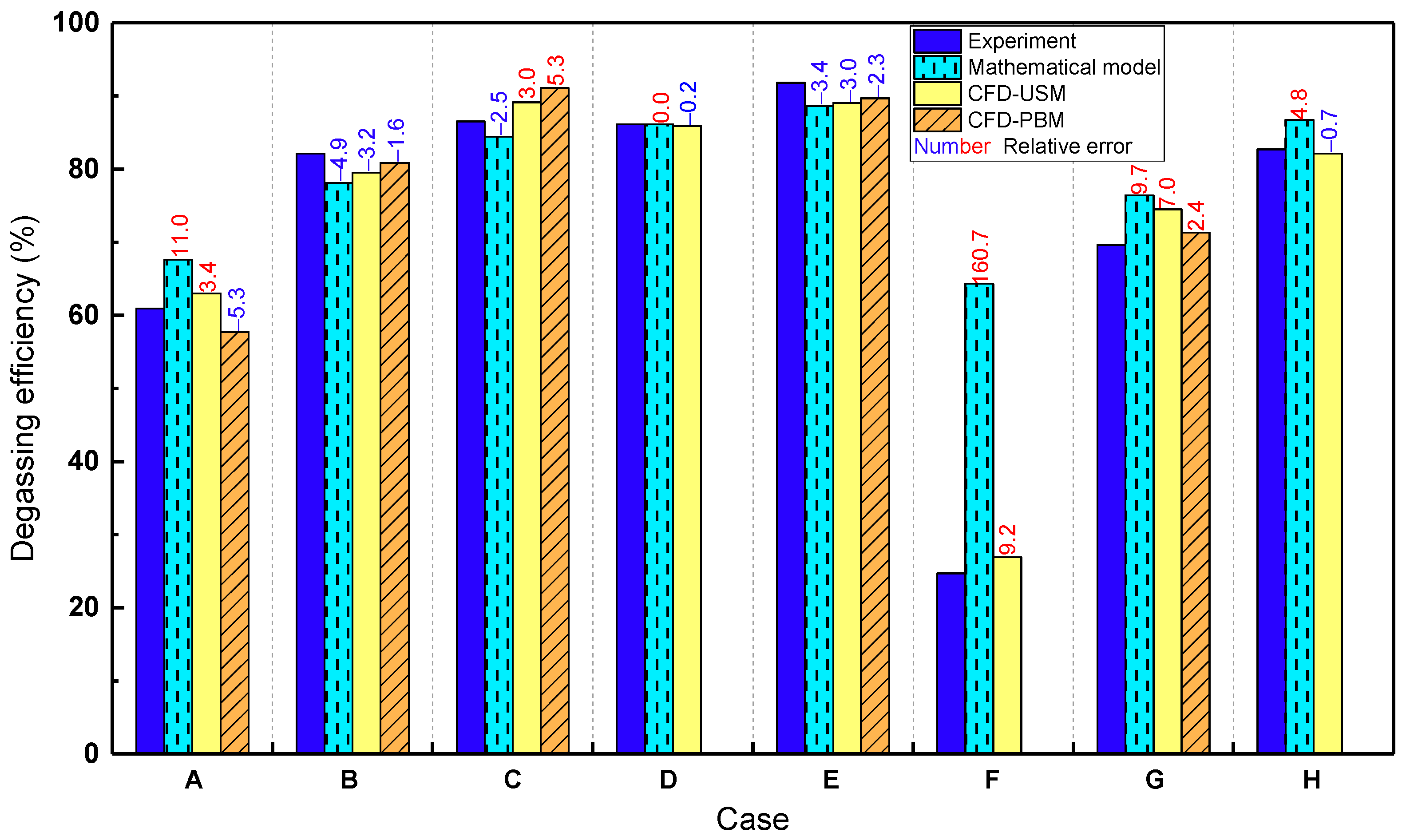

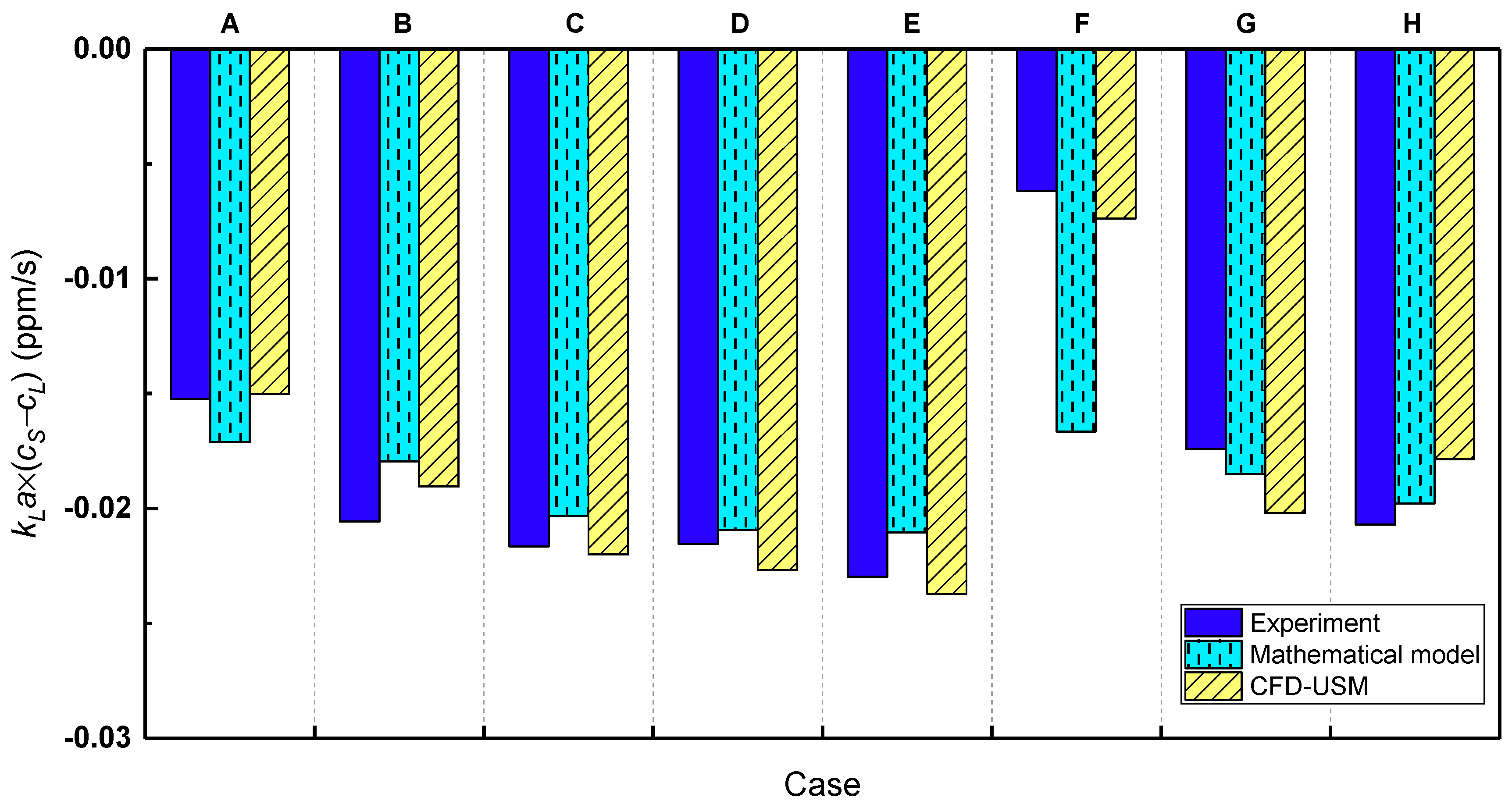

| Case No. | Reactor | Operating Conditions | Uniform Bubble Size (mm) | PBM | ||||
|---|---|---|---|---|---|---|---|---|
| Agitation Speed (Hz) | Gas Flow Rate (×10−4 m3∙s−1) | Inlet Bubble Size (mm) | Bubble Size Range (mm) | Factor | ||||
| Coalescence Kernel | Breakage Kernel | |||||||
| A | GIAT * | 8.3 | - | 3.5 | 4.0 | 1.0–8.0 | 1 | 0.5 |
| B | GIAT | 10.3 | - | 4.0 | 4.0 | 1.0–8.0 | 1 | 0.5 |
| C | GIAT | 13.3 | - | 5.0 | 4.0 | 1.0–8.0 | 1 | 0.5 |
| D | CSTR | 10.3 | 1.97 | 2.5 | - | - | - | - |
| E | CSTR | 10.3 | 5.90 | 2.5 | 1.0 | 0.5–4.0 | 0.05 | 0.1 |
| F | BC | - | 1.97 | 5.0 | - | - | - | - |
| G | BC | - | 3.93 | 1.5 | 1.0 | 0.5–4.0 | 0.03 | 0.03 |
| H | BC | - | 9.83 | 1.5 | - | - | - | - |
| Items | Grid I | Grid II | Grid III |
|---|---|---|---|
| Total number of cells | 38,950 | 76,362 | 145,979 |
| Maximum cell size (m3) | 3.45 × 10−6 | 2.25 × 10−6 | 1.00 × 10−6 |
| Minimum cell size (m3) | 2.81 × 10−7 | 1.41 × 10−7 | 8.04 × 10−8 |
| Items | Grid IV | Grid V | Grid VI |
|---|---|---|---|
| Total number of cells | 40,616 | 338,432 | 672,633 |
