Antibacterial Effect of Polyvinyl Alcohol/Biochar–Nano Silver/Sodium Alginate Gel Beads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Bacteria
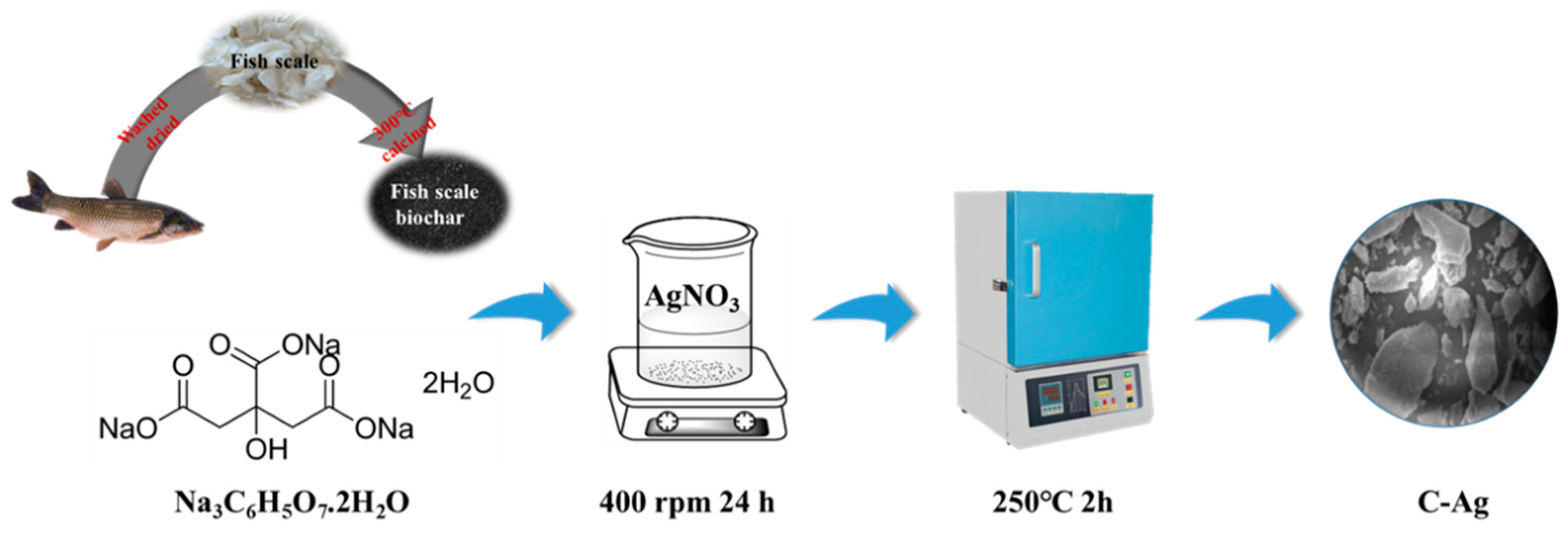
2.2. Preparation of C-Ag
2.3. Preparation of SA/PVA/C–Ag Gel Beads
2.4. Characterization of SA/PVA/C–Ag Gel Beads
2.5. Antibacterial Testing
2.6. The Materials and C-Ag Dosage Optimization of the SA/PVA/C–Ag Gel Beads
2.7. The Dosage and Time Optimization of the SA/PVA/C–Ag Gel Beads
2.8. Antibacterial Reusability and Stability
2.9. Swelling Ratio of SA/PVA/C-Ag Gel Beads
2.10. Morphological Properties, Water Loss, and Shrinkage of SA/PVA/C-Ag Gel Beads
2.11. Degradation of SA/PVA/C-Ag Gel Beads
3. Result and Discussion
3.1. Characterization of SA/PVA/C-Ag Gel Bead Composite
Fourier-Transform Infrared (FT-IR) Spectrometry Analysis
3.2. Antibacterial Properties
3.2.1. Effect of the Materials and C-Ag Dosage
3.2.2. The Dosage and Time Optimization of the SA/PVA/C–Ag Gel Beads
3.2.3. Antibacterial Reusability and Stability of SA/PVA/C–Ag Gel Beads
3.3. Physical Properties
3.3.1. Swelling Ratio of SA/PVA/C-Ag Gel Beads
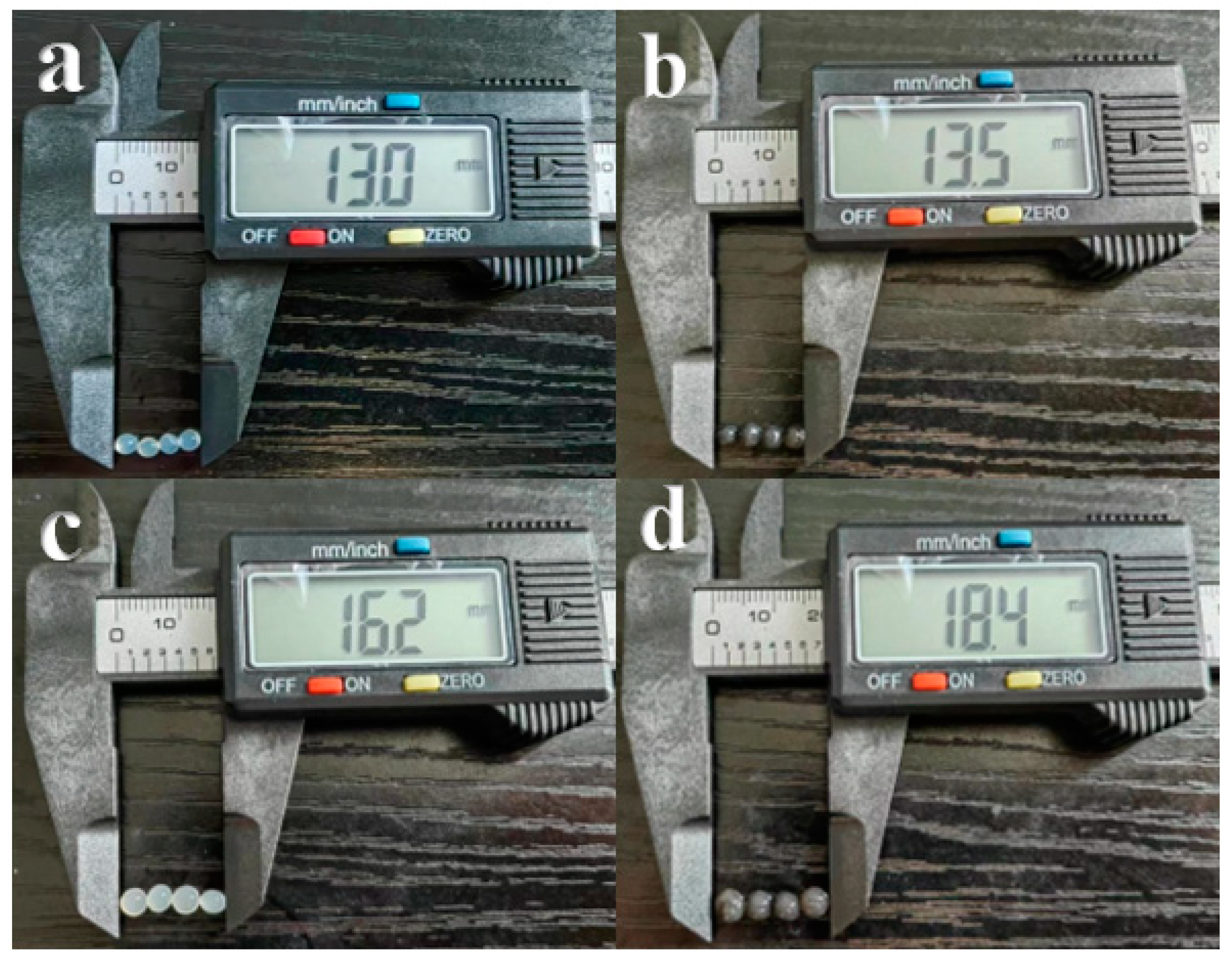
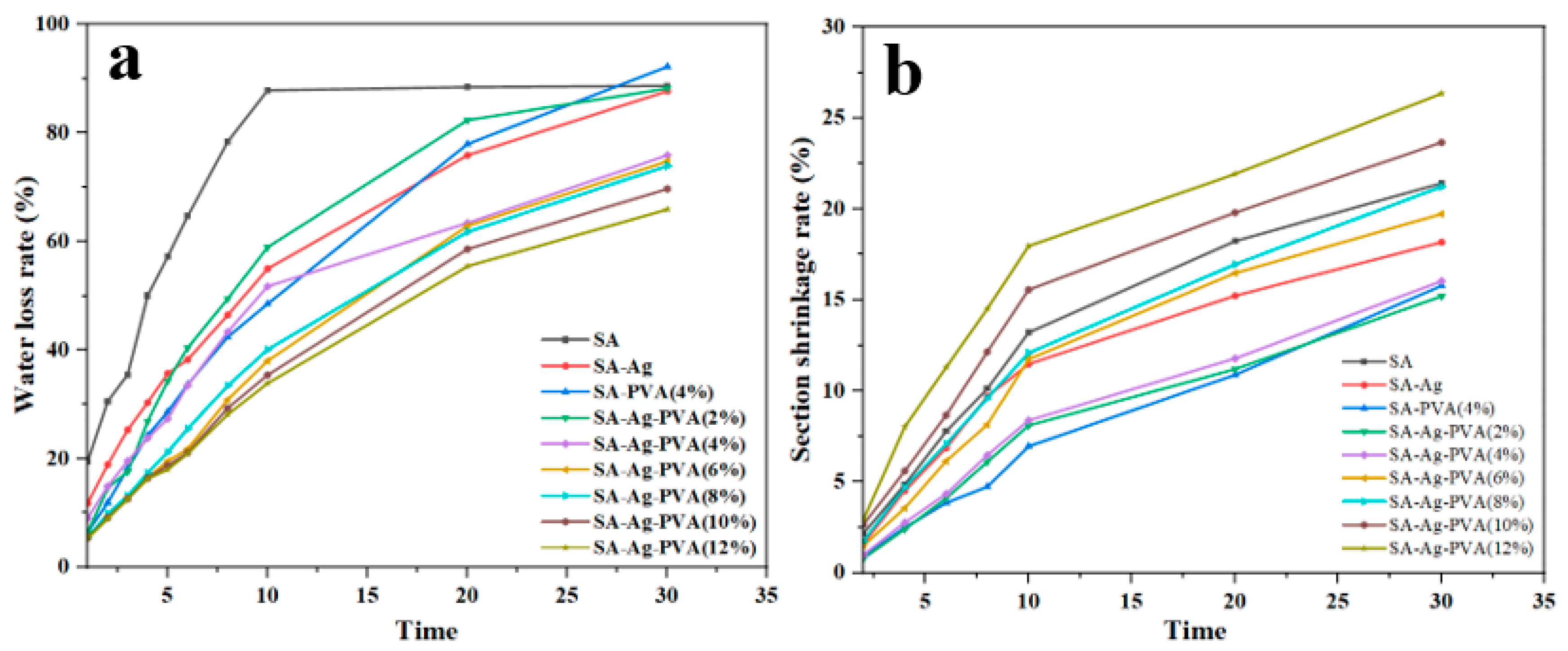
3.3.2. Morphological Properties, Shrinkage, and Water Loss of SA/PVA/C-Ag Gel Beads
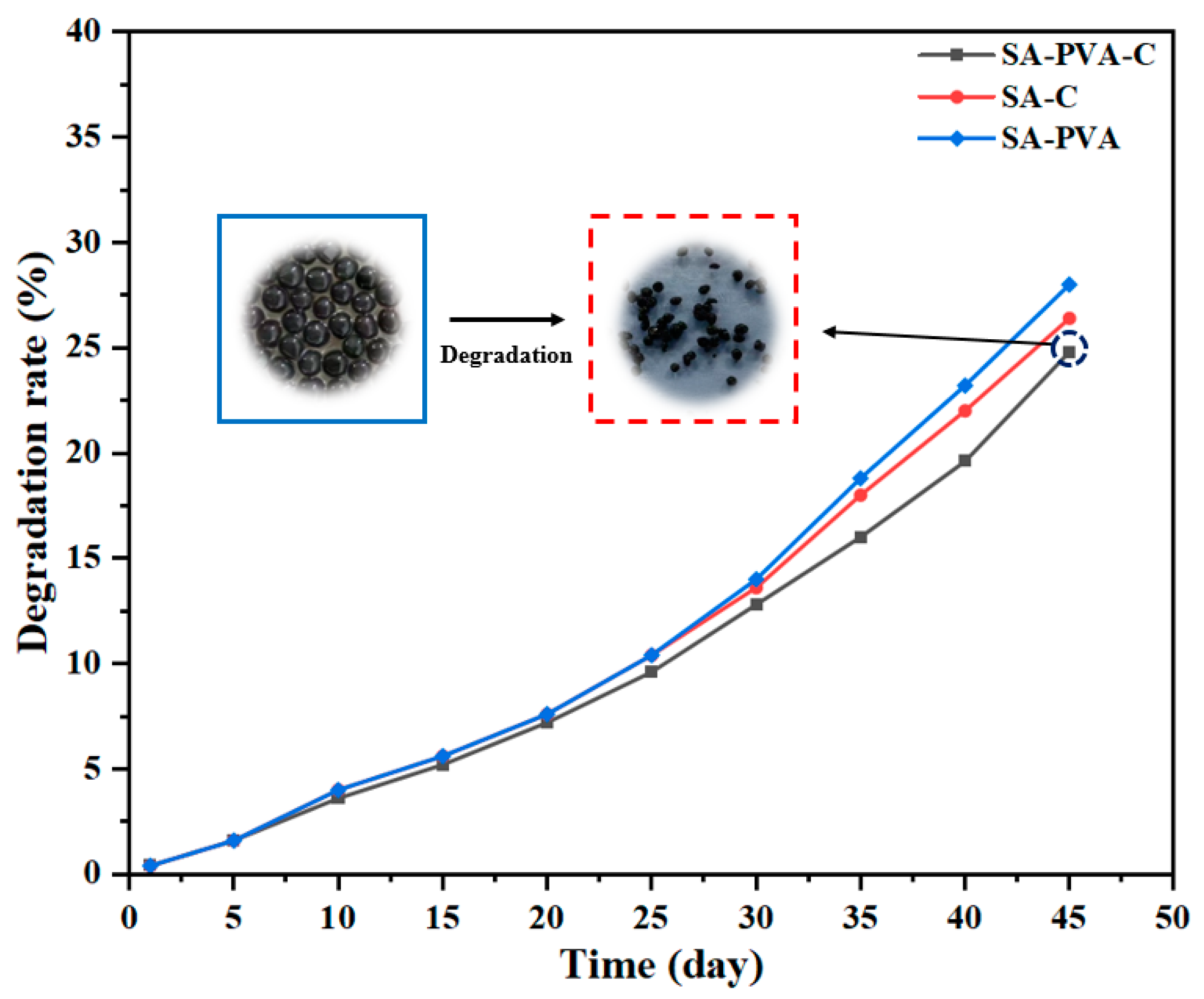
3.3.3. The Degradation of SA/PVA/C–Ag Gel Beads
3.4. The Mechanism of Bacterial Inhibition by SA/PVA/C-Ag Gel Beads
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Q.; Zhang, H.; Ma, M.; He, Y.; Jia, J.; Hu, Q.; Gong, Y. Critical assessment of protozoa contamination and control measures in mass culture of the diatom Phaeodactylum tricornutum. Bioresour. Technol. 2022, 359, 127460. [Google Scholar] [CrossRef]
- Yang, W.; Cai, C.; Guo, Y.; Wu, H.; Guo, Y.; Dai, X. Diversity and fate of human pathogenic bacteria, fungi, protozoa, and viruses in full-scale sludge treatment plants. J. Clean. Prod. 2022, 380, 134990. [Google Scholar] [CrossRef]
- Rodwihok, C.; Suwannakeaw, M.; Charoensri, K.; Wongratanaphisan, D.; Woo, S.W.; Kim, H.S. Alkali/zinc-activated fly ash nanocomposites for dye removal and antibacterial applications. Bioresour. Technol. 2021, 331, 125060. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biomass type effect on biochar surface characteristic and adsorption capacity relative to silver and copper. Fuel 2020, 78, 118168. [Google Scholar] [CrossRef]
- Kiehbadroudinezhad, M.; Merabet, A.; Hosseinzadeh-Bandbafha, H. A life cycle assessment perspective on biodiesel production from fish wastes for green microgrids in a circular bioeconomy. Bioresour. Technol. Rep. 2023, 21, 101303. [Google Scholar] [CrossRef]
