A Study on the Oil-Bearing Stability of Salt-Resistant Foam and an Explanation of the Viscoelastic Phenomenon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
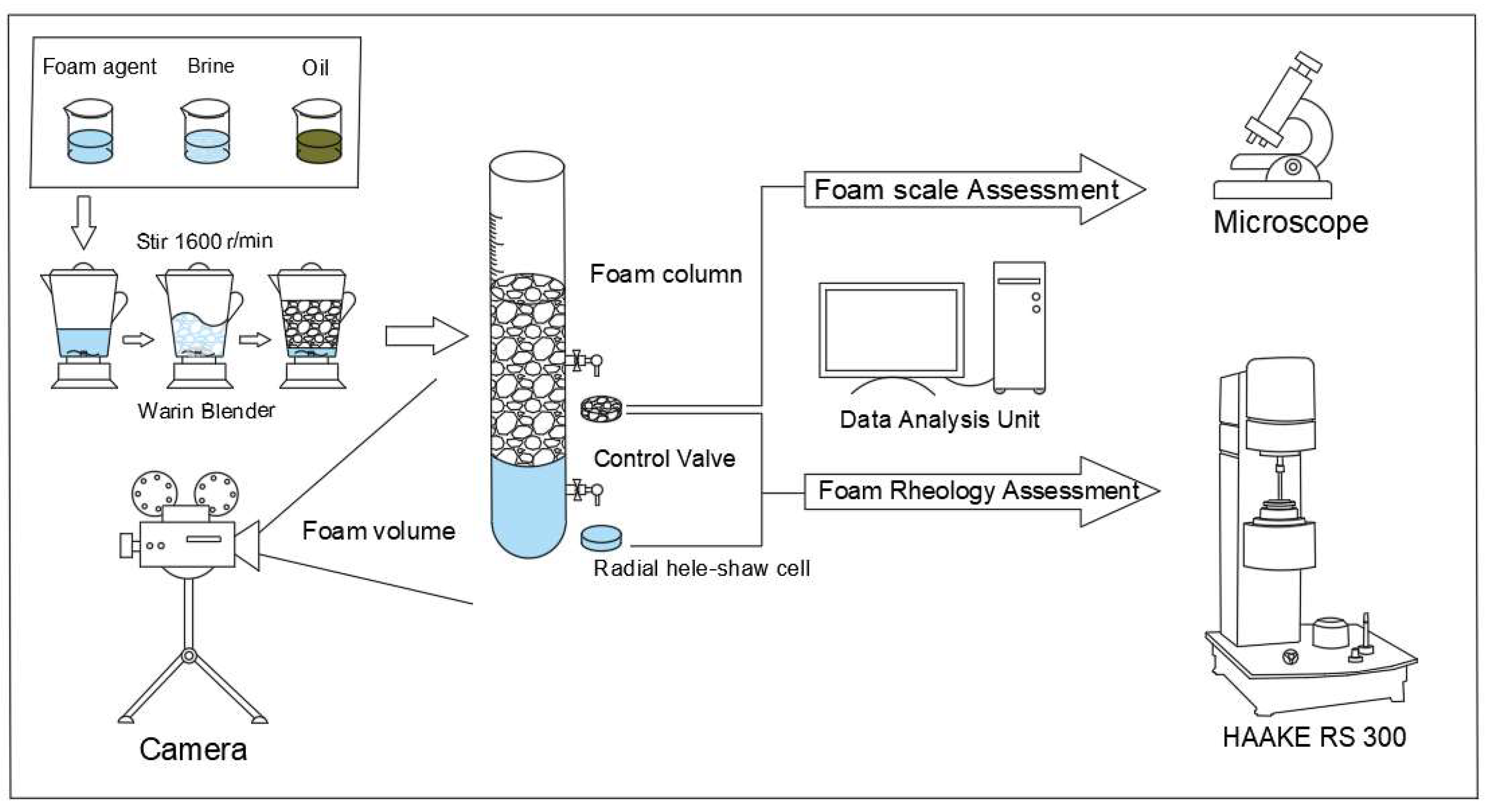
2.2. Methods
2.2.1. Salt-Resistant Foam System
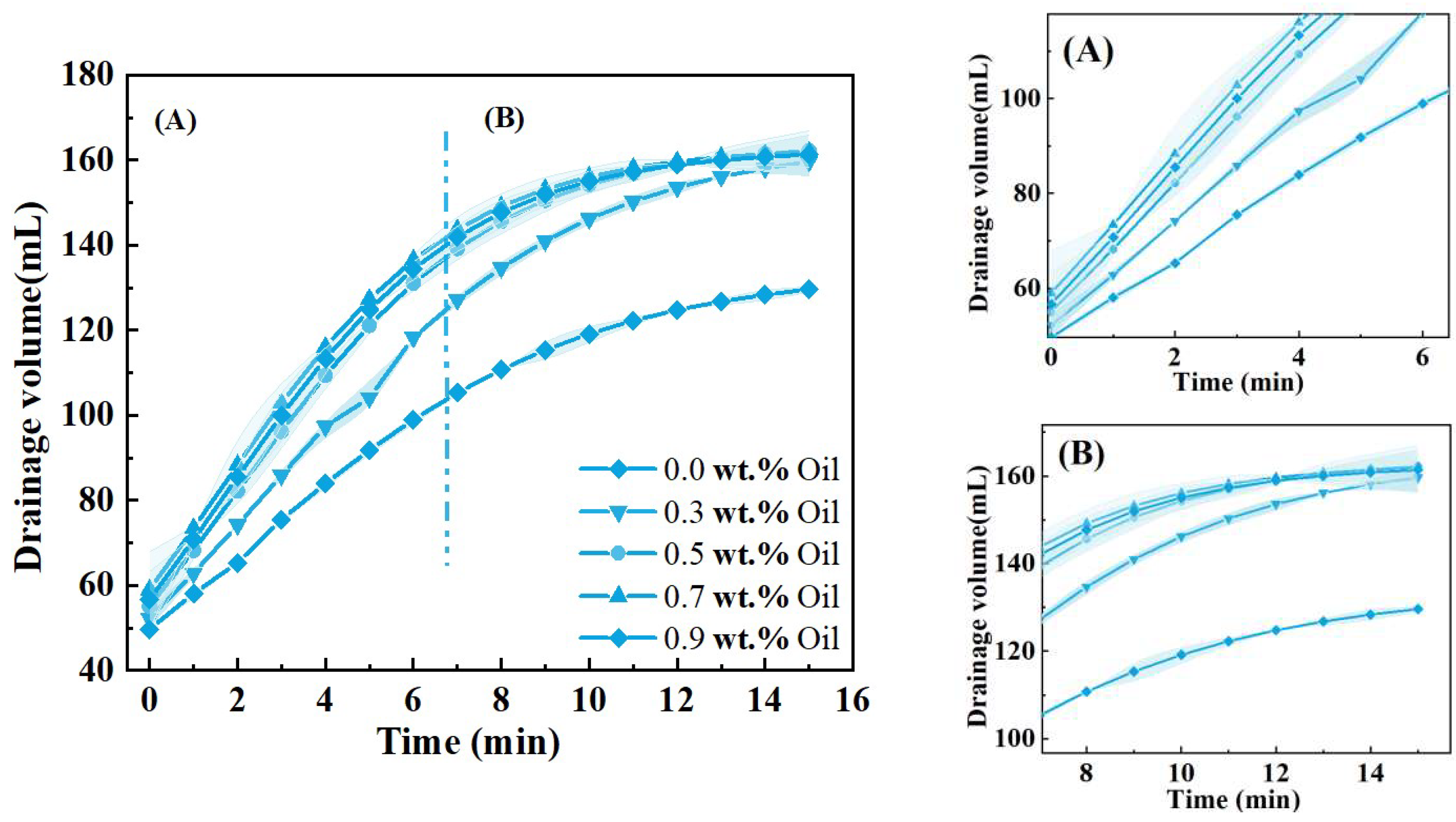
2.2.2. Oil Contained Stability
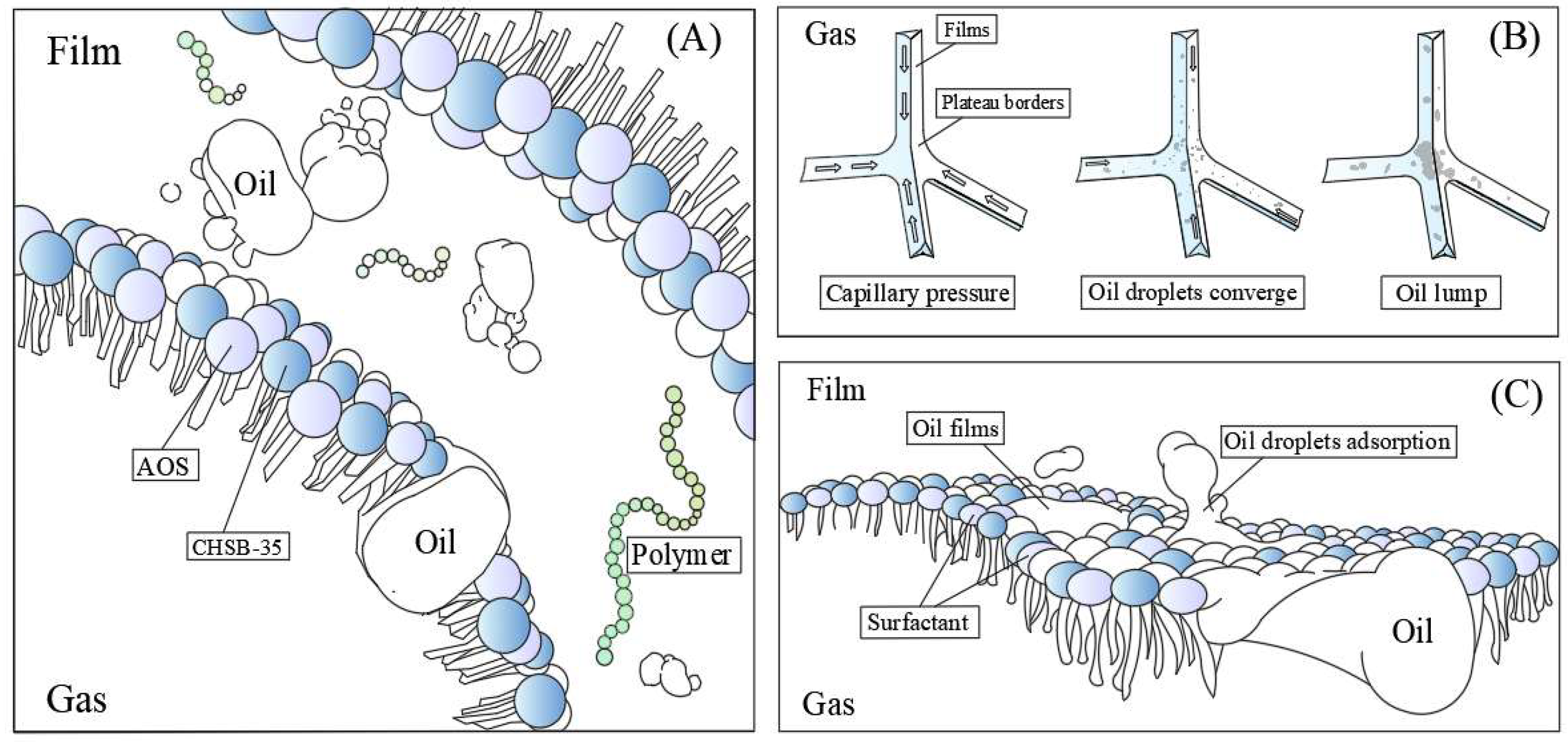
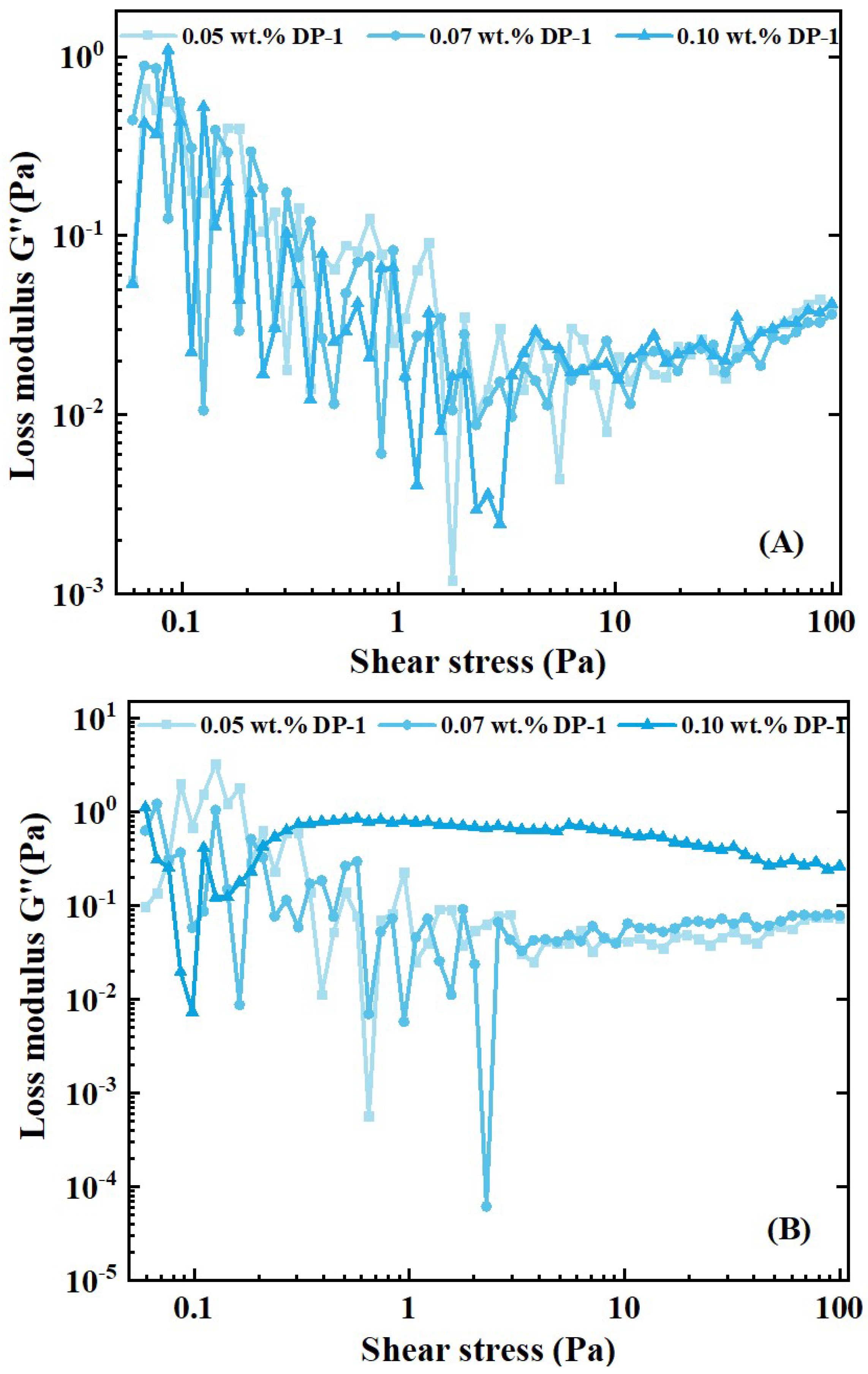
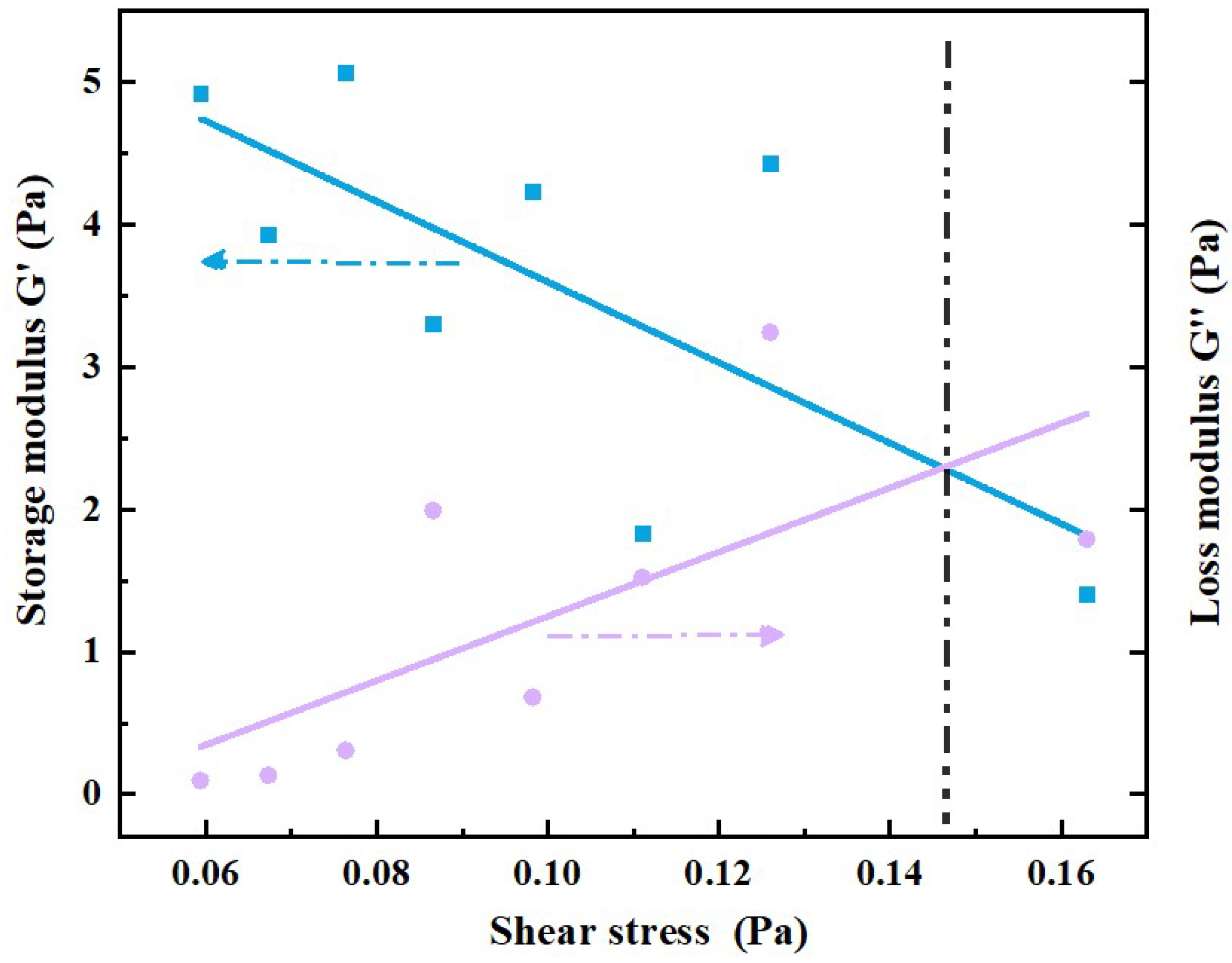
2.2.3. Viscoelastic Phenomenon
3. Results and Discussion
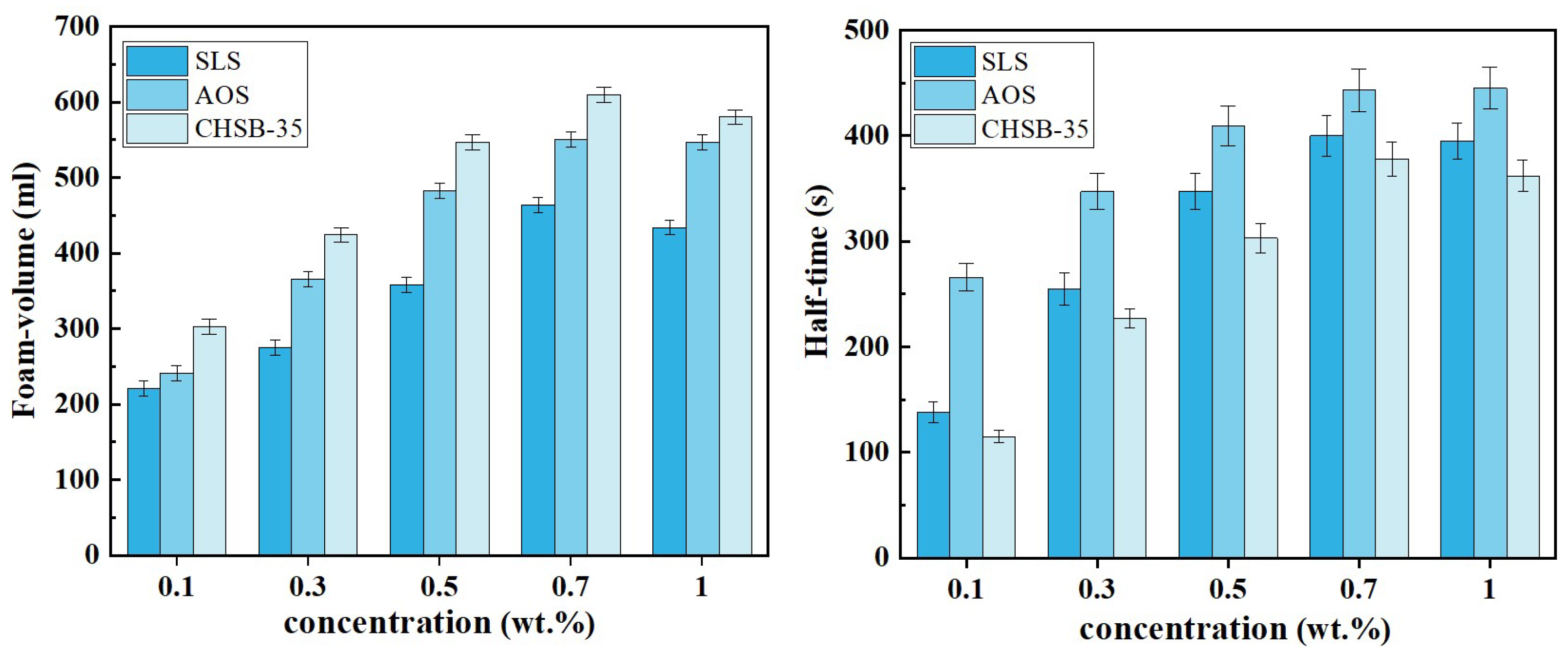
3.1. Salt-Resistant Foam System
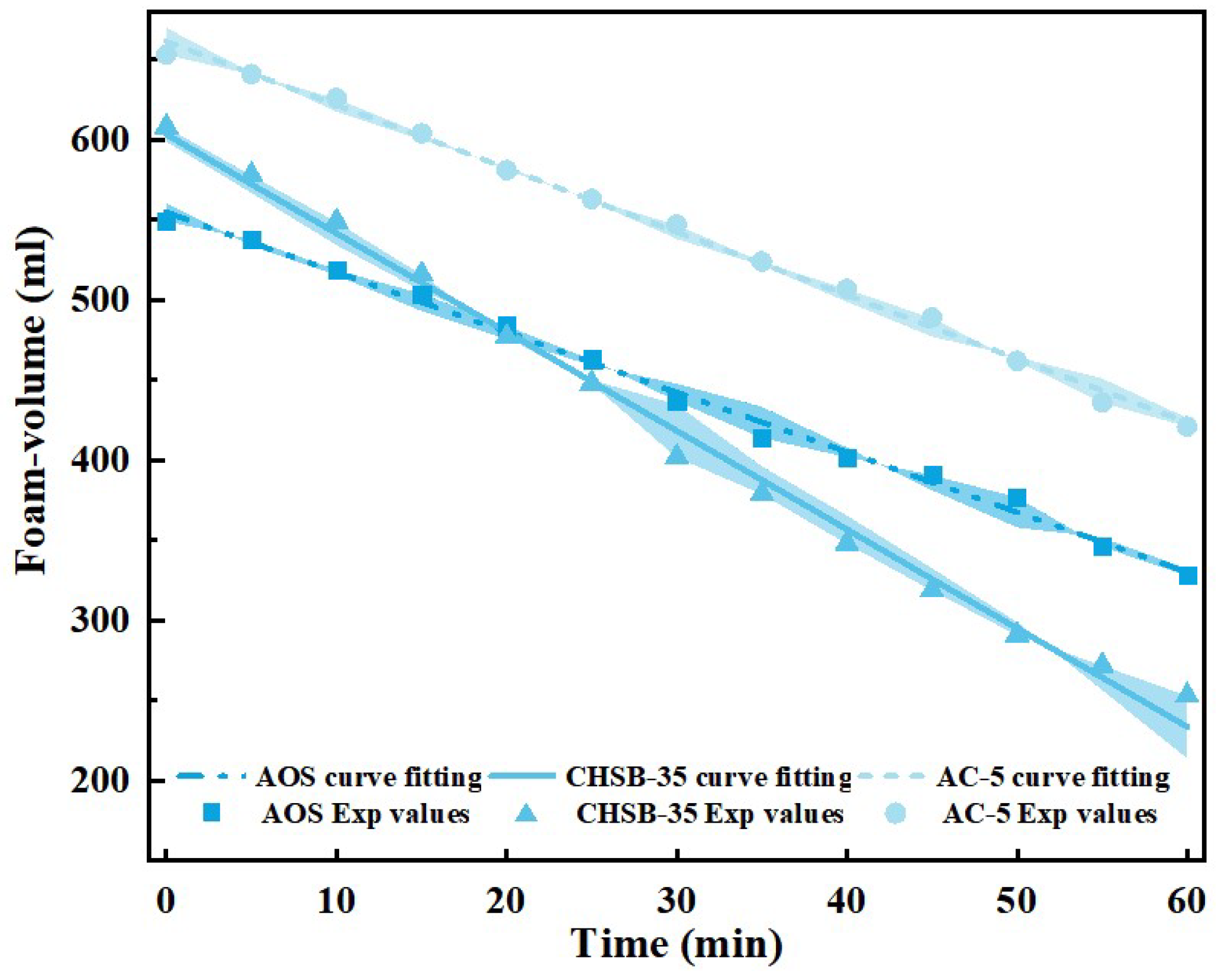
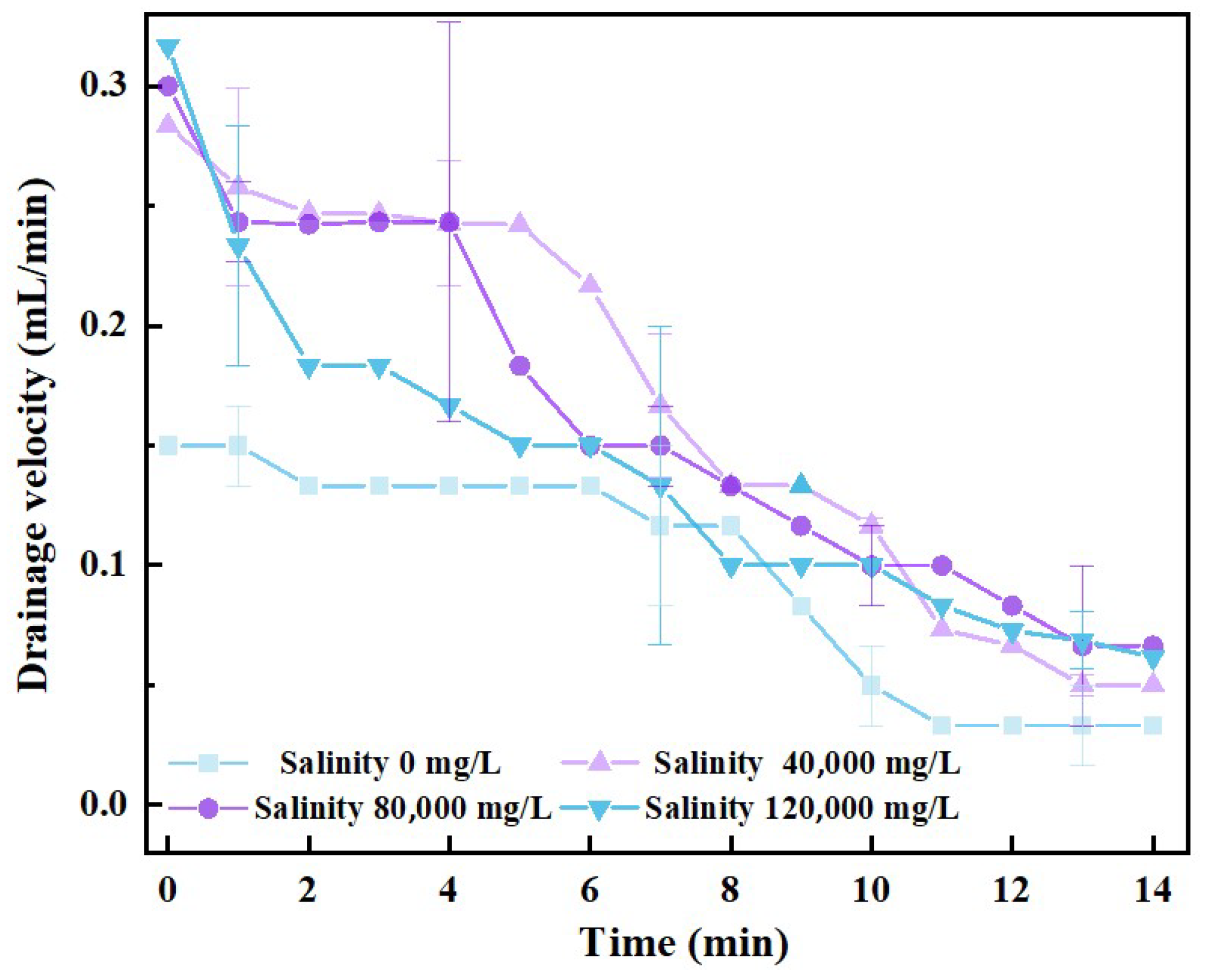
3.2. Oil-Containing Stability Analysis
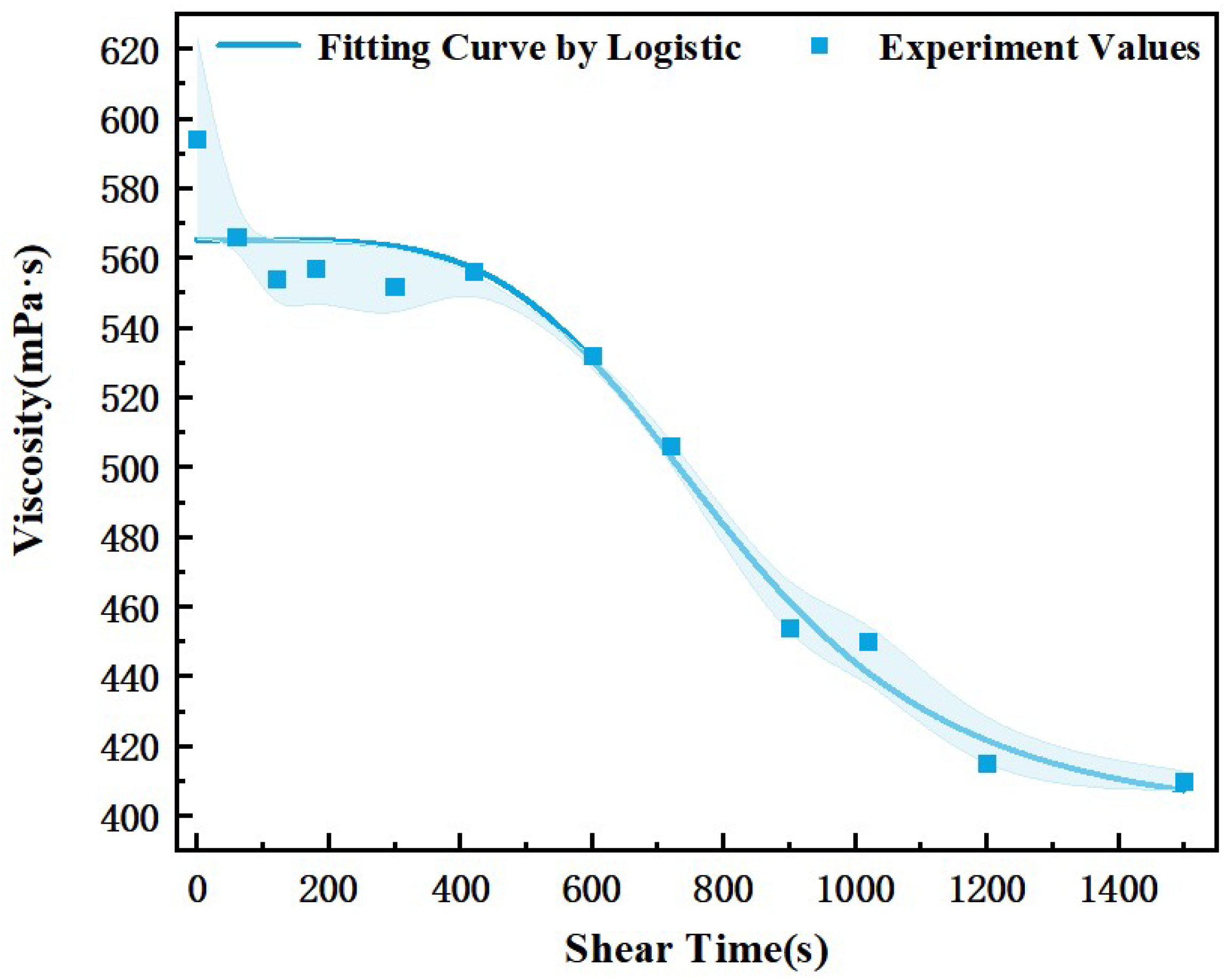
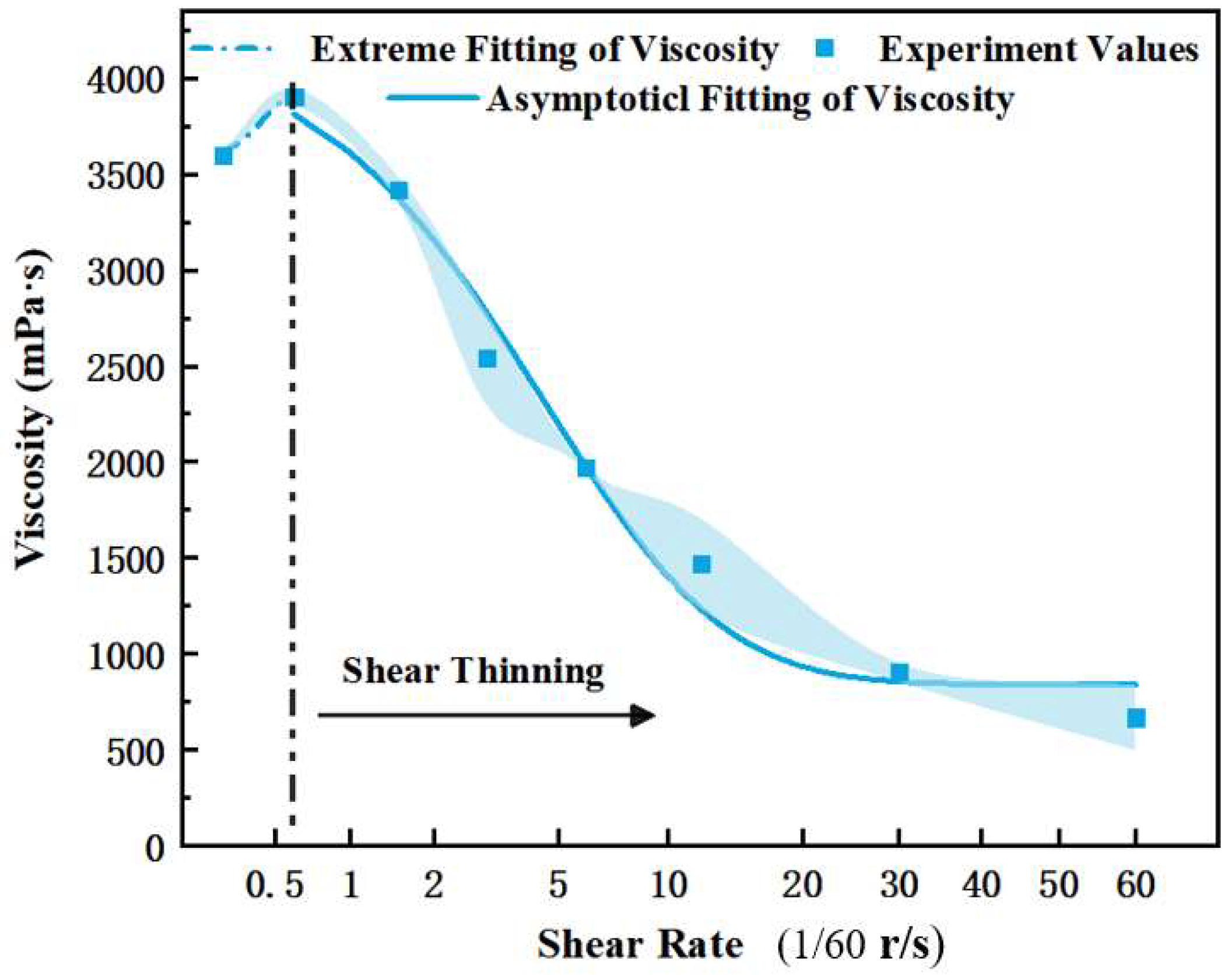
3.3. Interpretation of the Foam Fluid Viscoelasticity
4. Conclusions
- The draining process of foam films at a high degree of mineralization is complicated, and the film draining velocity is reduced by a “step-type” law; this process involves many factors, such as the curvature of the liquid films, the thickness of