Thermodynamic Analysis of Hydrogen Production from Bio-Oil Steam Reforming Utilizing Waste Heat of Steel Slag
Abstract
:1. Introduction
2. Methodology
2.1. Materials
2.2. Thermodynamic Method
2.3. Performance Evaluation
3. Results and Discussion
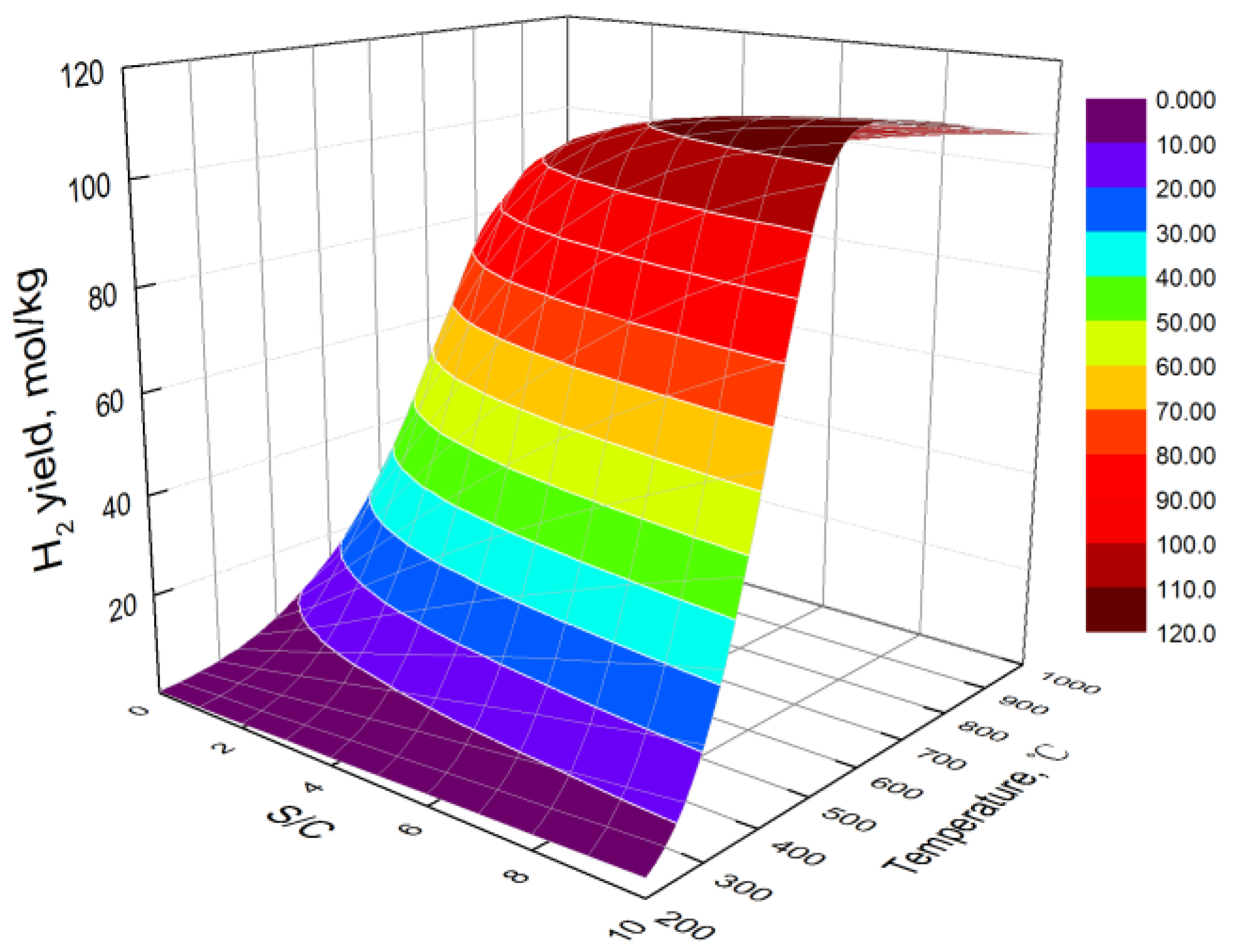
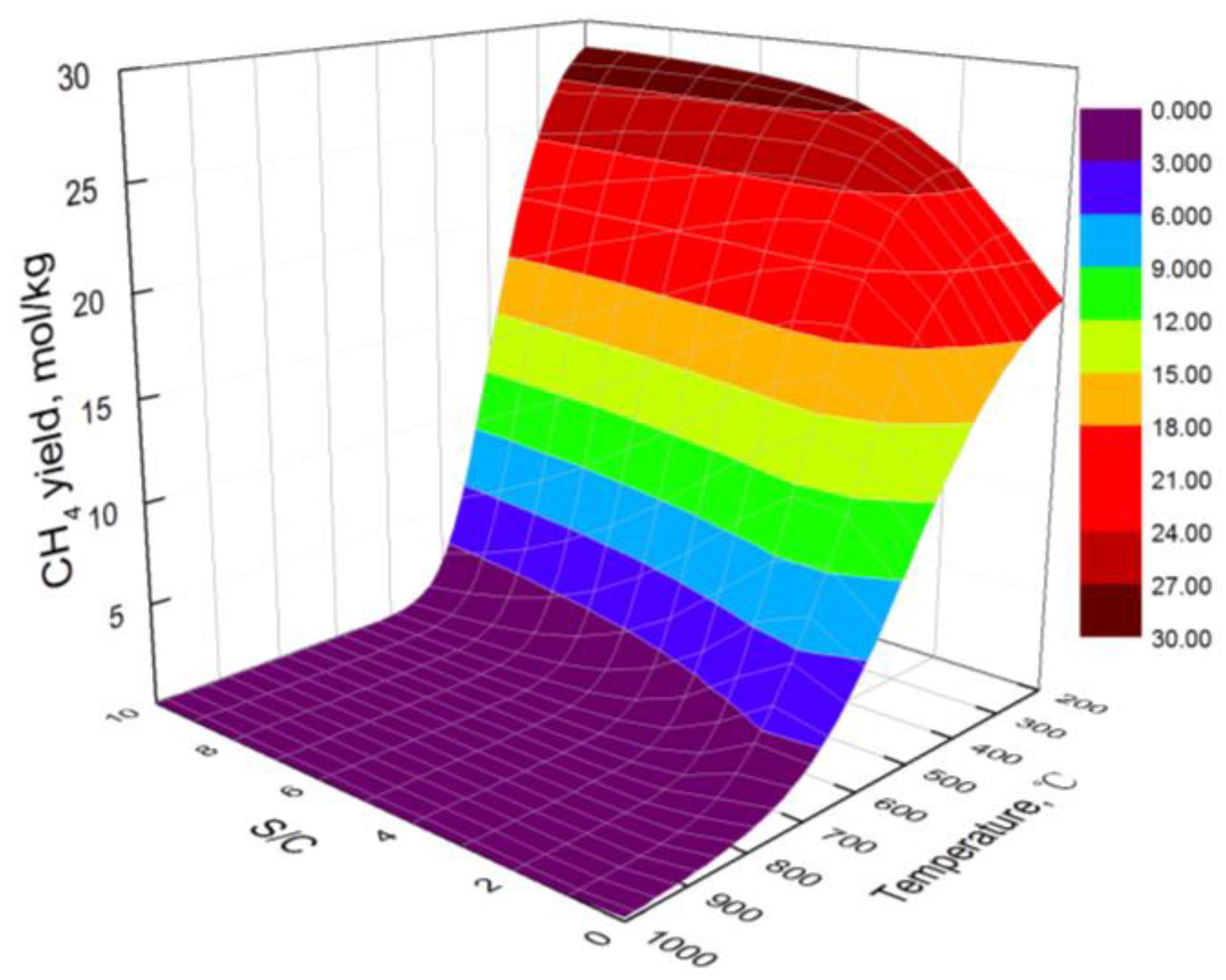
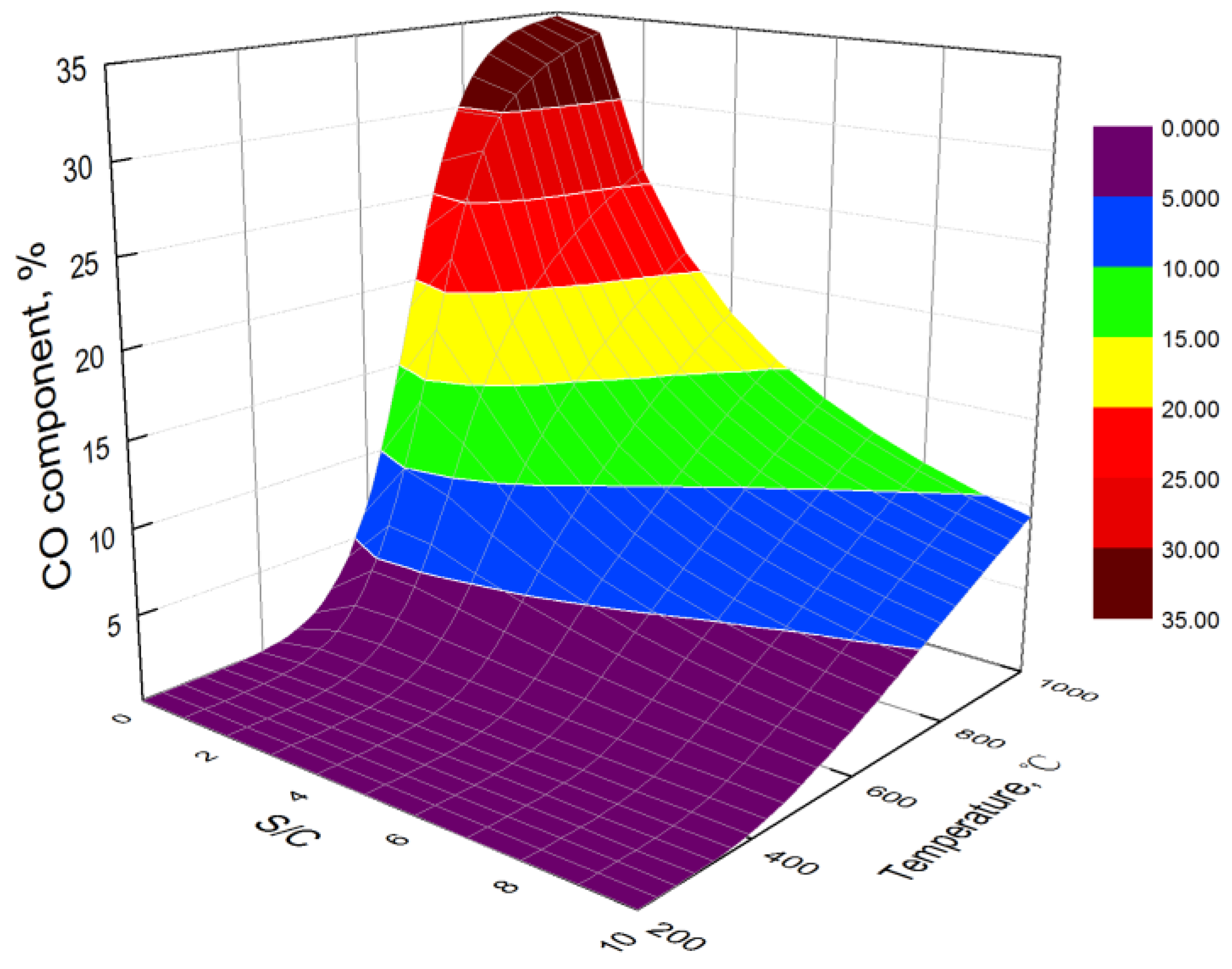
