The Effect of Electrolyzed Hydrogen-Rich Alkaline Reduced Water on Patients with Chronic Constipation—A Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Population
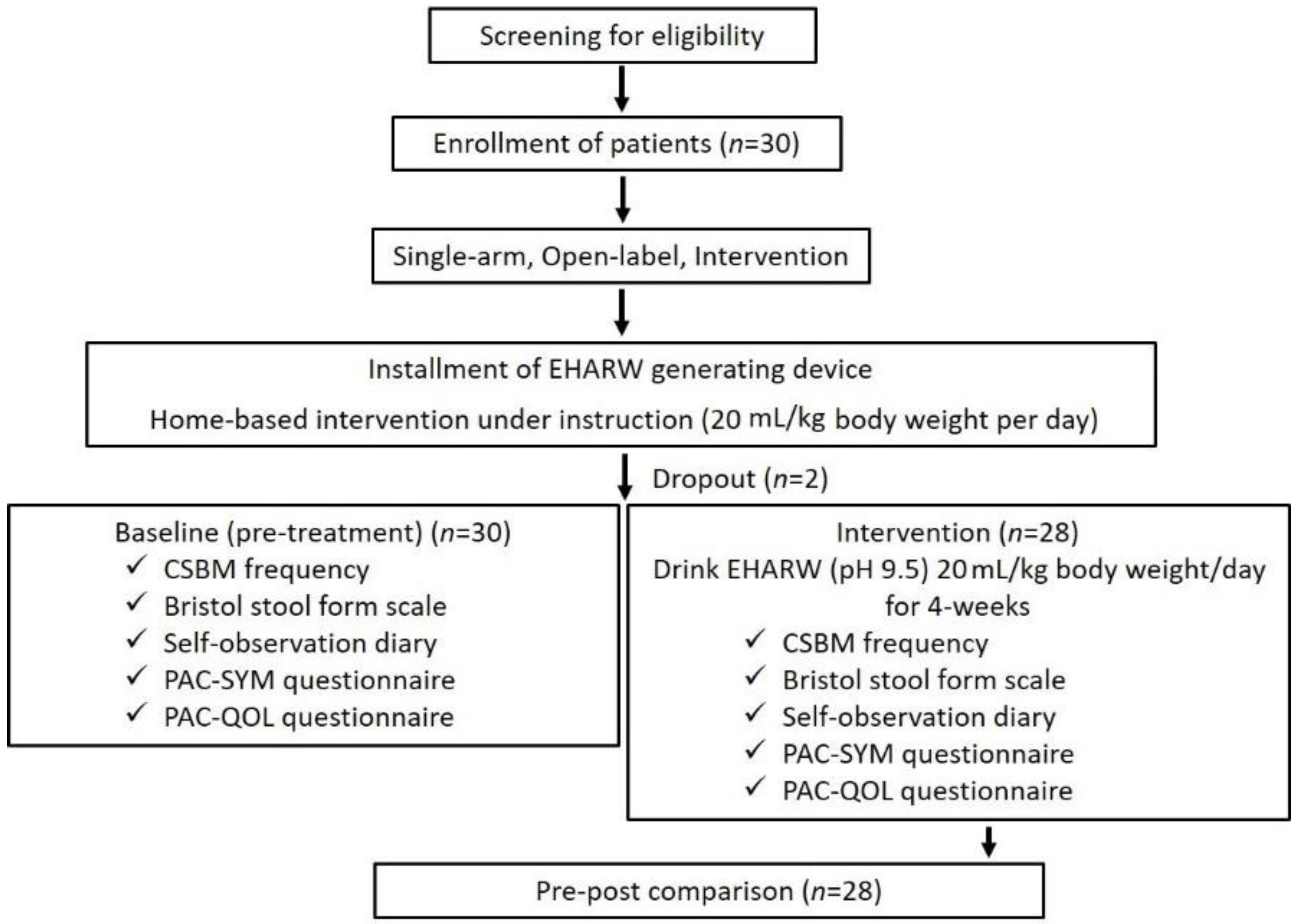
2.3. Study Design
2.4. Experimental Device and Water Characteristics
2.5. Interventions
2.6. Outcome Measures
2.7. Statistical Analysis
3. Results
3.1. Demographic Data of Pre- and Post-Intervention in Patients
3.2. Efficacy End Point
3.3. Changes in PAC-SYM and PAC-QOL Scores between Baseline and Post-Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| PAC-SYM | Patient assessment of constipation-symptoms |
| PAC-QOL | Patient assessment of constipation-quality of life |
| IBS | Intestinal bowel movement |
| H2 | Hydrogen |
| EHARW | Electrolyzed hydrogen-rich alkaline reduced water |
| ORP | Oxidation-reduction potential |
| GI | Gastrointestinal |
| CSBM | Complete spontaneous bowel movement |
| ITT | Intention-to-treat |
| PP | Per-protocol |
| SD | Standard deviation |
| ANOVA | Analysis of variance |
References
- Fabrizio, A.C.; Alimi, Y.; Kumar, A.S. Methods of evaluation of anorectal causes of obstructed defecation. Clin. Colon Rectal Surg. 2017, 30, 046–056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Liu, J.; Liu, B.; Cao, X.; Liu, Z.; Zhao, T.; Lv, X.; Guo, S.; Li, Y.; He, L. Efficacy of acupuncture in subpopulations with functional constipation: A protocol for a systematic review and individual patient data meta-analysis. PLoS ONE 2022, 17, e0266075. [Google Scholar] [CrossRef] [PubMed]
- Rajindrajith, S.; Devanarayana, N.M.; Weerasooriya, L.; Hathagoda, W.; Benninga, M.A. Quality of life and somatic symptoms in children with constipation: A school-based study. J. Pediatr. 2013, 163, 1069–1072.e1061. [Google Scholar] [CrossRef]
- Talley, N.J.; Jones, M.; Nuyts, G.; Dubois, D. Risk factors for chronic constipation based on a general practice sample. Am. J. Gastroenterol. 2003, 98, 1107–1111. [Google Scholar] [CrossRef]
- Sharma, A.; Rao, S.S.; Kearns, K.; Orleck, K.D.; Waldman, S.A. Diagnosis, management and patient perspectives of the spectrum of constipation disorders. Aliment. Pharmacol. Ther. 2021, 53, 1250–1267. [Google Scholar] [CrossRef]
- Lin, S.-R.; Ke, M.-Y.; Luo, J.-Y.; Yuan, Y.-Z.; Wang, J.-Y.; Walter, V.; Huang, J. A randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of tegaserod in patients from china with chronic constipation. World J. Gastroenterol. 2007, 13, 732. [Google Scholar] [CrossRef] [Green Version]