- Chowdhury, P.; Viraraghavan, T.; Srinivasan, A. Biological treatment processes for fish processing wastewater—A review. Bioresour. Technol. 2010, 101, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Ahmad, R.; Singh, R.; Sood, S.; Khare, S.K. Biologically synthesized silver nanoparticles by Streptomyces sp. EMB24 extracts used against the drug-resistant bacteria. Bioresour. Technol. Rep. 2021, 15, 100753. [Google Scholar] [CrossRef]
- Mobaraki, F.; Momeni, M.; Jahromi, M.; Kasmaie, F.M.; Barghbani, M.; Yazdi, M.E.T.; Meshkat, Z.; Shandiz, F.H.; Hosseini, S.M. Apoptotic, antioxidant and cytotoxic properties of synthesized AgNPs using green tea against human. Process Biochem. 2022, 119, 106–118. [Google Scholar] [CrossRef]
- Ashra, N.; Ahmad, F.; Lu, Y.; Yin, D.C. Bacterial extracellular protein interacts with silver ions to produce protein-encapsulated bactericidal AgNPs. Process Biochem. 2022, 106, 120–129. [Google Scholar] [CrossRef]
- Xia, D.; Liu, Y.; Cheng, X.; Gu, P.; Chen, Q.; Zhang, Z. Temperature-tuned fish-scale biochar with two-dimensional homogeneous porous structure: A promising uranium extractant. Appl. Surf. Sci. 2022, 591, 153136. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Maidannyk, V.; Miao, S. Effect of concentrations of alginate, soy protein isolate and sunflower oil on water loss, shrinkage, elastic and structural properties of alginate-based emulsion gel beads during gelation. Food Hydrocoll. 2020, 108, 105998. [Google Scholar] [CrossRef]
- Pal, A.; Khanum, F. Covalent immobilization of xylanase on glutaraldehyde activated alginate beads using response surface methodology: Characterization of immobilized enzyme. Process Biochem. 2011, 46, 1315–1322. [Google Scholar] [CrossRef]
- Bu, X.; Guan, M.; Dai, L.; Ji, N.; Qin, Y.; Xu, X.; Xiong, L.; Shi, R.; Sun, Q. Fabrication of starch-based emulsion gel beads by an inverse gelation technique for loading proanthocyanidin and curcumin. Food Hydrocoll. 2023, 137, 1083366. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, Y.T. Se(VI) reduction by continuous-flow reactors packed with Shigella fergusonii strain TB42616 immobilized by Ca2+-alginate gel beads. Process Biochem. 2020, 91, 46–56. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Miao, S. The impact of pH on mechanical properties, storage stability and digestion of alginate-based and soy protein isolate-stabilized emulsion gel beads with encapsulated lycopene. Food Chem. 2022, 372, 131262. [Google Scholar] [CrossRef]
