Construction of a Multifunctional PCM@Catalyst Composite and Its Application in the Fluid Catalytic Cracking Process
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
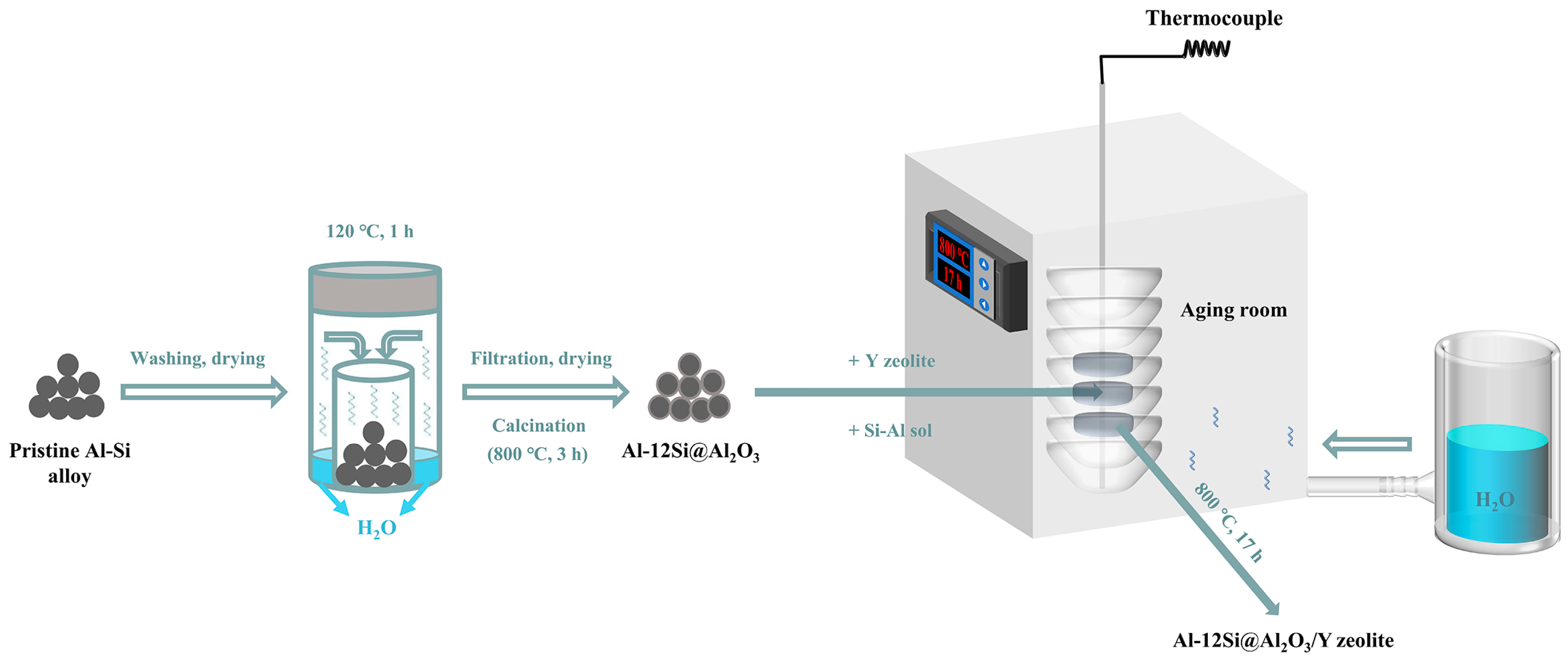
2.2. Synthesis of the Al-12wt%Si@Al2O3 Microcapsules
2.3. Synthesis of the Multifunctional Composite FCC Catalyst