- Talley, N.J.; Lasch, K.L.; Baum, C.L. A gap in our understanding: Chronic constipation and its comorbid conditions. Clin. Gastroenterol. Hepatol. 2009, 7, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Dennison, C.; Prasad, M.; Lloyd, A.; Bhattacharyya, S.K.; Dhawan, R.; Coyne, K. The health-related quality of life and economic burden of constipation. Pharmacoeconomics 2005, 23, 461–476. [Google Scholar] [CrossRef]
- Leppert, W. Emerging therapies for patients with symptoms of opioid-induced bowel dysfunction. Drug Des. Dev. Ther. 2015, 9, 2215. [Google Scholar] [CrossRef] [Green Version]
- Walters, J.B.; Montagnini, M. Current concepts in the management of opioid-induced constipation. J. Opioid Manag. 2010, 6, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Blake, M.; Raker, J.; Whelan, K. Validity and reliability of the bristol stool form scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 44, 693–703. [Google Scholar] [CrossRef] [Green Version]
- Nour-Eldein, H.; Salama, H.M.; Abdulmajeed, A.A.; Heissam, K.S. The effect of lifestyle modification on severity of constipation and quality of life of elders in nursing homes at ismailia city, egypt. J. Fam. Community Med. 2014, 21, 100. [Google Scholar] [CrossRef] [Green Version]
- Slappendel, R.; Simpson, K.; Dubois, D.; Keininger, D.L. Validation of the pac-sym questionnaire for opioid-induced constipation in patients with chronic low back pain. Eur. J. Pain. 2006, 10, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Whitehead, W.E.; Palsson, O.S.; Törnblom, H.; Simrén, M. An approach to the diagnosis and management of rome iv functional disorders of chronic constipation. Expert. Rev. Gastroenterol. Hepatol. 2020, 14, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.E.; Jung, H.-K.; Lee, T.H.; Jo, Y.; Lee, H.; Song, K.H.; Hong, S.N.; Lim, H.C.; Lee, S.J.; Chung, S.S. Guidelines for the diagnosis and treatment of chronic functional constipation in korea. J. Neurogastroenterol. Motil. 2016, 22, 383. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Liu, W.; Zeng, D.; Zhu, L.; Sun, X.; Sun, X. Effect of hydrogen-rich water on oxidative stress, liver function, and viral load in patients with chronic hepatitis b. Clin. Transl. Sci. 2013, 6, 372–375. [Google Scholar] [CrossRef] [Green Version]
- Ostojic, S.M. Targeting molecular hydrogen to mitochondria: Barriers and gateways. Pharmacol. Res. 2015, 94, 51–53. [Google Scholar] [CrossRef]
- Shen, N.-Y.; Bi, J.-B.; Zhang, J.-Y.; Zhang, S.-M.; Gu, J.-X.; Qu, K.; Liu, C. Hydrogen-rich water protects against inflammatory bowel disease in mice by inhibiting endoplasmic reticulum stress and promoting heme oxygenase-1 expression. World J. Gastroenterol. 2017, 23, 1375. [Google Scholar] [CrossRef]
