Abstract
The textile industry has become one of the largest producers of water pollution. The azo dyes used in the textile industry may present a serious environmental problem because of their high toxicity and chemical stability. In the present work, the cobalt/aluminum oxide-ceria (Co/Al2O3-CeO2) catalyst was synthesized, and the degradation of Reactive Red 195 (RR195) by catalytic ozonation was studied. The Co/Al2O3-CeO2 catalyst was synthesized via the incipient wetness method with the assistance of ultrasound. The presence of Co/Al2O3-CeO2 did not notably improve the degradation of RR195 compared to ozonation alone, but it was advantageous for RR195 mineralization. The effects of initial dye concentration (200–800 mg/L), catalyst dosage (1–4 g/L), and solution pH (4–10) on color, and COD removal were evaluated. The results indicate that the dye’s concentration significantly affects COD removal efficiency. The optimum catalyst dosage and pH values were determined to be 3 g/L and 8, respectively. Co/Al2O3-CeO2 catalyst shows good catalytic activity and stability based on four repeated tests during RR195 ozonation. Finally, a possible mechanism and a kinetic scheme of the catalytic ozonation of RR195 were proposed.
1. Introduction
The textile industry has become one of the largest producers of industrial wastewater, and dyes are major pollutants that come from these textile wastewaters [1]. The majority of dyes used in the textile industry are azo dyes, which are toxic, recalcitrant, chemically stable, and carcinogenic. Their release into the environment may pose many serious aesthetical, ecological, environmental, and health hazards [2]. Therefore, the discarding of these dyes must be controlled, and the effluents must be treated [3]. Different physical and biological methods, or combinations of them, have been investigated for color removal from dye-laden wastewater. However, they all suffer from specific disadvantages [2].
In recent years, chemical treatment processes, especially chemical oxidation, have become the method of choice due to their high efficiency and easy operation [4,5]. Ozone is one of the most effective oxidant agents used for decolorization of dyes owing to its extremely high redox potential (E0 = 2.07 V). During ozonation, conjugated double bonds, which are often associated with color, can be broken down by ozone either directly or indirectly [3,5,6]. Although single ozonation has been shown to be effective for color removal, the formed intermediates are frequently resistant to ozone attack and the mineralization extent is usually insufficient [5]. In order to improve efficiency, various advanced oxidation processes (AOPs, e.g., O3/H2O2, UV/O3, catalytic ozonation, etc.) have been investigated. Especially heterogeneous catalytic ozonation has received increasing attention in recent years due to its potentially high effectiveness in the degradation of harmful organic pollutants with low negative effects on the environment [7].
Different metals or their oxides (such as Co, Fe, Ni, and manganese oxides) deposited on porous materials (such as alumina, activated carbon, and MCM-41) were investigated as the catalysts for catalytic ozonation [8,9,10]. Ceria (CeO2), which can act as a reducible support or catalyst itself, has been extensively applied in heterogeneous catalysis due to its unique property of storing and releasing oxygen (redox property) and its excellent thermal and mechanical resistance [11,12,13]. Several works found that palladium oxide (PdO) loaded CeO2 catalyst could promote the degradation of pyruvic acid and oxalate in water by catalytic ozonation better than ozonation alone [11,14,15]. However, the CeO2 carrier suffers from the disadvantages of high cost and relatively low surface areas [13]. Therefore, deposition of ceria on a high surface area support, like alumina, was achieved. Adding cerium to alumina produces structural changes, improves the dispersion of the metal, and stabilizes the alumina to avoid thermal sinterization [16]. Due to the advantageous properties mentioned above, CeO2-Al2O3 has been used extensively as a potential support, such as in the generation of hydrogen by methane reforming and in the elimination of pollutants (such as NOx, CO, and hydrocarbons) in automobile exhausts [17]. Chen et al. [18] found that the Al2O3-CeO2 catalysts were active for CH2Cl2 catalytic combustion, and that the activity could be further promoted by the addition of Pt. Li et al. [19] found that the introduction of CeO2 into the CuO/ Al2O catalyst improved the dispersion of CuO on the catalyst surface. In addition, the specific surface area and pore volume of the samples gradually decreased with the increase in CeO2 content. The synergistic effect (Ce3+ + Cu2+ ↔ Ce4+ + Cu+) favurs the generation of oxygen vacancies and increases the activity of the catalyst. Zhou et al. [20] modified the conventional Pt/Al2O3 catalyst with CeO2 and increased the proportion of Pt0 from 74.5% to 82.1%. When the metal state Pt0 content is increased, the redox activity of the catalyst increases accordingly. Li et al. [17] systematically investigated the synergistic effect of Al2O3 and CeO2 on MIAA (monoiodoacetic acid)-catalyzed hydrodeiodination (HDI). Experimental characterization shows that the introduced CeO2 can improve the dispersion of Pt by forming a Ce-O-Pt bond and that the high zeta potential of the Al2O3 component can facilitate the adsorption of MIAA. Through kinetic experiments and characterization, the abundance of Pt sites and the synergistic interaction between CeO2 and Al2O3 allow Pt/CeO2-Al2O3 to exhibit excellent catalytic performance. In other studies, a Pt/Al2O3-CeO2 nanocatalyst was used to oxidize volatile organic compounds (VOCs) and toluene, it also showed good catalytic performance [12,13].
As above-mentioned, several catalysts based on Al2O3-CeO2 have been reported to be able to catalytic oxidation of organic compounds but seldom have been used in the catalytic ozonation of azo dyes. To better understand the potential use of this carrier, in this work, the Co/Al2O3-CeO2 catalyst was synthesized, and the degradation of Reactive Red 195 (RR195) by catalytic ozonation was studied. The influence of solution pH, initial dye concentration, ozone dosage, and catalyst dosage were assessed in catalytic ozonation of RR195. Finally, the mechanism and kinetics model of RR 195 degradation was established.
2. Materials and Methods
2.1. Materials
RR 195 was obtained from Quanzhou Anze Dyestuff Chemical Factory (Quanzhou, China). Cerium nitrate (AR) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Cobalt acetate (AR) was purchased from Shanghai Reagent Factory (Shanghai, China). γ-Al2O3 was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Other chemicals and reagents were purchased from Shantou Xilong Chemical Works (Shantou, China). Distilled water was used in preparing the solution for all the experiments.
2.2. Catalysts Preparation
The Al2O3-CeO2 support was prepared by impregnating γ-Al2O3 with cerium nitrate aqueous solutions following the incipient wetness method [21]. The γ-Al2O3 was doped with 20 wt% cerium nitrate. The samples were sonicated in ambient air for 3 h and then impregnated for 12 h. The impregnated samples were dried at 105 °C for 2 h and finally calcined in air at 450 °C for 2 h.
The Co/Al2O3-CeO2 catalyst was prepared by impregnating Al2O3-CeO2 with a cobalt acetate aqueous solution according to the incipient wetness method with the assistance of ultrasound [22]. The calculated Co content (wt.%) loaded on the catalyst was 4%. The samples were sonicated in ambient air for 3 h and impregnated for 12 h. After impregnation, the sample was dried at 105 °C for 2 h. Then the Co/Al2O3-CeO2 catalysts were formed by calcination at 450 °C for 2 h.
2.3. Experimental Setup
Ozonation and catalytic ozonation of RR195 were carried out in a semi-batch mode apparatus, which consisted of an oxygen cylinder, an ozone generator (CF-G-3-10g), a flow meter, an ozone reactor (8 cm internal diameter × 40 cm height) and an ozone off-gas destruction system, and the detailed description is shown in our previous report [23]. Before the start of the experiment, the reactor was filled with 500 mL RR195 solution, and the catalyst was introduced into the reactor. The mixtures were stirred for 10 min by a magnetic stirrer. After that, the ozonized oxygen, produced from the ozone generator, flowed into the reactor through a gas flow meter for 20 more minutes. Water samples were collected from the reactor at specific predetermined times to analyze RR195 and COD concentrations. A gas absorption bottle containing 10% Na2S2O3 solution was used to destroy the remaining ozone before leaving the reactor.
2.4. Analytical Methods
The crystal structure of the catalysts was emphasized by X-ray diffraction (XRD) with a BRUKER D8 Advanced analyzer (Bruker, Germany). The particle size distribution of Co/Al2O3-CeO2 was performed using a Mastersizer 2000 laser particle size analyzer (Malvern, USA). The concentrations of ozone in the gas phase were measured by iodometric titration [24]. The concentrations of ARB solution were determined by measuring the absorbance of the solution at 514 nm with a UNICO UV-4802H spectrophotometer (Unico, America).
CODcr was determined by a rapid microwave-sealed digestion method by K2Cr2O7. Using a pipette to draw 10 mL of water sample into the digestion tank, and 5 mL of K2Cr2O7 digestion solution and 10 mL of HNO3-AgNO3 were added. After shaking and sealing tightly, the tank was put into the digestive chamber for 15 min. After the digestion, the reaction solution was transferred to a 150 mL conical flask, and the indicator test ferroin and ferrous ammonium sulfate hexahydrate standard solution were added. When the color of the solution changed from yellow through blue-green to red-brown, that is the end of the titration.
