Ultrasound-Mediated DNA Transformation of Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Plasmids
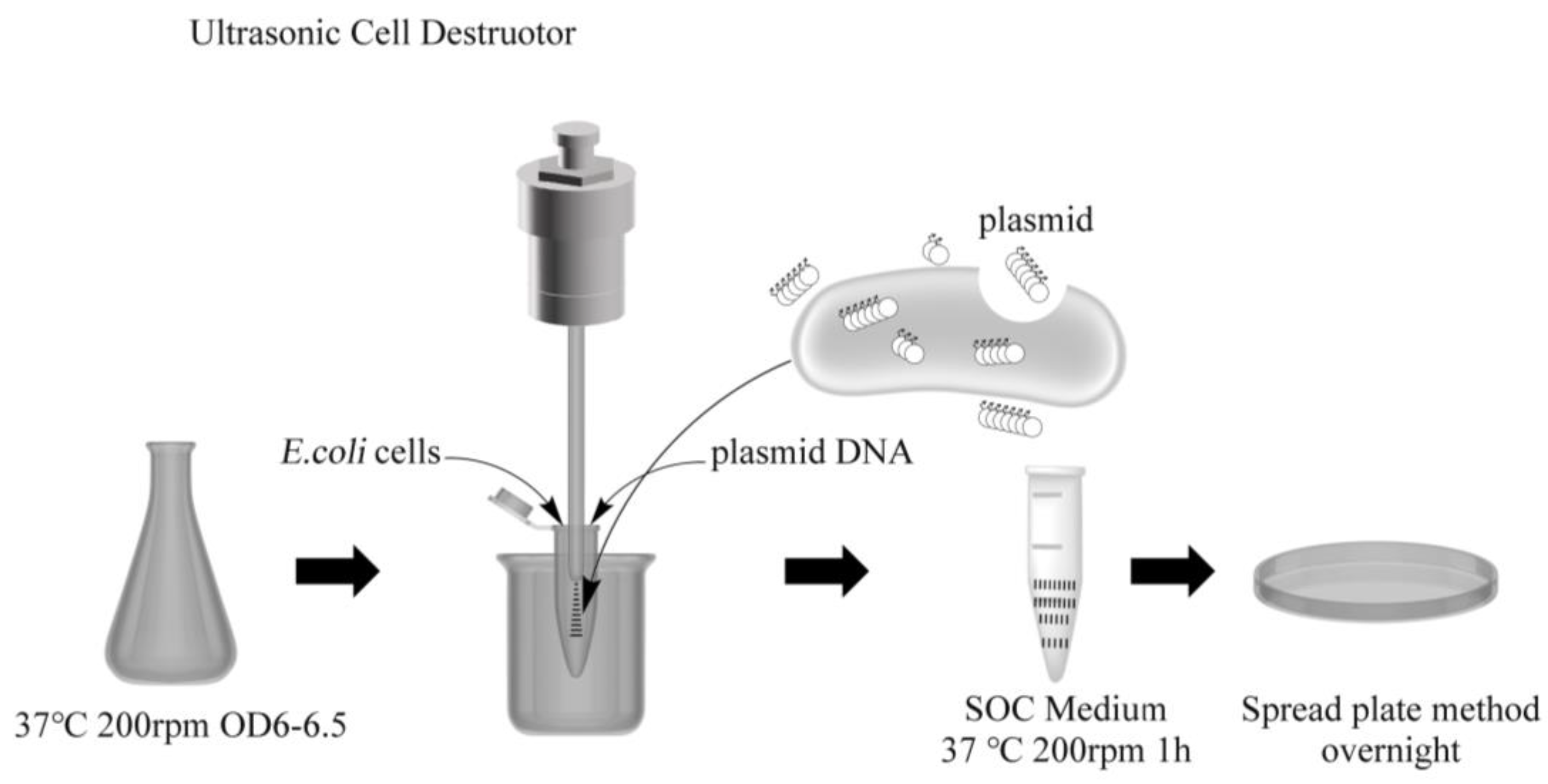
2.2. Cell Culture and Preparation of Receptive Cells
2.3. Ultrasonic Conversion Process
2.4. Transformant Identification
2.5. Electron Microscope Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Ultrasound-Mediated Plasmid Transformation of E. coli
3.2. Electron Microscopy
3.3. Ultrasound-Mediated Plasmid Transformation of Saccharomyces cerevisiae
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asif, A.; Mohsin, H.; Tanvir, R.; Rehman, Y. Revisiting the mechanisms involved in calcium chloride induced bacterial transformation. Front. Microbiol. 2017, 8, 2169. [Google Scholar] [CrossRef] [PubMed]
- Mandel, M.; Higa, A. Calcium-dependent bacteriophage DNA infection. J. Mol. Biol. 1970, 53, 159–162. [Google Scholar] [CrossRef]
- Cohen, S.N.; Chang, A.C.; Hsu, L. Nonchromosomal antibiotic resistance in bacteria: Genetic transformation of Escherichia coli by R-factor DNA. Proc. Natl. Acad. Sci. USA 1972, 69, 2110–2114. [Google Scholar] [CrossRef]
- Green, R.; Rogers, E.J. Transformation of chemically competent E. coli. Methods Enzymol. 2013, 529, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.; Yun, C.H. Trehalose increases chemical-induced transformation efficiency of Escherichia coli. Anal. Biochem. 2004, 333, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Nagamani, G.; Alex, S.; Soni, K.B.; Anith, K.N.; Viji, M.M.; Kiran, A.G. A novel approach for increasing transformation efficiency in E. coli DH5α cells using silver nanoparticles. 3 Biotech 2019, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Sambrook, J. Transformation of Escherichia coli by Electroporation. Cold Spring Harb. Protoc. 2020, 2020, 101220. [Google Scholar] [CrossRef]
- Neumann, E. The relaxation hysteresis of membrane electroporation. In Electroporation and Electrofusion in Cell Biology; Springer: Boston, MA, USA, 1989. [Google Scholar] [CrossRef]
- Przystupski, D.; Ussowicz, M. Landscape of cellular bioeffects triggered by ultrasound-induced sonoporation. Int. J. Mol. Sci. 2022, 23, 11222. [Google Scholar] [CrossRef]
- Escoffre, J.M.; Aya, Z.; Anthony, N.; Ayache, B. In-vivo gene delivery by sonoporation: Recent progress and prospects. Curr. Gene Ther. 2013, 1, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Thrall, B.D.; Miller, D.L. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound Med. Biol. 1997, 23, 953–959. [Google Scholar] [CrossRef]
