Oxidation Kinetics Analysis of Crude Oils with Different Viscosities
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Experimental Apparatus and Procedure
3. Results and Discussion
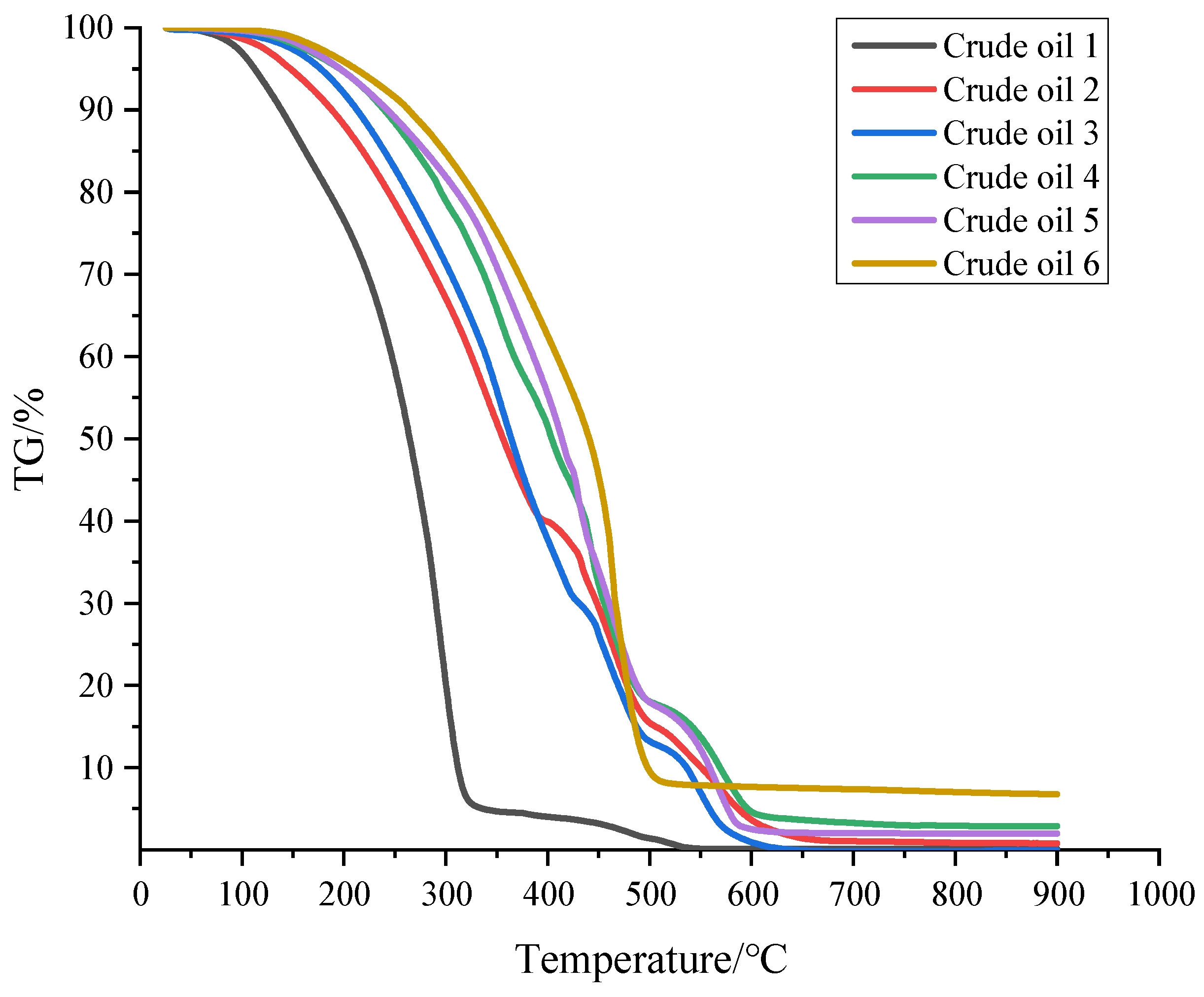