- Jiang, Z.; Zheng, Z.; Wu, J.; Li, X.; Yu, H.; Shen, J. Synthesis, characterization and performance of microorganism-embedded biocomposites of LDH-modified PVA/SA hydrogel beads for enhanced biological nitrogen removal process. Process Biochem. 2022, 121, 542–552. [Google Scholar] [CrossRef]
- Wang, J.; Liang, J.; Sun, L.; Li, G.; Temmink, H.; Rijnaarts, H.H.M. Granule-based immobilization and activity enhancement of anammox biomass via PVA/CS and PVA/CS/Fe gel beads. Bioresour. Technol. 2020, 309, 123448. [Google Scholar] [CrossRef]
- Jeong, D.; Jang, A. Mitigation of self-shading effect in embedded optical fiber in Chlorella sorokiniana immobilized polyvinyl alcohol gel beads. Chemosphere 2021, 283, 131195. [Google Scholar] [CrossRef]
- Wang, Y.; Su, J.; Ali, A.; Chang, Q.; Bai, Y.; Gao, Z. Enhanced nitrate; manganese, and phenol removal by polyvinyl alcohol/sodium alginate with biochar gel beads immobilized bioreactor: Performance, mechanism, and bacterial diversity. Bioresour. Technol. 2022, 348, 126818. [Google Scholar] [CrossRef] [PubMed]
- Uysal, U.; Hamamcı, H. Succinic acid production from cheese whey via fermentation by using alginate immobilized Actinobacillus succinogenes. Bioresour. Technol. Rep. 2021, 16, 100829. [Google Scholar] [CrossRef]
- Hazrati, R.; Davaran, S.; Omidi, Y. Bioactive functional scaffolds for stem cells delivery in wound healing and skin regeneration. React. Funct. Polym. 2022, 174, 105233. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X.; Zhang, L.; Hu, Z.; Zhong, L.; Xue, J. Preparation and bacteriostatic research of porous polyvinyl alcohol/biochar/nanosilver polymer gel for drinking water treatment. Sci. Rep. 2021, 11, 12205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, Y. Synthesis and Characteristics of a Fish Scale-Based Biochar–Nanosilver Antibacterial Material. Processes 2023, 11, 111992. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, S.; Hu, Z.; Zhong, L.; Wang, Y.; Zhang, X.; Xue, J. Preparation of polyvinyl alcohol/bacterial-cellulose-coated biochar–nanosilver antibacterial composite membranes. Appl. Sci. 2020, 10, 752. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhang, G.; Liu, J.; Gao, H.; Song, C.; Du, H.; Zhang, L.; Gong, Z.; LÜ, Y. Synthesis characteristics, and antibacterial activity of a rare-earth samarium/silver/titanium dioxide inorganic nanomaterials. J. Rare Earths 2014, 32, 727–732. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, L.; Zhong, L.; Zhou, Y.; Xue, J.; Li, Y. Preparation of an antibacterial chitosan-coated biochar-nanosilver composite for drinking water purification. Carbohydr. Polym. 2019, 219, 290–297. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, X.; Tian, H.; Zhong, L.; Ma, C.; Zhou, Y.; Chen, S.; Li, D. Synthesis of antibacterial film CTS/PVP/TiO2/Ag for drinking water system. Carbohydr. Polym. 2012, 89, 1060–1066. [Google Scholar] [CrossRef]
