Preparation of a New Adsorbent Material from Agro-Industrial Waste and Comparison with Commercial Adsorbent for Emerging Contaminant Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Biosorbent
2.3. Preparation of Hydrochar
2.4. Characterization of Adsorbents
2.5. Kinetic and Equilibrium Study
2.6. Production Cost Analysis
3. Results and Discussion
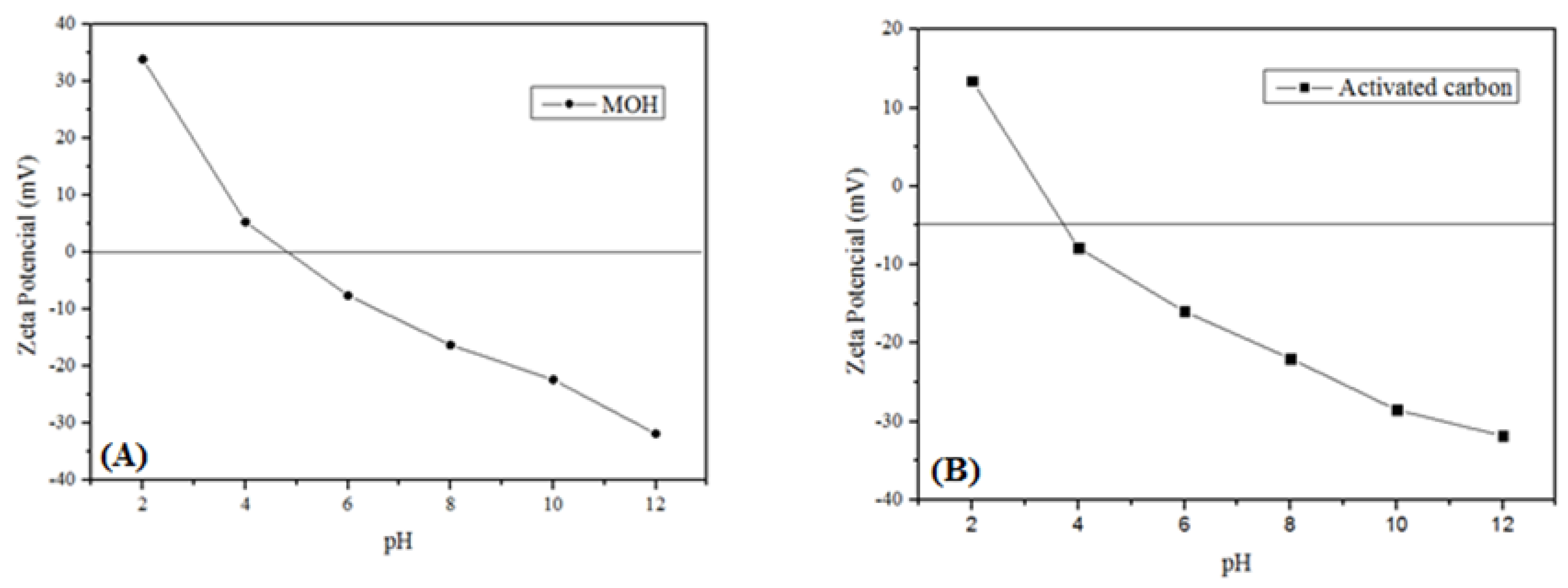
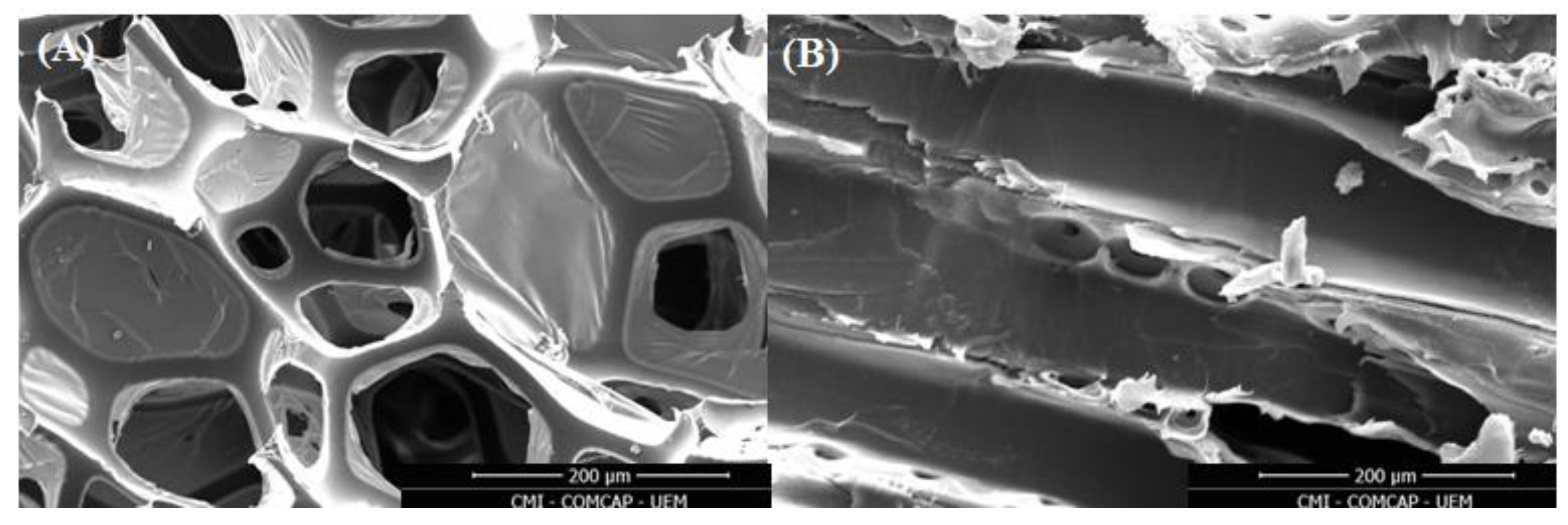
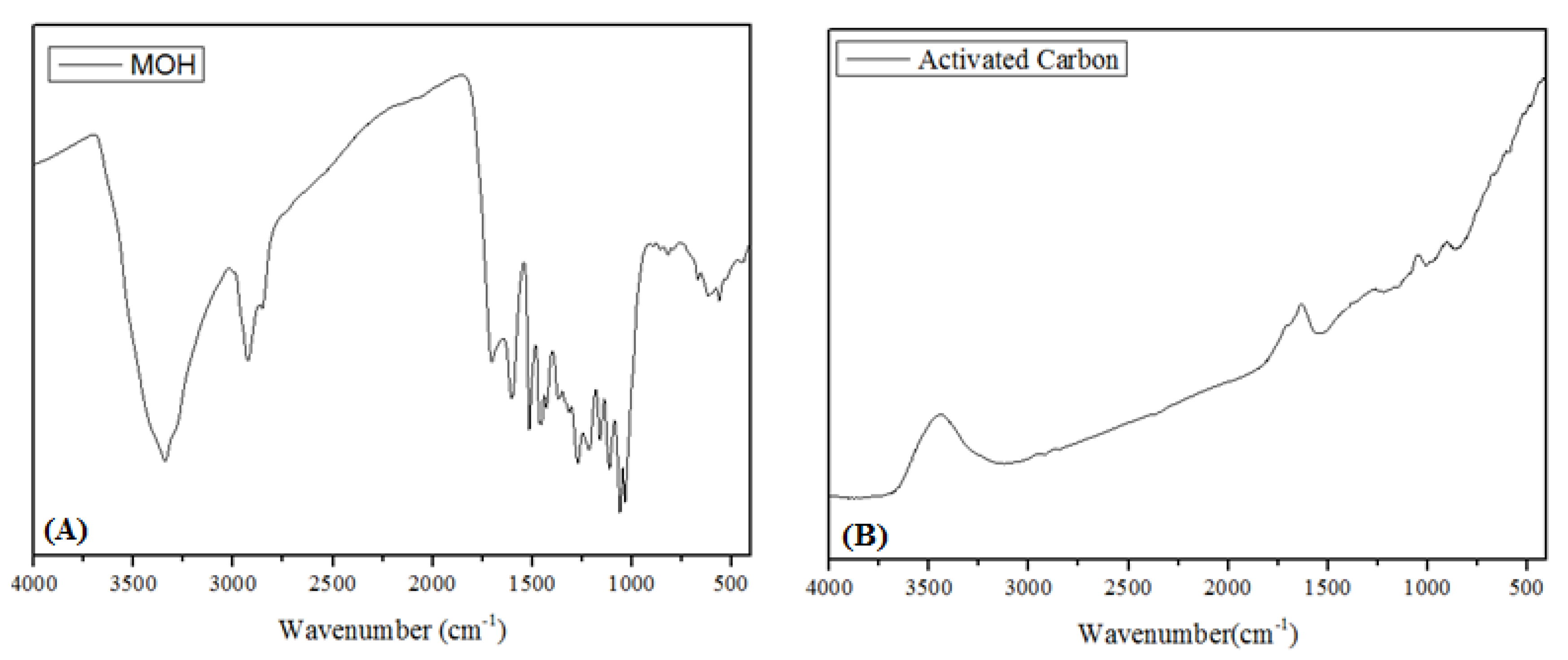
3.1. Adsorbent Characterizations
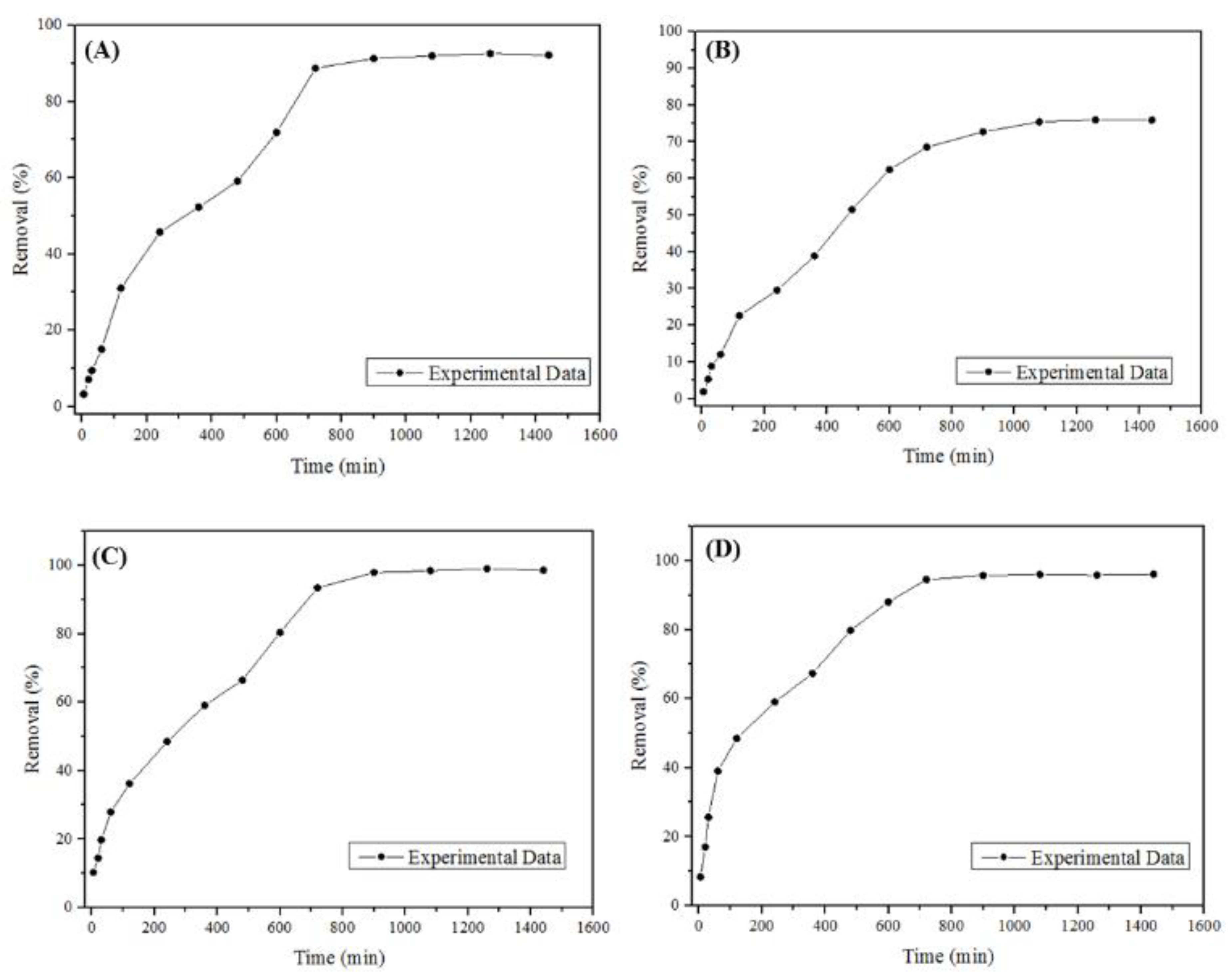
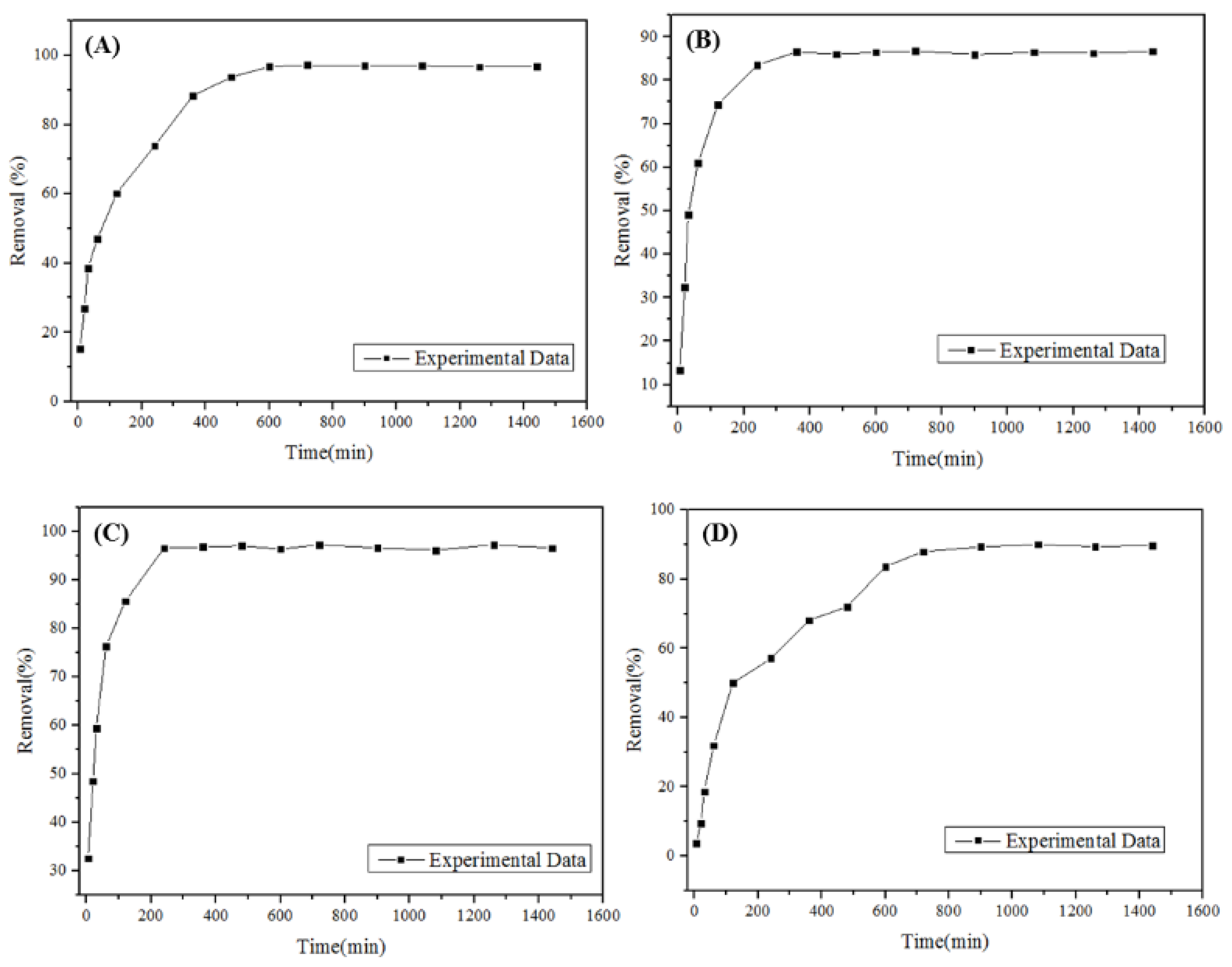
3.2. Kinetic and Equilibrium Study
3.3. Study Production Cost of Each Adsorbent
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naidoo, T.; Rajkaran, A. Sershen Impacts of Plastic Debris on Biota and Implications for Human Health: A South African Perspective. S. Afr. J. Sci. 2020, 116, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shakibaie, M.; Mohazab, N.S.; Ayatollahi Mousavi, S.A. Antifungal Activity of Selenium Nanoparticles Synthesized by Bacillus Species Msh-1 against Aspergillus Fumigatus and Candida Albicans. Jundishapur J. Microbiol. 2015, 8, e26381. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Badot, P.M. Application of Chitosan, a Natural Aminopolysaccharide, for Dye Removal from Aqueous Solutions by Adsorption Processes Using Batch Studies: A Review of Recent Literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Gülçek, B.; Tepe, O. Removal of Atrazine by Biogenic Manganese Oxide in Batch and Fixed-Bed Column Reactors. Geomicrobiol. J. 2022, 39, 17–27. [Google Scholar] [CrossRef]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; Bezerra, C.d.O.; Bergamasco, R. Surface Water Pollution by Pharmaceuticals and an Alternative of Removal by Low-Cost Adsorbents: A Review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef] [PubMed]
- Cusioli, L.F.; Quesada, H.B.; Barbosa de Andrade, M.; Gomes, R.G.; Bergamasco, R. Application of a Novel Low-Cost Adsorbent Functioned with Iron Oxide Nanoparticles for the Removal of Triclosan Present in Contaminated Water. Microporous Mesoporous Mater. 2021, 325, 111328. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A Review on the Biosynthesis of Metal and Metal Salt Nanoparticles by Microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef]