3.1. TG-DTG Analysis
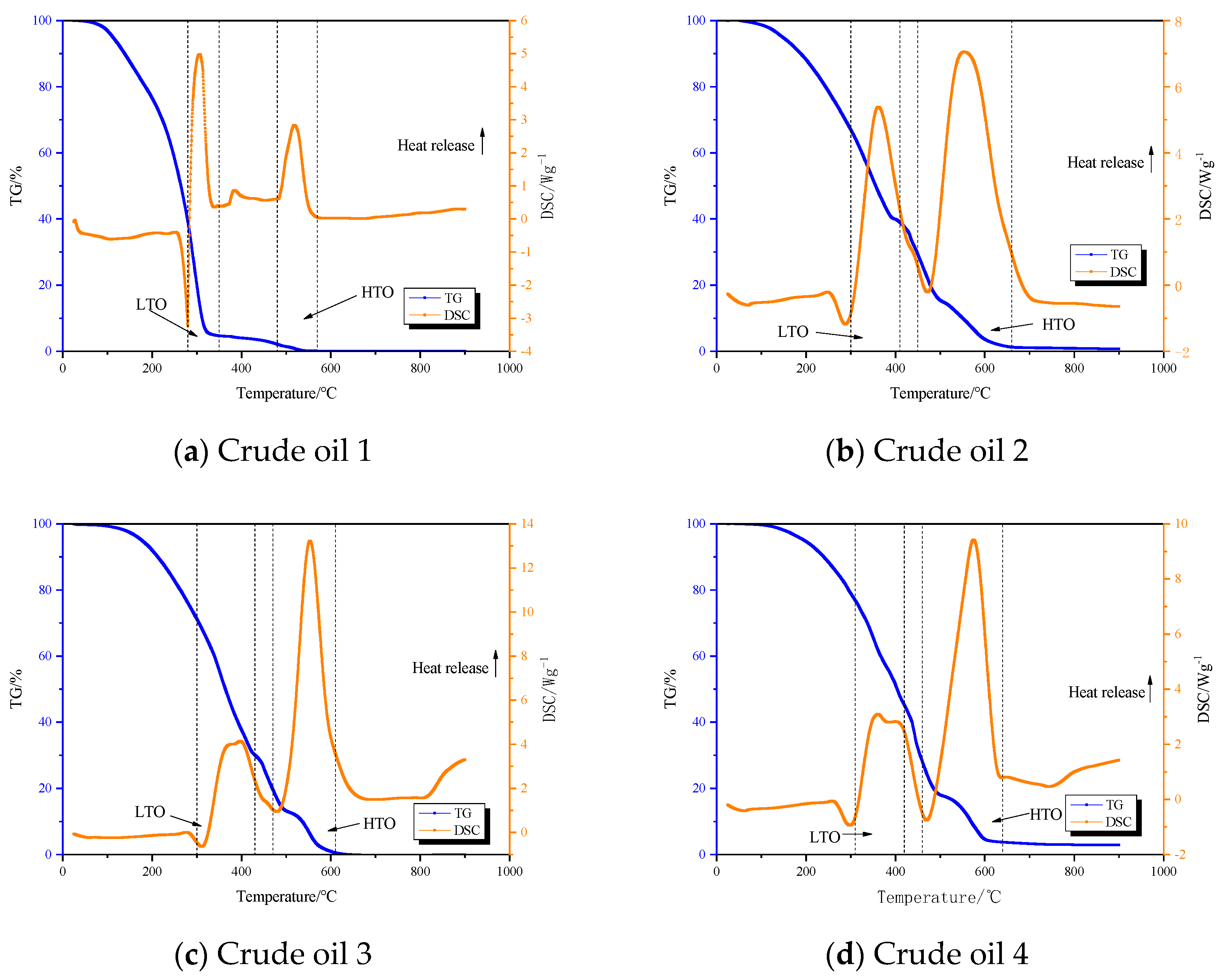
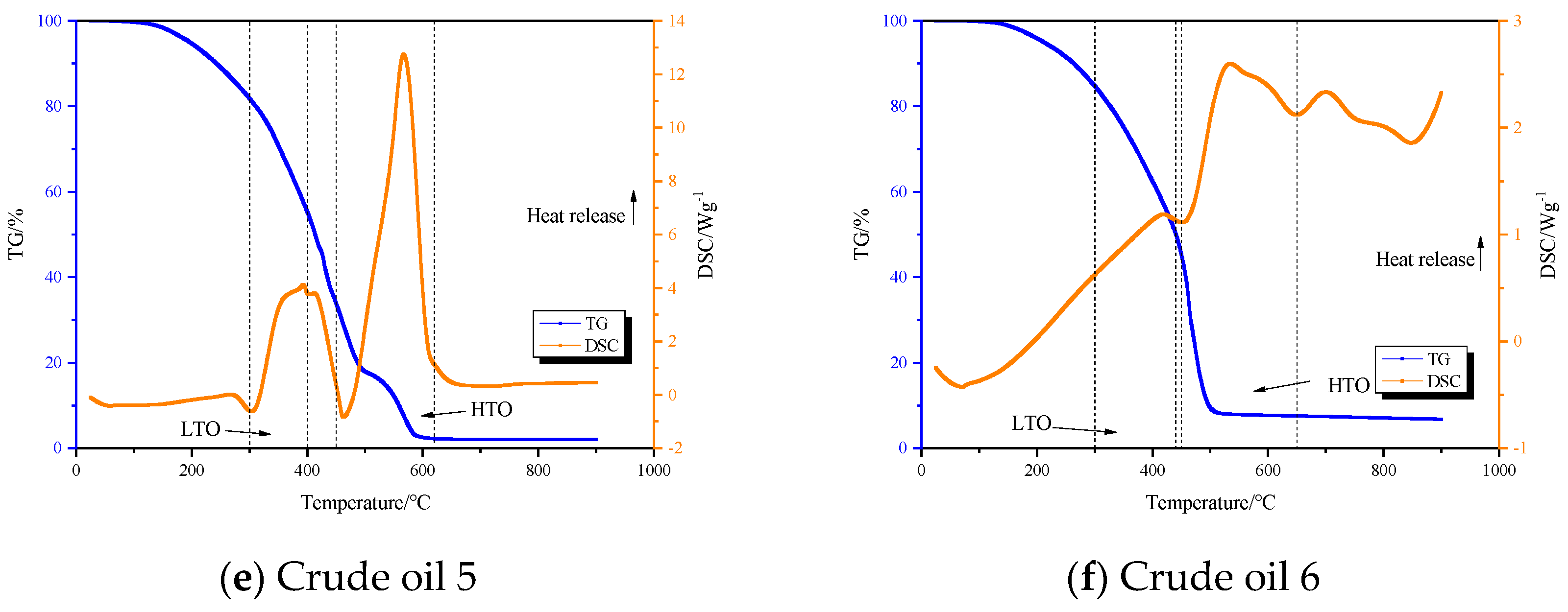
3.2. TG-DSC Analysis
3.3. Oxidation Reaction Models at Each Stage
3.3.1. Reaction Model for Low-Temperature Oxidation Stage
