Unraveling Oxygen Transfer Behavior in Submerged Arc Welding Using CaF2-SiO2-CaO Fluxes
Abstract
:1. Introduction
- CaF2 is the flux component free of O.
- The addition of CaO can effectively decrease the activity of SiO2.
2. Materials and Methods
2.1. Modeling Tools
2.2. Modeling Details
2.2.1. Droplet Reaction Zone
- The databases FToxid, Fstel, and FactPS were chosen. The molten slag and steel phases were simulated by selecting the solution phases of ASlag-liq all oxides, S (FToxid-SLAGH), and LIQUID (FStel-Liqu).
- The equilibrium temperature for the SAW process was set at 2500 °C, which corresponds to the temperature of the arc plasma. The input metal chemistries were obtained from the BM compositions.
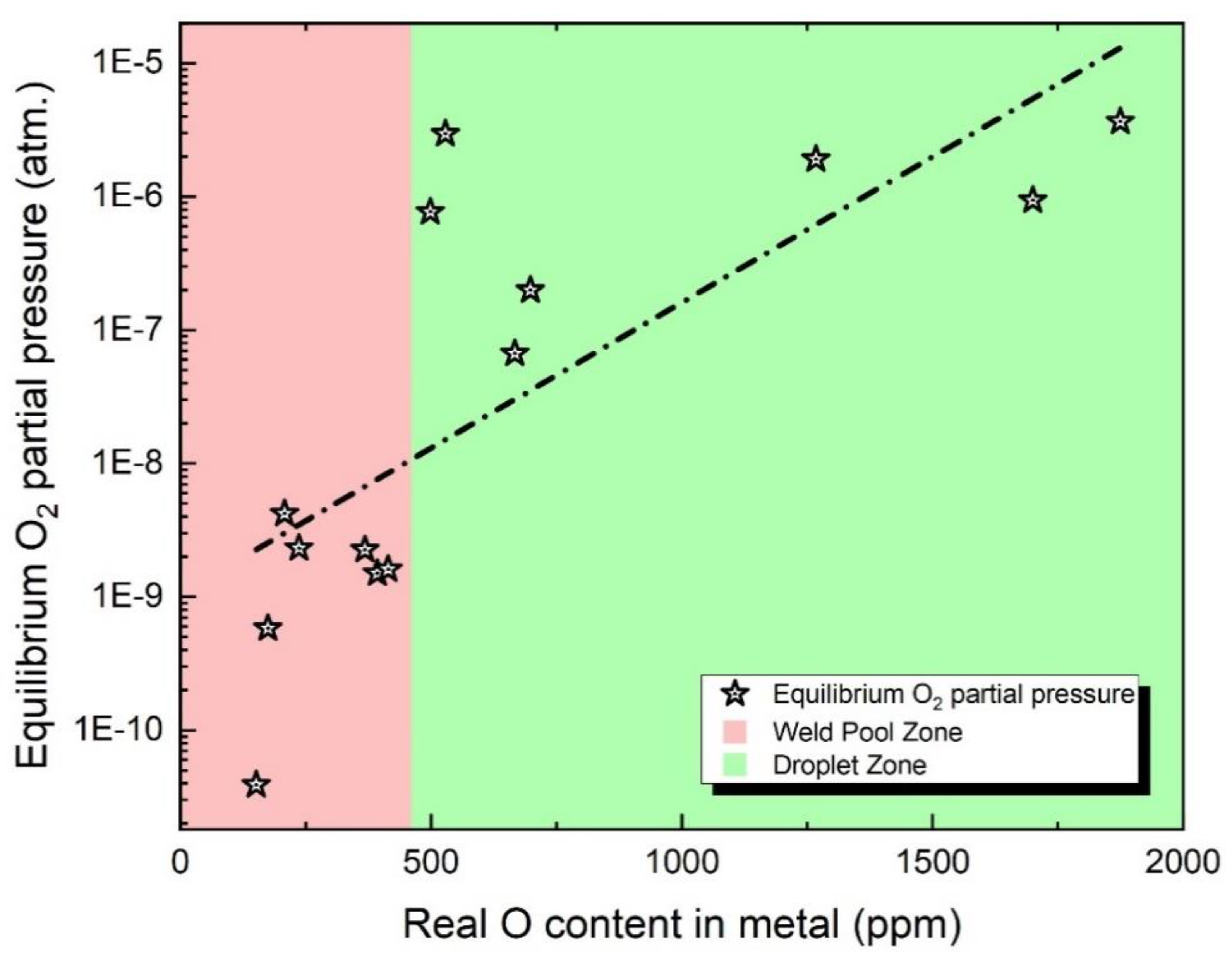
- To predict the O concentration in the droplet, an equilibrium calculation was conducted using iron (Fe) and O as the input metal constituents. The PO2 value provided in Table 3 was employed for this calculation. Table 3 presents the concentration of Oin molten droplets and the corresponding partial pressure of O2 in equilibrium.