the double-electric layer, chemical reactions, etc. It is difficult to use a single model for analysis and verification, and the main mechanism of ionics affecting foam stability and the liquid film draining process has not yet been clarified; it is necessary to analyze this in depth and establish a complete theoretical system.
- The influence of experimental oil depends on its presence of the liquid films. When oil droplets exist in the films at tiny particle sizes, they are gradually gathered into oil lumps. Under the driving effect of surface and volume pressure, oil lumps will accumulate in the Plateau borders; such behavior also increases the draining process of the liquid films. With the accumulation of oil droplets on the Plateau borders, large-volume oil droplets will hinder the film draining process. The population balance equation is used to described this process efficiently. Minimal oil droplets may be adsorbed on the foam film surface and disrupt the stress distribution of films, which will lead to a “stepwise” breakup mode of the foam column. This phenomenon has not yet received sufficient attention from scholars.
- Compared with the polymer solution, the foam fluid has a clearly elastic–plastic characteristic. At low values of shear and stress, foam liquids exhibit the characteristic of increasing viscosity in the shear; at high shear rates or stresses, the foam exhibits the rheological characteristics of the power law. These findings can extend the understanding of power-law liquids in the academic community, and help to further investigate the pressure distribution and fluid mechanics of foam liquid from the perspectives of interface mechanics and rheology.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kamal, M.S. A Novel Approach to Stabilize Foam Using Fluorinated Surfactants. Energies 2019, 12, 1163. [Google Scholar] [CrossRef]
- Ahmed, S.; Elraies, K.A.; Hashmet, M.R.; Alnarabiji, M.S. Empirical Modeling of the Viscosity of Supercritical Carbon Dioxide Foam Fracturing Fluid under Different Downhole Conditions. Energies 2018, 11, 782. [Google Scholar] [CrossRef]