- Medani, M.; Collins, D.; Docherty, N.G.; Baird, A.W.; O’Connell, P.R.; Winter, D.C. Emerging role of hydrogen sulfide in colonic physiology and pathophysiology. Inflamm. Bowel Dis. 2011, 17, 1620–1625. [Google Scholar] [CrossRef]
- He, J.; Xiong, S.; Zhang, J.; Wang, J.; Sun, A.; Mei, X.; Sun, X.; Zhang, C.; Wang, Q. Protective effects of hydrogen-rich saline on ulcerative colitis rat model. J. Surg. Res. 2013, 185, 174–181. [Google Scholar] [CrossRef]
- Abe, T.; Li, X.-K.; Yazawa, K.; Hatayama, N.; Xie, L.; Sato, B.; Kakuta, Y.; Tsutahara, K.; Okumi, M.; Tsuda, H. Hydrogen-rich university of wisconsin solution attenuates renal cold ischemia–reperfusion injury. Transplantation 2012, 94, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kiuchi, M.; Higashimura, Y.; Naito, Y.; Koyama, K. The effects of ingestion of hydrogen-dissolved alkaline electrolyzed water on stool consistency and gut microbiota: A double-blind randomized trial. Med. Gas Res. 2021, 11, 138. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Kura, B.; Kalocayova, B.; Tribulova, N.; Slezak, J. A new approach for the prevention and treatment of cardiovascular disorders. Molecular hydrogen significantly reduces the effects of oxidative stress. Molecules 2019, 24, 2076. [Google Scholar] [CrossRef] [Green Version]
- Bajgai, J.; Kim, C.-S.; Rahman, M.; Jeong, E.-S.; Jang, H.-Y.; Kim, K.-E.; Choi, J.; Cho, I.-Y.; Lee, K.-J.; Lee, M. Effects of alkaline-reduced water on gastrointestinal diseases. Processes 2022, 10, 87. [Google Scholar] [CrossRef]
- Rokhmalia, F.; Hermiyanti, P. A concentrations of ph, chromium, and mpn coliform parameters in alkaline water. Int. J. Adv. Health Sci. Technol. 2022, 2, 5. [Google Scholar] [CrossRef]
- Rane, M.D.; Shaikh, N.; Maniyar, F. Daily ingestion of structured alkaline water (organized/regularized) and it’s effect on health conditioning. Asian J. Adv. Res. 2022, 5, 606–618. [Google Scholar]
- Sharma, S.; Lee, K.-J.; Bajgai, J.; Trinh, T.T.; Antonio, J.M.; Rahman, M.H.; Vira, K.; Sofian, A.-N.; Cho, S.H.; Kim, C.-S. Anti-oxidative and anti-diabetic effects of electrolyzed weakly alkaline reduced water on renal proximal tubular epithelial cells. Processes 2022, 10, 2025. [Google Scholar] [CrossRef]
- Trinh, T.T.; Fadriquela, A.; Bajgai, J.; Sharma, S.; Rahman, M.H.; Goh, S.-H.; Khang, S.-S.; Khang, W.-R.; Kim, C.-S.; Lee, K.-J. Anti-oxidative effect of weak alkaline reduced water in raw 264.7 murine macrophage cells. Processes 2021, 9, 2062. [Google Scholar] [CrossRef]
- Shirahata, S.; Hamasaki, T.; Teruya, K. Advanced research on the health benefit of reduced water. Trends Food Sci. Technol. 2012, 23, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Tashiro, H.; Kitahora, T.; Fujiyama, Y.; Bammba, T. Clinical evaluation of alkali-ionized water for chronic diarrhea e placebocontrolled double-blind study. Dig. Absorpt. 2000, 23, 52–56. [Google Scholar]