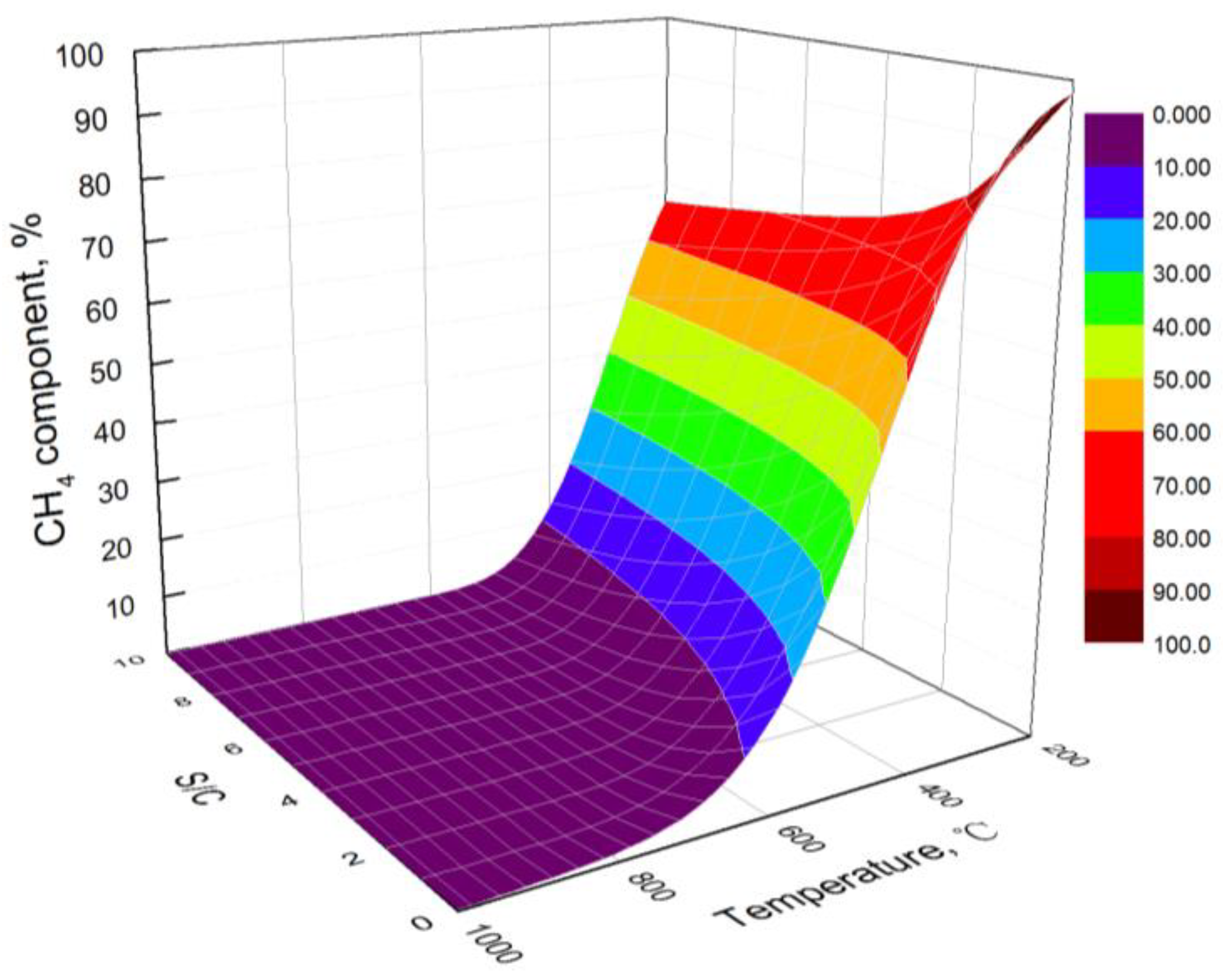
3.1. Effect of Temperature
3.2. Effect of S/C
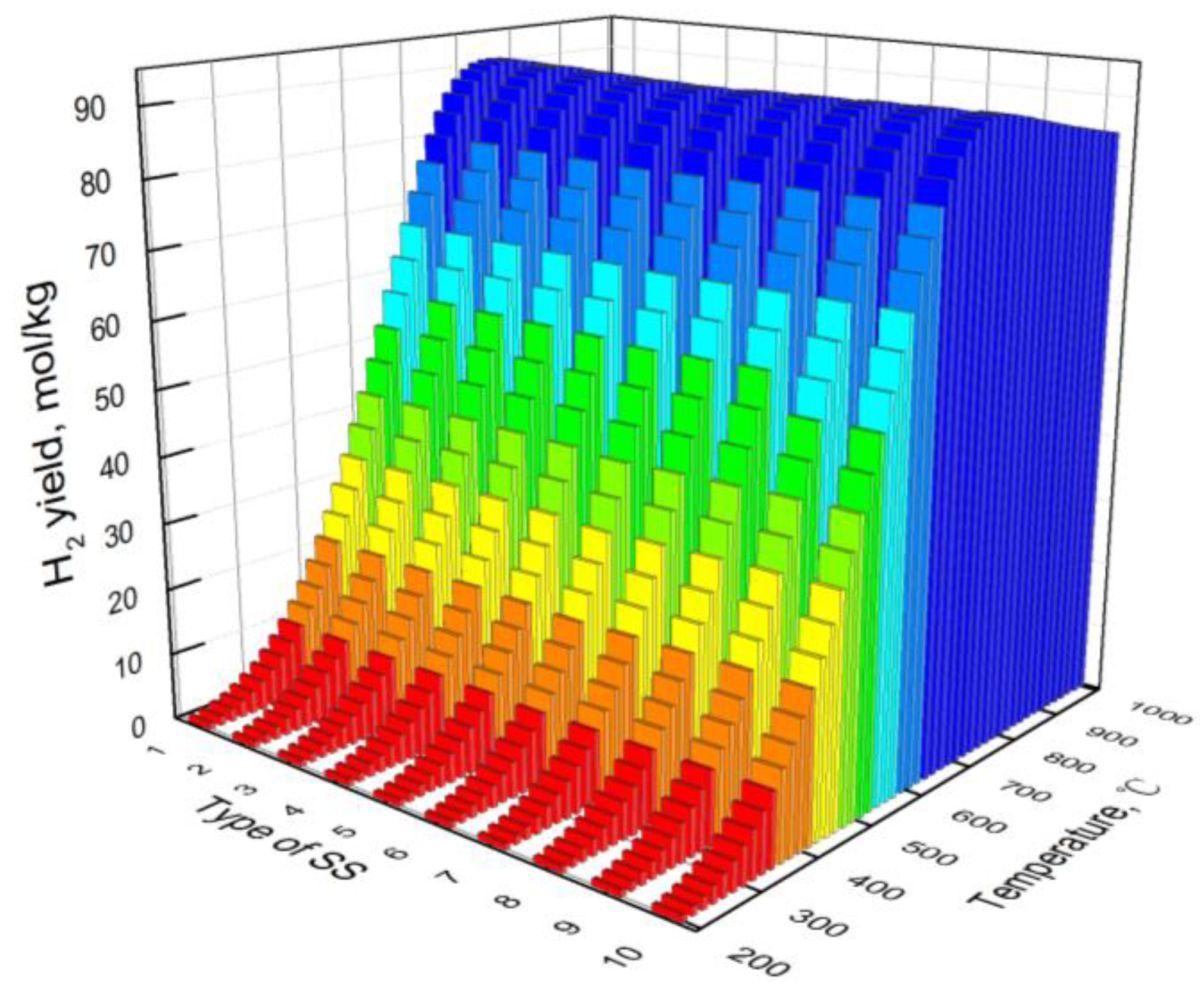
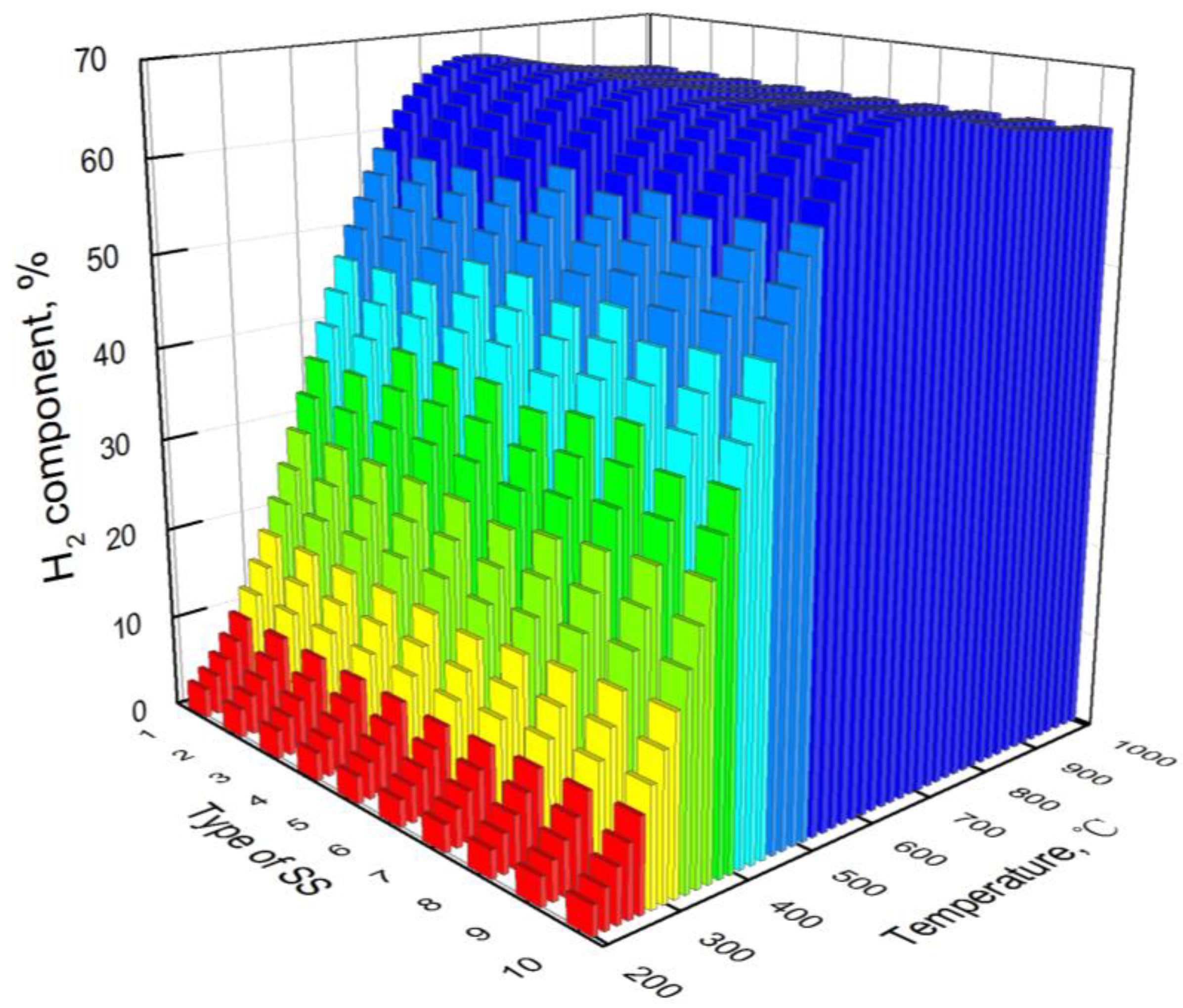
3.3. Effect of Type of Steel Slag
3.4. The Mechanism of Recovering Waste Heat from Steel Slag
4. Conclusions
- The novel method of utilizing bio-oil steam reforming to recover waste heat from steel slag is proposed in this study. It is proven that bio-oil steam reforming can be used to obtain hydrogen and utilize the waste heat of steel slag, and can provide an opportunity for green metallurgy technology.
- At low temperatures, the hydrogen yield of bio-oil steam reforming in steel slag increases with the increase in temperature. However, at high temperatures, the water gas shift reaction is the main reaction affecting the hydrogen yield, resulting in the decrease in the hydrogen yield with the increase in temperature. The carbon yield decreases with the increase in temperature. The hydrogen component increases first and then decreases with the increase in temperature.
- The higher the S/C, the higher the obtained hydrogen yield and the lower the obtained carbon yield. By combining the hydrogen yield and energy consumption, the optimal S/C is 6 and the optimal temperature is 706 °C. At the optimal condition, the hydrogen yield, hydrogen component, and carbon yield are 109.13 mol/kg, 70.21%, and 0.08 mol/kg, respectively.