3. Results and Discussion
3.1. Catalyst Characterization
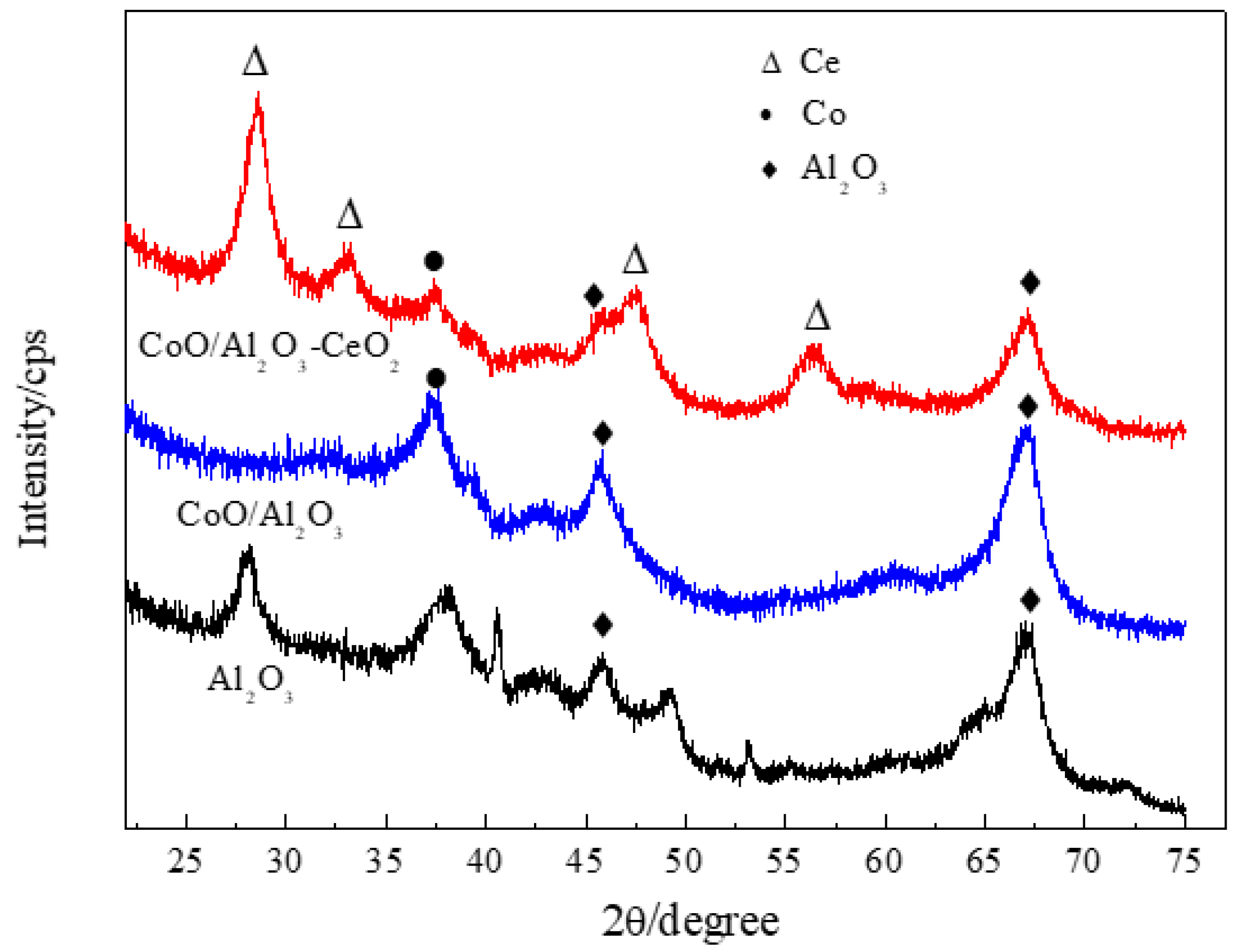
The particle size distribution of Co/Al2O3-CeO2 is given in Figure S1. The median particle diameter of Co/Al2O3-CeO2 was 22.472 μm, and the volume mean diameter of Co/Al2O3-CeO2 was 36.153 μm. The XRD patterns of γ-Al2O3 support, Co/Al2O3, and Co/Al2O3-CeO2 are shown in Figure 1. Three well-resolved reflections (at 2θ = 37.77°, 45.79° and 66.76°) were seen for γ-Al2O3. The diffraction peaks of cobalt species were observed at 2θ = 37.1° both in Co/Al2O3 and Co/Al2O3-CeO2 samples, indicating that cobalt species are dispersed across the supports. Moreover, the formation of CeO2, as indicated by the diffraction peak at 2θ = 28.7°, 33.2°, 47.7° and 56.6°, corresponds to CeO2 in the Co/Al2O3-CeO2 sample.
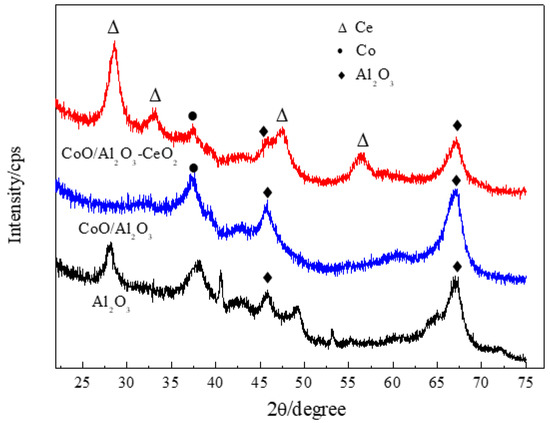
Figure 1.
The XRD pattern of Al2O3, Co/Al2O3, and Co/Al2O3-CeO2.
3.2. Catalytic Ozonation of RR 195
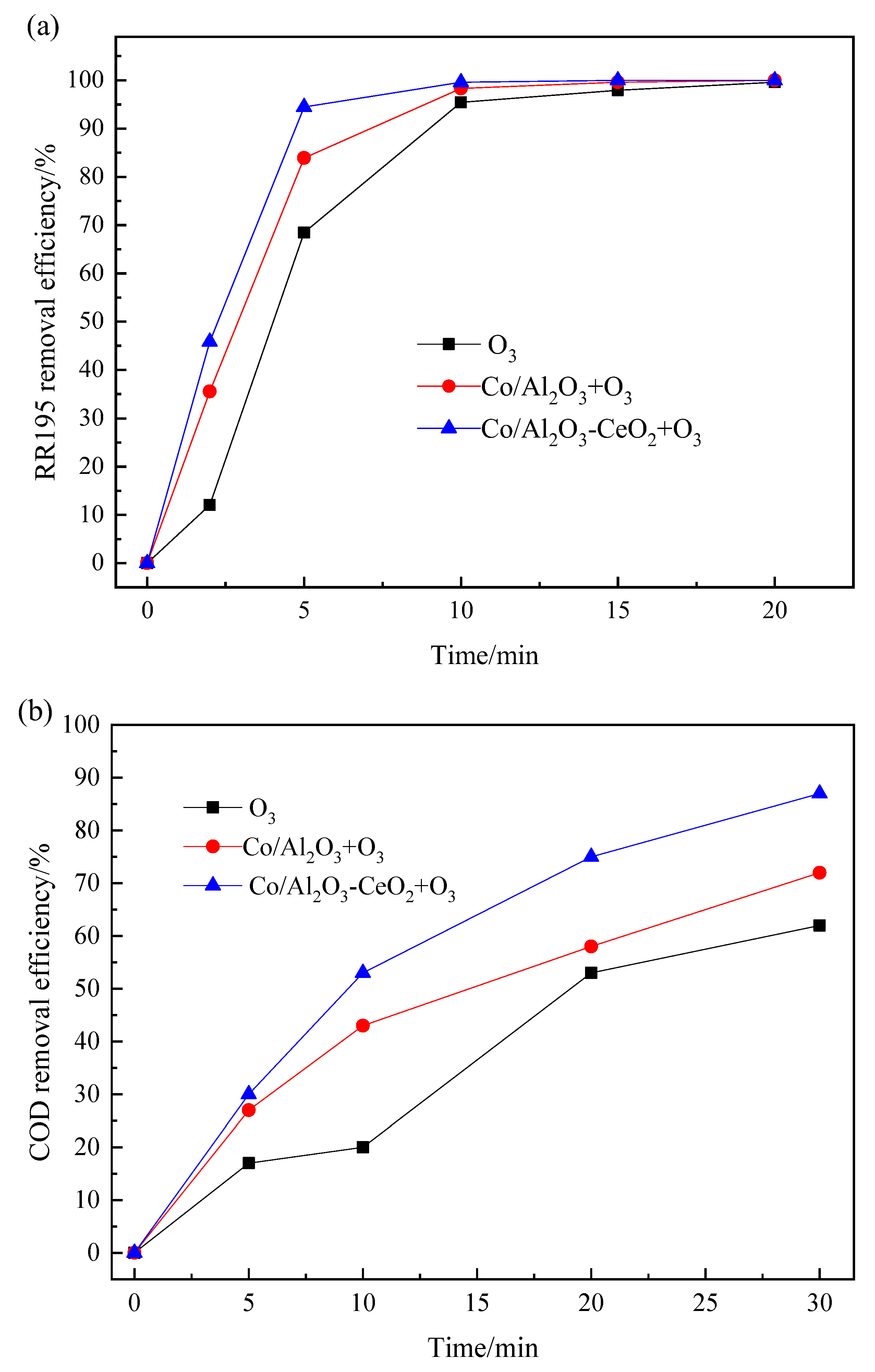
Figure 2 compares the color and COD degradation of RR195 for O3 alone, Co/Al2O3/O3, and Co/Al2O3-CeO2/O3 processes. As can be observed in Figure 2, the RR 195 removal efficiency of single ozonation was lower than that of catalytic ozonation at the first 15 min. However, the efficiencies all increased to almost 100% when the reaction time was extended to 20 min. This means that ozone is very efficient in RR195 removal. In addition, compared with single ozonation, it was found that both the Co/Al2O3-CeO2 catalyst and the Co/Al2O3 catalyst can improve COD removal efficiencies of RR195. However, the Co/Al2O3-CeO2/O3 process is the most efficient one. During the ozonation (200 mL/min ozone flow rate) of 400 mg/L of RR195 (pH 8.0), after 10 min, COD removal efficiency was only 20%, whereas the values reached 53% and 43%, respectively, after adding Co/Al2O3-CeO2 and Co/Al2O3 as a catalyst. After 30 min, the COD removal efficiencies achieved 87% and 72% by using Co/Al2O3-CeO2 and Co/Al2O3 catalysts, respectively, which were higher than that of the ozonation alone (62%).
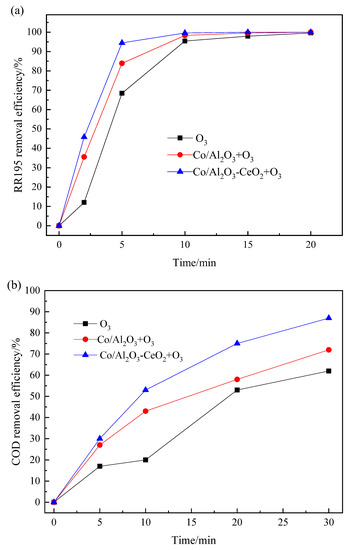
Figure 2.
Comparison of the RR195 removal with and without catalytic ozonation: (a) RR195 removal efficiency; (b) COD removal efficiency. (RR195 concentration 400 mg/L, pH 8, gas flow 200 mL/min, ozone flow 6 mg/min, catalyst dosage 3 g/L, magnetic stirring speed 1400 rpm).