- Islam, R.; Faysal, S.M.; Amin, R.; Juliana, F.M.; Islam, M.J.; Alam, J.; Hossain, M.N.; Asaduzzaman, M. Assessment of ph and total dissolved substances (tds) in the commercially available bottled drinking water. J. Nurs. Health Sci. 2017, 6, 35–40. [Google Scholar]
- LeBaron, T.W.; Sharpe, R.; Ohno, K. Electrolyzed–reduced water: Review ii: Safety concerns and effectiveness as a source of hydrogen water. Int. J. Mol. Sci. 2022, 23, 14508. [Google Scholar] [CrossRef]
- Tashiro, H.; Hokudo, T.; Ono, H.; Fujiyama, Y.; Baba, T. Clinical Evaluation of Alkaline Ionized Water for Abdominal Complaints: Placebo Controlled Double Blind Tests. Available online: https://wipalis.com/wp-content/uploads/2021/05/Clinical-evaluation-of-alkaline-ionized-water-for-abdominal-complaints.pdf (accessed on 1 July 2023).
- Johanson, J.; Kamm, M.; Shetzline, M.; Dunger-Baldauf, C.; Cohard-Radice, M. Association of complete spontaneous bowel movements (csbm) and symptom improvement in chronic constipation (cc) patients (pts): 931. Am. J. Gastroenterol. 2005, 100, S341. [Google Scholar] [CrossRef]
- Marquis, P.; De La Loge, C.; Dubois, D.; McDermott, A.; Chassany, O. Development and validation of the patient assessment of constipation quality of life questionnaire. Scand. J. Gastroenterol. 2005, 40, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Hale, M.; Morlion, B.; Tack, J.; Webster, L.; Wild, J. Naldemedine improves patient-reported outcomes of opioid-induced constipation in patients with chronic non-cancer pain in the compose phase 3 studies. J. Pain. Res. 2021, 14, 2179–2189. [Google Scholar] [CrossRef]
- Turan, N.; Atabek Aştı, T. The effect of abdominal massage on constipation and quality of life. Gastroenterol. Nurs. 2016, 39, 48–59. [Google Scholar] [CrossRef]
- Ignacio, R.M.C.; Joo, K.-B.; Lee, K.-J. Clinical effect and mechanism of alkaline reduced water. J. Food Drug Anal. 2012, 20, 33. [Google Scholar] [CrossRef]
- Henry, M.; Chambron, J. Physico-chemical, biological and therapeutic characteristics of electrolyzed reduced alkaline water (eraw). Water 2013, 5, 2094–2115. [Google Scholar] [CrossRef]
- Sharma, S.; Bajgai, J.; Fadriquela, A.; Rahman, M.H.; Thuy, T.T.; Goh, S.-H.; Kim, C.-S.; Yu, K.; Lee, K.-J. The Effect of a Granule-type Anti-Hangover Compound, Quechung, on Acute Alcohol-Induced Hangover in Healthy Subjects: A Randomized Crossover Study. Available online: https://www.earticle.net/Article/A385723 (accessed on 1 July 2023).