2.2.2. Weld Pool Reactions
- To simulate the molten slag and steel phases, the FToxid, Fstel, and FactPS databases were utilized, selecting the solution phases of ASlag-liq for all oxides, S (FTox-id-SLAGH) for slag, and LIQUID (FStel-Liqu) for steel.
- A modeling temperature of 2000 °C was chosen.
2.2.3. Solidification Zone
2.2.4. Viscosity Simulation
3. Results and Discussion
3.1. Transfer Behavior of O
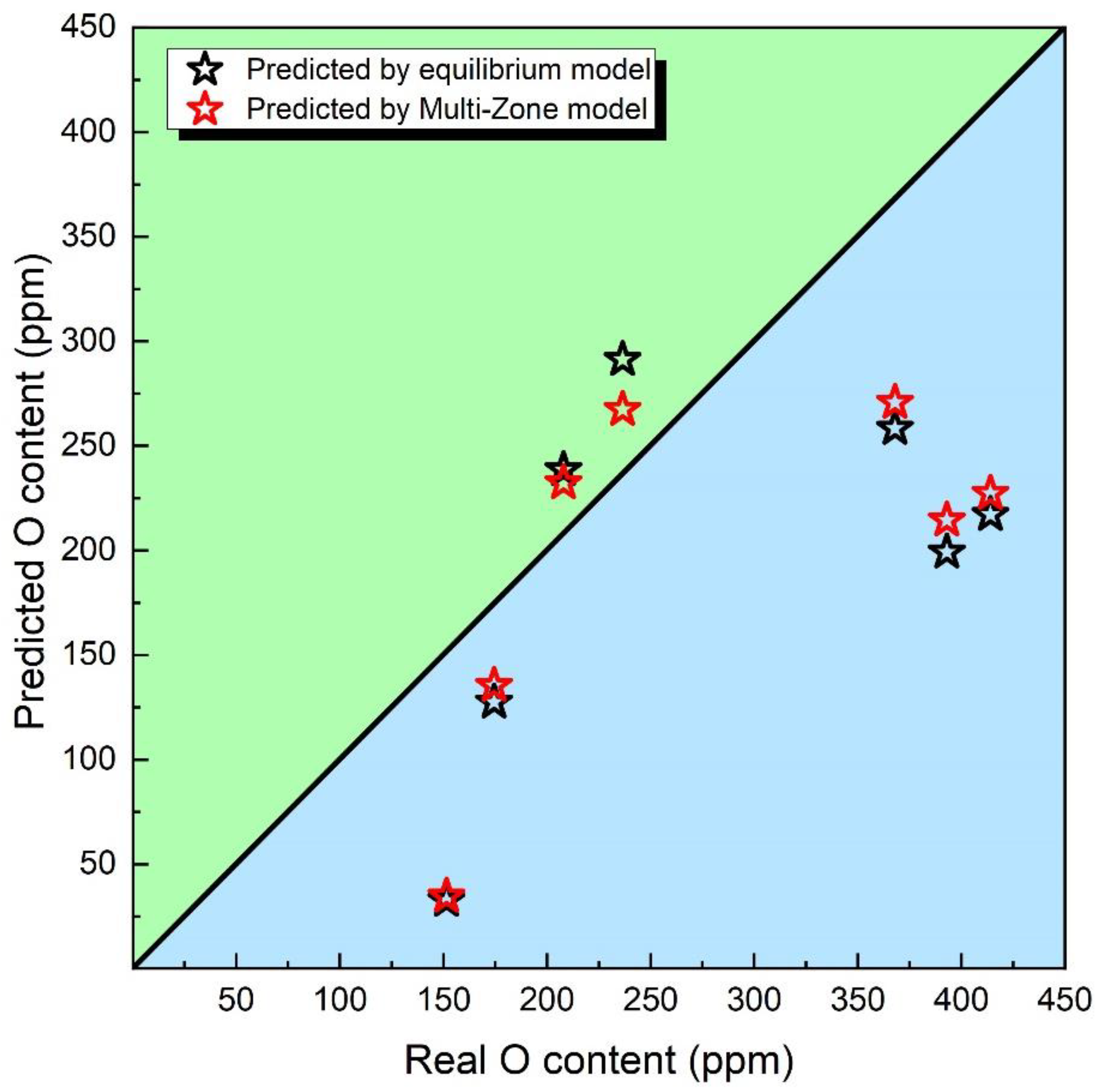
3.2. Prediction of O Content
3.3. Non-Equilibrium Status of O Transfer Behavior
3.4. Scientific Explanation
- SAW is a typical oxygen process. However, there has been debate about the oxygen mechanism in the SAW process. From the perspective of the BI model or the thermodynamic equilibrium model in the molten pool zone, O enhancement in the SAW process primarily occurs within the molten pool zone. However, experimental findings indicate that O enhancement in the SAW process predominantly takes place in the droplet reaction zone, and the molten pool region is essentially a deoxidation process [9,28]. Therefore, through comparative analysis with empirical data, this model identifies shortcomings in the traditional BI model and thermodynamic equilibrium model, revealing that the predicted O content trend of the Multi-Zone model aligns with the observed trend. Furthermore, the Multi-Zone model demonstrates higher accuracy in the ultimate prediction of O content composition.
- Regarding the non-equilibrium aspects of the SAW process, a simulation of ultra-high-temperature slag viscosity has been performed, validating the quantitative relationship between high-temperature viscosity and the non-equilibrium nature of the SAW process.