- Compared with CaO in steel slag, iron oxides have less effect on hydrogen production from bio-oil steam reforming in steel slag. The basicity of steel slag promotes hydrogen production. During industrial application, the hydrogen yield and hydrogen component of bio-oil steam reforming in steel slag can be promoted via an appropriate decrease in iron oxides and increase in basicity.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yao, X.; Yu, Q.; Wang, K.; Xie, H.; Qin, Q. Kinetic study on recovery heat of granulated blast-furnace slag through biomass gasification using CO2 as gasification agent. J. Therm. Anal. Calorim. 2018, 131, 1313–1321. [Google Scholar] [CrossRef]
- Yao, X.; Yu, Q.; Han, Z.; Xie, H.; Duan, W.; Qin, Q. Kinetic and experimental characterizations of biomass pyrolysis in granulated blast furnace slag. Int. J. Hydrogen Energy 2018, 43, 9246–9253. [Google Scholar] [CrossRef]
- Available online: https://worldsteel.org/steel-topics/statistics/world-steel-in-figures-2022/ (accessed on 2 April 2023).
- Lin, B.; Wu, R. Designing energy policy based on dynamic change in energy and carbon dioxide emission performance of China’s iron and steel industry. J. Clean. Prod. 2020, 256, 120412. [Google Scholar] [CrossRef]
- Yao, X.; Liu, Y.; Yu, Q.; Wang, S. Energy consumption of two-stage system of biomass pyrolysis and bio-oil reforming to recover waste heat from granulated BF slag. Energy 2023, 273, 127204. [Google Scholar] [CrossRef]
- Wang, R.Q.; Jiang, L.; Wang, Y.D.; Roskilly, A.P. Energy saving technologies and mass-thermal network optimization for decarbonized iron and steel industry: A review. J. Clean. Prod. 2020, 274, 122997. [Google Scholar] [CrossRef]
- Sun, Y.; Seetharaman, S.; Zhang, Z. Integrating biomass pyrolysis with waste heat recovery from hot slags via extending the C-loops: Product yields and roles of slags. Energy 2018, 149, 792–803. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Liu, L.; Wang, X. Integration of biomass/steam gasification with heat recovery from hot slags: Thermodynamic characteristics. Int. J. Hydrogen Energy 2016, 41, 5916–5926. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z. Disposal of High-Temperature Slags: A Review of Integration of Heat Recovery and Material Recycling. Metall. Mater. Trans. E 2016, 3, 114–122. [Google Scholar] [CrossRef]
- Li, P. Thermodynamic analysis of waste heat recovery of molten blast furnace slag. Int. J. Hydrogen Energy 2017, 42, 9688–9695. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Liu, L.; Wang, X. Heat Recovery from High Temperature Slags: A Review of Chemical Methods. Energies 2015, 8, 1917–1935. [Google Scholar] [CrossRef]
- Yao, X.; Yu, Q.; Xie, H.; Duan, W.; Han, Z.; Liu, S.; Qin, Q. Syngas production through biomass/CO2 gasification using granulated blast furnace slag as heat carrier. J. Renew. Sustain. Energy 2017, 9, 053101. [Google Scholar] [CrossRef]
- Yao, X.; Yu, Q.; Wang, K.; Xie, H.; Qin, Q. Kinetic characterizations of biomass char CO2-gasification reaction within granulated blast furnace slag. Int. J. Hydrogen Energy 2017, 42, 20520–20528. [Google Scholar] [CrossRef]
- Kasai, E.; Kitajima, T.; Akiyama, T.; Yagi, J.; Saito, F. Rate of methane-steam reforming reaction on the surface of molten BF slag—For heat recovery from molten slag by using a chemical reaction. ISIJ Int. 1997, 37, 1031–1036. [Google Scholar] [CrossRef]
- Duan, W.; Yu, Q.; Xie, H.; Liu, J.; Wang, K.; Qin, Q.; Han, Z. Thermodynamic analysis of synergistic coal gasification using blast furnace slag as heat carrier. Int. J. Hydrogen Energy 2016, 41, 1502–1512. [Google Scholar]
- Duan, W.; Yu, Q.; Xie, H.; Qin, Q.; Zuo, Z. Thermodynamic analysis of hydrogen-rich gas generation from coal/steam gasification using blast furnace slag as heat carrier. Int. J. Hydrogen Energy 2014, 39, 11611–11619. [Google Scholar] [CrossRef]
- Yao, X.; Yu, Q.; Xie, H.; Duan, W.; Han, Z.; Liu, S.; Qin, Q. The production of hydrogen through steam reforming of bio-oil model compounds recovering waste heat from blast furnace slag. J. Therm. Anal. Calorim. 2018, 131, 2951–2962. [Google Scholar] [CrossRef]