- Evans, D.; Hodgkinson, B.; Berry, J. Vital signs in hospital patients: A systematic review. Int. J. Nurs. Stud. 2001, 38, 643–650. [Google Scholar] [CrossRef]
- Mueller-Lissner, S.; Kamm, M.A.; Wald, A.; Hinkel, U.; Koehler, U.; Richter, E.; Bubeck, J. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of sodium picosulfate in patients with chronic constipation. Am. J. Gastroenterol. 2010, 105, 897–903. [Google Scholar] [CrossRef]
- Yiannakou, Y.; Tack, J.; Piessevaux, H.; Dubois, D.; Quigley, E.; Ke, M.; Da Silva, S.; Joseph, A.; Kerstens, R. The pac-sym questionnaire for chronic constipation: Defining the minimal important difference. Aliment. Pharmacol. Ther. 2017, 46, 1103–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, N.W.; Shorten, P.R.; Altermann, E.H.; Roy, N.C.; McNabb, W.C. Hydrogen cross-feeders of the human gastrointestinal tract. Gut Microbes 2019, 10, 270–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Modern Food Microbiology; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Jahng, J.; Jung, I.; Choi, E.; Conklin, J.; Park, H. The effects of methane and hydrogen gases produced by enteric bacteria on ileal motility and colonic transit time. J. Neurogastroenterol. Motil. 2012, 24, 185-e92. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Toyoda, Y.; Sharma, P.; Evans, M.; Guthrie, N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome—An open label pilot study. J. Clin. Biochem. Nutr. 2010, 46, 140–149. [Google Scholar] [CrossRef] [Green Version]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 1. Men and women aged 19 to 70 years old adult | 1. Those taking drugs such as opioid analgesics, antipsychotics, calcium blockers, and parasympathetic antagonists |
| 2. Those who meet Roma IV diagnostic criteria for chronic constipation | 2. Those with kidney disease (renal failure, potassium excretion disorder, etc.) |
| 3. Symptoms began six months prior and have lasted for >3 months | 3. Those with colon-related diseases (colon cancer, intestinal stenosis, rectal flow, anal fissure, rectal bleeding, irritable bowel syndrome, inflammatory bowel disease, recent gastrointestinal surgery, etc.) |
| 4. Those without structural abnormalities that may cause constipation | 4. Pregnancy or breastfeeding |
| 5. A patient who has personally signed the written informed consent | 5. Those who are judged to be difficult to conduct research with by the clinical trial director |
| 6. If all the criteria meet | 6. Those who meet at least one exclusion criteria mentioned above |
| Subscales | Questionnaires |
|---|---|
| Abdominal symptoms | 1. Stomach discomfort |
| 2. Stomach pain | |
| 3. Stomach bloating | |
| 4. Stomach cramps | |
| Rectal symptoms | 5. Painful bowel movements |
| 6. Rectal burning during or after a bowel movement | |
| 7. Rectal bleeding or tearing during or after a bowel movement | |
| Stool Symptoms | 8. Incomplete bowel movement |
| 9. Bowel movements were too hard | |
| 10. Bowel movements were too small | |
| 11. Straining or squeezing to try and pass bowel movements | |
| 12. Feeling like you had to pass a bowel movement, but you could not (False alarm) |
| Questionnaires | |
|---|---|
| Subscales | Questions |
| Physical discomfort | 1. Felt bloated to the point of bursting |
| 2. Felt heavy because of constipation | |
| 3. Felt any physical discomfort | |
| 4. Felt the need to have a bowel movement but was unable to do so | |
| Psychosocial discomfort | 5. Been embarrassed to be with other people |
| 6. Been eating less and less because of not being able to have bowel movements | |
| 7. Had to be careful what you eat | |
| 8. Had a decreased appetite | |
| 9. Been worried about not being able to choose what you eat | |
| 10. Been embarrassed about staying in the bathroom for so long when away from home | |
| 11. Been embarrassed about staying in the bathroom so often when away from home | |
| 12. Been worried about having to change your daily routine | |