- In terms of quantifying the elemental transfer behavior in SAW, the research findings indicate that the traditional quantification method (Δ) deviates from the actual O transfer process in the droplet zone due to its neglect of chemical reactions occurring there. Thus, it is necessary to separately consider O transfer in both the droplet and the molten pool zones, thereby achieving a more accurate prediction and simulation of the O transfer mechanism subject to the overall SAW process.
3.5. Plans for Further Research
- Both the Multi-Zone model and thermodynamic models are based on chemical thermodynamics theory. However, they do not account for the influence of physical factors on the transfer behavior of O, particularly the impact of physical slag entrapment on composition. Therefore, incorporating essential physical models will further enhance the overall predictive accuracy of the model.
- Currently, the Multi-Zone model still relies on local thermodynamic equilibrium as its modeling foundation. However, in reality, the SAW process exists in a global non-equilibrium state. While Mitra et al. [8,23,24,30] have proposed a dynamic model for the SAW process, this model only addresses the molten pool reaction zone. Therefore, developing metallurgical dynamic models for the droplet reaction zone and solidification reaction zone is imperative.
4. Conclusions
- The O transfer mechanism in SAW is elucidated as a dual-stage process, involving an initial oxidation in the droplet zone followed by deoxidation in the weld pool zone. Two new parameters, ΔdO and ΔwO, have been introduced to quantify the O transfer in droplet and weld pool zones.
- The Multi-Zone model is capable of capturing the metallurgical processes of oxidation and subsequent deoxidation during the SAW process yet demonstrates superior predictive accuracy in estimating the O content in the metal compared to the equilibrium model.
