Thermal Behavior Prediction of Sludge Co-Combustion with Coal: Curve Extraction and Artificial Neural Networks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Thermogravimetric Experiment
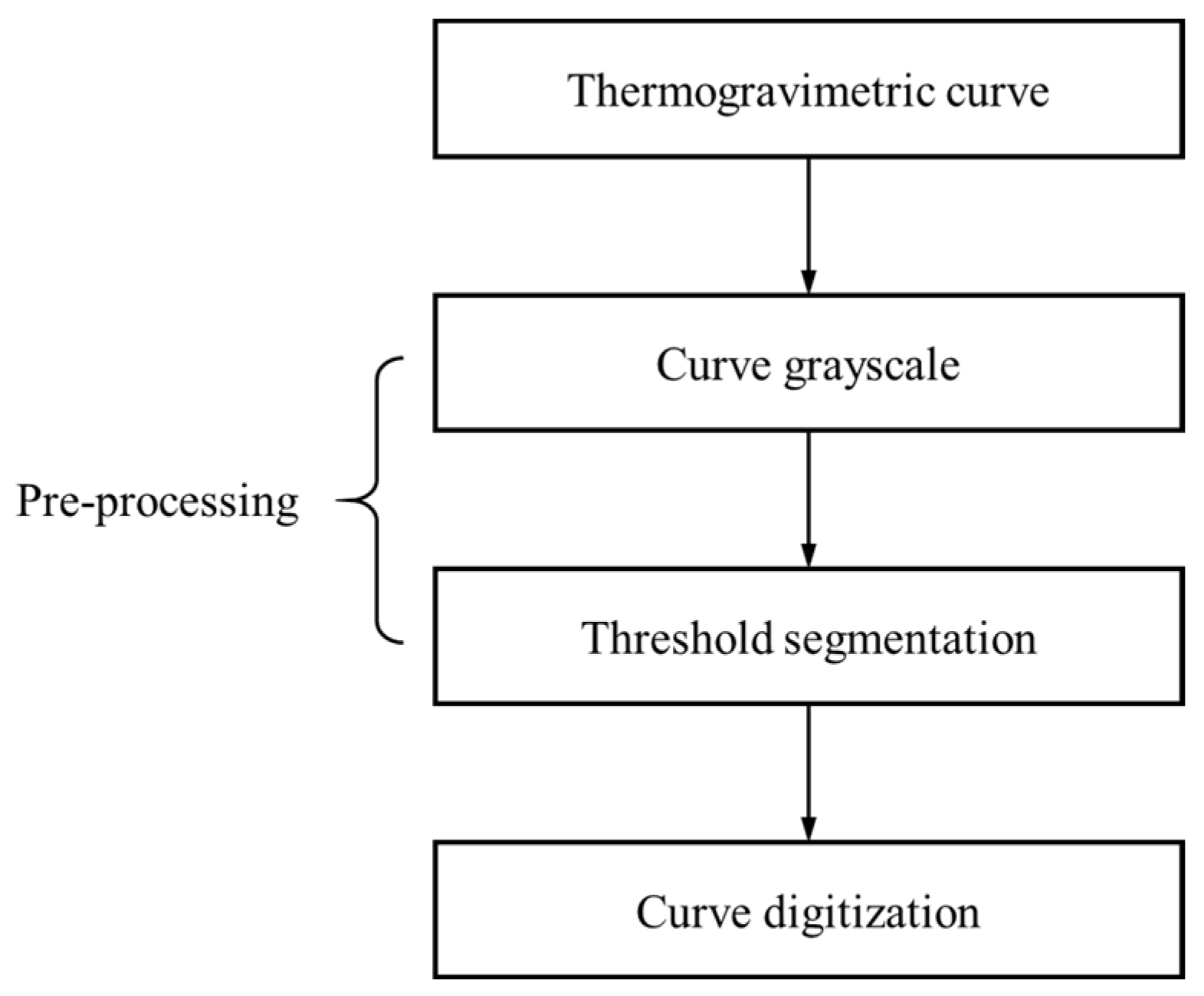
2.3. Thermogravimetric Curve Extraction
2.4. Artificial Neural Networks
3. Results and Discussion
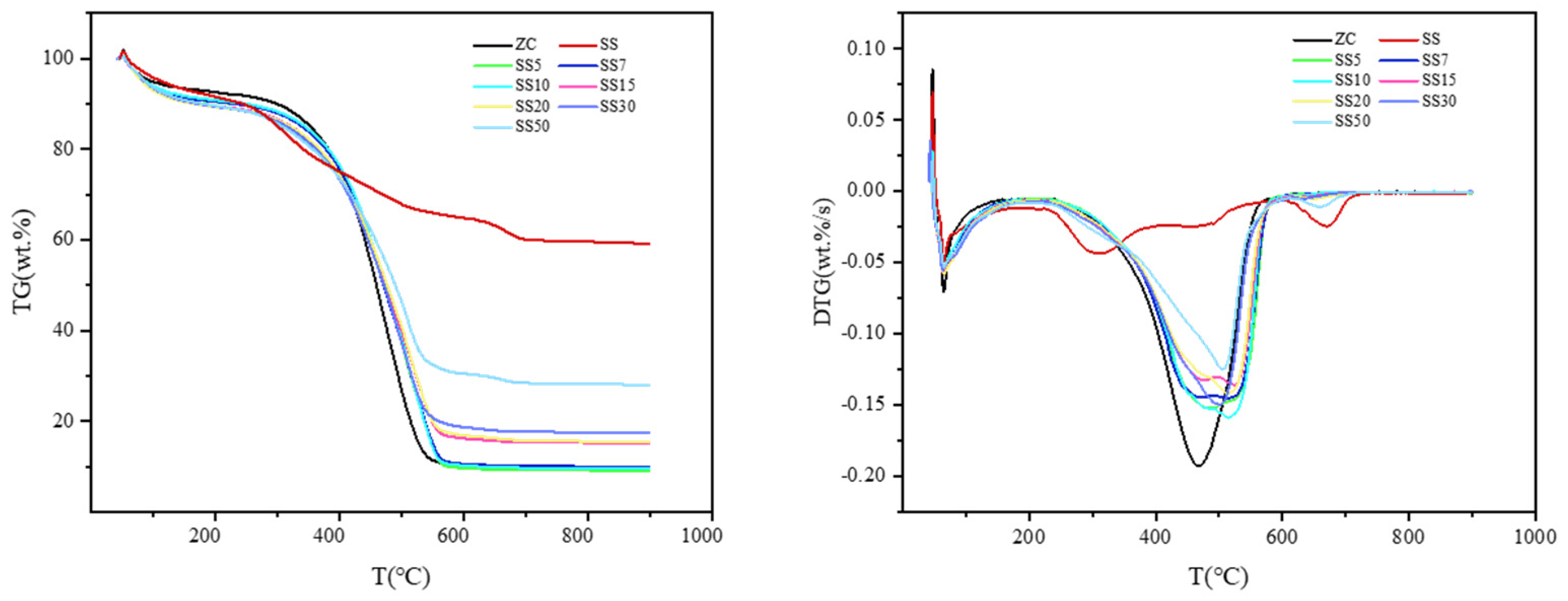
3.1. Co-Combustion Characteristics
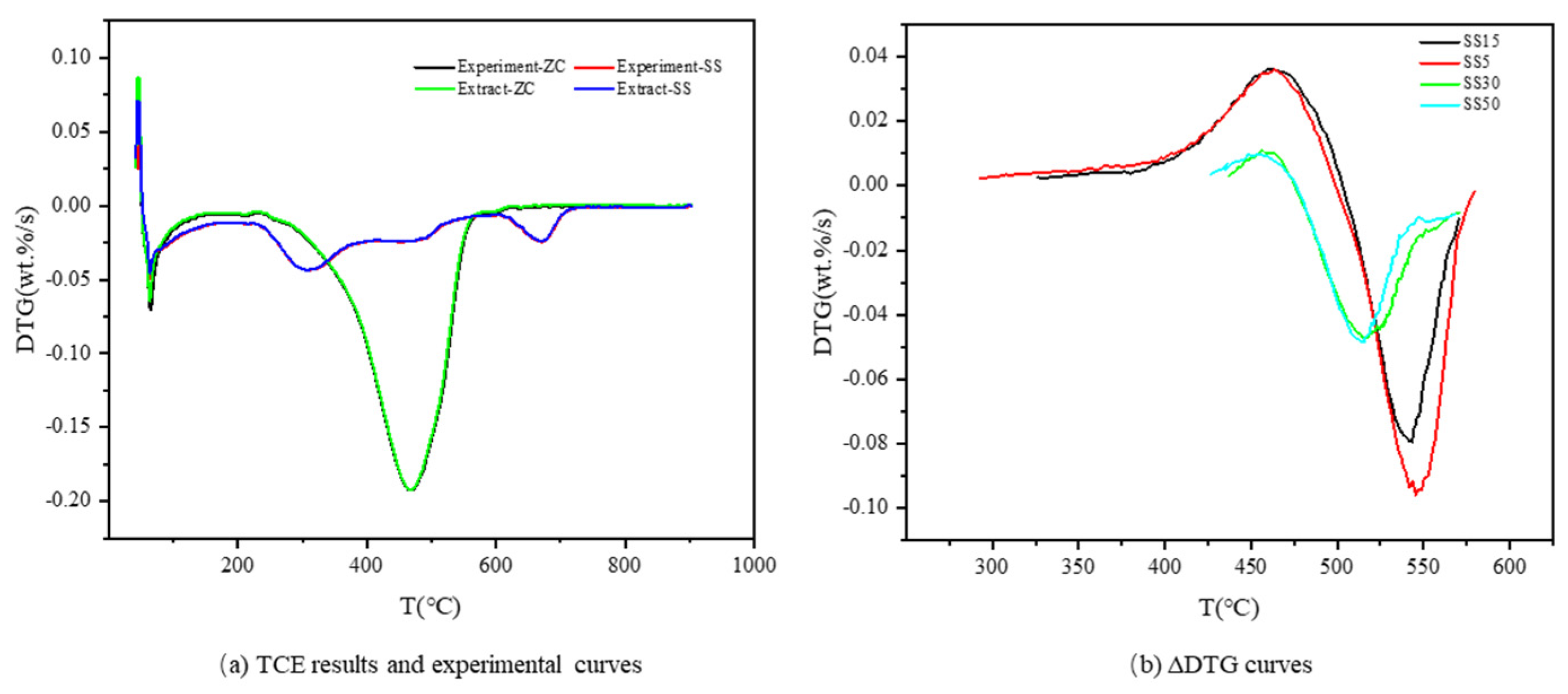
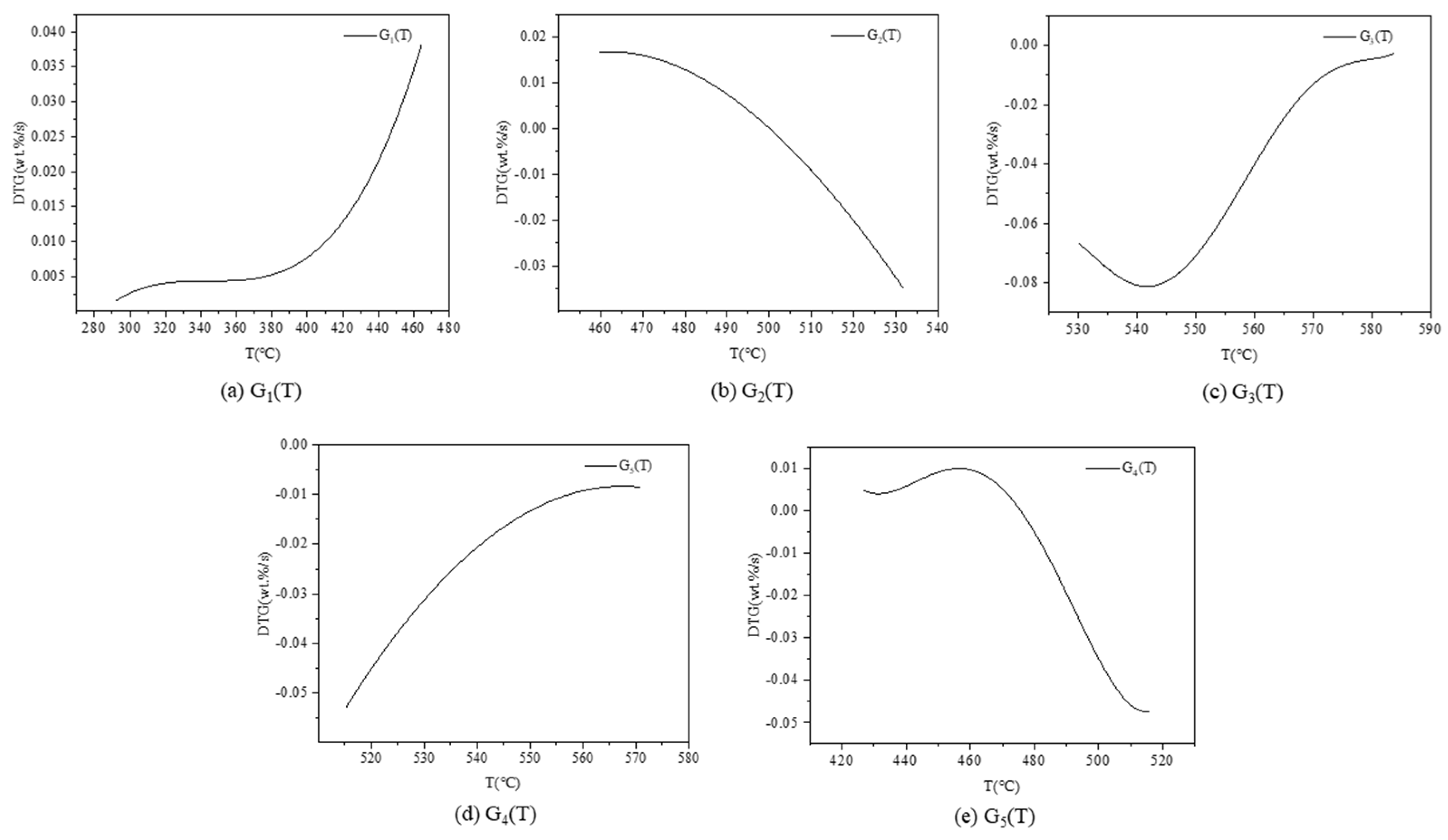
3.2. Thermogravimetric Curve Extraction
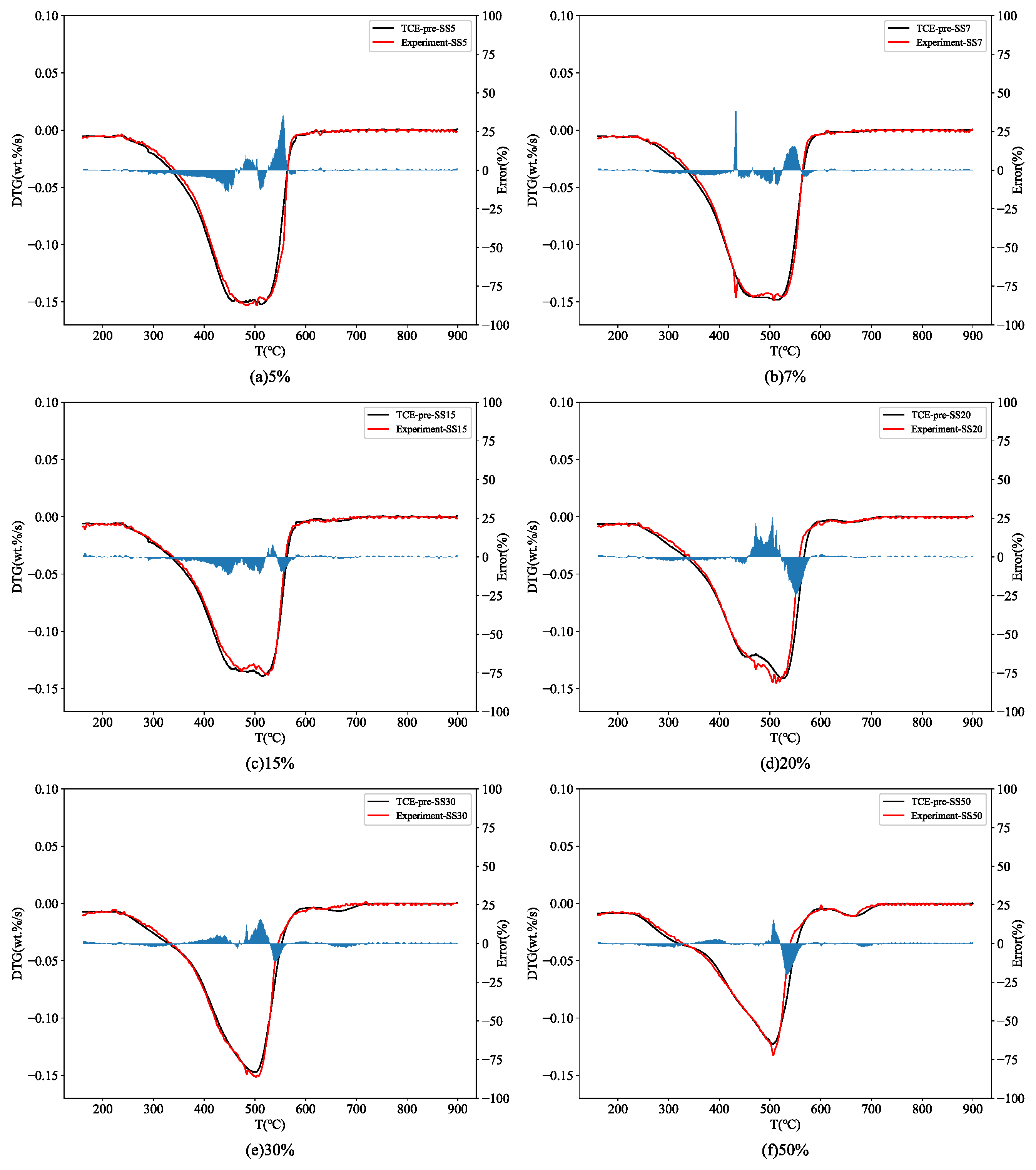
3.3. Thermal Behavior Prediction
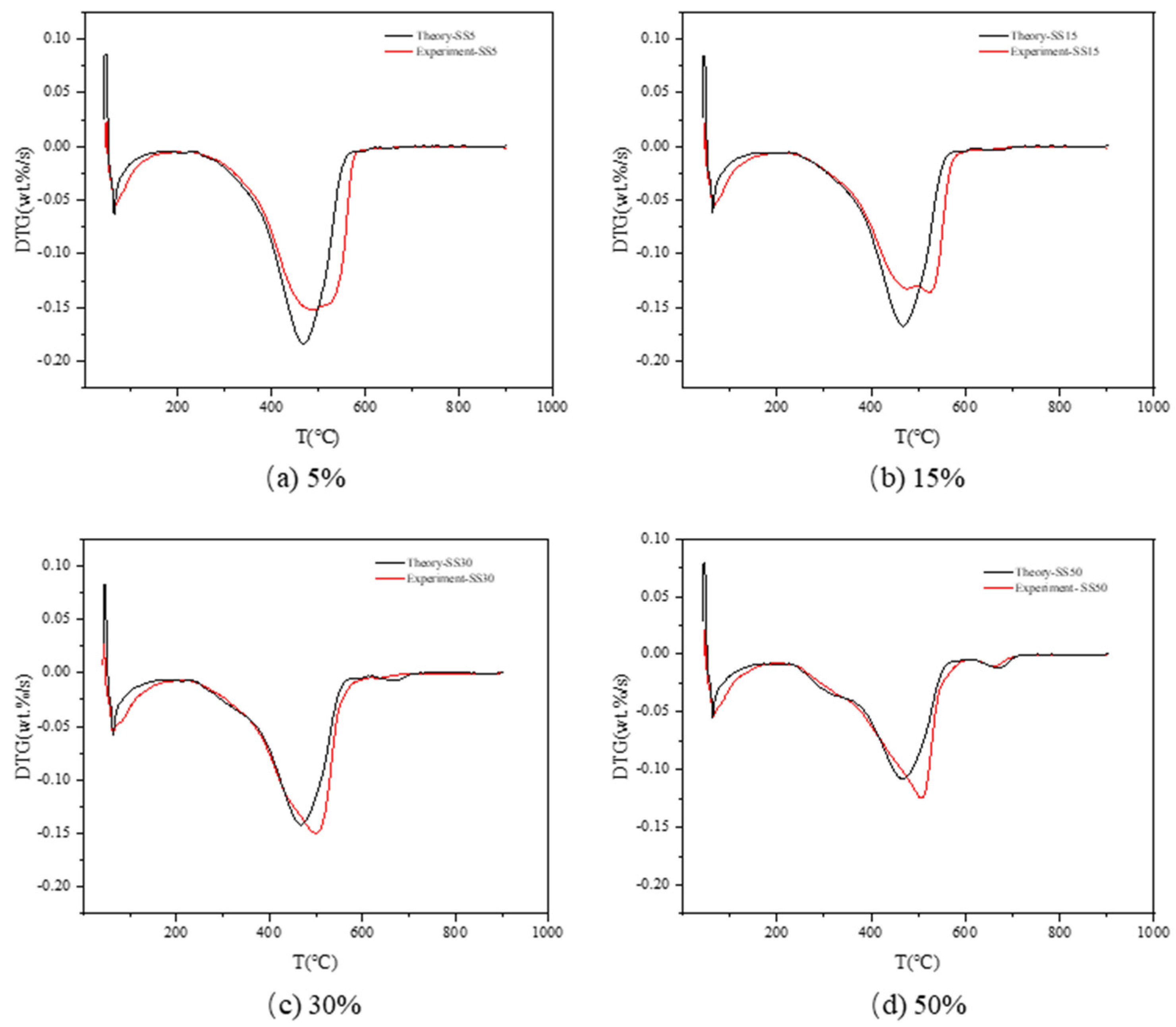
3.3.1. TCE
3.3.2. ANN Models
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| TGA | Thermogravimetric analysis | M | Moisture, wt% |
| TCE | Thermogravimetric curve extraction | A | Ash, wt% |
| ANN | Artificial neural networks | V | Volatile matter, wt% |
| DTG | The weight loss rate | FC | Fixed carbon, wt% |
| MAE | Mean absolute error | C | Carbon, wt% |
| MSE | Mean squared error | H | Hydrogen, wt% |
| TG | The total mass loss | N | Nitrogen, wt% |
| ZC | The mixture (0% sludge and 100% coal) | S | Sulfur, wt% |
| SS | The mixture (100% sludge and 0% coal) | O | Oxygen, wt% |
References
- Park, J.M.; Keel, S.; Yun, J.; Yun, J.H.; Lee, S.-S. Thermogravimetric study for the co-combustion of coal and dried sewage sludge. Korean J. Chem. Eng. 2017, 34, 2204–2210. [Google Scholar] [CrossRef]