From the above results, it can be seen that in a single ozonation process, RR 195 suffered a quick degradation achieving up to nearly 100%, but only a low mineralization degree was achieved with about 62% of final COD removal after 30 min. This may be due to the strong electrophilic nature of ozone molecule that reacts directly with nucleophilic positions of aromatic rings. Nevertheless, the accumulation of different refractory intermediates during the ozonation process leads to a low level of mineralization [25]. It was also found that Co/Al2O3-CeO2 shows higher catalytic activity for efficient mineralization of RR 195 than that of Co/Al2O3. As previously reported [26,27], cerium oxide prepared from Ce(III) salt aqueous solutions can result in CeO2 containing traces of Ce(III), which are thought to be necessary for promoting the decomposition of ozone into hydroxyl radicals by redox reactions on the catalyst surface. Another role that CeO2 played is to improve metal dispersion, which is believed to increase the active sites of the catalyst [16,26]. Due to these two reasons, in this case, the catalytic activity of the Co/Al2O3-CeO2 catalyst is higher than that of the Co/Al2O3, thus leading to a higher mineralization of RR 195.
3.3. Effect of Initial RR 195 Concentrations
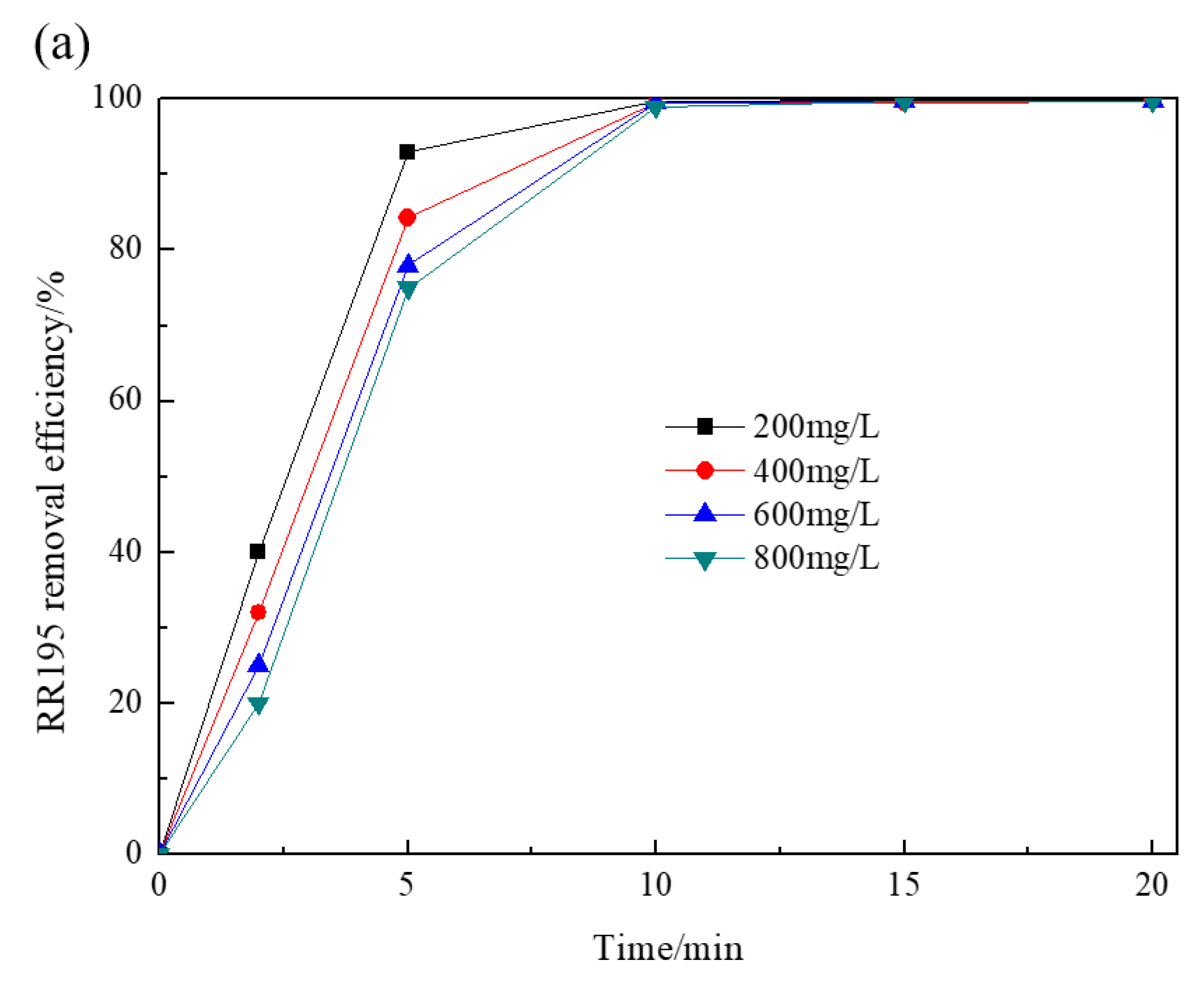
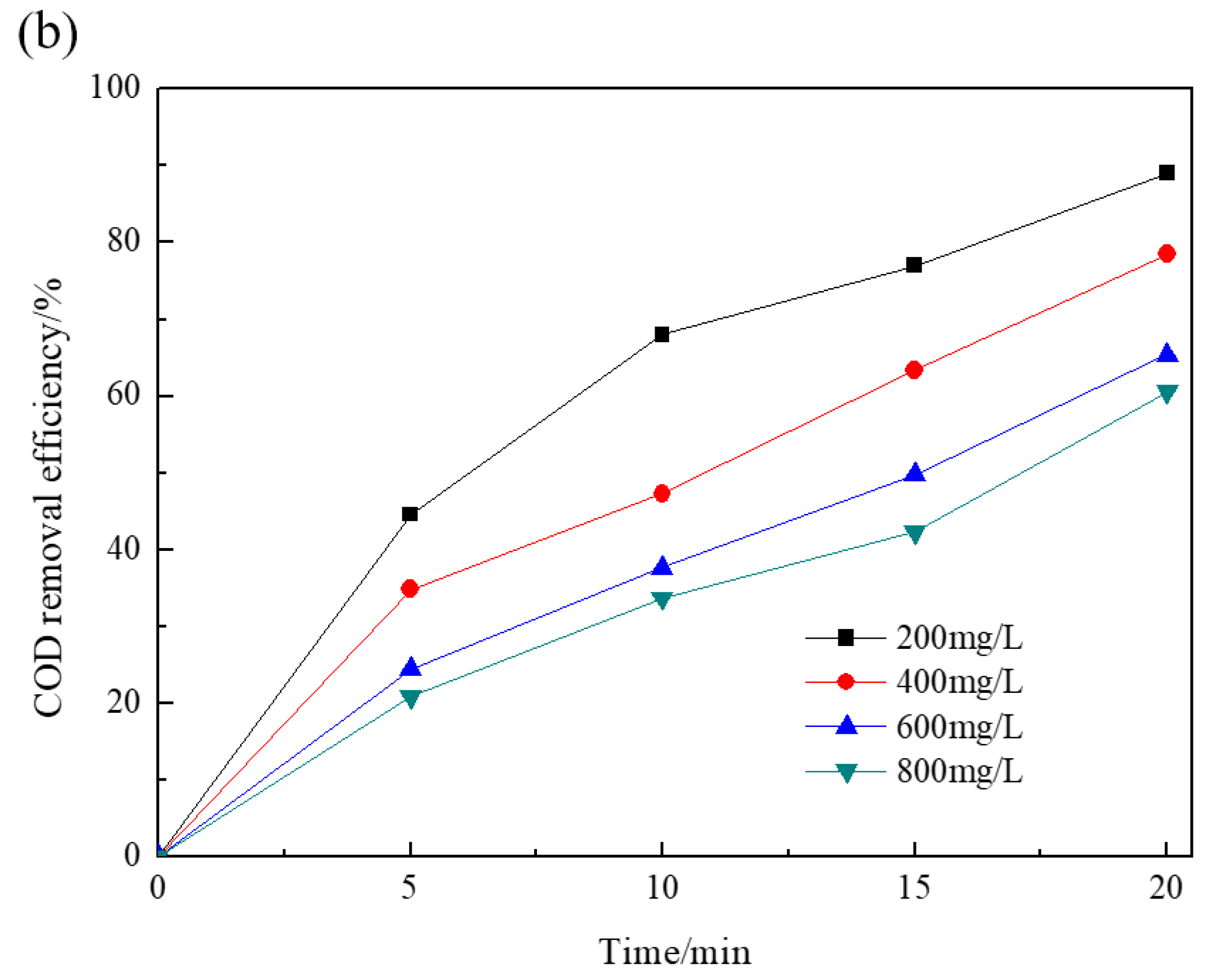
Figure 3 depicts the effect of initial RR195 concentrations on RR195 and COD removal by the Co/Al2O3-CeO2 catalytic ozonation process. It was observed that, after 5 min, RR195 removal efficiencies were 93%, 84.3%, 78%, and 75% when initial RR195 concentrations were 200, 400, 600 and 800 mg/L, respectively, indicating that the removal efficiencies decreased with increasing RR195 initial concentrations. However, after 20 min, the RR195 removal efficiencies were all close to 100%. In the case of COD removal efficiencies, the values were 89%, 78.5%, 65.4%, and 60.6% after 20 min with initial RR195 concentrations of 200, 400, 600, and 800 mg/L, respectively. With increasing RR195 concentrations, COD removal efficiency decreased more significantly than that of RR195 removal efficiency. These findings show that the dye’s concentration has no significant influence on RR195 removal within the tested range. However, it significantly affects COD removal efficiency. This may be because of a greater affinity of ozone with dye compounds than with their by-products, and thus, more organic intermediates may generate and accumulate in the reactor with increasing initial dye concentrations, which contributes to COD measurement other than RR195 measurement [28].
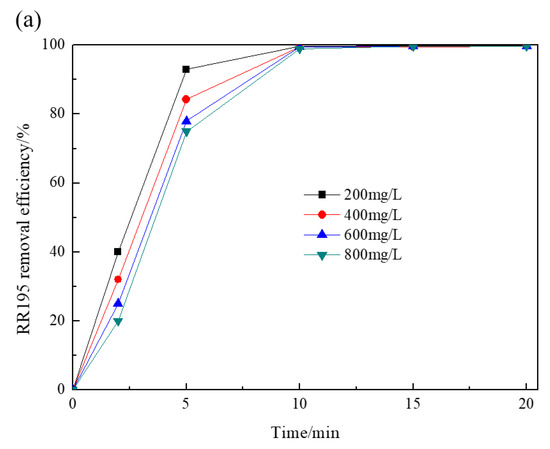
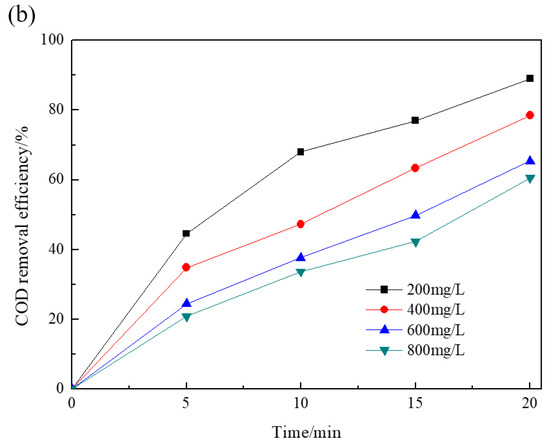
Figure 3.