- Cao, P.; Chen, Z.; Liu, M.; Cao, H.; Chen, B. Numerical and experimental study of a novel aerodynamic foam breaker for foam drilling fluid. Energy Sci. Eng. 2019, 7, 2410–2420. [Google Scholar] [CrossRef]
- Ni, C.; Xie, Y.; Liu, C.; Han, Z.; Shen, H.; Ran, W.; Xie, W.; Liang, Y. Exploring the separation mechanism of Gemini surfactant in scheelite froth flotation at low temperatures: Surface characterization, DFT calculations and kinetic simulations. Sep. Purif. Technol. 2023, 305, 122358. [Google Scholar] [CrossRef]
- Govindu, A.; Ahmed, R.; Shah, S.; Amani, M. The Effect of Inclination on the Stability of Foam Systems in Drilling and Well Operations. SPE Drill. Complet. 2020, 36, 263–280. [Google Scholar] [CrossRef]
- Maini, B.B.; Ma, V. Laboratory Evaluation of Foaming Agents for High-Temperature Applications—I. Measurements of Foam Stability at Elevated Temperatures and Pressures. J. Can. Pet. Technol. 1986, 25, PETSOC-86-06-05. [Google Scholar] [CrossRef]
- Lin, F.; Ng, J.K.; Huang, Y.; Huang, C.; Zeng, H. Formation and stability of oil-laden foam: Effect of surfactant and hydrocarbon solvent. Can. J. Chem. Eng. 2021, 99, 2658–2669. [Google Scholar] [CrossRef]