- Liu, J.-z.; Wang, R.-k.; Gao, F.-y.; Zhou, J.-H.; Cen, K.-F. Rheology and thixotropic properties of slurry fuel prepared using municipal wastewater sludge and coal. Chem. Eng. Sci. 2012, 76, 1–8. [Google Scholar] [CrossRef]
- Merdun, H.; Laougé, Z.B.; Çığgın, A.S. Synergistic effects on co-pyrolysis and co-combustion of sludge and coal investigated by thermogravimetric analysis. J. Therm. Anal. Calorim. 2021, 146, 2623–2637. [Google Scholar] [CrossRef]
- Kang, J.H.; Kang, J.Y.; Lee, S.H.; Kim, B.T.; Lee, N.-H. A Study on the Evaluation of Fuel Characteristic and Economic Benefit for Co-combustion of Dried Sewage Sludge with Coal. J. Korea Org. Resour. Recycl. Assoc. 2014, 22, 11–19. [Google Scholar]
- Yang, Z.; Zhang, Y.; Liu, L.; Wang, X.; Zhang, Z. Environmental investigation on co-combustion of sewage sludge and coal gangue: SO2, NOx and trace elements emissions. Waste Manag. 2016, 50, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Liu, G.; Mian, M.M.; Zhou, C.; Sun, M.; Wu, D.; Liu, Y. Co-combustion of industrial coal slurry and sewage sludge: Thermochemical and emission behavior of heavy metals. Chemosphere 2019, 233, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, L.; Guo, J.; Wang, B.; Zhang, L.; Xiang, J.; Jin, Y. Thermogravimetric analysis of co-combustion between municipal sewage sludge and coal slime: Combustion characteristics, interaction and kinetics. Thermochim. Acta 2021, 706, 179056. [Google Scholar] [CrossRef]
- Wu, J.; Wang, B.; Cheng, F. Thermal and kinetic characteristics of combustion of coal sludge. J. Therm. Anal. Calorim. 2017, 129, 1899–1909. [Google Scholar] [CrossRef]
