Abstract
Chronic constipation is a common symptom-based disorder that affects patient quality of life. Electrolyzed hydrogen-rich alkaline reduced water (EHARW) helps treat gastrointestinal disorders owing to its various bioactive properties. This single-arm, open-labelled study aimed to investigate the improvement of EHARW (pH 9.5; H2 ≈ 0.5 mg/L) in chronic constipation patients. Thirty patients with chronic constipation were enrolled after screening as intention-to-treat (ITT). During the intervention period, two patients dropped out, and 28 patients completed the study as per protocol (PP). The selected patients were instructed to drink EHARW (pH 9.5; H2 ≈ 0.5 mg/L) (20 mL/kg body weight/day) generated from a home medical device for four weeks. Complete spontaneous bowel movement (CSBM) frequency was measured as the primary outcome, and Bristol stool form, patient assessment of constipation–symptoms (PAC-SYM) score, and patient assessment of constipation–quality of life (PAC-QOL) score were measured as the secondary outcomes after the 4-week intervention compared to baseline. As a result of EHARW treatment, no adverse events were observed during the study period. Moreover, the frequency of CSBM/week (29.8%, p < 0.05) and Bristol stool form score (24.6%, p < 0.01) significantly increased compared to baseline. Finally, the overall and subscale scores of the PAC-SYM (58.0%) and PAC-QOL (54.2%) questionnaires significantly decreased (p < 0.001). These results suggest that daily ingestion of EHARW (pH 9.5; H2 ≈ 0.5 mg/L) can improve CSBM frequency as a primary outcome in chronic constipation patients. Likewise, EHARW (pH 9.5; H2 ≈ 0.5 mg/L) improved Bristol stool form score, symptoms and the quality of life as a secondary outcome in patients with chronic constipation through a home-based intervention.
1. Introduction
Constipation is a common health problem that medical practitioners and general surgeons encounter regularly. It is characterized by chronically incomplete, problematic, and irregular defecation [1]. Constipation is also a major social problem with a global prevalence rate of 10–15%, as it disturbs the quality of life [2,3]. Common risk factors for constipation include inadequate diet, concurrent diseases, medication, and bowel structure or function disorders [4]. The Rome IV criteria, clinical examination, and self-records of defecation habits can aid in the diagnosis and assessment of constipation severity [5]. In clinical trials, the frequency of bowel movements, stool consistency, and patient-rated symptoms have been used as objective outcomes to assess the effectiveness of interventions in patients with chronic constipation [6]. In addition, abdominal, rectal and stool-associated symptoms, such as feelings of incomplete evacuation, straining during defecation, abdominal discomfort or pain, are associated with chronic constipation, which might result in several significant complications such as anal hemorrhage, anal fissures, and rectal prolapse. These serious complications of long-term constipation can increase morbidity and affect the patient’s quality of life, which can be assessed by measuring the level of physical discomfort, psychosocial discomfort, worries/concern, and level of satisfaction [7,8,9,10,11]. Thus, a questionnaire-based assessment, such as the patient assessment of constipation symptoms (PAC-SYM) and patient assessment of constipation quality of life (PAC-QOL), provides reliable information to monitor the symptoms and QOL related to chronic constipation. The PAC-SYM consists of 3 subscales and 12-questions, whereas PAC-QOL consists of 4 subscales and 28-questions [12,13].
Moreover, depending on the cause of constipation, various therapies are currently available to relieve symptoms and complications: bulk-forming laxatives for fibre-deficient constipation; polyethylene glycol, which draws water into the intestine; stimulant laxatives; anorectal biofeedback therapies; and surgery [14,15]. These therapeutic approaches can alleviate symptoms; however, they have potential adverse effects and complications due to various etiological factors, especially over a long period. Therefore, an increasing demand for effective and well-tolerated chronic constipation interventions exists.