- Afshari, M.J.; Sabzi, M.; Jiang, L.; Behshad, Y.; Zanjanijam, A.R.; Mahdavinia, G.R.; Ahmadi, M. Incorporation of dynamic boronate links and Ag nanoparticles into PVA hydrogels for pH-Regulated and prolonged release of methotrexate. J. Drug Deliv. Sci. Technol. 2021, 63, 102502. [Google Scholar] [CrossRef]
- Fuhrmann, P.L.; Powell, J.; Rousseau, D. Structure and rheology of oil-continuous capillary suspensions containing water-swellable cellulose beads and fibres. Food Hydrocoll. 2023, 139, 108503. [Google Scholar] [CrossRef]
- Zeng, H.; Sun, S.; Xu, K.; Zhao, W.; Hao, R.; Zhang, J.; Li, D. Iron-loaded magnetic alginate-chitosan double-gel interpenetrated porous beads for phosphate removal from water: Preparation, adsorption behavior and pH stability. React. Funct. Polym. 2022, 177, 105328. [Google Scholar] [CrossRef]
- Min, C.; Zhang, C.; Cao, Y.; Li, H.; Pu, H.; Huang, J.; Xiong, Y.L. Rheological; textural, and water-immobilizing properties of mung bean starch and flaxseed protein composite gels as potential dysphagia food: The effect of Astragalus polysaccharide. Int. J. Biol. Macromol. 2023, 239, 124236. [Google Scholar] [CrossRef]
- Sun, G.; Cheng, L.; Tong, M.; Chen, L.; Luo, J.; Liu, R. Shrinkage stress of thermal cured epoxy resin reduced by addition of functional hollow microspheres. Prog. Org. Coat. 2023, 178, 107446. [Google Scholar] [CrossRef]
- Latif, A.; Maqbool, A.; Sun, K.; Si, Y. Immobilization of Trametes Versicolor laccase on Cu-alginate beads for biocatalytic degradation of bisphenol A in water: Optimized immobilization, degradation and toxicity assessment. J. Environ. Chem. Eng. 2022, 10, 107089. [Google Scholar] [CrossRef]
- Xiang, X.; Yi, X.; Zheng, W.; Li, Y.; Zhang, C.; Wang, X.; Chen, Z.; Huang, M.; Ying, G.G. Enhanced biodegradation of thiamethoxam with a novel polyvinyl alcohol (PVA)/sodium alginate (SA)/biochar immobilized Chryseobacterium sp. H5. J. Hazard. Mater. 2023, 443, 130247. [Google Scholar] [CrossRef] [PubMed]
- Ionita, M.; Pandele, M.A.; Iovu, H. Sodium alginate/graphene oxide composite films with enhanced thermal and mechanical properties. Carbohydr. Polym. 2013, 94, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Kuila, S.B.; Ray, S.K. Dehydration of dioxane by pervaporation using filled blend membranes of polyvinyl alcohol and sodium alginate. Carbohydr. Polym. 2014, 101, 1154–1165. [Google Scholar] [CrossRef]
- Anusuya, N.; Pragathiswaran, C.; Mary, J.V. A potential catalyst-TiO2/ZnO based chitosan gel beads for the reduction of nitro-aromatic compounds aggregated sodium borohydride and their antimicrobial activity. J. Mol. Struct. 2021, 1236, 130197. [Google Scholar] [CrossRef]