- Marmottant, P.; Hilgenfeldt, S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature 2003, 423, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Feril, L.B., Jr.; Kondo, T.; Zhao, Q.L.; Ogawa, R.; Tachibana, K.; Kudo, N.; Fujimoto, S.; Nakamura, S. Enhancement of ultrasound-induced apoptosis and cell lysis by echo-contrast agents. Ultrasound Med. Biol. 2003, 29, 331–337. [Google Scholar] [CrossRef]
- Kalli, C.; Teoh, W.C.; Leen, E. Introduction of genes via sonoporation and electroporation. Adv. Exp. Med. Biol. 2014, 818, 231–254. [Google Scholar] [CrossRef]
- Song, Y.; Hahn, T.; Thompson, I.P.; Mason, T.J.; Preston, G.M.; Li, G.; Paniwnyk, L.; Huang, W.E. Ultrasound-mediated DNA transfer for bacteria. Nucleic Acids Res. 2007, 35, e129. [Google Scholar] [CrossRef]
- Kimran, H. The effect of ultrasound exposure on the transformation efficiency of Escherichia coli HB101. Biosci. Horiz. 2010, 3, 2. [Google Scholar] [CrossRef]
- Lin, L.; Song, H.; Ji, Y.; He, Z.; Pu, Y.; Zhou, J.; Xu, J. Ultrasound-mediated plasmid transformation in thermophilic anaerobic bacteria. PLoS ONE 2010, 5, e12582. [Google Scholar] [CrossRef] [PubMed]
- Campos-Guillén, J.; Fernández, F.; Pastrana, X.; Loske, A.M. Relationship between plasmid size and shock wave-mediated bacterial transformation. Ultrasound Med. Biol. 2012, 38, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Wu, S.H.; Xie, Y.H.; Zhong, M.; Wei, M.L.; Li, Z.Y.; Long, X.F.; Niu, X.F. A high-throughput Screening Procedure (Py-Fe3+) for Enhancing Ethanol Production by Saccharomyces cerevisiae Using ARTP Random Mutagenesis. Processes 2022, 10, 2186. [Google Scholar] [CrossRef]
- Li, X.R.; Tian, G.Q.; Shen, H.J.; Liu, J.Z. Metabolic engineering of Escherichia coli to produce zeaxanthin. J. Ind. Microbiol. Biotechnol. 2015, 42, 627–636. [Google Scholar] [CrossRef]
- Harvey, E.N.; Loomis, A.L. The destruction of luminous bacteria by high frequency sound waves. J. Bacteriol. 1929, 5, 373–376. [Google Scholar] [CrossRef]
- Forbes, M.M.; O’Brien, W.D., Jr. Development of a theoretical model describing sonoporation activity of cells exposed to ultrasound in the presence of contrast agents. J. Acoust. Soc. Am. 2012, 131, 2723–2729. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.M.; Bettinger, T. Gene therapy progress and prospects: Ultrasound for gene transfer. Gene Ther. 2007, 14, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, K.; Pollard, R.; Borden, M. Ultrasound microbubble contrast agents: Fundamentals and application to gene and drug delivery. Annu. Rev. Biomed. Eng. 2007, 9, 415–447. [Google Scholar] [CrossRef]