Increasing evidence suggests that hydrogen (H2) is an anti-oxidative, anti-apoptotic, and anti-inflammatory substance with high potential for medical applications, including intestinal disorders [16,17]. Moreover, H2 is regarded as a novel antioxidant, as it can easily penetrate the cell membrane, diffuse into various organelles, and reduce free radicals (i.e., hydroxyl radicals and peroxynitrite) [18,19]. Previous studies on intestinal diseases have demonstrated that H2 may improve gastrointestinal symptoms, such as ulcerative colitis and chronic constipation [20,21,22,23]. The H2 also acts through various mechanistic pathways to rescue from oxidative stress or toxicity, including enhancement of antioxidants, anti-inflammation, signal modulation, and cytoprotection [24]. EHARW exhibits a negative oxidation-reduction potential (ORP) due to its alkaline pH and the dissolved H2 gas, which H2 is responsible for the antioxidant effects [25]. The EHARW is also considered an effective and convenient method for delivering H2 and is suitable for medical applications [19]. Moreover, an alkaline pH and high mineral content help to improve digestive function [26,27]. The Japanese Ministry of Health and Welfare approved using ERW for gastrointestinal (GI) disorders, such as hyperacidity, diarrhea, indigestion, and abnormal gastrointestinal fermentation [28,29,30]. Several GI symptoms can also be significantly improved with ERW [30,31]. The Korean Ministry of Food and Drug Safety guidelines categorize ARW as functional water from a pH of 8.6 to 10.0. Additionally, the ARW generator was authorized as a grade II home medical device, and the Korean and Japanese regulations mandate that alkaline water ionizer pH should not exceed pH 9.8 for drinking [28,29,32,33]. Furthermore, EARW (pH 9.5) has been shown to reduce the symptoms of GI disorders, including indigestion, diarrhea, and dyspepsia [25,34].
Considering the information above on EHARW and GI diseases, we hypothesized that four weeks of EHARW (pH 9.5; H2 ≈ 0.5 mg/L) consumption generated by a home medical device could relieve chronic constipation. The frequency of complete spontaneous bowel movements (CSBM), Bristol stool form scale, PAC-SYM, and PAC-QOL questionnaires were used to investigate the treatment effectiveness.
2. Materials and Methods
2.1. Ethical Approval
This clinical study was approved by the Research Review Board of Yonsei University Wonju Severance Christian Hospital (IRB number: CR222010) in Korea and registered with Clinical Trials.gov under the identifier NCT05734859. All the patients provided written informed consent before participating in the clinical trial. The study protocol was conducted in accordance with the Declaration of Helsinki (October 1996), Good Clinical Practice, and the applicable laws and regulations.
2.2. Study Population
Potential participants (ages 19–70 years) were screened to determine study eligibility, including baseline characteristics (age, height, and weight), vital signs (blood pressure and body temperature), medical history, medication use, and history of self-reported chronic constipation (an average of three or fewer defecations/week over the past 3-months). In addition, the physician confirmed that the patients met two or more of the following symptoms for at least three months: straining during defecation, lumpy or hard stools, the sensation of incomplete evacuation, the sensation of anorectal obstruction, and manual maneuvers to facilitate defecation at least 25% of the time, according to the Rome IV criteria for chronic constipation. The inclusion and exclusion criteria are presented in Table 1. A total of 30 patients were enrolled in this study.

Table 1.
Inclusion and exclusion criteria of patients.
2.3. Study Design
Thirty patients with chronic constipation who met the inclusion criteria were enrolled as intention-to-treat (ITT) based on an eligibility test performed by a physician. Before starting the intervention, a medical device generating EHARW (BTM-1200, Biontech, Gunpo-si, Republic of Korea) was installed in each patient’s house. Patients were instructed by the investigator on how to operate the device and drink the EHARW. The intervention period was four weeks. The primary efficacy endpoint was the difference in the mean number of CSBM between baseline and post-intervention. The secondary efficacy endpoints were self-observation records related to constipation symptoms (Bristol stool form score, straining at defecation, stiffness of the stool, sensation of incomplete evacuation, and sensation of obstruction) and PAC-SYM and PAC-QOL scores. Only twenty-eight patients who completed the clinical study as per protocol (PP) were included in the analysis. All data were collected at baseline and after four weeks of intervention (Figure 1).
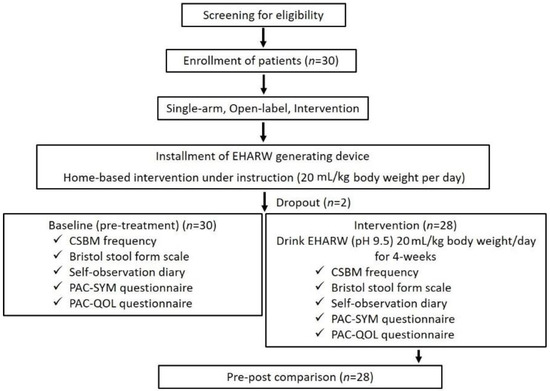
Figure 1.
Flowchart for the clinical study.