- Xiao, F.; Zhang, H.; Ma, P.; Wang, C. Fault diagnosis of variable rotating speed rolling bearing using generalized features based on MTFCE. Vib. Shock. 2022, 41, 152–159+188. [Google Scholar] [CrossRef]
- Yochum, M.; Renaud, C.; Jacquir, S. Automatic detection of P, QRS and T patterns in 12 leads ECG signal based on CWT. Biomed. Signal Process. Control. 2016, 25, 46–52. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; He, Y.; Huang, L.; Sun, S.; Sun, J.; Chang, K.; Kuo, J.; Huang, S.; Ning, X. Investigation of co-combustion characteristics of sewage sludge and coffee grounds mixtures using thermogravimetric analysis coupled to artificial neural networks modeling. Bioresour. Technol. 2017, 225, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Witek-Krowiak, A.; Chojnacka, K.; Podstawczyk, D.; Dawiec, A.; Pokomeda, K. Application of response surface methodology and artificial neural network methods in modelling and optimization of biosorption process. Bioresour. Technol. 2014, 160, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Aghbashlo, M.; Peng, W.X.; Tabatabaei, M.; Kalogirou, S.A.; Soltanian, S.; Hosseinzadeh-Bandbafha, H.; Mahian, O.; Lam, S.S. Machine learning technology in biodiesel research: A review. Prog. Energy Combust. Sci. 2021, 85, 100904. [Google Scholar] [CrossRef]
- Ni, Z.S.; Bi, H.B.; Jiang, C.L.; Sun, H.; Zhou, W.L.; Tian, J.J.; Lin, Q.Z. Investigation of co-combustion of sewage sludge and coffee industry residue by TG-FTIR and machine learning methods. Fuel 2022, 309, 122082. [Google Scholar] [CrossRef]