2.4. Synthesis of the Al2O3 Composite FCC Catalyst (Blank Sample)
2.5. Characterization
3. Results and Discussion
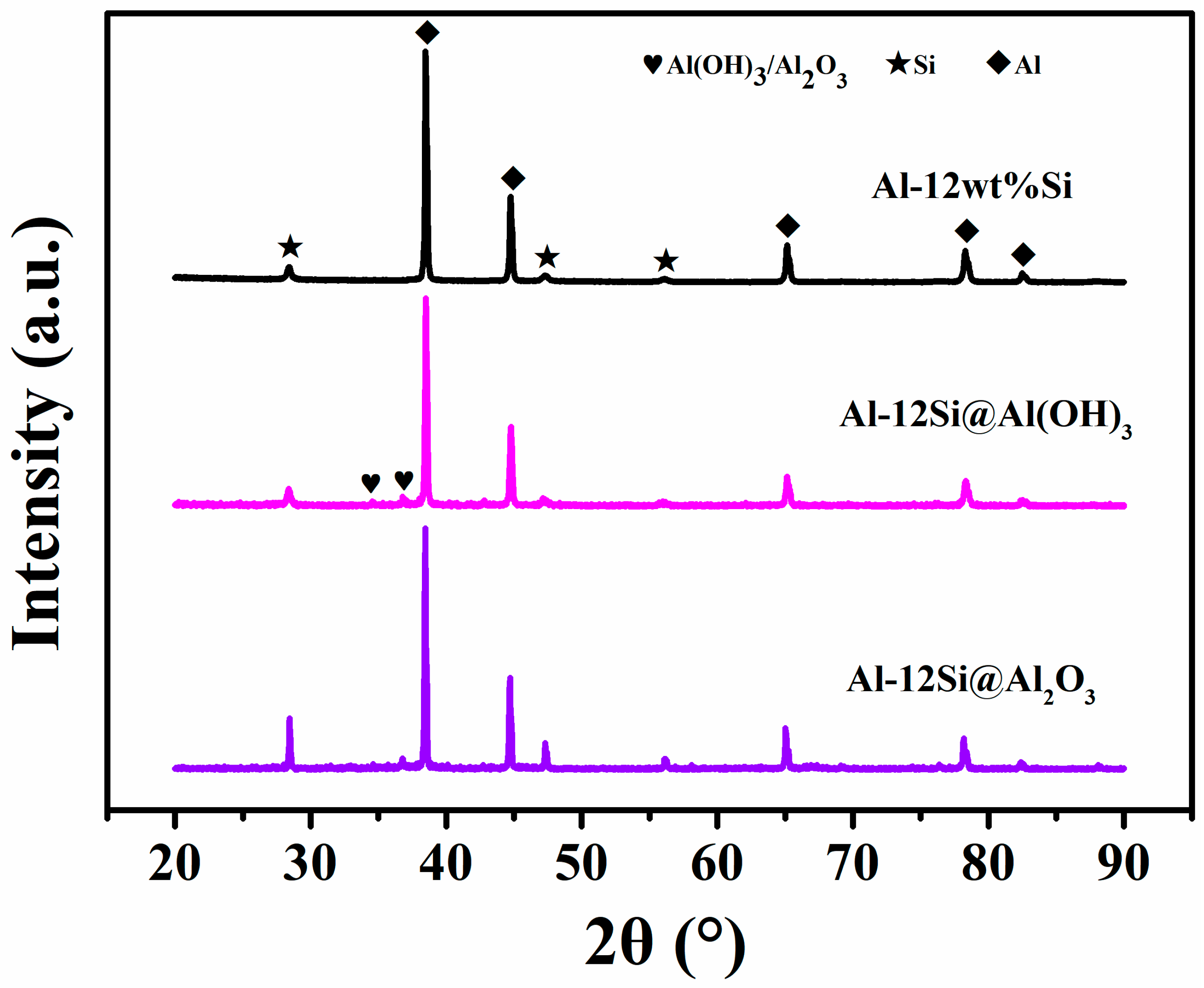
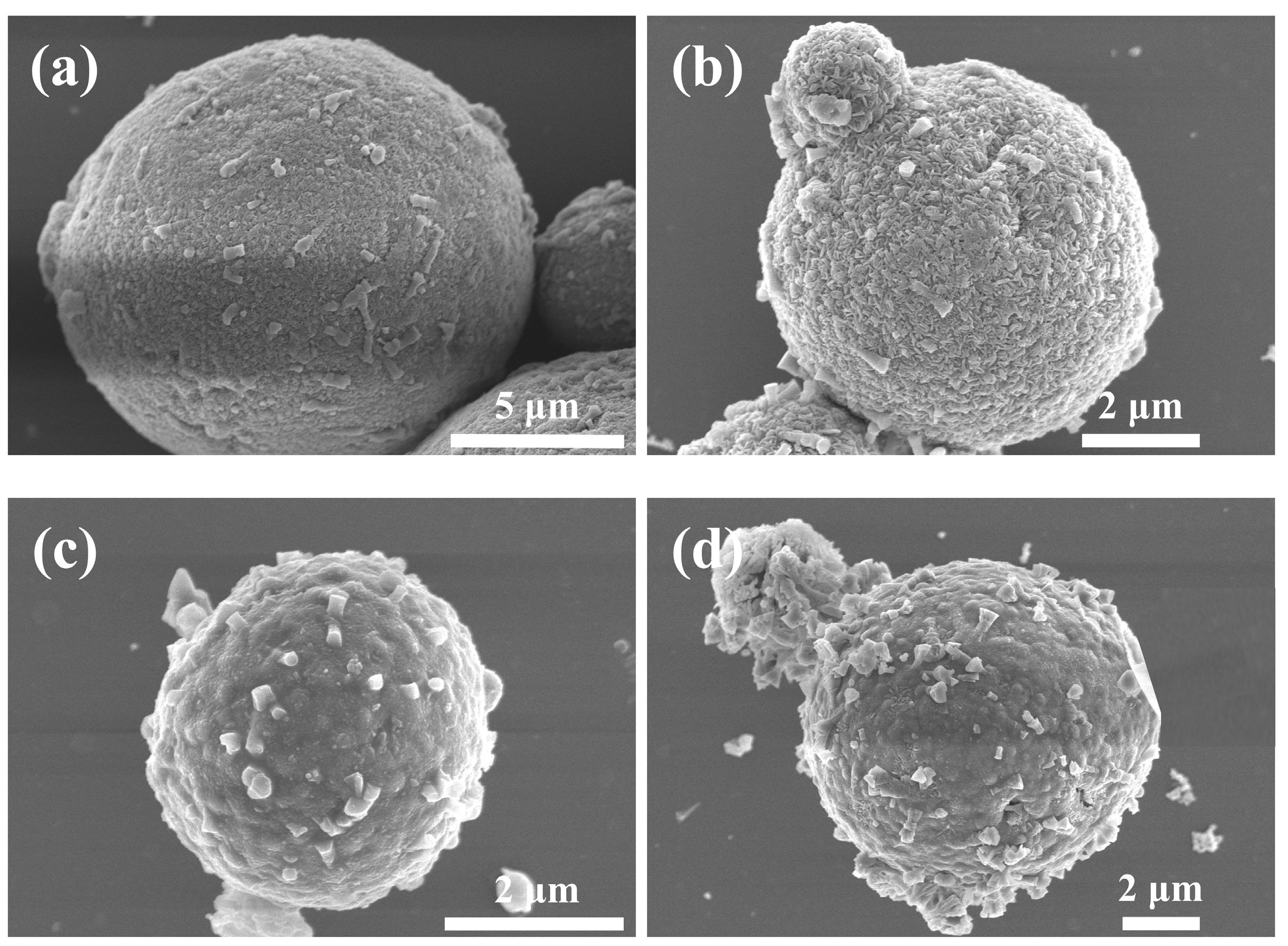
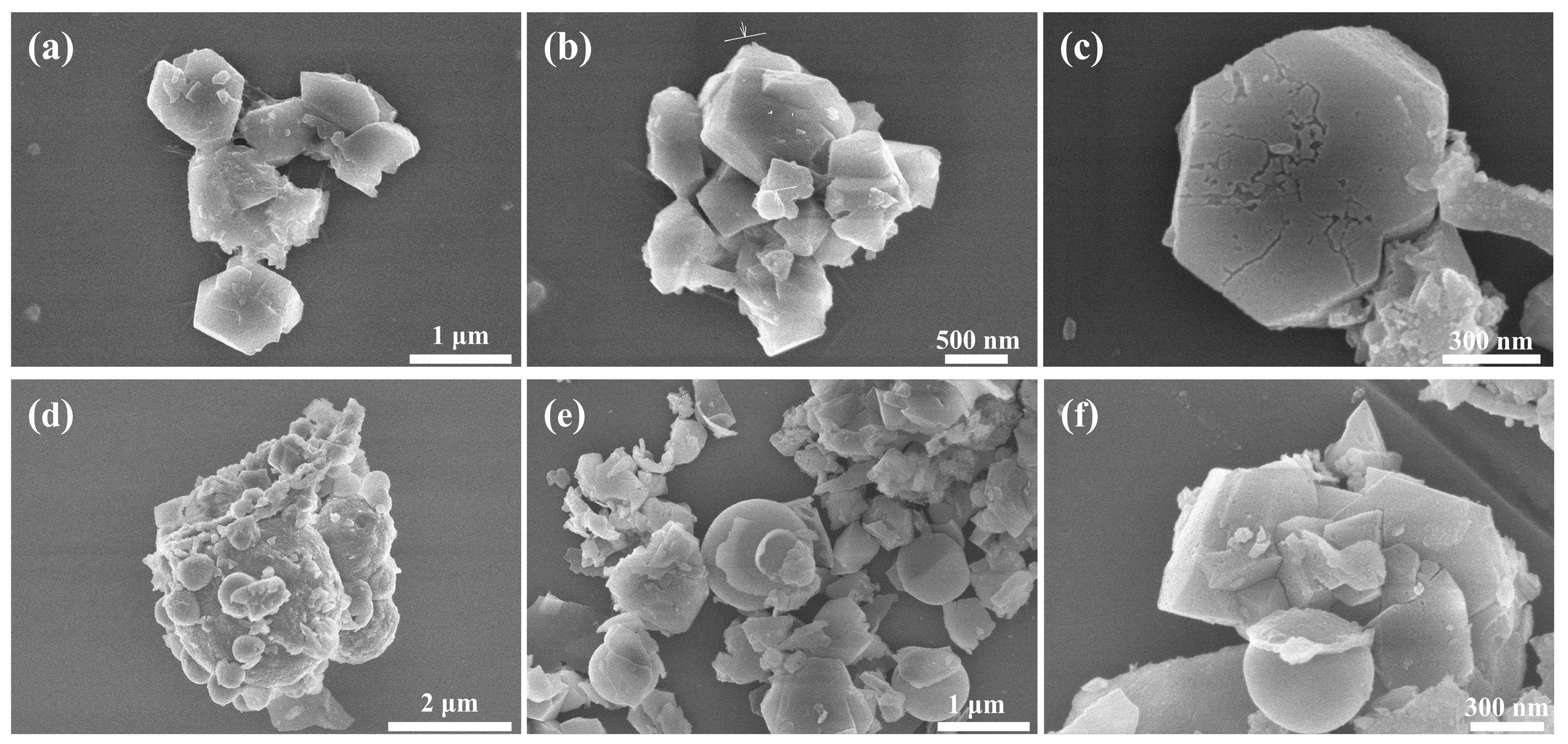
3.1. Structural Analysis of Al-12Si@Al2O3 Microcapsules