2.4. Experimental Device and Water Characteristics
EHARW (pH 9.5; H2 ≈ 0.5 mg/L) was produced by the electrolysis of water using an EHARW generator (BTM-1200, Biontech, Gunpo-si, Republic of Korea). This device has been approved as a Class II medical device by the Ministry of Food and Drug Safety of the Republic of Korea. EHARW had a pH of 9.5 (HM-31P, TOA DKK, Tokyo, Japan), oxidation-reduction potential value of −100 ± 5 mV (RM-30P, TOA DKK, Tokyo, Japan), and a hydrogen molecule (H2) content of approximately 0.5 mg/L (MARK-509, Hydrogen Meter, Nizhny Novgorod, Russia).
2.5. Interventions
For the home-based intervention, the medical device was installed in each patient’s house, and the patients were instructed on how to operate the device and drink the EHARW (pH 9.5; H2 ≈ 0.5 mg/L) generated by the experimental device. The total daily amount of EHARW was 20 mL/kg body weight, which was individually calculated based on the patient’s body weight. The patient was instructed to drink water three times a day, especially on an empty stomach after digestion of the meal, for four weeks during the intervention period.
2.6. Outcome Measures
As a primary efficacy endpoint, the frequency of CSBM was approached by the mean number of CSBMs per week, and the data were collected during the two weeks of pre-treatment (baseline) and the last two weeks of the total intervention period using the patient’s self-observation diary [11,35]. CSBM was defined as non-rescue medication-induced defecation with the sensation of complete evacuation.
The Bristol stool form scale and a self-observation diary were secondary outcome measures to evaluate constipation-related symptoms. The Bristol stool form was rated on a 7-point Likert scale ranging from 1 (hard lumps) to 7 (watery) at baseline and after four weeks of treatment [11]. Likewise, straining during defecation, stool stiffness, sensation of incomplete evacuation, and sensation of obstruction were assessed using a 5-point Likert scale ranging from 0 to 4, with a lower score indicating reduced symptom severity. In addition, the PAC-SYM questionnaire was used to assess the patient-rated severity of constipation symptoms using a 5-point Likert scale ranging from 0 to 4. This questionnaire consisted of 12 questions categorized into three subscales: abdominal, rectal, and stool symptoms (Table 2) [9,10]. Each subscore and overall score were presented as mean ± standard deviation (SD), which was analyzed with a paired t-test, and lower scores indicated the degree of symptom improvement. The PAC-QOL questionnaire measured the patient-rated impact of constipation on QOL. It consisted of 28 questions categorized into four subscales: physical discomfort, psychosocial discomfort, worries/concerns, and satisfaction, as shown in Table 3 [36]. PAC-QOL scores were presented as mean ± SD, and lower scores indicated better QOL. Questions 24–28 related to satisfaction were scored using inverse transformation, which contributed positively to the overall score.

Table 2.
Subscales of PAC-SYM questionnaire.

Table 3.
Subscales of PAC-QOL questionnaire.
2.7. Statistical Analysis
Statistical analyses were performed using the Prism software (version 8.0; GraphPad Software, San Diego, CA, USA). Data were presented as mean ± SD. The differences between baseline and post-treatment related to constipation (QOL, symptoms, Bristol stool form, and CSBM frequency) were analyzed using a parametric two-sample paired t-test, which was normally distributed and had equal variances. At p < 0.05, the findings were considered statistically significant.
3. Results
3.1. Demographic Data of Pre- and Post-Intervention in Patients
Of the 30 participants with chronic constipation, two patients withdrew from the clinical trial due to COVID-19 infection, and 28 completed the trial. Patient demographics (age, height, and body weight) and vital signs (blood pressure and body temperature) were recorded at baseline and after four weeks of treatment. By gender, females accounted for 66.7% of the participants. These parameters showed no remarkable differences between the baseline and post-treatment time points (Table 4).

Table 4.
Demographics and vital signs of the participants.
3.2. Efficacy End Point
The primary efficacy endpoint of EHARW in patients with chronic constipation was assessed based on CSBM frequency. At two weeks of the baseline, all patients showed ≤3 or less CSBM per week (1.81 0.59); however, after the EHARW treatment, the frequency of CSBMs significantly increased (2.58 1.72, F = 5.47, p < 0.05) compared to baseline (Table 5). The proportion of patients who reported higher CSBMs after treatment increased by 29.8%.

Table 5.
Assessment of constipation-related symptoms from patient’s self-observation diary.
As a secondary endpoint, we evaluated the Bristol stool form related to stool consistency using the patient’s self-observation diary. The score showed a significant increase after intervention (3.29 0.98, F = 9.18, p < 0.01) over the baseline (2.48 1.00). In addition, regarding constipation-related symptoms, straining during defecation (F = 9.91, p < 0.01), sensation of incomplete evacuation (F = 17.2, p < 0.001), and sensation of anorectal obstruction (F = 12.3, p < 0.01) showed a significant decrease compared with the baseline; however, stool stiffness did not differ significantly after EHARW treatment (Table 5).