| Maximum cell size (m3) | 1.41 × 10−6 | 2.78 × 10−7 | 1.27 × 10−7 |
| Minimum cell size (m3) | 3.02 × 10−8 | 1.81 × 10−9 | 8.08 × 10−10 |
| Case No. | Reactor | Uniform Bubble Size Used in CFD-USM (mm) | Mean Bubble Size Predicted by CFD-PBM (mm) |
|---|---|---|---|
| A | GIAT * | 3.5 | 3.4 |
| B | GIAT | 4.0 | 4.1 |
| C | GIAT | 5.0 | 4.4 |
| E | CSTR | 2.5 | 2.1 |
| G | BC | 1.5 | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Nie, Q.; Xie, G.; Tan, Z.; Yu, H. Mathematical Modelling and CFD Simulation for Oxygen Removal in a Multi-Function Gas-Liquid Contactor. Processes 2023, 11, 1780. https://doi.org/10.3390/pr11061780
Wang M, Nie Q, Xie G, Tan Z, Yu H. Mathematical Modelling and CFD Simulation for Oxygen Removal in a Multi-Function Gas-Liquid Contactor. Processes. 2023; 11(6):1780. https://doi.org/10.3390/pr11061780
Chicago/Turabian StyleWang, Mengdie, Qianqian Nie, Guangyuan Xie, Zhongchao Tan, and Hesheng Yu. 2023. "Mathematical Modelling and CFD Simulation for Oxygen Removal in a Multi-Function Gas-Liquid Contactor" Processes 11, no. 6: 1780. https://doi.org/10.3390/pr11061780