- Wang, J.; Liang, J.; Sun, L.; Shen, J.; He, Z. Enhancing anammox resistance to low operating temperatures with the use of PVA gel beads. Sci. Total Environ. 2021, 774, 144826. [Google Scholar] [CrossRef]
- Ingle, J.; Patel, U.D. Electrochemical reduction of nitrate in the presence of silver-coated polyvinyl alcohol beads as a spatially suspended catalyst. J. Water Process Eng. 2022, 49, 103082. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against Gram-Positive and Gram-Negative bacteria: A preliminary study. J. Nanomater. 2015, 2015, 720654. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Su, P.; Xu, T.; Yuan, L.; Qiao, M.; Yang, B.; Zhao, X. Comprehensive study on the role of reactive oxygen species and active chlorine species on the inactivation and subcellular damage of E. coli in electrochemical disinfection. Sep. Purif. Technol. 2023, 304, 122408. [Google Scholar] [CrossRef]
- Saha, S.; Malik, M.M.; Qureshi, M.S. Study of synergistic effects of antibiotics and triangular shaped silver nanoparticles, synthesized using UV-light irradiation, on S. Aureus and P. Aeruginosa. Mater. Today Proc. 2019, 18, 920–927. [Google Scholar] [CrossRef]
- Huang, J.-F.; Shi, Q.-S.; Feng, J.; Chen, M.-J.; Li, W.-R.; Li, L.-Q. Facile pyrolysis preparation of rosin-derived biochar for supporting silver nanoparticles with antibacterial activity. Compos. Sci. Technol. 2017, 145, 89–95. [Google Scholar] [CrossRef]
- Troïa, T.; Siad, J.; Di Giorgio, C.; Brunel, J.M. Design and synthesis of new polyamine quinoline antibiotic enhancers to fight resistant gram-negative P. aeruginosa bacteria. Eur. J. Med. Chem. Rep. 2022, 5, 100054. [Google Scholar] [CrossRef]
- Masilan, K.; Neethiselvan, N.; Shakila, R.J.; Muralidharan, N.; Karthy, A.; Ravikumar, T.; Parthiban, F. Investigation on the coacervation of fish scale gelatin hydrogel with seafood waste hydrolysates for the development of artificial fish bait: Physico-chemical, thermodynamic, and morpho-structural properties. J. Indian Chem. Soc. 2022, 99, 100783. [Google Scholar] [CrossRef]
- Thakur, K.; Kalia, S.; Kaith, B.S.; Pathania, D.; Kumar, A.; Thakur, P.; Knittel, C.E.; Schauer, C.L.; Totaro, G. The development of antibacterial and hydrophobic functionalities in natural fibers for fiber-reinforced composite materials. J. Environ. Chem. Eng. 2016, 4, 1743–1752. [Google Scholar] [CrossRef]
- Jin, F.; Liao, S.; Li, W.; Jiang, C.; Wei, Q.; Xia, X.; Wang, Q. Amphiphilic sodium alginate-polylysine hydrogel with high antibacterial efficiency in a wide pH range. Carbohydr. Polym. 2023, 299, 120195. [Google Scholar] [CrossRef]