- Muravyev, N.V.; Luciano, G.; Ornaghi, H.L.; Svoboda, R.; Vyazovkin, S. Artificial Neural Networks for Pyrolysis, Thermal Analysis, and Thermokinetic Studies: The Status Quo. Molecules 2021, 26, 3727. [Google Scholar] [CrossRef]
- Yildiz, Z.; Uzun, H.; Ceylan, S.; Topcu, Y. Application of artificial neural networks to co-combustion of hazelnut husk-lignite coal blends. Bioresour. Technol. 2016, 200, 42–47. [Google Scholar] [CrossRef]
- Mian, I.; Li, X.; Jian, Y.; Dacres, O.D.; Zhong, M.; Liu, J.; Ma, F.; Rahman, N. Kinetic study of biomass pellet pyrolysis by using distributed activation energy model and Coats Redfern methods and their comparison. Bioresour. Technol. 2019, 294, 122099. [Google Scholar] [CrossRef]
- SP, S.P.; Swaminathan, G.; Joshi, V.V. Energy conservation–A novel approach of co-combustion of paint sludge and Australian lignite by principal component analysis, response surface methodology and artificial neural network modeling. Environ. Technol. Innov. 2020, 20, 101061. [Google Scholar]
- Xie, C.; Liu, J.; Zhang, X.; Xie, W.; Sun, J.; Chang, K.; Kuo, J.; Xie, W.; Liu, C.; Sun, S. Co-combustion thermal conversion characteristics of textile dyeing sludge and pomelo peel using TGA and artificial neural networks. Appl. Energy 2018, 212, 786–795. [Google Scholar] [CrossRef]
- Yan, J.-d.; Cui, H.; Yang, J.; Liu, Z. Research on pyrolysis behavior of Yanzhou coal using TG/MS. Zhongguo Kuangye Daxue Xuebao 2003, 32, 102–106. [Google Scholar]
- Bifan, H.; Liao, W.; Chuan, H. Study on combustion characteristics and kinetics of Chongqing municipal sewage sludge. Proc. CSEE 2010, 30, 32–37. [Google Scholar]