- Majeed, T.; Sølling, T.I.; Kamal, M.S. Foamstability: The interplay between salt-, surfactant- and critical micelle concentration. J. Pet. Sci. Eng. 2019, 187, 106871. [Google Scholar] [CrossRef]
- Zhang, P.; Bai, G.; Cui, G.; Zhang, L.; Peng, X.; Pei, S.; Ren, S. Enhanced CO2 foam based on amide and amine surfactants and synergistically coupled with sodium dodecyl sulfate at high temperature and high pressure. J. Pet. Sci. Eng. 2019, 179, 266–275. [Google Scholar] [CrossRef]
- Harati, S.; Bayat, A.E.; Sarvestani, M.T. Assessing the effects of different gas types on stability of SiO2 nanoparticle foam for enhanced oil recovery purpose. J. Mol. Liq. 2020, 313, 113521. [Google Scholar] [CrossRef]
- Bashir, A.; Haddad, A.S.; Rafati, R. Nanoparticle/polymer-enhanced alpha olefin sulfonate solution for foam generation in the presence of oil phase at high temperature conditions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123875. [Google Scholar] [CrossRef]
- Telmadarreie, A.; Trivedi, J. CO2 Foam and CO2 Polymer Enhanced Foam for Heavy Oil Recovery and CO2 Storage. Energies 2020, 13, 5735. [Google Scholar] [CrossRef]