- Bezerra, C.d.O.; Cusioli, L.F.; Quesada, H.B.; Nishi, L.; Mantovani, D.; Vieira, M.F.; Bergamasco, R. Assessment of the Use of Moringa oleifera Seed Husks for Removal of Pesticide Diuron from Contaminated Water. Environ. Technol. 2018, 41, 191–201. [Google Scholar] [CrossRef]
- Grundgeiger, E.; Lim, Y.H.; Frost, R.L.; Ayoko, G.A.; Xi, Y. Application of Organo-Beidellites for the Adsorption of Atrazine. Appl. Clay Sci. 2015, 105–106, 252–258. [Google Scholar] [CrossRef]
- Al-Shaalan, N.H.; Ali, I.; ALOthman, Z.A.; Al-Wahaibi, L.H.; Alabdulmonem, H. High Performance Removal and Simulation Studies of Diuron Pesticide in Water on MWCNTs. J. Mol. Liq. 2019, 289, 111039. [Google Scholar] [CrossRef]
- Chen, H.; Luo, Y.; Potter, C.; Moran, P.J.; Grieneisen, M.L.; Zhang, M. Modeling Pesticide Diuron Loading from the San Joaquin Watershed into the Sacramento-San Joaquin Delta Using SWAT. Water Res. 2017, 121, 374–385. [Google Scholar] [CrossRef]
- Bhoi, Y.P.; Behera, C.; Majhi, D.; Equeenuddin, S.M.; Mishra, B.G. Visible Light-Assisted Photocatalytic Mineralization of Diuron Pesticide Using Novel Type II CuS/Bi2W2O9 Heterojunctions with a Hierarchical Microspherical Structure. New J. Chem. 2018, 42, 281–292. [Google Scholar] [CrossRef]
- Silambarasan, S.; Cornejo, P.; Vangnai, A.S. Biodegradation of 4-Nitroaniline by Novel Isolate Bacillus Sp. Strain AVPP64 in the Presence of Pesticides. Environ. Pollut. 2022, 306, 119453. [Google Scholar] [CrossRef]
- Velki, M.; Lackmann, C.; Barranco, A.; Ereño Artabe, A.; Rainieri, S.; Hollert, H.; Seiler, T.B. Pesticides Diazinon and Diuron Increase Glutathione Levels and Affect Multixenobiotic Resistance Activity and Biomarker Responses in Zebrafish (Danio Rerio) Embryos and Larvae. Environ. Sci. Eur. 2019, 31, 4. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Miao, X.S.; Koenig, B.G.; Struger, J. Distribution of Acidic and Neutral Drugs in Surface Waters near Sewage Treatment Plants in the Lower Great Lakes, Canada. Environ. Toxicol. Chem. 2003, 22, 2881–2889. [Google Scholar] [CrossRef]
- Daughton, C.G. Cradle-to-Cradle Stewardship of Drugs for Minimizing Their Environmental Disposition While Promoting Human Health. I. Rational for and Avenues toward a Green Pharmacy. Environ. Health Perspect. 2003, 111, 757–774. [Google Scholar] [CrossRef]
- Carmona, E.; Andreu, V.; Picó, Y. Multi-Residue Determination of 47 Organic Compounds in Water, Soil, Sediment and Fish—Turia River as Case Study. J. Pharm. Biomed. Anal. 2017, 146, 117–125. [Google Scholar] [CrossRef]