- Vityazev, F.V.; Khramova, D.S.; Saveliev, N.Y.; Ipatova, E.A.; Burkov, A.A.; Beloserov, V.S.; Belyi, V.A.; Kononov, L.O.; Martinson, E.A.; Litvinets, S.G.; et al. Pectin-glycerol gel beads: Preparation, characterization and swelling behaviour. Carbohydr. Polym. 2020, 238, 116166. [Google Scholar] [CrossRef]
- Tuyen, N.V.; Ryu, J.H.; Yae, J.B.; Kim, H.G.; Hong, S.W.; Ahn, D.H. Nitrogen removal performance of anammox process with PVA–SA gel bead crosslinked with sodium sulfate as a biomass carrier. J. Ind. Eng. Chem. 2018, 67, 326–332. [Google Scholar] [CrossRef]
- Chen, G.; He, L.; Zhang, P.; Zhang, J.; Mei, X.; Wang, D.; Zhang, Y.; Ren, X.; Chen, Z. Encapsulation of green tea polyphenol nanospheres in PVA/alginate hydrogel for promoting wound healing of diabetic rats by regulating PI3K/AKT pathway. Mater. Sci. Eng. C 2020, 110, 110686. [Google Scholar] [CrossRef]
- Deng, M.-J.; Wu, Y.-S. 2.2 V wearable asymmetric supercapacitors based on Co oxide//Mn oxide electrodes and a PVA-KOH-urea-LiClO4 alkaline gel electrolyte. J. Alloys Compd. 2023, 945, 169285. [Google Scholar] [CrossRef]
- Puguan, J.M.; Yu, X.; Kim, H. Characterization of structure, physico-chemical properties and diffusion behavior of Ca-Alginate gel beads prepared by different gelation methods. J. Colloid Interface Sci. 2014, 432, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Lević, S.; Lijaković, I.P.; Đorđević, V.; Rac, V.; Rakić, V.; Knudsen, T.Š.; Pavlović, V.; Bugarski, B.; Nedović, V. Characterization of sodium alginate/d-limonene emulsions and respective calcium alginate/d-limonene beads produced by electrostatic extrusion. Food Hydrocoll. 2015, 45, 111–123. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, D.; Liao, R.; Zhang, G.; Zhang, M.; Li, X. Study of adhesive self-degrading gel for wellbore sealing. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129567. [Google Scholar] [CrossRef]
- Li, K.; Zhang, B.; Yang, Z.; Jiang, X.; Li, X. Degradation behaviors of silicone gel encapsulation material with moisture intrusion. Polym. Degrad. Stab. 2022, 206, 110197. [Google Scholar] [CrossRef]
- Li, Y.; Shan, P.; Yu, F.; Li, H.; Peng, L. Fabrication and characterization of waste fish scale-derived gelatin/sodium alginate/carvacrol loaded ZIF-8 nanoparticles composite films with sustained antibacterial activity for active food packaging. Int. J. Biol. Macromol. 2023, 230, 123192. [Google Scholar] [CrossRef]
- Li, Q.; Di, J.; Liao, X.; Ni, J.; Li, Q.; He, Y.C.; Ma, C. Exploration of benign deep eutectic solvent–water systems for the highly efficient production of furfurylamine from sugarcane bagasse via chemoenzymatic cascade catalysis. Green Chem. 2021, 23, 8154–8168. [Google Scholar] [CrossRef]