- The role of flux viscosity in influencing the non-equilibrium O transfer behavior has been emphasized, showing that an increase in flux viscosity leads to a greater discrepancy from the equilibrium point.
Author Contributions
Funding
Conflicts of Interest
References
- Sengupta, V.; Havrylov, D.; Mendez, P. Physical Phenomena in the Weld Zone of Submerged Arc Welding—A Review. Weld. J. 2019, 98, 283–313. [Google Scholar] [CrossRef]
- Kou, S. Welding Metallurgy, 3rd ed.; JohnWiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 22–122. [Google Scholar]
- Olson, D.; Liu, S.; Frost, R.; Edwards, G.; Fleming, D. Nature and Behavior of Fluxes Used for Welding; ASM Handbook; ASM International: Detroit, MI, USA, 1993; Volume 6, pp. 55–63. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, G.; Fan, J.; Wang, L.; Zhang, D. A Review on Parallel Development of Flux Design and Thermodynamics Subject to Submerged Arc Welding. Processes 2022, 10, 2305. [Google Scholar] [CrossRef]
- Chai, C.-S. Slag-Metal Reactions during Flux Shielded Arc Welding. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1980. [Google Scholar]
- Mills, A.; Thewlis, G.; Whiteman, J. Nature of inclusions in steel weld metals and their influence on formation of acicular ferrite. Mater. Sci. Technol. 1987, 3, 1051–1061. [Google Scholar] [CrossRef]
- Natalie, C.A.; Olson, D.L.; Blander, M. Physical and Chemical Behavior of Welding Fluxes. Annu. Rev. Mater. Sci. 1986, 16, 389–413. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-metal Reactions during Welding: Part I. Evaluation and Reassessment of Existing Theories. Metall. Trans. B 1991, 22, 65–71. [Google Scholar] [CrossRef]
- Lau, T.; Weatherly, G.; McLean, A. The Sources of Oxygen and Nitrogen Contamination in Submerged Arc Welding using CaO-Al2O3 Based Fluxes. Weld. J. 1985, 64, 343–347. [Google Scholar]
- Zhang, J.; Zhang, D. Thermodynamic Simulation of O Content Variation Roadmap in Submerged Arc Welding Process: From Droplet to Weld Metal. Processes 2023, 11, 784. [Google Scholar] [CrossRef]
- Chai, C.; Eagar, T. Slag Metal Reactions in Binary CaF2-Metal Oxide Welding Fluxes. Weld. J. 1982, 61, 229–232. [Google Scholar]
- Chai, C.; Eagar, T. Slag-metal Equilibrium during Submerged Arc Welding. Metall. Trans. B 1981, 12, 539–547. [Google Scholar] [CrossRef]
- Dallam, C.; Liu, S.; Olson, D. Flux Composition Dependence of Microstructure and Toughness of Submerged Arc HSLA Weldments. Weld. J. 1985, 64, 140–151. [Google Scholar]
- Tuliani, S.; Boniszewski, T.; Eaton, N. Notch Toughness of Commercial Submerged Arc Weld Metal. Weld. Met. Fabr. 1969, 37, 327–339. [Google Scholar]
- Shao, G.; Liu, Z.; Fan, J.; Guo, Y.; Xu, Q.; Zhang, J. Evaluation of Flux Basicity Concept Geared toward Estimation for Oxygen Content in Submerged Arc Welded Metal. Metals 2022, 12, 1530. [Google Scholar] [CrossRef]
- Eagar, T. Sources of Weld Metal Oxygen Contamination during Submerged Arc Welding. Weld. J. 1978, 57, 76–80. [Google Scholar]