3.3. Changes in PAC-SYM and PAC-QOL Scores between Baseline and Post-Treatment
The overall PAC-SYM scores after the 4-week intervention significantly decreased compared to the baseline (58%, p < 0.001), indicating that constipation-related symptoms improved after drinking the EHARW. The subscales of the PAC-SYM, categorized by abdominal, rectal, and stool symptoms, also showed a significant decrease (p < 0.001 vs. baseline). Likewise, the PAC-QOL score also showed a significant decrease in the overall score (54.2%) as well as subscale scores after the intervention, such as physical discomfort, psychosocial discomfort, and worries (p < 0.001 vs. baseline in all cases). Patients reported high satisfaction (60.1%) after intervention (1.34 0.66, p < 0.001) over the baseline (3.36 0.58) with the EHARW (pH 9.5; H2 ≈ 0.5 mg/L) intervention in terms of constipation-related QOL (Table 6).

Table 6.
Assessment of PAC-SYM and PAC-QOL questionnaires.
4. Discussion
This clinical study investigated the effect of drinking EHARW (pH 9.5; H2 ≈ 0.5 mg/L) in patients with chronic constipation by measuring bowel function, constipation-related symptoms, and quality of life. As outcome measures, patients’ self-recorded diaries on CSBMs frequency and stool consistency and patient-rating questionnaires, such as PAC-SYM and PAC-QOL, have been used to evaluate the efficacy of treatments for chronic constipation [37,38]. Regarding the effect of EHARW, several studies on IBS, gastritis, and constipation have been conducted on gastrointestinal disease in vivo and in vitro [25,39]. However, few clinical studies have investigated the effects of EHARW (pH 9.5) on patients with chronic constipation. Hence, we performed a home-based interventional clinical trial to evaluate the effect of drinking EHARW (pH 9.5, 20 mL/kg body weight/day) over four weeks on chronic constipation [40].
First, we evaluated patients’ baseline characteristics and vital signs with chronic constipation before and during pre-post treatment, including age, height, weight, blood pressure, and temperature. Baseline characteristics and vital signs are essential in clinical studies to identify potential confounding factors, select patients, interpret study results, and monitor safety [41,42]. In this study, there were no significant differences in demographics, and vital signs between pre-and post-treatment, and no adverse effects were reported during the 4-week intervention period (Table 4).
In this study, we used the frequency of CSBM as the primary endpoint to investigate the effects of EHARW on chronic constipation, as CSBM frequency is associated with a complete sense of evacuation [43]. After four weeks of EHARW intervention, CSBM frequency significantly improved (29.8%, p < 0.05). In addition, the proportion of patients with ≥3 CSBMs/week and patients experiencing an increase of ≥1 CSBMs/week increased. The frequency of CSBM is related to bowel function in maintaining normal defecation habits. Complete bowel movements should result in a stool corresponding to a type 3 or 4 bristol stool form chart [7]. Categories 3 (sausage shape with cracks) and 4 (smooth and soft sausage shape) on the Bristol stool scale were considered normal bowel movements. In our result of Bristol stool form score, a 4-week treatment of EHARW scored 3.29 ± 0.98, which was indicated in normal stool formation, while baseline scored 2.48 ± 1.00. The improved Bristol stool score (24.6%, p < 0.01) is related to stool consistency. It supports the understanding of improving self-reported constipation symptoms, such as straining during defecation, sensation of incomplete evacuation, and sensation of anorectal obstruction (Table 5).
The PAC-SYM and PAC-QOL scores are reliable measurements for symptom monitoring and QOL of patients with chronic constipation [12,13,36]. Specifically, the PAC-SYM score questionnaire is frequently used to monitor improvements in constipation symptoms categorized into three subscales (abdominal, rectal, and stool symptoms) in clinical trials [44]. According to our results, four weeks of treatment with EHARW (pH 9.5; H2 ≈ 0.5 mg/L) showed significant improvement in the patient-rated chronic symptoms based on the overall score (58%) and each subscale score. Similarly, in the PAC-QOL, drinking the EHARW (pH 9.5; H2 ≈ 0.5 mg/L) significantly improved the constipation-related QOL of patients by 54.2%, and patients showed markedly high satisfaction (60.1%) with the EHARW (Table 6).