- Hosseini-Nasab, S.M.; Taal, M.; Zitha, P.L.J.; Sharifi, M. Effect of Newtonian and non-Newtonian viscosifying agents on stability of foams in enhanced oil recovery. Part I: Under bulk condition. Iran. Polym. J. 2019, 28, 291–299. [Google Scholar] [CrossRef]
- Parvaneh, R.; Riahi, S.; Lotfollahi, M.N. Experimental Evaluation of a Polymer Foam Agent on the Foam Stability, Concern to Surfactant, Nanoparticle, and Salinity. SPE J. 2022, 27, 1462–1479. [Google Scholar] [CrossRef]
- Scoggins, W.C.; Ke, M. Method of Increasing Lubricity of Brine-Based Drilling Fluids and Completion Brines. U.S. Patent 8,071,510, 6 December 2011. [Google Scholar]
- Roberto, P.-G.; Arturo, B.-T. Coalescence of air bubbles: Effect of the electrical double layer. Miner. Eng. 2020, 150, 106301. [Google Scholar] [CrossRef]
- Jin, F.-Y.; Wang, S.; Pu, W.-F.; Yuan, C.-D.; Wang, L.; Li, K.-X.; Gong, C. Emulsified oil foam for improving the flowability of heavy oil in wellbore under high salinity environments. J. Ind. Eng. Chem. 2016, 39, 153–161. [Google Scholar] [CrossRef]