Effect of initial RR195 concentrations on RR195 removal in the catalytic ozonation: (a) RR195 removal efficiency; (b) COD removal efficiency. (pH 7, gas flow 200 mL/min, ozone flow 6 mg/min, catalyst dosage 3 g/L, magnetic stirring speed 1400 rpm).
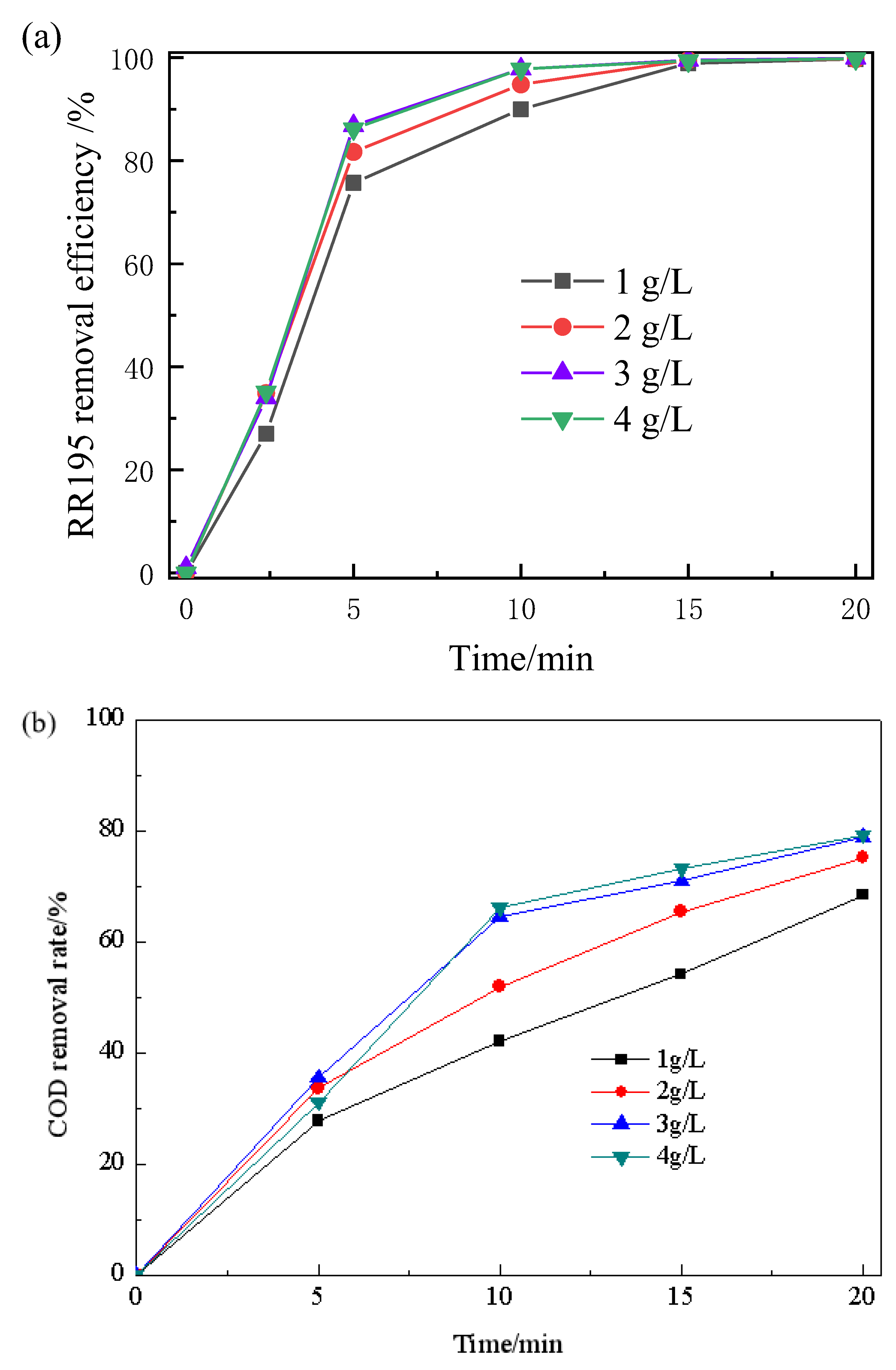
3.4. Effect of Catalyst Dosage
The catalytic ozonation of RR195 in the presence of various dosages of the Co/Al2O3-CeO2 catalysts (ranging between 1 g/L and 4 g/L) was evaluated. As shown in Figure 4, within 20 min of the reaction, COD removal efficiency of RR195 increased gradually, from 68.4% to 75.2%, 78.9%, and 79.2%, with increasing catalyst edge from 1 to 2, 3, and 4 g/L, respectively. These results further illustrate the effectiveness of Co/Al2O3-CeO2 as a catalyst in catalyzed ozonation. Moreover, as previously reported [29], increasing the catalyst loading could improve the organic compound removal efficiency. The results indicate that some other more effective oxidant (such as hydroxyl radical) may be generated when catalysts are used in the ozonation process. In addition, it was found that the removal efficiency increased faster at lower dosages (from 1 to 3 g/L) than at higher dosages (from 3 to 4 g/L). This phenomenon is possibly because, at higher catalyst concentrations, more hydroxyl radicals are produced, and more hydroxyl radicals will combine with each other [29]. Therefore, 3 g/L Co/Al2O3-CeO2 was determined as the optimum dosage for RR195 ozonation in the present work and was used in all remaining experiments.
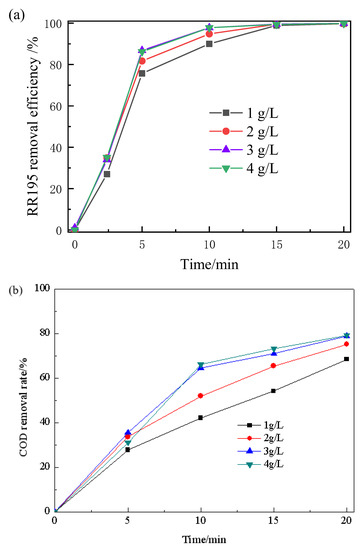
Figure 4.
Effect of Co/Al2O3-CeO2 dosage on RR195 removal in the catalytic ozonation: (a) RR195 removal efficiency; (b) COD removal rate. (pH 7, gas flow 200 mL/min, ozone flow 6 mg/min, RR195 concentration 400 mg/L, magnetic stirring speed 1400 rpm).
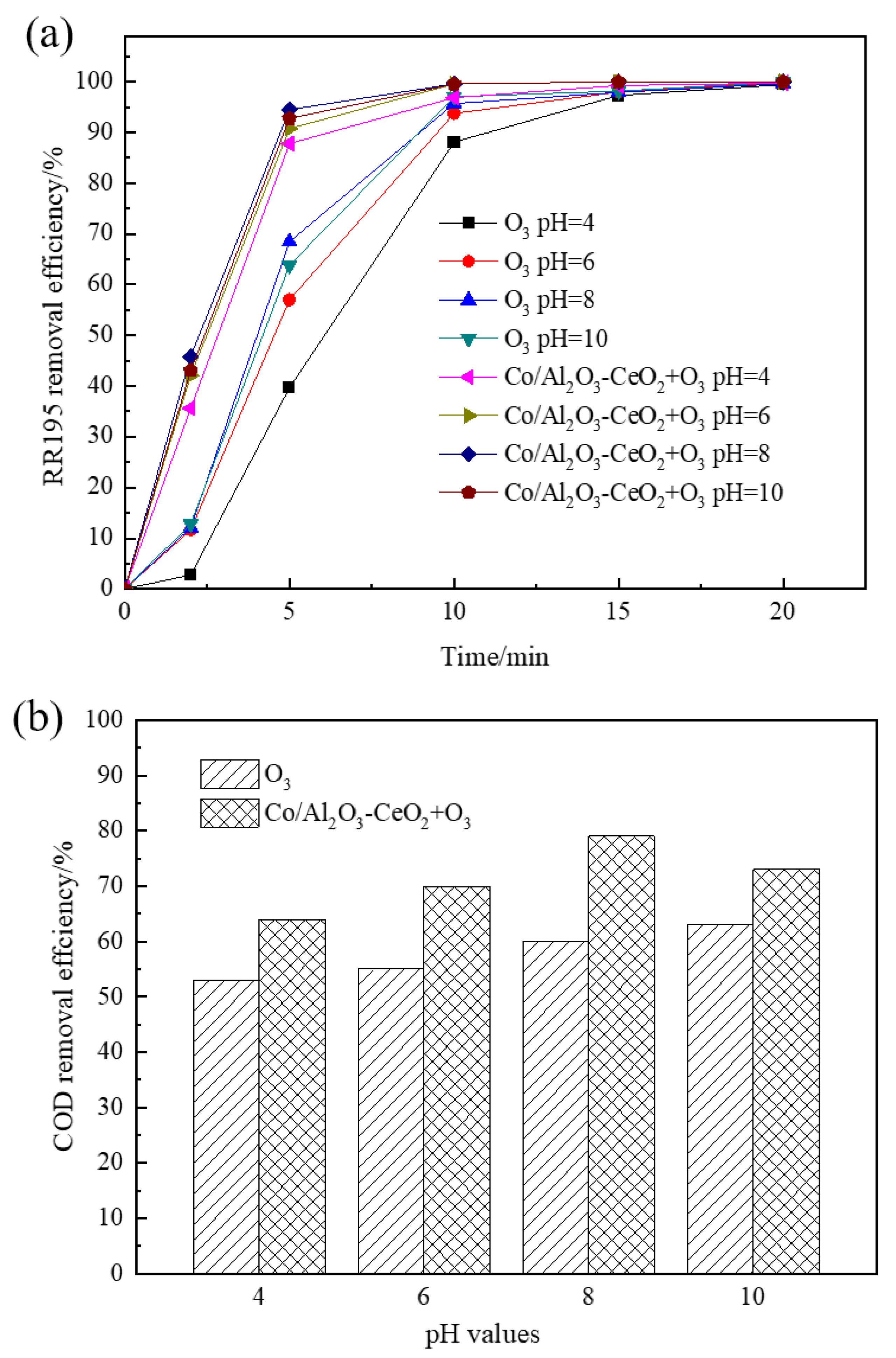
3.5. Effect of pH
The effect of solution pH on COD removal of RR195 was studied with pH ranging from 4 to 10. In a single ozonation process, it can be seen from Figure 5 that increasing the initial pH from acidity to basicity led to an enhancement in RR195 and COD removal. After 20 min, the RR195 removal efficiencies were nearly 100% for all pH. However, COD removal efficiencies were 53%, 55%, 60%, and 63% at pH 4, 6, 8, and 10, respectively. COD removal efficiency increases as pH increases from 4 to 10, and the reasons are as follows: Under acidic or neutral conditions, ozone directly attacks organic matter by its molecular form, and the molecular ozone has a high selectivity, which only reacts with the unsaturated aromatic compounds or some certain special groups. However, in the case of alkalinity condition, ozone produces hydroxyl radicals (•OH), which has a stronger oxidation ability and enhances COD removal efficiency [3].