- Jakubiec-Krzesniak, K.; Rajnisz-Mateusiak, A.; Guspiel, A.; Ziemska, J.; Solecka, J. Secondary Metabolites of Actinomycetes and Their Antibacterial, Antifungal and Antiviral Properties. Pol. J. Microbiol. 2018, 67, 259–272. [Google Scholar] [CrossRef]
- Scheurer, M.; Michel, A.; Brauch, H.J.; Ruck, W.; Sacher, F. Occurrence and Fate of the Antidiabetic Drug Metformin and Its Metabolite Guanylurea in the Environment and during Drinking Water Treatment. Water Res. 2012, 46, 4790–4802. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene Blue Dye: Toxicity and Potential Elimination Technology from Wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Katre, U.V.; Suresh, C.G.; Khan, M.I.; Gaikwad, S.M. Structure–Activity Relationship of a Hemagglutinin from Moringa oleifera Seeds. Int. J. Biol. Macromol. 2008, 42, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Ostaszewski, P.; Długosz, O.; Banach, M. Analysis of Measuring Methods of the Concentration of Methylene Blue in the Sorption Process in Fixed-Bed Column. Int. J. Environ. Sci. Technol. 2022, 19, 1–8. [Google Scholar] [CrossRef]
- Zein, R.; Satrio Purnomo, J.; Ramadhani, P.; Safni; Alif, M.F.; Putri, C.N. Enhancing Sorption Capacity of Methylene Blue Dye Using Solid Waste of Lemongrass Biosorbent by Modification Method. Arab. J. Chem. 2023, 16, 104480. [Google Scholar] [CrossRef]
- Saeed, A.A.H.; Harun, N.Y.; Sufian, S.; Siyal, A.A.; Zulfiqar, M.; Bilad, M.R.; Vagananthan, A.; Al-Fakih, A.; Ghaleb, A.A.S.; Almahbashi, N. Eucheuma Cottonii Seaweed-Based Biochar for Adsorption of Methylene Blue Dye. Sustainability 2020, 12, 10318. [Google Scholar] [CrossRef]
- Soria-Aguilar, M.d.J.; Martínez-Luévanos, A.; Sánchez-Castillo, M.A.; Carrillo-Pedroza, F.R.; Toro, N.; Narváez-García, V.M. Removal of Pb(II) from Aqueous Solutions by Using Steelmaking Industry Wastes: Effect of Blast Furnace Dust’s Chemical Composition. Arab. J. Chem. 2021, 14, 103061. [Google Scholar] [CrossRef]
- Khedr, R.F. Synthesis of Amidoxime Adsorbent by Radiation-Induced Grafting of Acrylonitrile/Acrylic Acid on Polyethylene Film and Its Application in Pb Removal. Polymers 2022, 14, 3136. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. Lead (Pb) Removal from Contaminated Water Using Constructed Wetland Planted with Scirpus Grossus: Optimization Using Response Surface Methodology (RSM) and Assessment of Rhizobacterial Addition. Chemosphere 2022, 291, 132952. [Google Scholar] [CrossRef]