- Polar, A.; Indacochea, J.; Blander, M. Electrochemically generated oxygen contamination in submerged arc welding. Weld. J. 1990, 69, 69–74. [Google Scholar]
- Indacochea, J.E.; Blander, M.; Christensen, N.; Olson, D.L. Chemical Reactions During Submerged Arc Welding with FeO-MnO-SiO2 Fluxes. Metall. Trans. B 1985, 16, 237–245. [Google Scholar] [CrossRef]
- Chai, C.; Eagar, T. Prediction of Weld-metal Composition during Flux-shielded Welding. J. Mater. Energy Syst. 1983, 5, 160–164. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.; Eriksson, G.; Gheribi, A.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J. Reprint of: FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 55, 1–19. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, P.; Zhang, D. Advancing Manganese Content Prediction in Submerged Arc Welded Metal: Development of a Multi-Zone Model via the Calphad Technique. Processes 2023, 11, 1265. [Google Scholar] [CrossRef]
- Jung, I.-H. Overview of the Applications of Thermodynamic Databases to Steelmaking Processes. Calphad 2010, 34, 332–362. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-metal Reactions During Welding: Part II. Theory. Metall. Trans. B 1991, 22, 73–81. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T. Slag-metal Reactions during Welding: Part III. Verification of the Theory. Metall. Trans. B 1991, 22, 83–100. [Google Scholar] [CrossRef]
- Saifutdinov, A. Numerical study of various scenarios for the formation of atmospheric pressure DC discharge characteristics in argon: From glow to arc discharge. Plasma Sources Sci. Technol. 2022, 31, 094008. [Google Scholar] [CrossRef]
- Baeva, M.; Loffhagen, D.; Becker, M.M.; Siewert, E.; Uhrlandt, D. Plasma parameters of microarcs towards minuscule discharge gap. Contrib. Plasma Phys. 2020, 60, e202000033. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Shi, H.; Huang, L.; Mao, Z. Element Loss Behavior and Compensation in Additive Manufacturing of Memory Alloys. Trans. China Weld. Inst. 2022, 43, 50–55. [Google Scholar] [CrossRef]
- Lau, T.; Weatherly, G.; McLean, A. Gas/metal/slag Reactions in Submerged Arc Welding Using CaO-Al2O3 Based Fluxes. Weld. J. 1986, 65, 31–38. [Google Scholar]
- Zhang, J.; Shao, G.; Guo, Y.; Xu, Q.; Liu, Z. Facilitating flux design process geared towards submerged arc welding via thermodynamic approach: Case study into CaF2–SiO2–Na2O–Al2O3–TiO2 agglomerated flux. Calphad 2022, 79, 102483. [Google Scholar] [CrossRef]
- Mitra, U. Kinetics of Slag Metal Reactions during Submerged Arc Welding of Steel; Massachusetts Institute of Technology: Cambridge, MA, USA, 1984. [Google Scholar]
- Eagar, T.W. Thermochemistry of joining. In Proceedings of the Elliott Symposium on Chemical Process Metallurgy, Cambridge, MA, USA, 10–13 June 1991. [Google Scholar]



| Flux | WM | CaF2 | SiO2 | CaO |
|---|---|---|---|---|
| F-1 | WM-1 | 10 | 40 | 50 |
| F-2 | WM-2 | 20 | 40 | 40 |