Our results suggest that drinking EHARW (pH 9.5; H2 ≈ 0.5 mg/L) has beneficial effects on chronic constipation, as indicated by the patient-reported data. On average, ERW has an H2 concentration of less than 0.01 mg/L to nearly 2 mg/L over the pH range of 8 to 12. This concentration of H2 depends on the machine, electrode material, water source, TDS, flow rate, surface area, applied voltage, and minerals in the water source [33]. Our study measured H2 concentration as approximately 0.5 mg/L. Here, our results explained the therapeutic properties of the EHARW, including high pH (9.5) and rich in H2 (≈0.5 mg/L), which results in negative ORP (−100 ± 5 mV). H2 is also produced by many human microbiota and has a beneficial role in the human GI tract that impacts human nutrition, health, and well-being. Carbohydrates are normally broken down in the GI tract via glycolysis, which leads to the generation of H2 molecules. These produced H2 molecules are essential for bacterial metabolism and play a role in maintaining the GI environment [45]. Thus, the addition of H2 through EHARW is effective in maintaining the GI environment during chronic constipation.
Moreover, changes in the pH and ORP of the gut can significantly affect the growth and composition of the microbiota, resulting in changes in the intestinal environment [46]. In an animal study using guinea pig ileum, H2 shortened the colonic transit time by 47% in the proximal colon and 10% in the distal colon compared to the baseline [47]. One of the potential adverse effects of hydrogen water has been the tendency of loose stools and increased frequency of bowel movements [48]. The favorable effects of H2 on the microbiome and the shortened colonic transit time indicate that the dissolved H2 is most likely responsible for the observed therapeutic effects. However, more research is needed to determine if the effects are exclusively due to H2 as indicated by a recent review of ERW [45].
Based on several studies, it is speculated that the improvements in constipation symptoms seen in our study may be because of the antioxidant efficacy of EHARW due to H2 molecules that are dissolved in the water. This dissolved H2 may positively affect the gut environment by lowering ORP, decreasing oxidative stress, increasing beneficial bacteria, and improving patients’ constipation-related symptoms and QOL. However, beyond the numerous benefits of this study, there are some limitations. In this study, patients were instructed to drink specifically 20 mL/kg body weight/day of EHARW (pH 9.5; H2 ≈ 0.5 mg/L) for four weeks. Hence, it would be preferable to investigate the therapeutic mechanism of EHARW in more detail, using a higher volume, time, and biochemical examinations. In addition, further clinical research is also needed to determine if the biological benefits observed in this study are exclusively due to the molecular H2 also from the alkaline pH or a combination of them.
5. Conclusions
In this clinical study, drinking EHARW (pH 9.5; H2 ≈ 0.5 mg/L) over four weeks alleviated symptoms related to chronic constipation, as evident by the frequency of CSBM, stool consistency, and self-rated PAC-SYM scores, and the patients showed high satisfaction in their disease-related QOL. In addition, there were no adverse effects during the study period. These results suggest that daily ingestion of EHARW can be an effective and safe adjuvant treatment for managing chronic constipation, especially through home-based administration. However, further in-depth clinical studies are required to confirm the efficacy of EHARW in treating chronic constipation, considering the long-time treatment and elaborating on various mechanisms.
Author Contributions
Conceptualization, K.-J.L.; Methodology, S.S., J.B., H.I.K., M.H.R. and C.-S.K.; Software, S.S. and J.B.; Validation, Y.K., C.-S.K. and H.I.K.; Formal Analysis, Y.K. and H.I.K.; Investigation, Y.K., C.-S.K. and S.H.G.; Resources, C.-S.K., Y.K. and S.H.G.; Data Curation, S.S., J.B. and M.H.R.; Writing—Original Draft Preparation, S.S.; Writing—Review and Editing, S.S., Y.K., J.B., M.H.R., Y.J.J., S.H.G., H.J.P., C.-S.K. and H.I.K.; Visualization, Y.K., J.B. and H.I.K.; Supervision, C.-S.K.; Project Administration, K.-J.L.; Funding Acquisition, K.-J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant (2021-51-0662) from Biontech Co., Ltd.
Institutional Review Board Statement
Ethical approval was granted by the Institutional Research Ethics Review Board, Yonsei University, Wonju Severance Christian Hospital (IRBN: CR222010).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article (tables and figures).