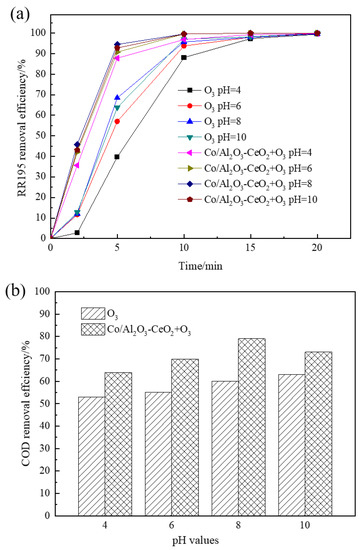
Figure 5.
Effect of solution pH on removal of RR195 (a) and COD (b) in the single ozonation and catalytic ozonation (gas flow 200 mL/min, ozone flow 6 mg/min, catalyst dosage 3 g/L, RR195 concentration 400 mg/L, magnetic stirring speed 1400 rpm).
Under catalytic ozonation, the RR195 and the COD removal efficiency increased when pH increased from 4 to 8 and then decreased with a further increase to 10. Due to the acceleration of the ozone decomposition rate with increasing pH from 4 to 8, RR 195 and COD removal efficiency improved. This, in turn, leads to the formation of the highly reactive radical of •OH [30], which is beneficial to catalytic ozonation. Other research also found that a higher pH resulted in the formation of stronger oxidative radicals of •OH [31]. •OH are mainly generated at alkalinity conditions (pH above 10). Thus, in the single ozonation process, the COD and RR195 removal efficiency reaching the highest value at pH 10 can be explained by the formation of radical species other than hydroxyls. However, under catalytic ozonation, the maximum COD and RR195 removal obtained at pH of 8 may be explained by the fact that the Co/Al2O3-CeO2 can reduce the optimum pH for hydroxyl radical generation. It also can be further inferred that the Co/Al2O3-CeO2 effectively catalyzes ozone decomposition and accelerates oxidative radical formation, resulting in an increased oxidation rate.
It has been reported that the enhancement of catalytic ozonation involves the adsorption of ozone or pollutant or both of them on the catalyst surface, leading to the formation of free radicals, which react with non-adsorbed species in the bulk liquid. Therefore, the surface characteristics of the catalyst play an important role in the oxidation [32]. Solution pH is one of the most important factors affecting oxide surface properties. According to previous studies, the hydroxyl group on the surface of the metal oxides is thought to be the active site providing the catalytic effect and has zero charge when the solution pH is close to the pHpzc of the catalyst. The surface hydroxyl group with zero charge may be the site for catalytic ozone decomposition and for catalytic ozonation of organic compounds [33]. It can be seen that, in the catalytic ozonation process, the COD removal efficiency reached a maximum value when pH was 8, which is very close to the pHzpc of the Co/Al2O3-CeO2 (about 8.45, seen in Figure S2). At that point, the hydroxyl group on the Co/Al2O3-CeO2 surface with zero charge is conducive to catalytic ozone decomposition and catalytic ozonation of RR195. When increasing pH to 10, the strong alkaline condition may affect the density of the surface hydroxyl groups, which causes the loss of the catalytic activity [33]. Therefore, in the catalytic ozonation process, the highest color, and COD removal efficiencies of RR195 are reached at pH 8.
3.6. Stability of the Catalyst
From a practical point of view, an important characteristic of a catalyst is its deactivation or potential reuse. To evaluate the stability of the Co/Al2O3-CeO2 catalyst, it was reused four times without any modifications after the catalyzed ozonation. The COD removal efficiency was determined after each experiment, and the results are shown in Figure S3. In the four series of recycling, the COD removal efficiencies of RR195 were 78.4%, 75.3%, 75%, and 74.8%, respectively. The catalytic activity decreases slightly, which demonstrates that the Co/Al2O3-CeO2 catalyst is effective and stable in the catalytic ozonation of RR195.
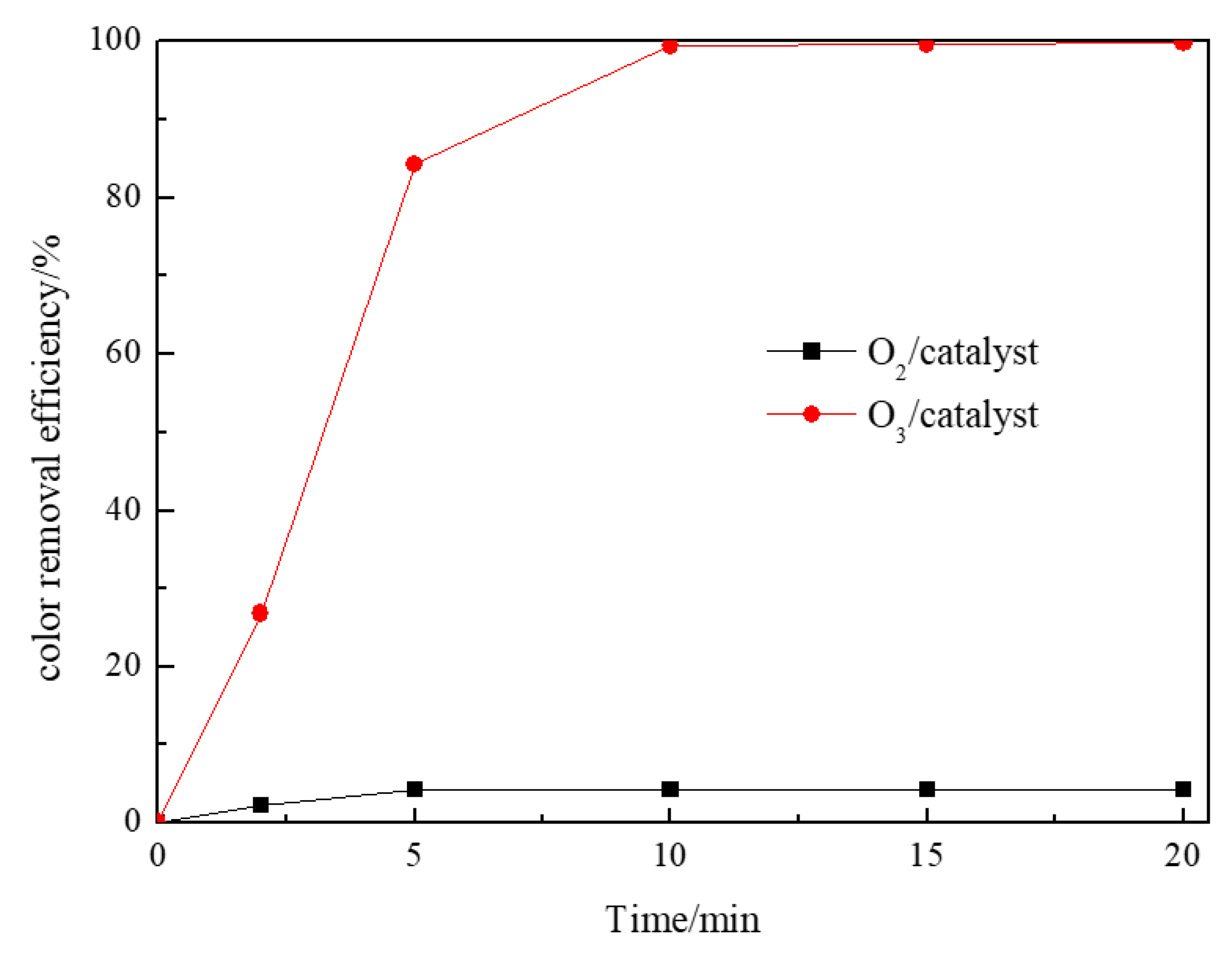
3.7. Mechanism of RR195 Degradation
According to Wang and Chen [34], the adsorption of organic molecule on the catalyst is critical for catalytic ozonation. Yuan et al. [35] also found that the adsorption of organics onto the catalyst was an important step which would have a direct influence on the effectiveness of the heterogeneous catalytic oxidation. Therefore, the adsorption capacity of the catalyst needs to be analyzed. Figure 6 shows the adsorption of RR195 on the Co/Al2O3-CeO2 catalyst at pH 8. From Figure 6, it can be seen that, after 20 min, 4.35% of the RR 195 was adsorbed by the Co/Al2O3-CeO2. However, the removal efficiency of RR 195 was 99.8% by catalytic ozonation. This result indicates that adsorption of RR195 on the catalyst’s surface occurs, even though it is not obvious when compared with the removal efficiency by the catalytic ozonation process. From the discussion above, it can be assumed that the mechanisms of catalytic ozonation by Co/Al2O3-CeO2 may involve two steps. One is the adsorption of ozone over the catalyst with the formation of hydroxyl radicals, which will react with the pollutants. The other is the adsorption of the organic pollutant and reaction with ozone molecule (aqueous or gaseous) or adsorption of both reactants with further surface reaction.
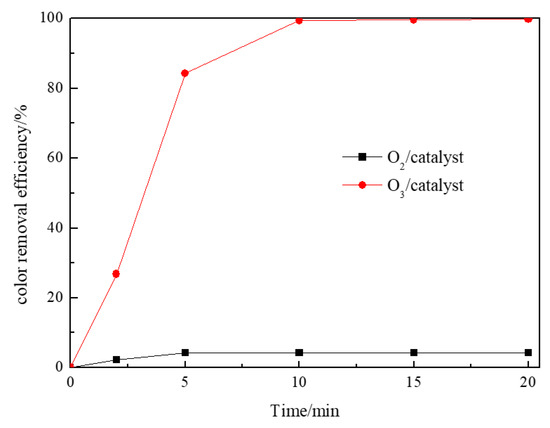
Figure 6.