3.3.2. Reaction Model for Fuel Deposition Stage
3.3.3. Reaction Model for High-Temperature Oxidation Stage
3.4. Kinetics of Oxidation of Crude Oil
3.4.1. Kinetic Mechanism of Oxidation Reaction
3.4.2. Calculation of Oxidation Kinetic Parameters
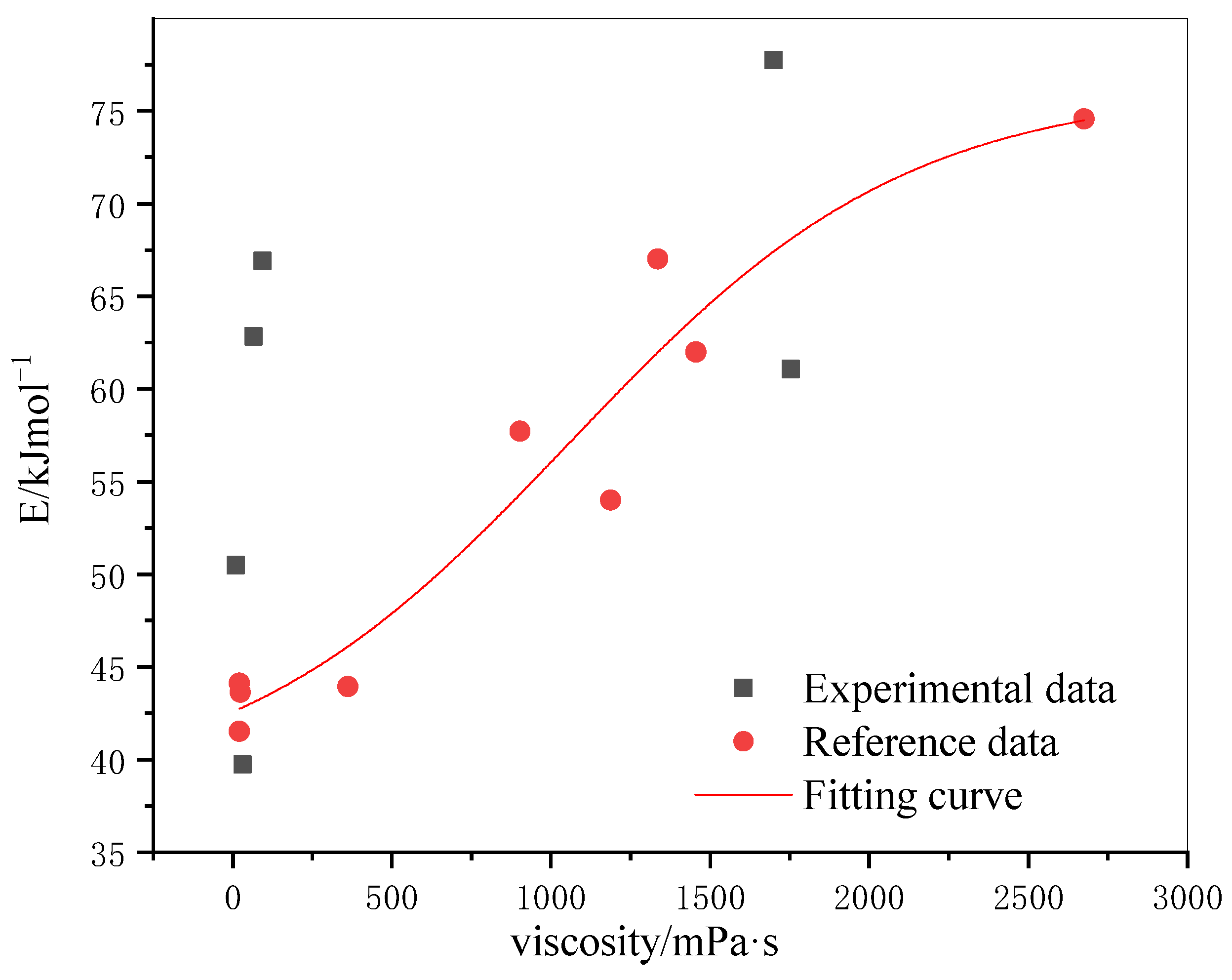
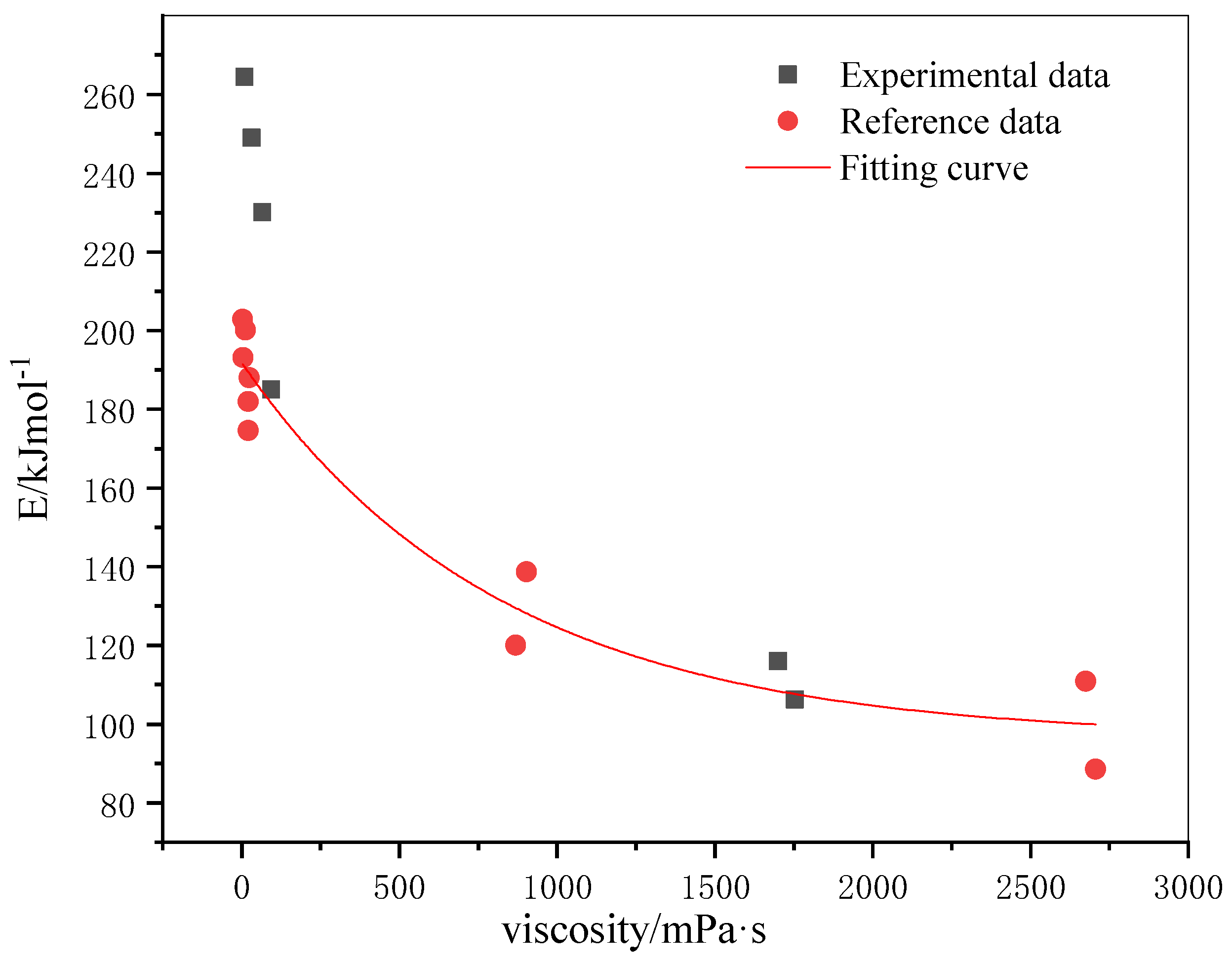
3.5. Effect of Viscosity on Oxidation Kinetics of Crude Oil
4. Conclusions
- Light oil, ordinary heavy oil I, and ordinary heavy oil II can be divided into three reaction stages during the oxidation process: low-temperature oxidation stage, fuel deposition stage, and high-temperature oxidation stage.