Acknowledgments
The authors thank Biontech Co., Ltd. for providing the EHARW-producing medical device for the clinical trial (BTM-1200, Biontech, Gunpo-si, Republic of Korea). We also thank all the participants for their support in performing this study.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviation
| PAC-SYM | Patient assessment of constipation-symptoms |
| PAC-QOL | Patient assessment of constipation-quality of life |
| IBS | Intestinal bowel movement |
| H2 | Hydrogen |
| EHARW | Electrolyzed hydrogen-rich alkaline reduced water |
| ORP | Oxidation-reduction potential |
| GI | Gastrointestinal |
| CSBM | Complete spontaneous bowel movement |
| ITT | Intention-to-treat |
| PP | Per-protocol |
| SD | Standard deviation |
| ANOVA | Analysis of variance |
References
- Fabrizio, A.C.; Alimi, Y.; Kumar, A.S. Methods of evaluation of anorectal causes of obstructed defecation. Clin. Colon Rectal Surg. 2017, 30, 046–056. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, J.; Liu, B.; Cao, X.; Liu, Z.; Zhao, T.; Lv, X.; Guo, S.; Li, Y.; He, L. Efficacy of acupuncture in subpopulations with functional constipation: A protocol for a systematic review and individual patient data meta-analysis. PLoS ONE 2022, 17, e0266075. [Google Scholar] [CrossRef] [PubMed]
- Rajindrajith, S.; Devanarayana, N.M.; Weerasooriya, L.; Hathagoda, W.; Benninga, M.A. Quality of life and somatic symptoms in children with constipation: A school-based study. J. Pediatr. 2013, 163, 1069–1072.e1061. [Google Scholar] [CrossRef]
- Talley, N.J.; Jones, M.; Nuyts, G.; Dubois, D. Risk factors for chronic constipation based on a general practice sample. Am. J. Gastroenterol. 2003, 98, 1107–1111. [Google Scholar] [CrossRef]
- Sharma, A.; Rao, S.S.; Kearns, K.; Orleck, K.D.; Waldman, S.A. Diagnosis, management and patient perspectives of the spectrum of constipation disorders. Aliment. Pharmacol. Ther. 2021, 53, 1250–1267. [Google Scholar] [CrossRef]
- Lin, S.-R.; Ke, M.-Y.; Luo, J.-Y.; Yuan, Y.-Z.; Wang, J.-Y.; Walter, V.; Huang, J. A randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of tegaserod in patients from china with chronic constipation. World J. Gastroenterol. 2007, 13, 732. [Google Scholar] [CrossRef]
- Talley, N.J.; Lasch, K.L.; Baum, C.L. A gap in our understanding: Chronic constipation and its comorbid conditions. Clin. Gastroenterol. Hepatol. 2009, 7, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Dennison, C.; Prasad, M.; Lloyd, A.; Bhattacharyya, S.K.; Dhawan, R.; Coyne, K. The health-related quality of life and economic burden of constipation. Pharmacoeconomics 2005, 23, 461–476. [Google Scholar] [CrossRef]
- Leppert, W. Emerging therapies for patients with symptoms of opioid-induced bowel dysfunction. Drug Des. Dev. Ther. 2015, 9, 2215. [Google Scholar] [CrossRef]
- Walters, J.B.; Montagnini, M. Current concepts in the management of opioid-induced constipation. J. Opioid Manag. 2010, 6, 435–444. [Google Scholar] [CrossRef]
- Blake, M.; Raker, J.; Whelan, K. Validity and reliability of the bristol stool form scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 44, 693–703. [Google Scholar] [CrossRef]
- Nour-Eldein, H.; Salama, H.M.; Abdulmajeed, A.A.; Heissam, K.S. The effect of lifestyle modification on severity of constipation and quality of life of elders in nursing homes at ismailia city, egypt. J. Fam. Community Med. 2014, 21, 100. [Google Scholar] [CrossRef]
- Slappendel, R.; Simpson, K.; Dubois, D.; Keininger, D.L. Validation of the pac-sym questionnaire for opioid-induced constipation in patients with chronic low back pain. Eur. J. Pain. 2006, 10, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Whitehead, W.E.; Palsson, O.S.; Törnblom, H.; Simrén, M. An approach to the diagnosis and management of rome iv functional disorders of chronic constipation. Expert. Rev. Gastroenterol. Hepatol. 2020, 14, 39–46. [Google Scholar] [CrossRef]
- Shin, J.E.; Jung, H.-K.; Lee, T.H.; Jo, Y.; Lee, H.; Song, K.H.; Hong, S.N.; Lim, H.C.; Lee, S.J.; Chung, S.S. Guidelines for the diagnosis and treatment of chronic functional constipation in korea. J. Neurogastroenterol. Motil. 2016, 22, 383. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Liu, W.; Zeng, D.; Zhu, L.; Sun, X.; Sun, X. Effect of hydrogen-rich water on oxidative stress, liver function, and viral load in patients with chronic hepatitis b. Clin. Transl. Sci. 2013, 6, 372–375. [Google Scholar] [CrossRef]