- Prince, M.J.; Blanch, H.W. Bubble coalescence and break-up in air-sparged bubble columns. AIChE J. 1990, 36, 1485–1499. [Google Scholar] [CrossRef]
- Fichthorn, K.A.; Weinberg, W.H. Theoretical foundations of dynamical Monte Carlo simulations. J. Chem. Phys. 1991, 95, 1090–1096. [Google Scholar] [CrossRef]
- Huang, B.; Nan, X.; Fu, C.; Liu, W.; Guo, W.; Wang, S.; Zhang, L. Probing the Coalescence Mechanism of Oil Droplets in Fluids Produced by Oil Wells and the Microscopic Interaction between Molecules in Oil Films. Energies 2022, 15, 4274. [Google Scholar] [CrossRef]
- Bashir, A.; Haddad, A.S.; Sherratt, J.; Rafati, R. An investigation of viscous oil displacement in a fractured porous medium using polymer-enhanced surfactant alternating foam flooding. J. Pet. Sci. Eng. 2022, 212, 110280. [Google Scholar] [CrossRef]
- An Oil-Tolerant and Salt-Resistant Aqueous Foam System for Heavy Oil Transportation. All Databases n.d. Available online: https://www.webofscience.com/wos/alldb/summary/798e1c0c-5233-495d-b1aa-ec3b697dd04e-7e3d3302/relevance/1 (accessed on 1 April 2023).