- Ji, L.; Tang, Z.; Yang, D.; Ma, C.; He, Y.C. Improved one-pot synthesis of furfural from corn stalk with heterogeneous catalysis using corn stalk as biobased carrier in deep eutectic solvent-water system. Bioresour. Technol. 2021, 340, 125691. [Google Scholar] [CrossRef] [PubMed]
- Zha, J.; Fan, B.; He, J.; He, Y.C.; Ma, C. Valorization of biomass to furfural by chestnut shell-based solid acid in methyl isobutyl ketone-water-sodium chloride system. Appl. Biochem. Biotechnol. 2022, 194, 2021–2035. [Google Scholar] [CrossRef]
- Shen, J.; Gao, R.; He, Y.C.; Ma, C. Efficient synthesis of furfural from waste biomasses by sulfonated crab shell-based solid acid in a sustainable approach. Ind. Crops Prod. 2023, 202, 116989. [Google Scholar] [CrossRef]
- Gong, L.; Zha, J.; Pan, L.; Ma, C.; He, Y.C. Highly efficient conversion of sunflower stalk-hydrolysate to furfural by sunflower stalk residue-derived carbonaceous solid acid in deep eutectic solvent/organic solvent system. Bioresour. Technol. 2022, 351, 126945. [Google Scholar] [CrossRef]
- Yang, D.; Yang, L.; Li, Q.; Fan, B.; Ma, C.; He, Y.C. Preparation of a biobased polyelectrolyte complex from chitosan and sodium carboxymethyl cellulose and its antibacterial characteristics. Int. J. Biol. Macromol. 2023, 227, 524–534. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; He, Y.C.; Ma, C. Synthesis of furoic acid from biomasses by sequential catalysis with fish scale-rice husk-based heterogeneous chemocatalyst and dehydrogenase biocatalyst. Ind. Crops Prod. 2023, 202, 117033. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Tripathi, A.; Shweta; Singh, S.; Singh, Y.; Vishwakarma, K.; Yadav, G.; Sharma, S.; Singh, V.K.; Mishra, R.K.; et al. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: A concentric review. Front. Microbiol. 2017, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.; Liang, Y.; Yang, D.; Liu, Y. Facile fabrication of rice husk based silicon dioxide nanospheres loaded with silver nanoparticles as a rice antibacterial agent. Sci. Rep. 2016, 6, 21423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, B.; Hashmi, A.; Khan, M.S.; Musarrat, J. ROS mediated destruction of cell membrane, growth and biofilms of human bacterial pathogens by stable metallic AgNPs functionalized from bell pepper extract and quercetin. Adv. Powder Technol. 2018, 29, 1601–1616. [Google Scholar] [CrossRef]