- Ostojic, S.M. Targeting molecular hydrogen to mitochondria: Barriers and gateways. Pharmacol. Res. 2015, 94, 51–53. [Google Scholar] [CrossRef]
- Shen, N.-Y.; Bi, J.-B.; Zhang, J.-Y.; Zhang, S.-M.; Gu, J.-X.; Qu, K.; Liu, C. Hydrogen-rich water protects against inflammatory bowel disease in mice by inhibiting endoplasmic reticulum stress and promoting heme oxygenase-1 expression. World J. Gastroenterol. 2017, 23, 1375. [Google Scholar] [CrossRef]
- Medani, M.; Collins, D.; Docherty, N.G.; Baird, A.W.; O’Connell, P.R.; Winter, D.C. Emerging role of hydrogen sulfide in colonic physiology and pathophysiology. Inflamm. Bowel Dis. 2011, 17, 1620–1625. [Google Scholar] [CrossRef]
- He, J.; Xiong, S.; Zhang, J.; Wang, J.; Sun, A.; Mei, X.; Sun, X.; Zhang, C.; Wang, Q. Protective effects of hydrogen-rich saline on ulcerative colitis rat model. J. Surg. Res. 2013, 185, 174–181. [Google Scholar] [CrossRef]
- Abe, T.; Li, X.-K.; Yazawa, K.; Hatayama, N.; Xie, L.; Sato, B.; Kakuta, Y.; Tsutahara, K.; Okumi, M.; Tsuda, H. Hydrogen-rich university of wisconsin solution attenuates renal cold ischemia–reperfusion injury. Transplantation 2012, 94, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kiuchi, M.; Higashimura, Y.; Naito, Y.; Koyama, K. The effects of ingestion of hydrogen-dissolved alkaline electrolyzed water on stool consistency and gut microbiota: A double-blind randomized trial. Med. Gas Res. 2021, 11, 138. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Kura, B.; Kalocayova, B.; Tribulova, N.; Slezak, J. A new approach for the prevention and treatment of cardiovascular disorders. Molecular hydrogen significantly reduces the effects of oxidative stress. Molecules 2019, 24, 2076. [Google Scholar] [CrossRef]
- Bajgai, J.; Kim, C.-S.; Rahman, M.; Jeong, E.-S.; Jang, H.-Y.; Kim, K.-E.; Choi, J.; Cho, I.-Y.; Lee, K.-J.; Lee, M. Effects of alkaline-reduced water on gastrointestinal diseases. Processes 2022, 10, 87. [Google Scholar] [CrossRef]
- Rokhmalia, F.; Hermiyanti, P. A concentrations of ph, chromium, and mpn coliform parameters in alkaline water. Int. J. Adv. Health Sci. Technol. 2022, 2, 5. [Google Scholar] [CrossRef]
- Rane, M.D.; Shaikh, N.; Maniyar, F. Daily ingestion of structured alkaline water (organized/regularized) and it’s effect on health conditioning. Asian J. Adv. Res. 2022, 5, 606–618. [Google Scholar]
- Sharma, S.; Lee, K.-J.; Bajgai, J.; Trinh, T.T.; Antonio, J.M.; Rahman, M.H.; Vira, K.; Sofian, A.-N.; Cho, S.H.; Kim, C.-S. Anti-oxidative and anti-diabetic effects of electrolyzed weakly alkaline reduced water on renal proximal tubular epithelial cells. Processes 2022, 10, 2025. [Google Scholar] [CrossRef]
- Trinh, T.T.; Fadriquela, A.; Bajgai, J.; Sharma, S.; Rahman, M.H.; Goh, S.-H.; Khang, S.-S.; Khang, W.-R.; Kim, C.-S.; Lee, K.-J. Anti-oxidative effect of weak alkaline reduced water in raw 264.7 murine macrophage cells. Processes 2021, 9, 2062. [Google Scholar] [CrossRef]
- Shirahata, S.; Hamasaki, T.; Teruya, K. Advanced research on the health benefit of reduced water. Trends Food Sci. Technol. 2012, 23, 124–131. [Google Scholar] [CrossRef]
- Tashiro, H.; Kitahora, T.; Fujiyama, Y.; Bammba, T. Clinical evaluation of alkali-ionized water for chronic diarrhea e placebocontrolled double-blind study. Dig. Absorpt. 2000, 23, 52–56. [Google Scholar]
- Islam, R.; Faysal, S.M.; Amin, R.; Juliana, F.M.; Islam, M.J.; Alam, J.; Hossain, M.N.; Asaduzzaman, M. Assessment of ph and total dissolved substances (tds) in the commercially available bottled drinking water. J. Nurs. Health Sci. 2017, 6, 35–40. [Google Scholar]
- LeBaron, T.W.; Sharpe, R.; Ohno, K. Electrolyzed–reduced water: Review ii: Safety concerns and effectiveness as a source of hydrogen water. Int. J. Mol. Sci. 2022, 23, 14508. [Google Scholar] [CrossRef]
- Tashiro, H.; Hokudo, T.; Ono, H.; Fujiyama, Y.; Baba, T. Clinical Evaluation of Alkaline Ionized Water for Abdominal Complaints: Placebo Controlled Double Blind Tests. Available online: https://wipalis.com/wp-content/uploads/2021/05/Clinical-evaluation-of-alkaline-ionized-water-for-abdominal-complaints.pdf (accessed on 1 July 2023).