- Liu, J.; Jiang, X.; Shen, J.; Zhang, H. Chemical properties of superfine pulverized coal particles. Part 1. Electron paramagnetic resonance analysis of free radical characteristics. Adv. Powder Technol. 2014, 25, 916–925. [Google Scholar] [CrossRef]
- Folgueras, M.B.; Díaz, R.M. Influence of FeCl3 and lime added to sludge on sludge–coal pyrolysis. Energy 2010, 35, 5250–5259. [Google Scholar] [CrossRef]
- Xiao, P.; Xu, L.; Wang, X.; Chang, Z. Co-pyrolysis characteristics of coal and sludge blends using thermogravimetric analysis. Environ. Prog. Sustain. Energy 2015, 34, 1780–1789. [Google Scholar] [CrossRef]
- Coimbra, R.N.; Paniagua, S.; Escapa, C.; Calvo, L.F.; Otero, M. Thermogravimetric analysis of the co-pyrolysis of a bituminous coal and pulp mill sludge. J. Therm. Anal. Calorim. 2015, 122, 1385–1394. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, D.-K. An experimental study of oil recovery from sewage sludge by low-temperature pyrolysis in a fluidised-bed. Fuel 2003, 82, 465–472. [Google Scholar] [CrossRef]
- Lutz, H.; Romeiro, G.A.; Damasceno, R.N.; Kutubuddin, M.; Bayer, E. Low temperature conversion of some Brazilian municipal and industrial sludges. Bioresour. Technol. 2000, 74, 103–107. [Google Scholar] [CrossRef]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef] [PubMed]
- Hippert, H.S.; Pedreira, C.E.; Souza, R.C. Neural networks for short-term load forecasting: A review and evaluation. IEEE Trans. Power Syst. 2001, 16, 44–55. [Google Scholar] [CrossRef]