- Ramola, S.; Rawat, N.; Shankhwar, A.K.; Srivastava, R.K. Fixed Bed Adsorption of Pb and Cu by Iron Modified Bamboo, Bagasse and Tyre Biochar. Sustain. Chem. Pharm. 2021, 22, 100486. [Google Scholar] [CrossRef]
- Narayana, P.L.; Lingamdinne, L.P.; Karri, R.R.; Devanesan, S.; AlSalhi, M.S.; Reddy, N.S.; Chang, Y.Y.; Koduru, J.R. Predictive Capability Evaluation and Optimization of Pb(II) Removal by Reduced Graphene Oxide-Based Inverse Spinel Nickel Ferrite Nanocomposite. Environ. Res. 2022, 204, 112029. [Google Scholar] [CrossRef]
- de Souza, R.M.; Seibert, D.; Quesada, H.B.; de Jesus Bassetti, F.; Fagundes-Klen, M.R.; Bergamasco, R. Occurrence, Impacts and General Aspects of Pesticides in Surface Water: A Review. Process Saf. Environ. Prot. 2020, 135, 22–37. [Google Scholar] [CrossRef]
- Alizadeh Fard, M.; Barkdoll, B. Magnetic Activated Carbon as a Sustainable Solution for Removal of Micropollutants from Water. Int. J. Environ. Sci. Technol. 2019, 16, 1625–1636. [Google Scholar] [CrossRef]
- Eniola, J.O.; Kumar, R.; Barakat, M.A.; Rashid, J. A Review on Conventional and Advanced Hybrid Technologies for Pharmaceutical Wastewater Treatment. J. Clean. Prod. 2022, 356, 131826. [Google Scholar] [CrossRef]
- Metz, F.; Glaus, A. Integrated Water Resources Management and Policy Integration: Lessons from 169 Years of Flood Policies in Switzerland. Water 2019, 11, 1173. [Google Scholar] [CrossRef]
- Reck, I.M.; Paixão, R.M.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S. Removal of Tartrazine from Aqueous Solutions Using Adsorbents Based on Activated Carbon and Moringa oleifera Seeds. J. Clean. Prod. 2018, 171, 85–97. [Google Scholar] [CrossRef]
- Baptista, A.T.A.; Coldebella, P.F.; Cardines, P.H.F.; Gomes, R.G.; Vieira, M.F.; Bergamasco, R.; Vieira, A.M.S. Coagulation-Flocculation Process with Ultrafiltered Saline Extract of Moringa oleifera for the Treatment of Surface Water. Chem. Eng. J. 2015, 276, 166–173. [Google Scholar] [CrossRef]
- Ueda Yamaguchi, N.; Cusioli, L.F.; Quesada, H.B.; Camargo Ferreira, M.E.; Fagundes-Klen, M.R.; Salcedo Vieira, A.M.; Gomes, R.G.; Vieira, M.F.; Bergamasco, R. A Review of Moringa oleifera Seeds in Water Treatment: Trends and Future Challenges. Process Saf. Environ. Prot. 2021, 147, 405–420. [Google Scholar] [CrossRef]