- Velidandi, A.; Pabbathi, N.P.P.; Dahariya, S.; Baadhe, R.R. Green synthesis of novel Ag–Cu and Ag–Znbimetallic nanoparticles and their in vitro biological, eco-toxicity and catalytic studies. Nano-Struct. Nano-Objects 2021, 26, 100687. [Google Scholar] [CrossRef]
- Zhang, Y.; Dang, Q.; Liu, C.; Yan, J.; Cha, D.; Liang, S.; Li, X.; Fan, B. Synthesis characterization, and evaluation of poly(aminoethyl) modified chitosan and its hydrogel used as antibacterial wound dressing. Int. J. Biol. Macromol. 2017, 102, 457–467. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Z.; Wei, X.; Sui, Z.; Geng, J.; Xiao, J.; Huang, D. Antibacterial and antioxidant water-degradable food packaging chitosan film prepared from American cockroach. Food Biosci. 2022, 49, 101893. [Google Scholar] [CrossRef]
- Ma, G.; Qian, B.; Yang, J.; Hu, C.; Nie, J. Synthesis and properties of photosensitive chitosan derivatives. Int. J. Biol. Macromol. 2010, 46, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; An, Q.; Qian, L.; He, B.; Xiao, H. Synergistic effects of chitosan–guanidine complexes on enhancing antimicrobial activity and wet-strength of paper. Bioresour. Technol. 2010, 101, 5693–5700. [Google Scholar] [CrossRef] [PubMed]
- Khalaki, M.A.; Moameri, M.; Ghorbani, A.; Alagoz, S.M.; Dolatabadi, N.; Lajayer, B.A.; van Hullebusch, E.D. Chapter 8—Effects, uptake and translocation of Ag-based nanoparticles in plants. In Toxicity of Nanoparticles in Plants; Rajput, V.D., Minkina, T., Sushkova, S., Mandzhieva, S.S., Rensing, C., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 171–192. [Google Scholar]
- Xie, W.; Zhao, K.; Xu, L.; Gao, N.; Zhao, H.; Gong, Z.; Yu, L.; Jiang, J. Oxalic acid cross-linked sodium alginate and carboxymethyl chitosan hydrogel membrane for separation of dye/NaCl at high NaCl concentration. Chin. Chem. Lett. 2022, 33, 1951–1955. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, Z.; Zhao, L.; Huang, M.; Dai, L.; Tian, D.; Zou, J.; Zeng, Y.; Hu, J.; Shen, F. Tailoring biochar by PHP towards the oxygenated functional groups (OFGs)-rich surface to improve adsorption performance. Chin. Chem. Lett. 2022, 33, 3097–3100. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Zhang, Z.; He, Y. Antibacterial Effect of Polyvinyl Alcohol/Biochar–Nano Silver/Sodium Alginate Gel Beads. Processes 2023, 11, 2330. https://doi.org/10.3390/pr11082330
Xie L, Zhang Z, He Y. Antibacterial Effect of Polyvinyl Alcohol/Biochar–Nano Silver/Sodium Alginate Gel Beads. Processes. 2023; 11(8):2330. https://doi.org/10.3390/pr11082330
Chicago/Turabian StyleXie, Licheng, Zhichao Zhang, and Yucai He. 2023. "Antibacterial Effect of Polyvinyl Alcohol/Biochar–Nano Silver/Sodium Alginate Gel Beads" Processes 11, no. 8: 2330. https://doi.org/10.3390/pr11082330