- Xu, T.; Wang, C.B.; Hong, D.K.; Li, S.; Yue, S. The synergistic effect during co-combustion of municipal sludge and coal: Experimental and ReaxFF molecular dynamic study. Energy 2023, 262, 125553. [Google Scholar] [CrossRef]
- Lee, J.G.; Jun, S.; Cho, Y.W.; Lee, H.; Kim, G.B.; Seo, J.B.; Kim, N. Deep Learning in Medical Imaging: General Overview. Korean J. Radiol. 2017, 18, 570–584. [Google Scholar] [CrossRef] [PubMed]

| Sample | Proximate Analysis (wt.%) | Ultimate Analysis (wt.%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | A | V | FC | V/FC | C | H | N | S | O | C/H | |
| SS | 2.60 | 63.63 | 33.57 | 0.20 | 167.85 | 12.07 | 1.86 | 1.41 | 0.23 | 18.20 | 6.49 |
| ZC | 1.68 | 3.63 | 44.06 | 50.63 | 0.87 | 70.75 | 4.65 | 1.57 | 0.55 | 17.17 | 15.22 |
| Condition | Value |
|---|---|
| Temperature | room temperature ~900 °C |
| Temperature accuracy | ±5 °C |
| Sample quality | 8 mg |
| Heating rate | 20 °C/min. |
| Air flow rate | 50 mL/min |
| Sample | ||||||
|---|---|---|---|---|---|---|
| ZC | 10.67 | 0.193 | 0.053 | 393.53 | 617.17 | 470.62 |
| SS5 | 8.53 | 0.152 | 0.054 | 391.93 | 615.88 | 481.23 |
| SS7 | 8.26 | 0.143 | 0.054 | 389.88 | 608.80 | 492.82 |
| SS10 | 9.14 | 0.158 | 0.054 | 389.19 | 612.35 | 511.66 |
| SS15 | 6.83 | 0.136 | 0.045 | 384.19 | 612.03 | 525.77 |
| SS20 | 7.77 | 0.140 | 0.050 | 383.04 | 611.71 | 513.61 |
| SS30 | 7.23 | 0.149 | 0.048 | 380.76 | 682.63 | 497.50 |
| SS50 | 4.56 | 0.123 | 0.037 | 374.64 | 703.64 | 512.11 |
| SS | 1.56 | 0.044 | 0.019 | 266.76 | 750.21 | 312.21 |
| Sample | (|dw/dt|)max (wt.%/s) | Tmax (°C) | R2 | |