- Beluci, N.d.C.L.; Mateus, G.A.P.; Miyashiro, C.S.; Homem, N.C.; Gomes, R.G.; Fagundes-Klen, M.R.; Bergamasco, R.; Vieira, A.M.S. Hybrid Treatment of Coagulation/Flocculation Process Followed by Ultrafiltration in TiO2-Modified Membranes to Improve the Removal of Reactive Black 5 Dye. Sci. Total Environ. 2019, 664, 222–229. [Google Scholar] [CrossRef]
- Suarez, M.; Haenni, M.; Canarelli, S.; Fisch, F.; Chodanowski, P.; Servis, C.; Michielin, O.; Freitag, R.; Moreillon, P.; Mermod, N. Structure-Function Characterization and Optimization of a Plant-Derived Antibacterial Peptide. Antimicrob. Agents Chemother. 2005, 49, 3847–3857. [Google Scholar] [CrossRef]
- Cusioli, L.F.; Mantovani, D.; Quesada, H.B.; Gomes, R.G.; Bergamasco, R. Adsorption of COVID-19-Related Drug from Contaminated Water Using Activated Carbon. Desalination Water Treat. 2022, 277, 85–89. [Google Scholar] [CrossRef]
- Cusioli, L.F.; Bezerra, C.d.O.; Quesada, H.B.; Alves Baptista, A.T.; Nishi, L.; Vieira, M.F.; Bergamasco, R. Modified Moringa oleifera Lam. Seed Husks as Low-Cost Biosorbent for Atrazine Removal. Environ. Technol. 2019, 42, 1092–1103. [Google Scholar] [CrossRef]
- Wernke, G.; Fagundes-Klen, M.R.; Vieira, M.F.; Suzaki, P.Y.R.; de Souza, H.K.S.; Shimabuku, Q.L.; Bergamasco, R. Mathematical Modelling Applied to the Rate-Limiting Mass Transfer Step Determination of a Herbicide Biosorption onto Fixed-Bed Columns. Environ. Technol. 2018, 41, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Yang, L.; Leng, S.; Zhang, W.; Zhou, Y.; Peng, H.; Li, H.; Hu, Y.; Jiang, S.; Li, H. A Review on Nitrogen Transformation in Hydrochar during Hydrothermal Carbonization of Biomass Containing Nitrogen. Sci. Total Environ. 2021, 756, 143679. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A Review of the Hydrothermal Carbonization of Biomass Waste for Hydrochar Formation: Process Conditions, Fundamentals, and Physicochemical Properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Liu, H.; Basar, I.A.; Nzihou, A.; Eskicioglu, C. Hydrochar Derived from Municipal Sludge through Hydrothermal Processing: A Critical Review on Its Formation, Characterization, and Valorization. Water Res. 2021, 199, 117186. [Google Scholar] [CrossRef]