- Li, P.; Yu, Q.; Xie, H.; Qin, Q.; Wang, K. CO2 gasification rate analysis of Datong coal using slag granules as heat carrier for heat recovery from blast furnace slag by using a chemical reaction. Energy Fuels 2013, 27, 4810–4817. [Google Scholar] [CrossRef]
- Li, P.; Yu, Q.; Qin, Q.; Lei, W. Kinetics of CO2/coal gasification in molten blast furnace slag. Ind. Eng. Chem. Res. 2012, 51, 15872–15883. [Google Scholar] [CrossRef]
- Yao, X.; Yu, Q.; Han, Z.; Xie, H.; Duan, W.; Qin, Q. Kinetics of CO2 gasification of biomass char in granulated blast furnace slag. Int. J. Hydrogen Energy 2018, 43, 12002–12012. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Liu, L.; Wang, X. Two-stage high temperature sludge gasification using the waste heat from hot blast furnace slags. Bioresour. Technol. 2015, 198, 364–371. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Liu, L.; Wang, X. Integrated carbon dioxide/sludge gasification using waste heat from hot slags: Syngas production and sulfur dioxide fixation. Bioresour. Technol. 2015, 181, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Purwanto, H.; Akiyama, T. Hydrogen production from biogas using hot slag. Int. J. Hydrogen Energy 2006, 31, 491–495. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Qing, S.; Liu, H. Characteristics of gaseous product from municipal solid waste gasification with hot blast furnace slag. J. Nat. Gas Chem. 2010, 19, 403–408. [Google Scholar] [CrossRef]
- Duan, W.; Yu, Q.; Liu, J.; Wu, T.; Yang, F.; Qin, Q. Experimental and kinetic study of steam gasification of low-rank coal in molten blast furnace slag. Energy 2016, 111, 859–868. [Google Scholar] [CrossRef]
- Duan, W.; Yu, Q.; Wu, T.; Yang, F.; Qin, Q. Experimental study on steam gasification of coal using molten blast furnace slag as heat carrier for producing hydrogen-enriched syngas. Energy Convers. Manag. 2016, 117, 513–519. [Google Scholar] [CrossRef]
- Luo, S.; Guo, J.; Feng, Y. Hydrogen-rich gas production from pyrolysis of wet sludge in situ steam agent. Int. J. Hydrogen Energy 2017, 42, 18309–18314. [Google Scholar] [CrossRef]
- Luo, S.; Fu, J.; Zhou, Y.; Yi, C. The production of hydrogen-rich gas by catalytic pyrolysis of biomass using waste heat from blast-furnace slag. Renew. Energy 2017, 101, 1030–1036. [Google Scholar] [CrossRef]
- Wang, P.; Xie, H.; Zhang, J.; Jia, L.; Yu, Z.; Li, R. Optimization of two bio-oil steam reforming processes for hydrogen production based on thermodynamic analysis. Int. J. Hydrogen Energy 2022, 47, 9853–9863. [Google Scholar] [CrossRef]
- Jin, K.; Ji, D.; Xie, Q.; Nie, Y.; Yu, F.; Ji, J. Hydrogen production from steam gasification of tableted biomass in molten eutectic carbonates. Int. J. Hydrogen Energy 2019, 44, 22919–22925. [Google Scholar] [CrossRef]
- Krzywanski, J.; Fan, H.; Feng, Y.; Shaikh, A.; Fang, M.; Wang, Q. Genetic algorithms and neural networks in optimization of sorbent enhanced H2 production in FB and CFB gasifiers. Energy Convers. Manag. 2018, 171, 1651–1661. [Google Scholar] [CrossRef]
- Bizkarra, K.; Bermudez, J.; Arcelus-Arrillaga, P.; Barrio, V.L.; Cambra, J.; Millan, M. Nickel based monometallic and bimetallic catalysts for synthetic and real bio-oil steam reforming. Int. J. Hydrogen Energy 2018, 46, 11706–11718. [Google Scholar] [CrossRef]
- Valle, B.; Aramburu, B.; Benito, P.; Bilbao, J.; Gayubo, A.G. Biomass to hydrogen-rich gas via steam reforming of raw bio-oil over Ni/La2O3-αAl2O3 catalyst: Effect of space-time and steam-to-carbon ratio. Fuel 2018, 216, 445–455. [Google Scholar] [CrossRef]
- Rodrigues, C.; Alonso, C.; Machado, G.; de Souza, T. Optimization of bio-oil steam reforming process by thermodynamic analysis. Int. J. Hydrogen Energy 2020, 45, 28350–28360. [Google Scholar] [CrossRef]
- Yao, X.; Yu, Q.; Xu, G.; Han, Z.; Qin, Q. Production of syngas from dry reforming of bio-oil model compound in granulated blast furnace slag. Korean J. Chem. Eng. 2019, 36, 722–728. [Google Scholar] [CrossRef]