|---|---|---|---|---|
| SS5 | Predicted value | −0.148 | 487.12 | 99.3% |
| Error (%) | 2.76 | 1.22 | ||
| SS7 | Predicted value | −0.146 | 495.39 | 99.7% |
| Error (%) | 2.01 | 2.48 | ||
| SS15 | Predicted value | −0.137 | 517.72 | 99.6% |
| Error (%) | 1.03 | 1.53 | ||
| SS20 | Predicted value | −0.141 | 524.50 | 97.4% |
| Error (%) | 0.50 | 2.21 | ||
| SS30 | Predicted value | −0.147 | 503.67 | 99.7% |
| Error (%) | 2.65 | 0.31 | ||
| SS50 | Predicted value | −0.122 | 506.68 | 96.5% |
| Error (%) | 0.89 | 1.06 |
| Model | Layers Size | MSE | MAE | R2 |
|---|---|---|---|---|
| ANN 1 | (2, 8, 1) | 0.00104 | 0.022132 | 0.9433 |
| ANN 2 | (2, 16, 1) | 0.001064 | 0.019928 | 0.942 |
| ANN 3 | (2, 32, 1) | 0.017427 | 0.09442 | 0.0501 |
| ANN 4 | (2, 64, 1) | 0.017925 | 0.096915 | 0.023 |
| ANN 5 | (2, 128, 1) | 0.013364 | 0.072531 | 0.2716 |
| ANN 6 | (4, 8, 1) | 0.000991 | 0.020615 | 0.946 |
| ANN 7 | (4, 16, 1) | 0.001184 | 0.019918 | 0.9355 |
| ANN 8 | (4, 32, 1) | 0.001295 | 0.021509 | 0.9294 |
| ANN 9 | (4, 64, 1) | 0.001787 | 0.022526 | 0.9026 |
| ANN 10 | (4, 128, 1) | 0.00101 | 0.018309 | 0.945 |
| ANN 11 | (8, 8, 1) | 0.00115 | 0.021255 | 0.9373 |
| ANN 12 | (8, 16, 1) | 0.000723 | 0.015966 | 0.9606 |
| ANN 13 | (8, 32, 1) | 0.001363 | 0.020865 | 0.9257 |
| ANN 14 | (8, 64, 1) | 0.000961 | 0.0179 | 0.9476 |
| ANN 15 | (8, 128, 1) | 0.001038 | 0.017388 | 0.9434 |
| ANN 16 | (16, 8, 1) | 0.00126 | 0.019521 | 0.9313 |
| ANN 17 | (16, 16, 1) | 0.001354 | 0.019959 | 0.9262 |
| ANN 18 | (16, 32, 1) | 0.000931 | 0.016625 | 0.9492 |