- Regalbuto, J.R.; Robles, J.O. The Engineering of Pt/Carbon Catalyst Preparation; University of Illinois: Chicago, IL, USA, 2004; Volume 1, pp. 1–14. [Google Scholar]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Chibowski, E.; Szcześ, A. Zeta Potential and Surface Charge of DPPC and DOPC Liposomes in the Presence of PLC Enzyme. Adsorption 2016, 22, 755–765. [Google Scholar] [CrossRef]
- Kiwumulo, H.F.; Muwonge, H.; Ibingira, C.; Lubwama, M.; Kirabira, J.B.; Ssekitoleko, R.T. Green Synthesis and Characterization of Iron-Oxide Nanoparticles Using Moringa oleifera: A Potential Protocol for Use in Low and Middle Income Countries. BMC Res. Notes 2022, 15, 149. [Google Scholar] [CrossRef]
- Wibawa, P.J.; Nur, M.; Asy’ari, M.; Nur, H. SEM, XRD and FTIR Analyses of Both Ultrasonic and Heat Generated Activated Carbon Black Microstructures. Heliyon 2020, 6, e03546. [Google Scholar] [CrossRef]
- Adebayo, G.B.; Jamiu, W.; Okoro, H.K.; Okeola, F.O.; Adesina, A.K.; Feyisetan, O.A. Kinetics, Thermodynamics and Isothermal Modelling of Liquid Phase Adsorption of Methylene Blue onto Moringa Pod Husk Activated Carbon. S. Afr. J. Chem. 2019, 72, 263–273. [Google Scholar] [CrossRef]
- Saka, C. BET, TG-DTG, FT-IR, SEM, Iodine Number Analysis and Preparation of Activated Carbon from Acorn Shell by Chemical Activation with ZnCl2. J. Anal. Appl. Pyrolysis 2012, 95, 21–24. [Google Scholar] [CrossRef]
- Cruz-Espinoza, J.E.; Orduña-Díaz, A.; Rosales-Perez, M.; Zaca-Morán, O.; Delgado-Macuil, R.; Gayou, V.L.; Rojas-López, M. FTIR Analysis of Phenolic Extracts from Moringa oleifera Leaves. J. Biom. Biostat. 2012, 11, 2802. [Google Scholar]
- Hashim, F.J.; Mathkor, T.H.; Hussein, H.; Shawkat, M.S. GC-MS and FTIR Analysis of Moringa oleifera Leaves Different Extracts and Evaluation of Their Antioxidant Activity. Asian J. Microbiol. Biotechnol. Environ. Sci. 2017, 19, 861–871. [Google Scholar]
- Coldebella, P.F.; Fagundes-klen, R.; Valverde, K.C.; Cavalcanti, E.B.; Aparecida, O. Potential Effect of Chemical and Thermal Treatment on the Kinetics, Equilibrium, and Thermodynamic Studies for Atrazine Biosorption by the Moringa oleifera Pods. Can. J. Chem. Eng. 2017, 95, 961–973. [Google Scholar] [CrossRef]
- Sya’banah, N.; Yulianti, E.; Istighfarini, V.N.; Lutfia, F.N.L. Characterization and Effectiveness of Moringa oleifera Seeds Extract as a Phosphate Coagulant. J. Neutrino 2020, 12, 57–64. [Google Scholar] [CrossRef]