- Johanson, J.; Kamm, M.; Shetzline, M.; Dunger-Baldauf, C.; Cohard-Radice, M. Association of complete spontaneous bowel movements (csbm) and symptom improvement in chronic constipation (cc) patients (pts): 931. Am. J. Gastroenterol. 2005, 100, S341. [Google Scholar] [CrossRef]
- Marquis, P.; De La Loge, C.; Dubois, D.; McDermott, A.; Chassany, O. Development and validation of the patient assessment of constipation quality of life questionnaire. Scand. J. Gastroenterol. 2005, 40, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Hale, M.; Morlion, B.; Tack, J.; Webster, L.; Wild, J. Naldemedine improves patient-reported outcomes of opioid-induced constipation in patients with chronic non-cancer pain in the compose phase 3 studies. J. Pain. Res. 2021, 14, 2179–2189. [Google Scholar] [CrossRef]
- Turan, N.; Atabek Aştı, T. The effect of abdominal massage on constipation and quality of life. Gastroenterol. Nurs. 2016, 39, 48–59. [Google Scholar] [CrossRef]
- Ignacio, R.M.C.; Joo, K.-B.; Lee, K.-J. Clinical effect and mechanism of alkaline reduced water. J. Food Drug Anal. 2012, 20, 33. [Google Scholar] [CrossRef]
- Henry, M.; Chambron, J. Physico-chemical, biological and therapeutic characteristics of electrolyzed reduced alkaline water (eraw). Water 2013, 5, 2094–2115. [Google Scholar] [CrossRef]
- Sharma, S.; Bajgai, J.; Fadriquela, A.; Rahman, M.H.; Thuy, T.T.; Goh, S.-H.; Kim, C.-S.; Yu, K.; Lee, K.-J. The Effect of a Granule-type Anti-Hangover Compound, Quechung, on Acute Alcohol-Induced Hangover in Healthy Subjects: A Randomized Crossover Study. Available online: https://www.earticle.net/Article/A385723 (accessed on 1 July 2023).
- Evans, D.; Hodgkinson, B.; Berry, J. Vital signs in hospital patients: A systematic review. Int. J. Nurs. Stud. 2001, 38, 643–650. [Google Scholar] [CrossRef]
- Mueller-Lissner, S.; Kamm, M.A.; Wald, A.; Hinkel, U.; Koehler, U.; Richter, E.; Bubeck, J. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of sodium picosulfate in patients with chronic constipation. Am. J. Gastroenterol. 2010, 105, 897–903. [Google Scholar] [CrossRef]
- Yiannakou, Y.; Tack, J.; Piessevaux, H.; Dubois, D.; Quigley, E.; Ke, M.; Da Silva, S.; Joseph, A.; Kerstens, R. The pac-sym questionnaire for chronic constipation: Defining the minimal important difference. Aliment. Pharmacol. Ther. 2017, 46, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.W.; Shorten, P.R.; Altermann, E.H.; Roy, N.C.; McNabb, W.C. Hydrogen cross-feeders of the human gastrointestinal tract. Gut Microbes 2019, 10, 270–288. [Google Scholar] [CrossRef] [PubMed]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Modern Food Microbiology; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Jahng, J.; Jung, I.; Choi, E.; Conklin, J.; Park, H. The effects of methane and hydrogen gases produced by enteric bacteria on ileal motility and colonic transit time. J. Neurogastroenterol. Motil. 2012, 24, 185-e92. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Toyoda, Y.; Sharma, P.; Evans, M.; Guthrie, N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome—An open label pilot study. J. Clin. Biochem. Nutr. 2010, 46, 140–149. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).