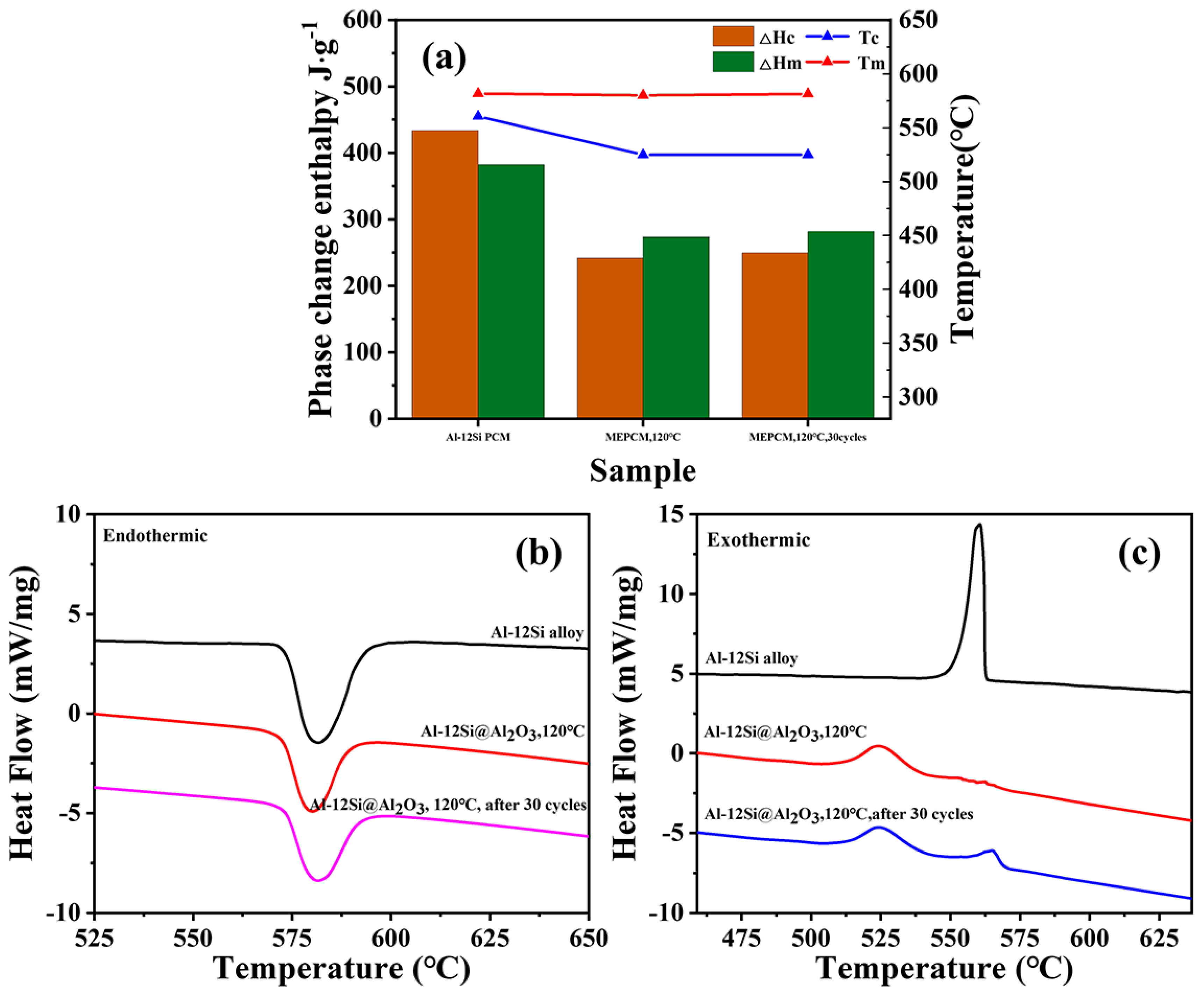
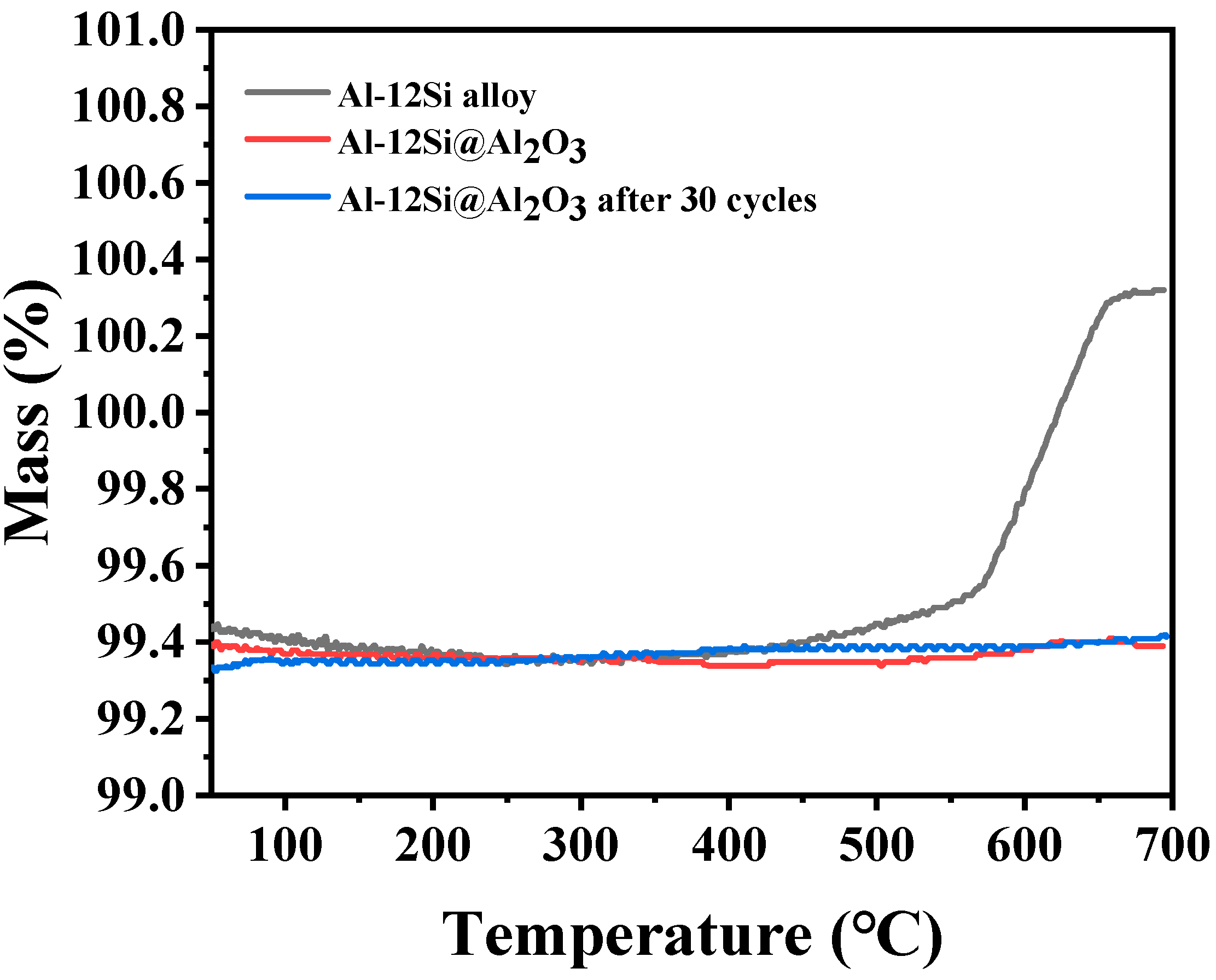
3.2. Thermal Properties and Stability of Al-12Si@Al2O3 Microcapsules