The color removal efficiency of Co/Al2O3-CeO2 adsorption (oxygen gas flow 200 mL/min, RR195 concentration 400 mg/L, magnetic stirring speed 1400 rpm, pH 8) and Co/Al2O3-CeO2 catalytic ozonation processes (gas flow 200 mL/min, ozone flow 6 mg/min, catalyst dosage 3 g/L, RR195 concentration 400 mg/L, magnetic stirring speed 1400 rpm, pH 8).
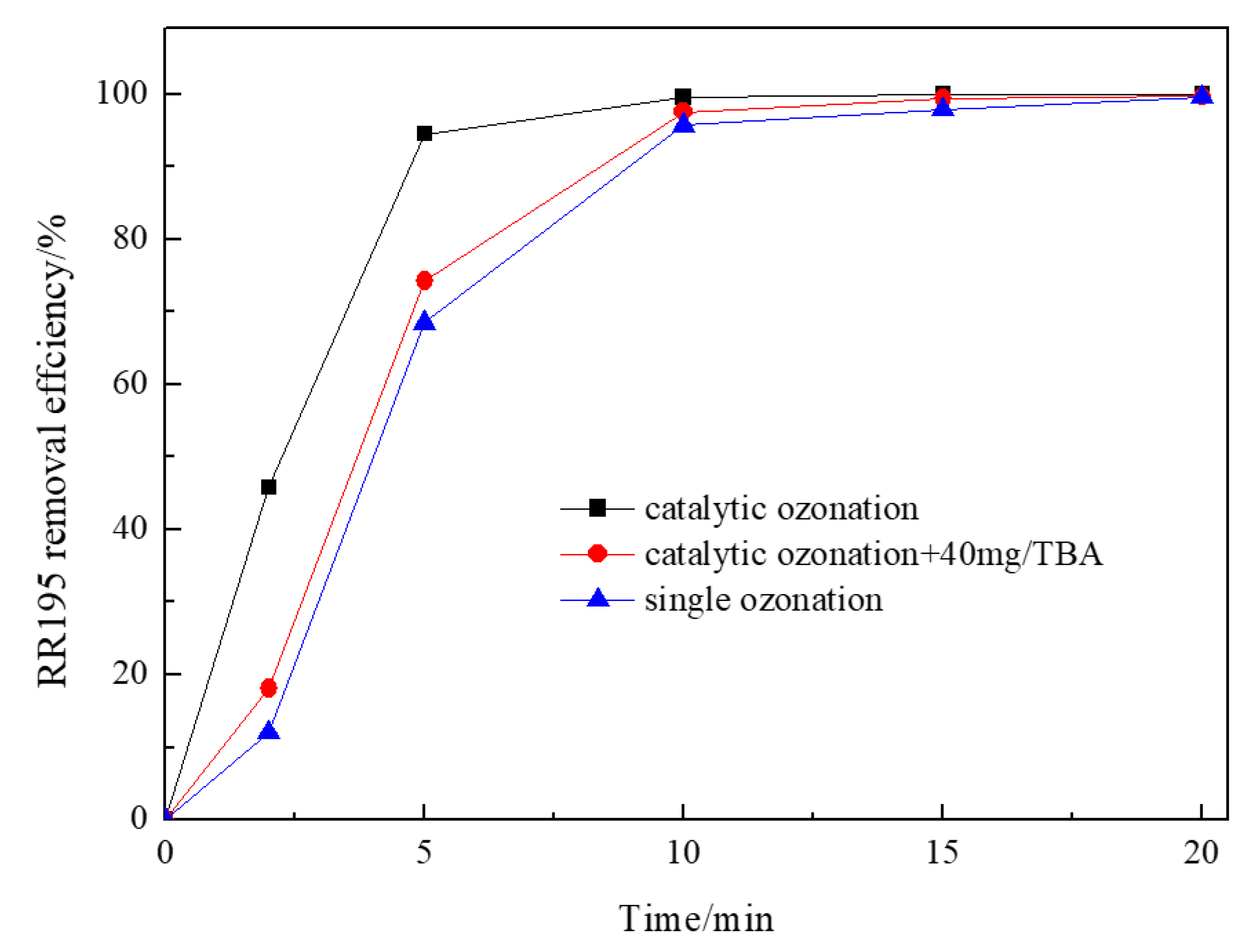
In order to verify whether the generation of hydroxyl radicals is mainly responsible for the improvement of catalytic ozonation, the influence of radical scavengers was investigated. Tert-butanol (TBA), an organic radical scavenger, was used to indirectly evidence indirectly the transformation of ozone into hydroxyl radicals in Co/Al2O3-CeO2/O3 process. It has the reaction rate constants of 6 × 108 M−1S−1 with hydroxyl radicals and 3 × 10−3 M−1S−1 with ozone, in addition, it cannot be adsorbed on the surface of the catalyst because of its physical–chemical properties [36]. Figure 7 shows the influence of TBA on the degradation of RR195 in catalytic ozonation with Co/Al2O3-CeO2. As illustrated in Figure 7, the presence of TBA inhibited the degradation of RR195 in the Co/Al2O3-CeO2 catalyzed ozonation process, indicating that hydroxyl radicals are formed during the process of Co/Al2O3-CeO2 catalyzed ozonation. The generation of hydroxyl radicals in the Co/Al2O3-CeO2 catalyzed ozonation process may be because the presence of a heterogeneous surface increases the dissolution of ozone and acts as an initiator of the ozone decomposition reaction in the aqueous phase [36]. In addition, a previous study also found that the interaction of ozone with the metal oxide surface results in the formation of free radicals, which can initiate a radical chain-type reaction both on the surface of the catalyst and in the liquid phase [37], the hydroxyl radicals may also be produced through this reaction. However, the removal efficiency of the catalytic ozonation in the presence of TBA was still higher than that of the single ozonation, which implies the existence of another reaction pathway: O3 directly oxidation of RR 195 after both of them are adsorbed on the catalyst.
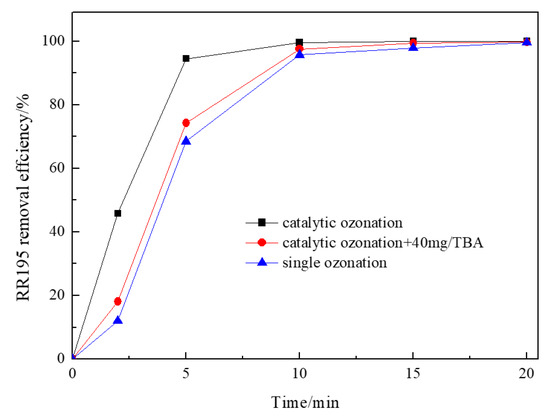
Figure 7.
Effect of tert-butanol on ozonation and Co/Al2O3-CeO2 catalytic ozonation processes (gas flow 200 mL/min, ozone flow 6 mg/min, catalyst dosage 3 g/L, RR195 concentration 400 mg/L, magnetic stirring speed 1400 rpm, pH 8).
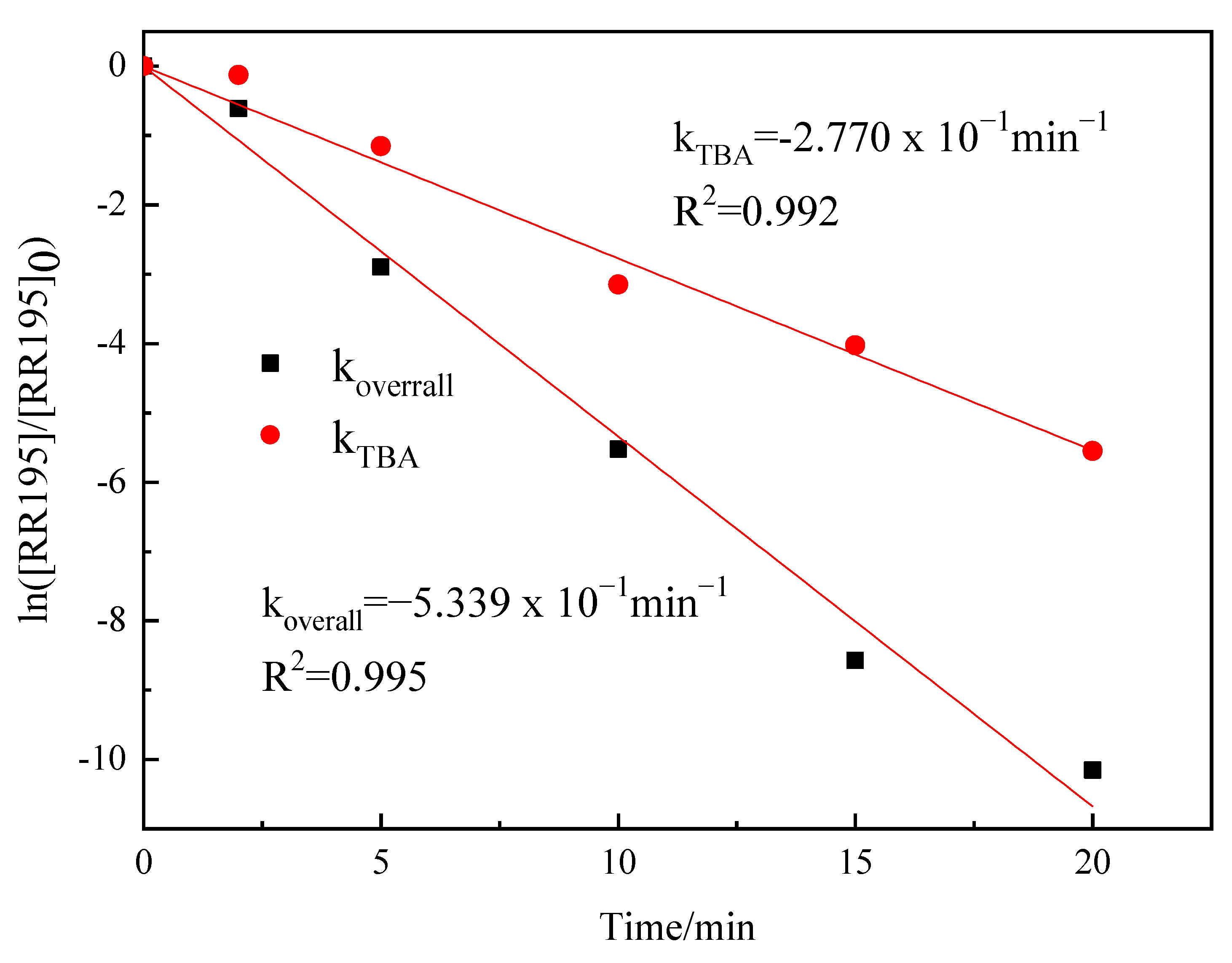
The catalytic ozonation of organic compounds involves a number of complex reactions. From the discussion above, it can be assumed that, in Co/Al2O3-CeO2 catalytic ozonation, both surface and liquid bulk reactions occur, involving ozone and •OH radicals. In order to fractionate the contribution of the ozone molecule (δO3) and hydroxyl radicals (δ·OH) in the degradation of RR195, kinetic constants were studied and compared between the catalytic ozonation with and without the presence of TBA. This can be described by a simplified and unbalanced reaction mechanism as follows [38]:
Homogeneous reactions:
Heterogeneous reactions:
where k1 and k2 represent the rate constants of RR195 in homogeneous reaction with molecular ozone and •OH, respectively, M−1min−1, k3 represents the adsorption reaction constant between RR195 and the catalyst surface, M−1min−1, k4 and k5 represent the rate constants of RR195 in heterogeneous reaction with molecular ozone and •OH, respectively, M−1min−1.