- Pu, W.; Jiang, R.; Pang, S.; Qiu, T.; Sun, Z.; Shen, C.; Wei, P. Experimental investigation of surfactant-stabilized foam stability in the presence of light oil. J. Dispers. Sci. Technol. 2019, 41, 1596–1606. [Google Scholar] [CrossRef]
- Rezaeiakmal, F.; Parsaei, R. Visualization study of polymer enhanced foam (PEF) flooding for recovery of waterflood residual oil: Effect of cross flow. J. Pet. Sci. Eng. 2021, 203, 108583. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Yu, Z. A Study on the Oil-Bearing Stability of Salt-Resistant Foam and an Explanation of the Viscoelastic Phenomenon. Processes 2023, 11, 2598. https://doi.org/10.3390/pr11092598
Yang C, Yu Z. A Study on the Oil-Bearing Stability of Salt-Resistant Foam and an Explanation of the Viscoelastic Phenomenon. Processes. 2023; 11(9):2598. https://doi.org/10.3390/pr11092598
Chicago/Turabian StyleYang, Changhua, and Zhenye Yu. 2023. "A Study on the Oil-Bearing Stability of Salt-Resistant Foam and an Explanation of the Viscoelastic Phenomenon" Processes 11, no. 9: 2598. https://doi.org/10.3390/pr11092598