- After the analysis of the TG-DTG-DSC curve, different crude oils show obvious differences. The mass loss rate of light crude oil in the low-temperature oxidation stage is the largest, and the mass loss rate of ordinary heavy crude oil is smaller than that of light crude oil.
- Arrhenius method calculated that the activation energy of oil samples with different properties was 39.73–77.74 kJ/mol in the low-temperature oxidation stage and 106.27–264.47 kJ/mol in the high-temperature oxidation stage.
- The activation energy of crude oil increases with the increase of viscosity in the low-temperature oxidation stage and decreases with the increase of viscosity in the high-temperature oxidation stage.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ado, M.R. A detailed approach to up-scaling of the Toe-to-Heel Air Injection (THAI) In-Situ Combustion enhanced heavy oil recovery process. J. Pet. Sci. Eng. 2020, 187, 106740. [Google Scholar] [CrossRef]
- Huang, S.; Sheng, J.J. Feasibility of spontaneous ignition during air injection in light oil reservoirs. Fuel 2018, 226, 698–708. [Google Scholar] [CrossRef]
- Gutierrez, D.; Kumar, V.; Moore, R.G.; Mehta, S.A. Case history and appraisal of the west buffalo red river unit high-pressure air injection project. In Proceedings of the Hydrocarbon Economics and Evaluation Symposium, Dallas, TX, USA, 1–3 April 2007; pp. 178–187. [Google Scholar] [CrossRef]
- Mehrabi-Kalajahi, S.; Varfolomeev, M.A.; Yuan, C.; Zinnatullin, A.L.; Rodionov, N.O.; Vagizov, F.G.; Yakimova, L.S. Improving heavy oil oxidation performance by oil-dispersed CoFe2O4 nanoparticles in In-situ combustion process for enhanced oil recovery. Fuel 2021, 285, 119216. [Google Scholar] [CrossRef]
- Bartlett, G.; Vossoughi, S.; Willhite, G.P. Development of a Kinetic Model for In-Situ Combustion and Prediction of the Process Variables using TGA/DSC Techniques. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 26 September 1982. [Google Scholar]
- Kovscek, A.R.R.; Castanier, L.M.M.; Gerritsen, M.G.G. Improved Predictability of In-Situ-Combustion Enhanced Oil Recovery. SPE Reserv. Eval. Eng. 2013, 16, 172–182. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Liu, Y.; Liu, C.; Kovscek, A.R. In-Situ Combustion Frontal Stability Analysis. In Proceedings of the SPE Western Regional Meeting, San Jose, CA, USA, 23–26 April 2019; Volume 26, pp. 2271–2286. [Google Scholar] [CrossRef]
- Vossoughi, S.; Ei-Shoubary, Y. Kinetics of Crude-Oil Coke Combustion. In Proceedings of the SPE International Symposium on Oilfield Chemistry, San Antonio, TX, USA, 4–6 February 1987; pp. 271–278. [Google Scholar] [CrossRef]
- Srinivasa Reddy, D.; Suresh Kumar, G. Numerical Simulation of Heavy Crude Oil Combustion in Porous Combustion Tube. Combust. Sci. Technol. 2015, 187, 1905–1921. [Google Scholar] [CrossRef]
- Tirado, A.; Yuan, C.; Varfolomeev, M.A.; Ancheyta, J. Kinetic modeling of aquathermolysis for upgrading of heavy oils. Fuel 2022, 310, 122286. [Google Scholar] [CrossRef]