- Dalhoumi, W.; Guesmi, F.; Bouzidi, A.; Akermi, S.; Hfaiedh, N.; Saidi, I. Therapeutic Strategies of Moringa oleifera Lam. (Moringaceae) for Stomach and Forestomach Ulceration Induced by HCl/EtOH in Rat Model. Saudi J. Biol. Sci. 2022, 29, 103284. [Google Scholar] [CrossRef]
- Joga Rao, H. Characterization Studies on Adsorption of Lead and Cadmium Using Activated Carbon Prepared from Waste Tyres. Nat. Environ. Pollut. Technol. 2021, 20, 561–568. [Google Scholar] [CrossRef]
- Dittmann, D.; Saal, L.; Zietzschmann, F.; Mai, M.; Altmann, K.; Al-Sabbagh, D.; Schumann, P.; Ruhl, A.S.; Jekel, M.; Braun, U. Characterization of Activated Carbons for Water Treatment Using TGA-FTIR for Analysis of Oxygen-Containing Functional Groups. Appl. Water Sci. 2022, 12, 203. [Google Scholar] [CrossRef]
- González-González, R.B.; González, L.T.; Madou, M.; Leyva-Porras, C.; Martinez-Chapa, S.O.; Mendoza, A. Synthesis, Purification, and Characterization of Carbon Dots from Non-Activated and Activated Pyrolytic Carbon Black. Nanomaterials 2022, 12, 298. [Google Scholar] [CrossRef]
- Hussain, O.A.; Hathout, A.S.; Abdel-Mobdy, Y.E.; Rashed, M.M.; Abdel Rahim, E.A.; Fouzy, A.S.M. Preparation and Characterization of Activated Carbon from Agricultural Wastes and Their Ability to Remove Chlorpyrifos from Water. Toxicol. Rep. 2023, 10, 146–154. [Google Scholar] [CrossRef]
- Mistar, E.M.; Alfatah, T.; Supardan, M.D. Synthesis and Characterization of Activated Carbon from Bambusa Vulgaris Striata Using Two-Step KOH Activation. J. Mater. Res. Technol. 2020, 9, 6278–6286. [Google Scholar] [CrossRef]
- Osborne, S.P.; Nasi, G.; Powell, M. Beyond Co-Production: Value Creation and Public Services. Public Adm. 2021, 99, 641–657. [Google Scholar] [CrossRef]
- Bahşi, N.; Çetin, E. Determining of Agricultural Credit Impact on Agricultural Production Value in Turkey. Cienc. Rural 2020, 50, e20200003. [Google Scholar] [CrossRef]
| MOH | Activated Carbon | |
|---|---|---|
| BET specific surface area (m2 g−1) | 38.25 | 205.71 |
| Average pore diameter (Å) | 26.44 | 32.57 |
| Total pore volume (cm3 g−1) | 0.1293 | 0.4899 |
| Micropore volume (cm3 g−1) | 0.0996 | 0.4012 |
| Mesopore volume (cm3 g−1) | 0.0297 | 0.0887 |
| Models | Parameters | Metformin | Diuron | Methylene Blue | Lead |
|---|---|---|---|---|---|
| PFO | qe (mg g−1) | 29.65 | 27.13 | 31.07 | 30.20 |
| k1 (min−1) | 0.008 | 0.013 | 0.009 | 0.015 | |