- Yao, X.; Yu, Q.; Xu, G.; Han, Z.; Xie, H.; Duan, W.; Qin, Q. The characteristics of syngas production from bio-oil dry reforming utilizing the waste heat of granulated blast furnace slag. Int. J. Hydrogen Energy 2018, 43, 22108–22115. [Google Scholar] [CrossRef]
- Shimada, T.; Kochura, V.; Akiyama, T.; Kasai, E.; Yagi, J. Effects of slag compositions on the rate of methane–steam reaction. ISIJ Int. 2000, 41, 111–115. [Google Scholar] [CrossRef]
- Xie, H.; Yu, Q.; Zuo, Z.; Han, Z.; Yao, X.; Qin, Q. Hydrogen production via sorption-enhanced catalytic steam reforming of bio-oil. Int. J. Hydrogen Energy 2016, 41, 2345–2353. [Google Scholar] [CrossRef]
- Zuo, Z.; Yu, Q.; Xie, H.; Yang, F.; Qin, Q. Thermodynamic analysis of reduction in copper slag by biomass molding compound based on phase equilibrium calculating model. J. Therm. Anal. Calorim. 2018, 132, 1277–1289. [Google Scholar] [CrossRef]
- Xie, H.; Yu, Q.; Zhang, Y.; Zhang, J.; Liu, J.; Qin, Q. New process for hydrogen production from raw coke oven gas via sorption-enhanced steam reforming: Thermodynamic analysis. Int. J. Hydrogen Energy 2017, 42, 2914–2923. [Google Scholar] [CrossRef]
- Xie, H.; Yu, Q.; Wei, M.; Duan, W.; Yao, X.; Qin, Q.; Zuo, Z. Hydrogen production from steam reforming of simulated bio-oil over Ce–Ni/Co catalyst with in continuous CO2 capture. Int. J. Hydrogen Energy 2015, 40, 1420–1428. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, J.; Yu, Q.; Zuo, Z.; Liu, J.; Qin, Q. Study on Steam Reforming of Tar in Hot Coke Oven Gas for Hydrogen Production. Energy Fuels 2016, 30, 2336–2344. [Google Scholar] [CrossRef]
- Luo, S.; Feng, Y. The production of fuel oil and combustible gas by catalytic pyrolysis of waste tire using waste heat of blast-furnace slag. Energy Convers. Manag. 2017, 136, 27–35. [Google Scholar] [CrossRef]
- Huang, B.-S.; Chen, H.-Y.; Chuang, K.-H.; Yang, R.-X.; Wey, M.-Y. Hydrogen production by biomass gasification in a fluidized-bed reactor promoted by an Fe/CaO catalyst. Int. J. Hydrogen Energy 2012, 37, 6511–6518. [Google Scholar] [CrossRef]




| Type of Steel Slag | CaO | SiO2 | Al2O3 | MgO | Fe2O3 | FeO | Fe2O3 + FeO | R |
|---|---|---|---|---|---|---|---|---|
| 1 | 54.00 | 18.00 | 8.00 | 5.00 | 4.75 | 10.25 | 15.00 | 3 |
| 2 | 50.25 | 16.75 | 8.00 | 5.00 | 6.33 | 13.67 | 20.00 | 3 |
| 3 | 46.50 | 15.50 | 8.00 | 5.00 | 7.92 | 17.08 | 25.00 | 3 |
| 4 | 42.75 | 14.25 | 8.00 | 5.00 | 9.50 | 20.50 | 30.00 | 3 |
| 5 | 39.00 | 13.00 | 8.00 | 5.00 | 11.08 | 23.92 | 35.00 | 3 |
| 6 | 41.33 | 20.67 | 8.00 | 5.00 | 7.92 | 17.08 | 25.00 | 2 |
| 7 | 44.29 | 17.71 | 8.00 | 5.00 | 7.92 | 17.08 | 25.00 | 2.5 |
| 8 | 46.50 | 15.50 | 8.00 | 5.00 | 7.92 | 17.08 | 25.00 | 3 |
| 9 | 48.22 | 13.78 | 8.00 | 5.00 | 7.92 | 17.08 | 25.00 | 3.5 |
| 10 | 49.60 | 12.40 | 8.00 | 5.00 | 7.92 | 17.08 | 25.00 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Z.; Liu, Y.; Yao, X.; Xue, Y.; Li, C.; Li, Z.; Wang, S.; Wu, J. Thermodynamic Analysis of Hydrogen Production from Bio-Oil Steam Reforming Utilizing Waste Heat of Steel Slag. Processes 2023, 11, 2342. https://doi.org/10.3390/pr11082342
Ding Z, Liu Y, Yao X, Xue Y, Li C, Li Z, Wang S, Wu J. Thermodynamic Analysis of Hydrogen Production from Bio-Oil Steam Reforming Utilizing Waste Heat of Steel Slag. Processes. 2023; 11(8):2342. https://doi.org/10.3390/pr11082342
Chicago/Turabian StyleDing, Zhijun, Yang Liu, Xin Yao, Yuekai Xue, Chenxiao Li, Zhihui Li, Shuhuan Wang, and Jianwei Wu. 2023. "Thermodynamic Analysis of Hydrogen Production from Bio-Oil Steam Reforming Utilizing Waste Heat of Steel Slag" Processes 11, no. 8: 2342. https://doi.org/10.3390/pr11082342
APA StyleDing, Z., Liu, Y., Yao, X., Xue, Y., Li, C., Li, Z., Wang, S., & Wu, J. (2023). Thermodynamic Analysis of Hydrogen Production from Bio-Oil Steam Reforming Utilizing Waste Heat of Steel Slag. Processes, 11(8), 2342. https://doi.org/10.3390/pr11082342