- Zinser, E.R.; Kolter, R. Escherichia coli evolution during stationary phase. Res. Microbiol. 2004, 155, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Anderson, T.; Zhang, H.; Flotte, T.J.; Doukas, A.G. Alteration of cell membrane by stress waves in vitro. Ultrasound Med. Biol. 1996, 22, 1285–1293. [Google Scholar] [CrossRef]
- Deng, X.C.; Sieling, F.; Hua, C.; Jian, M. Ultrasound-induced cell membrane porosity. Ultrasound Med. Biol. 2004, 30, 519–526. [Google Scholar] [CrossRef]
- Loske, A.M.; Campos-Guillen, J.; Fernández, F.; Castaño-Tostado, E. Enhanced shock wave-assisted transformation of Escherichia coli. Ultrasound Med. Biol. 2011, 37, 502–510. [Google Scholar] [CrossRef]
- James, C.B. CHAPTER 19-Gene Cloning. In Guide to Biochemistry; Butterworth: Oxford, UK; Waltham, MA, USA, 1989; pp. 230–241. [Google Scholar] [CrossRef]
- Minagawa, S.; Ogasawara, H.; Kato, A.; Yoko, Y.; Eguchi, Y.; Oshima, T.; Mori, H.; Ishihama, A.; Utsumi, R. Identification and molecular characterization of the Mg2+ stimulon of Escherichia coli. J. Bacteriol. 2003, 185, 3696–3702. [Google Scholar] [CrossRef]
- Gerdesmeyer, L.; Eiff, C.V.; Horn, C.; Henne, M.; Gollwitzer, H. Antibacterial effects of extracorporeal shock waves. Ultrasound Med. Biol. 2005, 31, 115–119. [Google Scholar] [CrossRef]







| Name | Description | Reference/Sources |
|---|---|---|
| STRAIN | ||
| E. coli DH5α | supE44 Δ(lacZYA-argF) U169 (Φ80lacZ ΔM15) hsdR17 recA endA1 | Invitrogen |
| S. cerevisiae S288C | MATα SUC2 gal2 mal2 mel flo1 flo8-1 hap1 ho bio1 bio6 | ATCC 204508 |
| S. cerevisiae NGT-F1 | derived from S. cerevisiae S288C, and tolerance to 381 g/L source | [19] |
| PLASMID | ||
| pZEABP | constitute expression vector, pBR322 ori, P37 promoter, Ampr, BglBrick, ePathBrick containing four isocaudomers (AvrII, NheI, SpeI, and XbaI) | [20] |
| pUG6 | Shuttle plasmid, Ampr in E. coli, G418r in S. cerevisiae | Novagen VT1696 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.-P.; Yuan, Y.-M.; Yang, S.; Xu, Y.; Liao, C.-Y.; Niu, F.-X. Ultrasound-Mediated DNA Transformation of Bacteria. Processes 2023, 11, 2163. https://doi.org/10.3390/pr11072163
Wang B-P, Yuan Y-M, Yang S, Xu Y, Liao C-Y, Niu F-X. Ultrasound-Mediated DNA Transformation of Bacteria. Processes. 2023; 11(7):2163. https://doi.org/10.3390/pr11072163
Chicago/Turabian StyleWang, Bei-Ping, Yue-Mei Yuan, Sheng Yang, Yun Xu, Chun-Yan Liao, and Fu-Xing Niu. 2023. "Ultrasound-Mediated DNA Transformation of Bacteria" Processes 11, no. 7: 2163. https://doi.org/10.3390/pr11072163
APA StyleWang, B.-P., Yuan, Y.-M., Yang, S., Xu, Y., Liao, C.-Y., & Niu, F.-X. (2023). Ultrasound-Mediated DNA Transformation of Bacteria. Processes, 11(7), 2163. https://doi.org/10.3390/pr11072163