- Pu, W.; Chen, Y.; Li, Y.; Zou, P.; Li, D. Comparison of Different Kinetic Models for Heavy Oil Oxidation Characteristic Evaluation. Energy Fuels 2017, 31, 12665–12676. [Google Scholar] [CrossRef]
- Zhao, S.; Pu, W.; Sun, B.; Gu, F.; Wang, L. Comparative evaluation on the thermal behaviors and kinetics of combustion of heavy crude oil and its SARA fractions. Fuel 2019, 239, 117–125. [Google Scholar] [CrossRef]
- Kok, M.V. Characterization of medium and heavy crude oils using thermal analysis techniques. Fuel Process. Technol. 2011, 92, 1026–1031. [Google Scholar] [CrossRef]
- Kok, M.V. Thermal behavior and kinetics of crude oils at low heating rates by differential scanning calorimeter. Fuel Process. Technol. 2012, 96, 123–127. [Google Scholar] [CrossRef]
- Gundogar, A.S.; Kok, M.V. Thermal characterization, combustion and kinetics of different origin crude oils. Fuel 2014, 123, 59–65. [Google Scholar] [CrossRef]
- Hu, F.; Xiong, B.; Huang, X.; Liu, Z. Theoretical analysis and experimental verification of diminish the diffusion influence on determination of char oxidation kinetics by thermo-gravimetric analysis. Energy 2023, 275, 127460. [Google Scholar] [CrossRef]
- Liu, P.-G.; Pu, W.-F.; Ni, J.-H.; Ma, X.-P.; Zhang, J.; Liu, M. Thermal investigation on crude oil oxidation kinetics through TG/DTG and DTA tests. Pet. Sci. Technol. 2016, 34, 685–692. [Google Scholar] [CrossRef]
- Pu, W.-F.; Wang, L.-L.; Peng, X.-Q.; Li, N.; Zhao, S. Effects of aromatics, resins, and asphaltenes on oxidation behavior and kinetics of heavy crude oil. Pet. Sci. Technol. 2020, 38, 815–822. [Google Scholar] [CrossRef]
- Yuan, C.; Emelianov, D.A.; Varfolomeev, M.A.; Rodionov, N.O.; Suwaid, M.A.; Vakhitov, I.R. Mechanistic and kinetic insight into catalytic oxidation process of heavy oil in in-situ combustion process using copper (Ⅱ) stearate as oil soluble catalyst. Fuel 2021, 284, 118981. [Google Scholar] [CrossRef]
- Kök, M.V.; Varfolomeev, M.A.; Nurgaliev, D.K. Thermal and mass spectrometer analysis (TG–DTG–MS) of different oAPI gravity crude oils from new oil fields in Turkey. Geoenergy Sci. Eng. 2023, 224, 211598. [Google Scholar] [CrossRef]
- Wang, L.; Wang, T.; Bai, Z.; Yuan, C.; Song, W.; Chen, Y.; Wang, J. Thermo-oxidative behavior and kinetics analysis of light and heavy oils based on TG, DSC, and FTIR. Geoenergy Sci. Eng. 2023, 223, 211525. [Google Scholar] [CrossRef]
- Kök, M.V.; Pokol, G.; Keskin, C.; Madarász, J.; Bagci, S. Light Crude Oil Combustion in the Presence of Limestone Matrix. J. Therm. Anal. Calorim. 2004, 75, 781–789. [Google Scholar] [CrossRef]
- Zhao, S.; Pu, W.; Gao, H.; Wang, R.; Varfolomeev, M.A.; Jiang, Q.; Yuan, C. Isothermal low-temperature oxidation kinetics of heavy crude oil and its oxidized oils. Fuel 2023, 344, 128146. [Google Scholar] [CrossRef]
- Clara, C.; Durandeau, M.; Quenault, G. Laboratory Studies for Light-Oil Air Injection Projects: Potential Application in Handil Field. SPE Res. Eval. Eng. 2000, 3, 239–248. [Google Scholar] [CrossRef]