| R2 | 0.962 | 0.9703 | 0.991 | 0.957 | |
| χ2 | 0.189 | 0.234 | 0.218 | 0.245 | |
| PSO | qe (mg g−1) | 25.17 | 21.22 | 30.78 | 27.45 |
| k2 (g mg−1 min−1) | 0.012 | 0.023 | 0.011 | 0.033 | |
| R2 | 0.907 | 0.921 | 0.934 | 0.893 | |
| χ2 | 0.267 | 0.328 | 0.437 | 0.578 |
| Models | Parameters | Metformin | Diuron | Methylene Blue | Lead |
|---|---|---|---|---|---|
| PFO | qe (mg g−1) | 32.34 | 27.76 | 32.10 | 27.86 |
| k1 (min−1) | 0.004 | 0.009 | 0.005 | 0.008 | |
| R2 | 0.973 | 0.981 | 0.994 | 0.975 | |
| χ2 | 0.109 | 0.156 | 0.153 | 0.147 | |
| PSO | qe (mg g−1) | 30.72 | 24.55 | 28.83 | 23.67 |
| k2 (g mg−1 min−1) | 0.009 | 0.013 | 0.009 | 0.025 | |
| R2 | 0.942 | 0.954 | 0.962 | 0.913 | |
| χ2 | 0.203 | 0.293 | 0.374 | 0.278 |
| Activated Carbon | |
|---|---|
| Production | Cost (BRL/kg) |
| Activated carbon (commercial) | 35.00 |
| Total cost | 35.00 |
| MOH | |
| Production | Cost (BRL/kg) |
| Raw material (waste) | 0.00 |
| Water wash | 1.25 |
| Material drying | 1.05 |
| Crushing of the material | 2.00 |
| Hydrothermal reactor processing | 3.25 |
| Material drying | 1.05 |
| Granulometry separation | 0.80 |
| Total cost | 9.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cusioli, L.F.; Mantovani, D.; Bergamasco, R.; Tusset, A.M.; Lenzi, G.G. Preparation of a New Adsorbent Material from Agro-Industrial Waste and Comparison with Commercial Adsorbent for Emerging Contaminant Removal. Processes 2023, 11, 2478. https://doi.org/10.3390/pr11082478
Cusioli LF, Mantovani D, Bergamasco R, Tusset AM, Lenzi GG. Preparation of a New Adsorbent Material from Agro-Industrial Waste and Comparison with Commercial Adsorbent for Emerging Contaminant Removal. Processes. 2023; 11(8):2478. https://doi.org/10.3390/pr11082478
Chicago/Turabian StyleCusioli, Luís Fernando, Daniel Mantovani, Rosângela Bergamasco, Angelo Marcelo Tusset, and Giane Gonçalves Lenzi. 2023. "Preparation of a New Adsorbent Material from Agro-Industrial Waste and Comparison with Commercial Adsorbent for Emerging Contaminant Removal" Processes 11, no. 8: 2478. https://doi.org/10.3390/pr11082478
APA StyleCusioli, L. F., Mantovani, D., Bergamasco, R., Tusset, A. M., & Lenzi, G. G. (2023). Preparation of a New Adsorbent Material from Agro-Industrial Waste and Comparison with Commercial Adsorbent for Emerging Contaminant Removal. Processes, 11(8), 2478. https://doi.org/10.3390/pr11082478