Then, the overall RR195 ozonation rate in the presence of Co/Al2O3-CeO2 could be expressed as a sum of (1)–(5):
where the reactions of the ozonation and catalytic ozonation are expressed with an apparent first-order kinetics constant koverall: , M−1min−1.
According to Equation (6), koverall can be obtained from the slope of ln([RR195]/[RR195]0) vs. reaction time. When enough inhibitors ([TBA] = 50 mg/L in this study) are introduced into the catalytic reaction system, the reaction referring to the •OH oxidation (indirect oxidation) will be terminated. The reaction kinetic after the addition of TBA to the catalytic process can be described in the following equation:
The fraction of •OH (δ OH) contributing to the depletion of RR195 can be quantitatively determined by Equation (8):
koverall and kTBA were calculated by the plots of ln([RR195]/[RR195]0) versus reaction time (Figure 8). kTBA was found to be 0.277 min−1, and koverall was 0.5339 min−1, which makes δ OH = 48.7%, indicating that •OH play an important role in the oxidation.
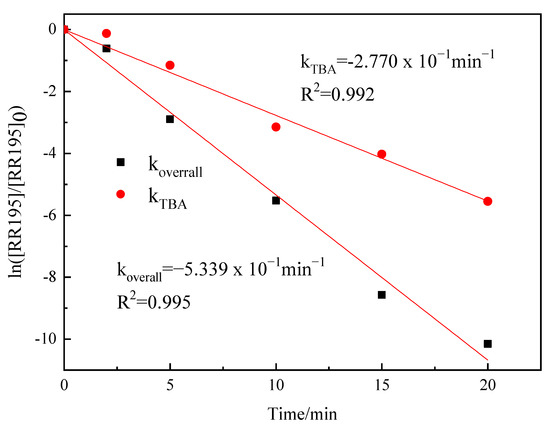
Figure 8.
Pseudo-first-order plots of RR195 by catalytic ozonation and catalytic ozonation + TBA (gas flow 200 mL/min, ozone flow 6 mg/min, catalyst dosage 3 g/L, RR195 concentration 400 mg/L, magnetic stirring speed 1400 rpm, pH 8).
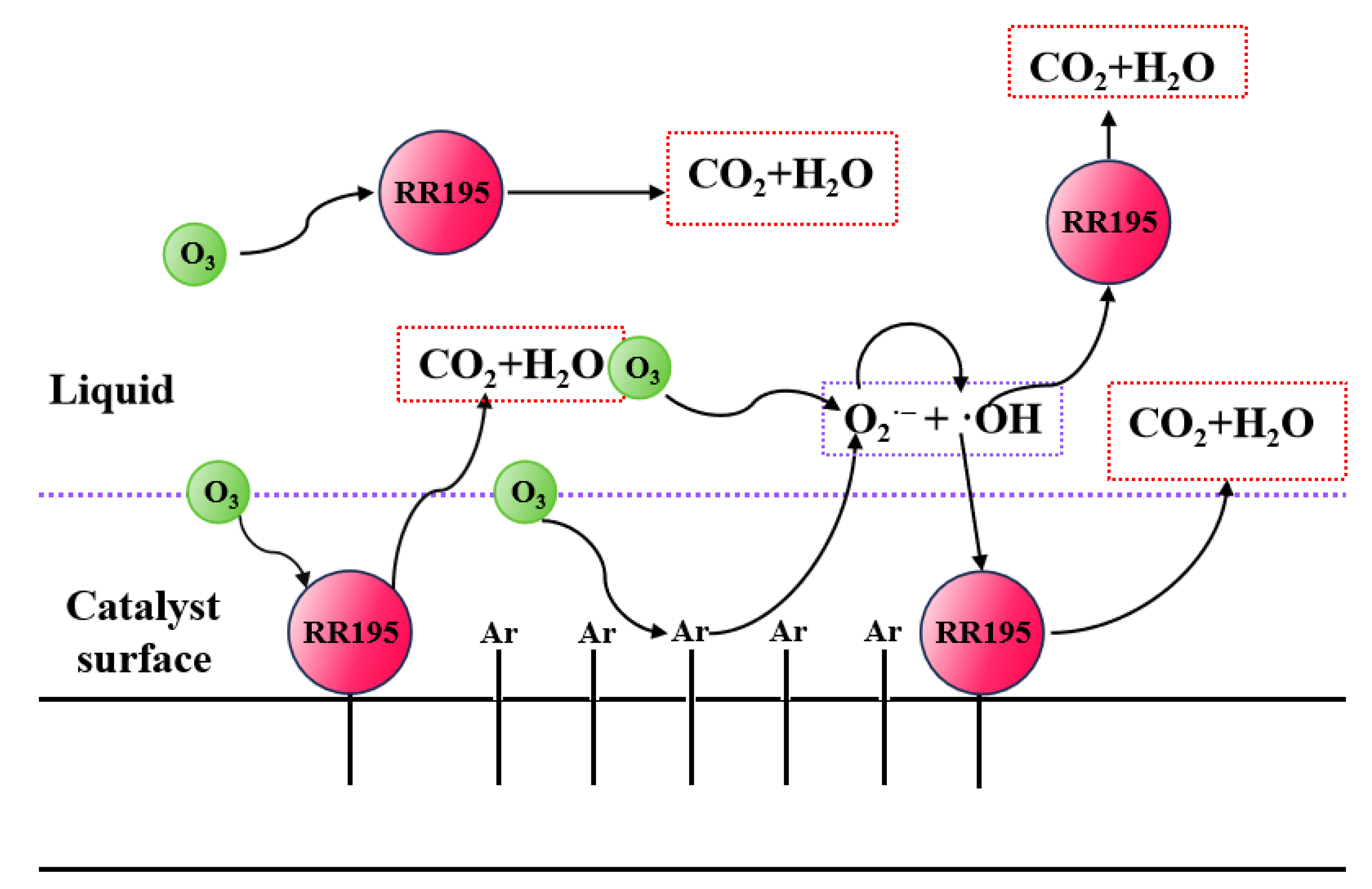
From the above experiments, it can be found that the ozonation of RR195 catalyzed by cerium oxide is almost completely inhibited in the presence of TBA, which confirms that hydroxyl radicals play an important role in the reaction mechanism. However, adsorption of RR195 on the catalysts was observed, and it can be accepted as one of the reaction mechanism steps. The possible mechanism of catalytic ozonation includes an indirect oxidative reaction by hydroxyl radicals and a direct oxidation reaction by O3 after the ozone and RR195 adsorbed on the surface of the catalyst (Figure 9).
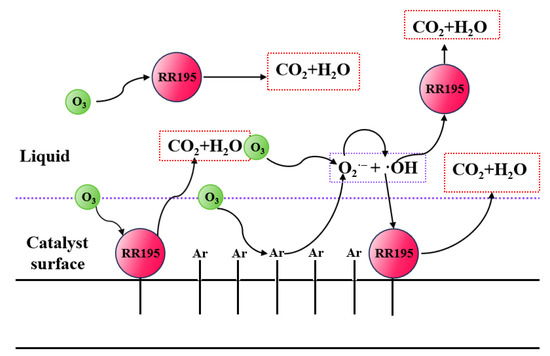
Figure 9.
Mechanism of catalytic ozonation RR195.
4. Conclusions
This study shows that the Co/Al2O3-CeO2 catalyst is efficient in the catalytic ozonation of RR 195 solution, and it accelerates the color and COD removal more in comparison with single ozonation. The optimum pH was determined to be 8, and the optimum catalyst dosage was 3 g/L. The RR 195 removal efficiency decreased with increasing RR 195 concentrations from 200 to 800 mg/L. The Co/Al2O3-CeO2 catalyst had good stability after four successive recycles. The amount of RR 195 adsorbed by Co/Al2O3-CeO2, which was negligible compared with the removal rate of the catalytic ozonation process. The presence of TBA was shown to inhibit the degradation of RR 195 by Co/Al2O3-CeO2 catalytic ozonation. The experimental results indicate that the possible mechanism of catalytic ozonation includes an indirect oxidative reaction by hydroxyl radicals and a direct oxidation reaction after the ozone and RR195 adsorbed on the surface of the catalyst.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr11072141/s1, Figure S1. The particle size distribution of the catalyst. Figure S2. The pHPZC value of catalyst. Figure S3. The effect of catalyst recycle times on the ozonation of RR 195. (RR 195 concentration 400 mg/L, catalyst dosage 3 g/L, gas flow 200 mL/min, ozone flow 15 mg/min, pH 8, reaction time 20 min).
Author Contributions
Methodology, validation, writing-original draft, formal analysis, B.-H.L.; background investigation and experimental analysis, X.-Y.L.; supervision, funding acquisition, review & editing, Z.-M.Z.; software operation guidance, G.-H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the MOE Key Laboratory of Resources and Environmental System Optimization (KLRE-KF202205), and the Youth Innovation Grant of Xiamen, Fujian Province (Nos. 3502Z20206006) and the cooperate program (20221HH493).
Data Availability Statement
Not applicable.