- Zhang, H. Kinetics characteristics of the oxidation for different oils in air injection process. Pet. Geol. Oilfield Dev. Daqing 2019, 38, 105–111. [Google Scholar] [CrossRef]
- Murugan, P.; Mahinpey, N.; Mani, T.; Freitag, N. Pyrolysis and combustion kinetics of Fosterton oil using thermogravimetric analysis. Fuel 2009, 88, 1708–1713. [Google Scholar] [CrossRef]
- Yuan, C.; Varfolomeev, M.A.; Emelianov, D.A.; Eskin, A.A.; Nagrimanov, R.N.; Kok, M.V.; Kopylova, E.V. Oxidation Behavior of Light Crude Oil and Its SARA Fractions Characterized by TG and DSC Techniques: Differences and Connections. Energy Fuels 2017, 32, 801–808. [Google Scholar] [CrossRef]
- Kok, M.V.; Gundogar, A.S. DSC study on combustion and pyrolysis behaviors of Turkish crude oils. Fuel Process. Technol. 2013, 116, 110–115. [Google Scholar] [CrossRef]
- Kök, M.V.; Varfolomeev, M.A.; Nurgaliev, D.K. TGA and DSC investigation of different clay mineral effects on the combustion behavior and kinetics of crude oil from Kazan region, Russia. J. Pet. Sci. Eng. 2021, 200, 108364. [Google Scholar] [CrossRef]




| Sample No. | Viscosity/(mPa·s) | API Gravity |
|---|---|---|
| 1# | 9.6 | 31.2 |
| 2# | 66 | 22.9 |
| 3# | 105 | 22.1 |
| 4# | 574 | 21.4 |
| 5# | 2053 | 19.6 |
| 6# | 2927 | 18.3 |
| Sample Name | Initial Mass/mg | Final Mass/mg | Mass Loss Rate/% |
|---|---|---|---|
| Crude oil 1 | 26.595 | 0.378 | 98.58 |
| Crude oil 2 | 10.3 | 0.1085 | 98.95 |
| Crude oil 3 | 13.295 | 0.1604 | 98.79 |
| Crude oil 4 | 16.7 | 0.6361 | 96.19 |
| Crude oil 5 | 18.5 | 0.382 | 97.94 |
| Crude oil 6 | 16.1 | 0.415 | 97.42 |
| Sample Name | LTO/°C | Fuel Deposition Stage/°C | HTO/°C |
|---|---|---|---|
| Crude oil 1 | 280–350 | 350–480 | 480–570 |
| Crude oil 2 | 300–410 | 410–450 | 450–660 |
| Crude oil 3 | 300–360 | 360–450 | 450–610 |
| Crude oil 4 | 310–420 | 420–450 | 450–640 |
| Crude oil 5 | 300–400 | 400–450 | 450–620 |
| Crude oil 6 | 300–410 | 410–420 | 420–650 |
| Sample Name | E/kJmol−1 | A/min−1 | ||
|---|---|---|---|---|
| LTO | HTO | LTO | HTO | |
| Crude oil 1 | 50.49 | 264.47 | 2.5785 | 18.5098 |
| Crude oil 2 | 39.73 | 249.04 | 1.2812 | 12.0165 |
| Crude oil 3 | 62.84 | 230.05 | 0.2628 | 12.4100 |
| Crude oil 4 | 66.91 | 185.02 | 2.3762 | 4.6598 |
| Crude oil 5 | 61.08 | 106.27 | 1.8582 | 0.0876 |
| Crude oil 6 | 77.74 | 115.97 | 2.8013 | 0.2923 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, B.; Yu, C.; Chen, Y.; Yue, K.; Yue, L.; Chen, T. Oxidation Kinetics Analysis of Crude Oils with Different Viscosities. Processes 2023, 11, 2361. https://doi.org/10.3390/pr11082361
Luo B, Yu C, Chen Y, Yue K, Yue L, Chen T. Oxidation Kinetics Analysis of Crude Oils with Different Viscosities. Processes. 2023; 11(8):2361. https://doi.org/10.3390/pr11082361
Chicago/Turabian StyleLuo, Bincheng, Chunsheng Yu, Yuehao Chen, Kunhai Yue, Lifan Yue, and Tao Chen. 2023. "Oxidation Kinetics Analysis of Crude Oils with Different Viscosities" Processes 11, no. 8: 2361. https://doi.org/10.3390/pr11082361