| ANN 19 | (16, 64, 1) | 0.000983 | 0.018556 | 0.9464 |
| ANN 20 | (16, 128, 1) | 0.001179 | 0.021205 | 0.9357 |
| ANN 21 | (32, 8, 1) | 0.001418 | 0.022844 | 0.9227 |
| ANN 22 | (32, 16, 1) | 0.000711 | 0.015996 | 0.9613 |
| ANN 23 | (32, 32, 1) | 0.001004 | 0.01887 | 0.9453 |
| ANN 24 | (32, 64, 1) | 0.000467 | 0.010857 | 0.9746 |
| ANN 25 | (32, 128, 1) | 0.000948 | 0.018843 | 0.9483 |
| Sample | (wt.%/s) | (°C) | R2 | |
|---|---|---|---|---|
| SS7 | Predicted value | −0.153 | 516.15 | 99.1% |
| Experimental value | −0.149 | 507.99 | ||
| Error (%) | 2.68 | 1.61 | ||
| SS30 | Predicted value | −0.136 | 488.77 | 94.9% |
| Experimental value | −0.151 | 502.13 | ||
| Error (%) | 9.93 | 2.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, C.; Lu, J.; Lin, X.; Ying, Y.; Ma, Y.; Yu, H.; Yu, W.; Huang, Q.; Li, X.; Yan, J. Thermal Behavior Prediction of Sludge Co-Combustion with Coal: Curve Extraction and Artificial Neural Networks. Processes 2023, 11, 2275. https://doi.org/10.3390/pr11082275
Wen C, Lu J, Lin X, Ying Y, Ma Y, Yu H, Yu W, Huang Q, Li X, Yan J. Thermal Behavior Prediction of Sludge Co-Combustion with Coal: Curve Extraction and Artificial Neural Networks. Processes. 2023; 11(8):2275. https://doi.org/10.3390/pr11082275
Chicago/Turabian StyleWen, Chaojun, Junlin Lu, Xiaoqing Lin, Yuxuan Ying, Yunfeng Ma, Hong Yu, Wenxin Yu, Qunxing Huang, Xiaodong Li, and Jianhua Yan. 2023. "Thermal Behavior Prediction of Sludge Co-Combustion with Coal: Curve Extraction and Artificial Neural Networks" Processes 11, no. 8: 2275. https://doi.org/10.3390/pr11082275