Acknowledgments
We also thank the Instrumental Analysis Center of Huaqiao University for analysis support.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Azanaw, A.; Birlie, B.; Teshome, B.; Jemberie, M. Textile effluent treatment methods and eco-friendly resolution of textile wastewater. Case Stud. Chem. Environ. Eng. 2022, 6, 100230. [Google Scholar] [CrossRef]
- Adane, T.; Adugna, A.T.; Alemayehu, E. Textile industry effluent treatment techniques. J. Chem. 2021, 2021, 5314404. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Rai, B.N.; Singh, R.S.; Jaiswal, R.P. A comprehensive review on the integration of advanced oxidation processes with biodegradation for the treatment of textile wastewater containing azo dyes. Rev. Chem. Eng. 2022, 38, 617–639. [Google Scholar] [CrossRef]
- Verma, R.K.; Sankhla, M.S.; Rathod, N.V.; Sonone, S.S.; Parihar, K.; Singh, G.K. Eradication of fatal textile industrial dyes by wastewater treatment. Biointerface Res. Appl. Chem. 2021, 12, 567–587. [Google Scholar]
- Eren, H.A.; Yiğit, İ.; Eren, S.; Avinc, O. Ozone: An alternative oxidant for textile applications. In Sustainability in the Textile and Apparel Industries: Production Process Sustainability; Springer: Cham, Switzerland, 2020; pp. 81–98. [Google Scholar]
- Epelle, E.I.; Macfarlane, A.; Cusack, M.; Burns, A.; Okolie, J.A.; Mackay, W.; Rateb, M.; Yaseen, M. Ozone application in different industries: A review of recent developments. Chem. Eng. J. 2023, 454, 140188. [Google Scholar] [CrossRef]
- Li, X.; Fu, L.; Chen, F.; Zhao, S.; Zhu, J.; Yin, C. Application of heterogeneous catalytic ozonation in wastewater treatment: An overview. Catalysts 2023, 13, 342. [Google Scholar] [CrossRef]
- Huang, R.; Lan, B.; Chen, Z.; Yan, H.; Zhang, Q.; Bing, J.; Li, L. Catalytic ozonation of p-chlorobenzoic acid over MCM-41 and Fe loaded MCM-41. Chem. Eng. J. 2012, 180, 19–24. [Google Scholar] [CrossRef]
- Li, R.; Xiong, J.; Zhang, Y.; Wang, S.; Zhu, H.; Lu, L. Catalytic Ozonation of Norfloxacin Using Co-Mn/CeO2 as a Multi-Component Composite Catalyst. Catalysts 2022, 12, 1606. [Google Scholar] [CrossRef]
- Rodríguez, J.L.; Valenzuela, M.A. Ni-based catalysts used in heterogeneous catalytic ozonation for organic pollutant degradation: A minireview. Environ. Sci. Pollut. Res. 2022, 29, 84056–84075. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Sagawa, E.; Nakayama, A.; Nakagawa, Y.; Tomishige, K. Hydrogen Atom Abstraction by Heterogeneous–Homogeneous Hybrid Catalyst of CeO2 and 2-Cyanopyridine via Redox of CeO2 for C–H Bond Oxidation with Air. ACS Catal. 2021, 11, 11867–11872. [Google Scholar] [CrossRef]
- Li, Z.; Ma, C.; Qi, M.; Li, Y.; Qu, Y.; Zhang, Y.; Zhou, L.; Yun, J. CeO2 from pyrolysis of MOFs for efficient catalytic combustion of VOCs. Mol. Catal. 2023, 535, 112857. [Google Scholar] [CrossRef]
- Raza, J.; Khoja, A.H.; Naqvi, S.R.; Mehran, M.T.; Shakir, S.; Liaquat, R.; Tahir, M.; Ali, G. Methane decomposition for hydrogen production over biomass fly ash-based CeO2 nanowires promoted cobalt catalyst. J. Environ. Chem. Eng. 2021, 9, 105816. [Google Scholar] [CrossRef]
- Chen, X.; Yang, H.; Au, C.; Tian, S.; Xiong, Y.; Chang, Y. Efficiency and mechanism of pollutant degradation and bromate inhibition by faceted CeO2 catalyzed ozonation: Experimental and theoretical study. Chem. Eng. J. 2020, 390, 124480. [Google Scholar] [CrossRef]
- Guzmán, I.C.; Rodríguez, J.L.; Poznyak, T.; Chairez, I.; Hernández, I.; Hernández, R.T. Catalytic ozonation of 4-chlorophenol and 4-phenolsulfonic acid by CeO2 films. Catal. Commun. 2020, 133, 105827. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Palcheva, R.; Karakirova, Y.; Sanchez, M.C.; Tyuliev, G.; Gaigneaux, E.; Fierro, J.L. Structure and surface properties of ceria-modified Ni-based catalysts for hydrogen production. Appl. Catal. B Environ. 2018, 225, 340–353. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Yang, Y.; Yu, D.; Lin, J.; Wan, R.; Zhu, H. Remarkably efficient Pt/CeO2–Al2O3 catalyst for catalytic hydrodeiodination of monoiodoacetic acid: Synergistic effect of Al2O3 and CeO2. Chemosphere 2023, 327, 138515. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Li, N.; Luo, M.-F.; Lu, J.-Q. Catalytic oxidation of dichloromethane over Pt/CeO2–Al2O3 catalysts. Appl. Catal. B Environ. 2012, 127, 159–166. [Google Scholar] [CrossRef]
- Li, F.; Zhao, B.; Tan, Y.; Chen, W.; Tian, M. Preparation of Al2O3–CeO2 by Hydrothermal Method Supporting Copper Oxide for the Catalytic Oxidation of CO and C3H8. Ind. Eng. Chem. Res. 2022, 61, 4739–4751. [Google Scholar] [CrossRef]
- Zhou, B.; Ke, Q.; Wen, M.; Ying, T.; Cui, G.; Zhou, Y.; Gu, Z.; Lu, H. Catalytic combustion of toluene on Pt/Al2O3 and Pd/Al2O3 catalysts with CeO2, CeO2–Y2O3 and La2O3 as coatings. J. Rare Earths, 2022; in press. [Google Scholar]
- Ji, Y.; Zhao, Z.; Duan, A.; Jiang, G.; Liu, J. Comparative study on the formation and reduction of bulk and Al2O3-supported cobalt oxides by H2-TPR technique. J. Phys. Chem. C 2009, 113, 7186–7199. [Google Scholar] [CrossRef]
- Şayan, E. Ultrasound-assisted preparation of activated carbon from alkaline impregnated hazelnut shell: An optimization study on removal of Cu2+ from aqueous solution. Chem. Eng. J. 2006, 115, 213–218. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Z.; Jing, G.; Fang, J. Catalytic ozonation of Acid Red B in aqueous solution over a Fe–Cu–O catalyst. Sep. Purif. Technol. 2013, 115, 129–135. [Google Scholar] [CrossRef]
- Birdsall, C.M.; Jenkins, A.C.; Spadinger, E. Iodometric determination of ozone. Anal. Chem. 1952, 24, 662–664. [Google Scholar] [CrossRef]
- Hu, J.; Lim, F.Y.; Hu, J. Ozonation facilitates the aging and mineralization of polyethylene microplastics from water: Behavior, mechanisms, and pathways. Sci. Total Environ. 2023, 866, 161290. [Google Scholar] [CrossRef]
- Li, Y.; Wu, L.; Wang, Y.; Ke, P.; Xu, J.; Guan, B. γ-Al2O3 doped with cerium to enhance electron transfer in catalytic ozonation of phenol. J. Water Process Eng. 2020, 36, 101313. [Google Scholar] [CrossRef]
- Ershov, B.G.; Shilov, B.P. Reactions of ozone and intermediate products of its decomposition with actinides, lanthanides and transition metals in aqueous solutions. Radiochim. Acta 2021, 109, 583–601. [Google Scholar] [CrossRef]
- Bilińska, L.; Gmurek, M. Novel trends in AOPs for textile wastewater treatment. Enhanced dye by-products removal by catalytic and synergistic actions. Water Resour. Ind. 2021, 26, 100160. [Google Scholar] [CrossRef]
- Fu, X.; Huang, Y.; Wang, Y.; Liang, M.; Yang, Y.; Jin, Z.; Yang, J.; Hu, S.; Li, L. Ozonation catalyzed by CoxFe1 layered double hydroxide for the degradation of P-toluenesulfonic acid. Ozone Sci. Eng. 2021, 43, 163–172. [Google Scholar] [CrossRef]
- Mu, J.; Li, S.; Wang, J.; Li, X.; Chen, W.; Tong, X.; Tang, Y.; Li, L. Efficient catalytic ozonation of bisphenol A by three-dimensional mesoporous CeOx-loaded SBA-16. Chemosphere 2021, 278, 130412. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, S.; Lin, X.; Ganiyu, S.O.; Kamaraj, S.-K.; Thiam, A.; Liu, D. Insight into BDD electrochemical oxidation of florfenicol in water: Kinetics, reaction mechanism, and toxicity. Chemosphere 2022, 288, 132433. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zhai, Y.; Zhang, L.; Xiang, L.; Lin, F. Low-Temperature Catalytic Ozonation of Multitype VOCs over Zeolite-Supported Catalysts. Int. J. Environ. Res. Public Health 2022, 19, 14515. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Bao, H.; Su, W.; Jiang, Y.; Tong, S. Manganese containing oxides catalytic ozonation in aqueous solution: Catalytic mechanism on acid sites. Sep. Purif. Technol. 2022, 282, 120053. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Y.; Guo, Y.; Wang, Z. Enhanced degradation of dimethyl phthalate in wastewater via heterogeneous catalytic ozonation process: Performances and mechanisms. RSC Adv. 2022, 12, 31024–31031. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Lv, Y.; Wei, J.; Zhang, J.; Song, Y. Efficient removal of sulfamethazine by a magnetic recoverable CeO2/Fe3O4/natural zeolite catalyst in catalytic ozonation process. Front. Environ. Sci. 2023, 11, 317. [Google Scholar] [CrossRef]
- Ma, J.; Cao, R.; Dang, Y.; Wang, J. A recent progress of room–temperature airborne ozone decomposition catalysts. Chin. Chem. Lett. 2021, 32, 2985–2993. [Google Scholar] [CrossRef]
- Shokouhi, S.B.; Dehghanzadeh, R.; Aslani, H.; Shahmahdi, N. Activated carbon catalyzed ozonation (ACCO) of Reactive Blue 194 azo dye in aqueous saline solution: Experimental parameters, kinetic and analysis of activated carbon properties. J. Water Process Eng. 2020, 35, 101188. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).