| F-3 | WM-3 | 30 | 40 | 30 |
| F-4 | WM-4 | 40 | 20 | 40 |
| F-5 | WM-5 | 50 | 20 | 30 |
| F-6 | WM-6 | 60 | 20 | 20 |
| F-7 | WM-7 | 70 | 20 | 10 |
| C | O | Si | Mn | Fe | |
|---|---|---|---|---|---|
| Base metal (BM) | 0.04 | 0.005 | 0.03 | 0.37 | Balanced |
| Electrode | 0.09 | 0.02 | 0.58 | 1.18 | Balanced |
| WM | PO2 (atm.) | Droplet O Content (wt pct) |
|---|---|---|
| WM-1 | 9.39 × 10−7 | 0.128 |
| WM-2 | 1.91 × 10−6 | 0.182 |
| WM-3 | 3.66 × 10−6 | 0.252 |
| WM-4 | 6.69 × 10−8 | 0.034 |
| WM-5 | 1.99 × 10−7 | 0.059 |
| WM-6 | 7.68 × 10−7 | 0.115 |
| WM-7 | 2.96 × 10−6 | 0.227 |
| WM | C | Si | Mn | O | Fe |
|---|---|---|---|---|---|
| WM-1 | 0.065 | 0.19 | 0.4 | 0.064 | Balanced |
| WM-2 | 0.065 | 0.19 | 0.4 | 0.091 | Balanced |
| WM-3 | 0.065 | 0.19 | 0.4 | 0.126 | Balanced |
| WM-4 | 0.065 | 0.19 | 0.4 | 0.017 | Balanced |
| WM-5 | 0.065 | 0.19 | 0.4 | 0.030 | Balanced |
| WM-6 | 0.065 | 0.19 | 0.4 | 0.058 | Balanced |
| WM-7 | 0.065 | 0.19 | 0.4 | 0.113 | Balanced |
| WM | C | Si | Mn | O | Fe |
|---|---|---|---|---|---|
| WM-1 | 0.07 | 0.31 | 0.78 | 0.01 | Balanced |
| WM-2 | 0.07 | 0.31 | 0.78 | 0.01 | Balanced |
| WM-3 | 0.07 | 0.31 | 0.78 | 0.01 | Balanced |
| WM-4 | 0.07 | 0.31 | 0.78 | 0.01 | Balanced |
| WM-5 | 0.07 | 0.31 | 0.78 | 0.01 | Balanced |
| WM-6 | 0.07 | 0.31 | 0.78 | 0.01 | Balanced |
| WM-7 | 0.07 | 0.31 | 0.78 | 0.01 | Balanced |
| Flux | CaF2 | SiO2 | CaO | Viscosity |
|---|---|---|---|---|
| F-1 | 10 | 40 | 50 | 0.064 |
| F-2 | 20 | 40 | 40 | 0.059 |
| F-3 | 30 | 40 | 30 | 0.056 |
| F-4 | 40 | 20 | 40 | 0.02 |
| F-5 | 50 | 20 | 30 | 0.019 |
| F-6 | 60 | 20 | 20 | 0.018 |
| F-7 | 70 | 20 | 10 | 0.017 |
| WM | Multi-Zone Model | Equilibrium Model | Real Data | |||
|---|---|---|---|---|---|---|
| ΔDO | ΔWO | ΔDO | ΔWO | ΔDO | ΔWO | |
| WM-1 | 1275 | −1061 | 0 | 199 | 1700 | −1307 |
| WM-2 | 1819 | −1592 | 0 | 217 | 1268 | −854 |
| WM-3 | 2519 | −2248 | 0 | 258 | 1875 | −1507 |
| WM-4 | 340 | −305 | 0 | 33 | 667 | −516 |
| WM-5 | 590 | −455 | 0 | 127 | 698 | −524 |
| WM-6 | 1153 | −886 | 0 | 291 | 499 | −262 |
| WM-7 | 1265 | −1033 | 0 | 339 | 529 | −321 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Fan, J.; Zhang, D. Unraveling Oxygen Transfer Behavior in Submerged Arc Welding Using CaF2-SiO2-CaO Fluxes. Processes 2023, 11, 2622. https://doi.org/10.3390/pr11092622
Zhang J, Fan J, Zhang D. Unraveling Oxygen Transfer Behavior in Submerged Arc Welding Using CaF2-SiO2-CaO Fluxes. Processes. 2023; 11(9):2622. https://doi.org/10.3390/pr11092622
Chicago/Turabian StyleZhang, Jin, Jun Fan, and Dan Zhang. 2023. "Unraveling Oxygen Transfer Behavior in Submerged Arc Welding Using CaF2-SiO2-CaO Fluxes" Processes 11, no. 9: 2622. https://doi.org/10.3390/pr11092622
APA StyleZhang, J., Fan, J., & Zhang, D. (2023). Unraveling Oxygen Transfer Behavior in Submerged Arc Welding Using CaF2-SiO2-CaO Fluxes. Processes, 11(9), 2622. https://doi.org/10.3390/pr11092622