| Worries /Concerns | 13. Felt irritable because of your condition |
| 14. Been upset by your condition | |
| 15. Felt obsessed by your condition | |
| 16. Felt stressed by your condition | |
| 17. Felt less self-confident because of your condition | |
| 18. Felt in control of your situation | |
| 19. Been worried about not knowing when you can have a bowel movement | |
| 20. Been worried about not being able to have a bowel movement | |
| 21. Been increasingly bothered by not being able to have a bowel movement | |
| 22. Been worried that your condition will get worse | |
| 23. Felt that your body was not working properly | |
| Satisfaction | 24. Fewer bowel movements than desired |
| 25. Satisfied with how often you have a bowel movement | |
| 26. Satisfied with the regularity of your bowel movements | |
| 27. Satisfied with the time it takes for food to pass through the intestines | |
| 28. Satisfied with your treatment | |
| Parameters | Baseline | Post-Treatment | Mean Difference (Paired t-Test) | p-Value |
|---|---|---|---|---|
| Female | 20 (66.7%) | 19 (67.9%) | 1 | |
| Male | 10 (33.3%) | 9 (32.1%) | 1 | |
| Age (years) | 48.79 ± 9.63 | 48.79 ± 9.63 | 0 | - |
| Height (cm) | 161.48 ± 7.93 | 161.50 ±7.91 | −0.02 | p = 0.692 |
| Weight (kg) | 62.93 ± 7.93 | 62.64 ± 10.84 | 0.27 | p = 0.93 |
| Systolic blood pressure (mmHg) | 121.07 ±10.58 | 123.64 ± 10.75 | −2.57 | p = 0.160 |
| Diastolic blood pressure (mmHg) | 79.32 ± 7.33 | 81.54 ± 7.45 | −2.21 | p = 0.141 |
| Body temperature (°C) | 36.21 ± 0.19 | 36.26 ± 0.19 | −0.05 | p = 0.215 |
| Outcomes | Baseline | Normal Distribution (Area) | Post-Treatment | Normal Distribution (Area) | F-Test | Mean Difference (Paired t-Test) | p-Value |
|---|---|---|---|---|---|---|---|
| CSBM frequency/week | 1.81 0.59 | 0.3997 | 2.58 1.72 * | 0.6408 | 5.47 | −0.771 | p < 0.05 |
| Bristol stool form score | 2.48 1.00 | 0.6985 | 3.29 0.98 ** | 0.9126 | 9.18 | −0.804 | p < 0.01 |
| Straining at defecation | 3.32 1.31 | 0.8504 | 2.36 0.99 ** | 0.6569 | 9.91 | 0.964 | p < 0.01 |
| Stiffness of the stool | 3.14 1.27 | 0.8236 | 2.61 1.13 | 0.7174 | 2.44 | 0.536 | p = 0.130 |
| The sensation of incomplete evacuation | 3.11 0.92 | 0.8944 | 2.11 1.13 *** | 0.5528 | 17.2 | 1.000 | p < 0.001 |
| Sensation of obstruction | 2.96 1.29 | 0.7809 | 2.00 0.98 ** | 0.5163 | 12.3 | 0.964 | p < 0.01 |
| Subscales | Baseline | Post-Treatment | p-Value |
|---|---|---|---|
| PAC-SYM: | |||
| 1. Abdominal | 2.36 0.55 | 0.83 0.54 *** | p < 0.001 |
| 2. Rectal | 2.48 0.72 | 0.81 0.16 *** | p < 0.001 |
| 3. Stools | 3.57 0.58 | 1.86 0.20 *** | p < 0.001 |
| Overall | 9.92 1.38 | 4.17 2.09 *** | p < 0.001 |
| PAC-QOL: | |||
| 1. Physical | 2.68 0.74 | 1.30 0.56 *** | p < 0.001 |
| 2. Psychosocial | 2.08 0.64 | 0.96 0.51 *** | p < 0.001 |
| 3. Worries | 2.31 0.61 | 0.89 0.65 *** | p < 0.001 |
| 4. Satisfaction | 3.36 0.58 | 1.34 0.66 *** | p < 0.001 |
| Overall | 17.7 3.90 | 8.10 5.39 *** | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Kim, Y.; Bajgai, J.; Rahman, M.H.; Jeong, Y.J.; Goh, S.H.; Park, H.J.; Kim, C.-S.; Kim, H.I.; Lee, K.-J. The Effect of Electrolyzed Hydrogen-Rich Alkaline Reduced Water on Patients with Chronic Constipation—A Clinical Trial. Processes 2023, 11, 2142. https://doi.org/10.3390/pr11072142
Sharma S, Kim Y, Bajgai J, Rahman MH, Jeong YJ, Goh SH, Park HJ, Kim C-S, Kim HI, Lee K-J. The Effect of Electrolyzed Hydrogen-Rich Alkaline Reduced Water on Patients with Chronic Constipation—A Clinical Trial. Processes. 2023; 11(7):2142. https://doi.org/10.3390/pr11072142
Chicago/Turabian StyleSharma, Subham, Yundeok Kim, Johny Bajgai, Md. Habibur Rahman, Yun Ju Jeong, Seong Hoon Goh, Hong Jun Park, Cheol-Su Kim, Hyun Il Kim, and Kyu-Jae Lee. 2023. "The Effect of Electrolyzed Hydrogen-Rich Alkaline Reduced Water on Patients with Chronic Constipation—A Clinical Trial" Processes 11, no. 7: 2142. https://doi.org/10.3390/pr11072142