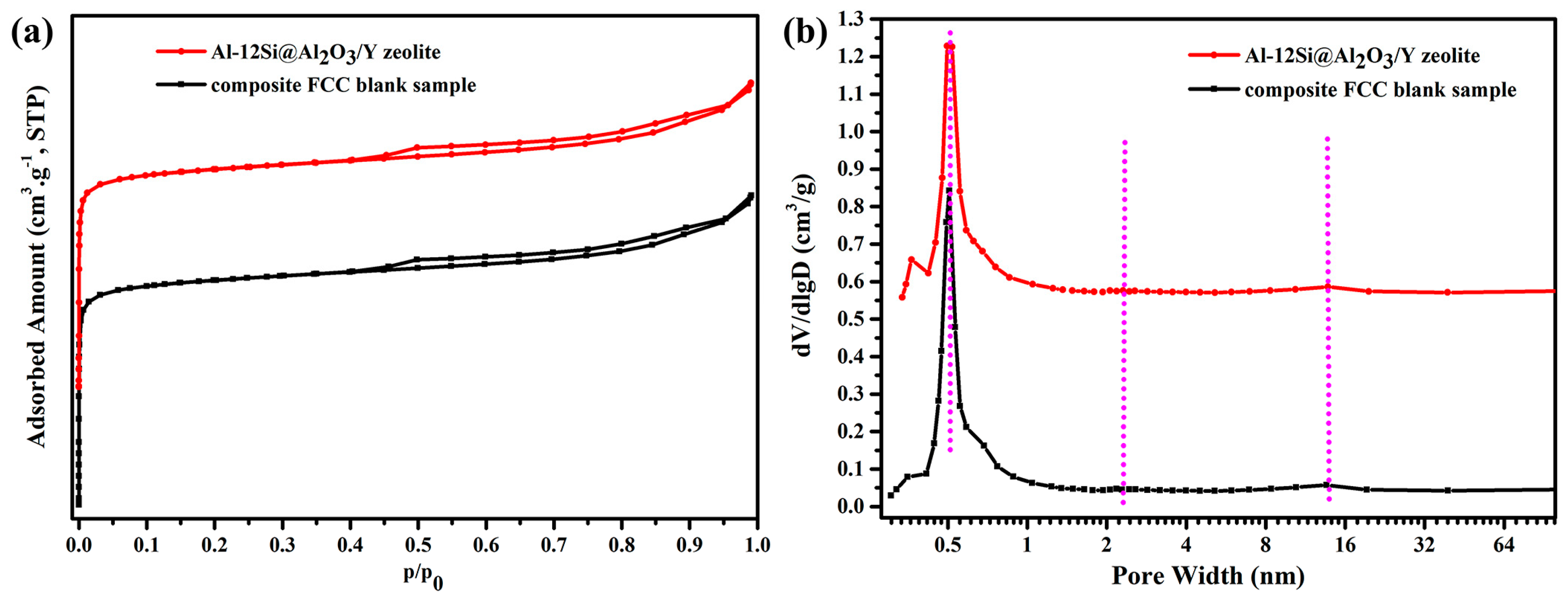
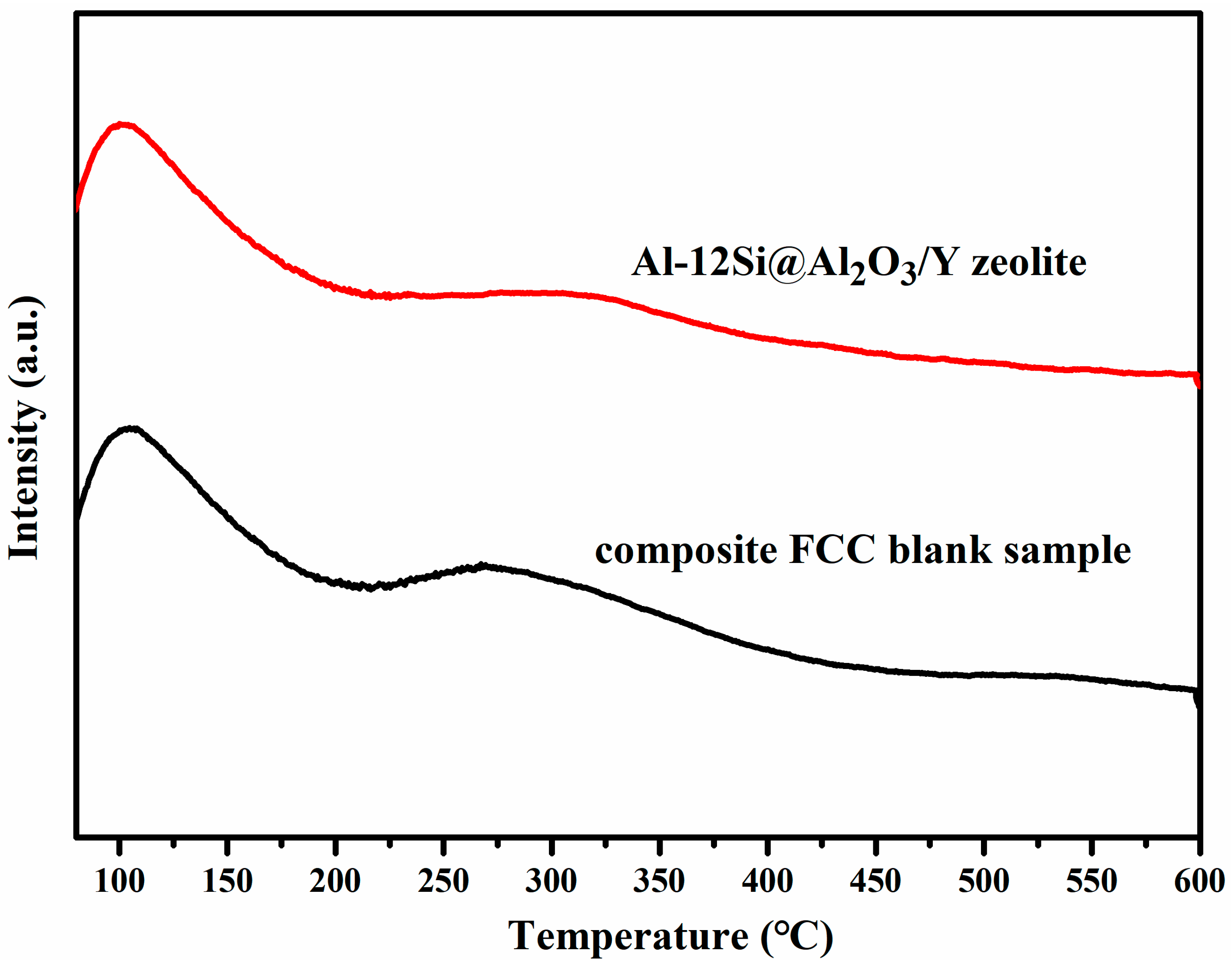
3.3. Structure and Performance Analysis for Al-12Si@Al2O3/Y Zeolite
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dupain, X.; Makkee, M.; Moulijn, J.A. Optimal conditions in fluid catalytic cracking: A mechanistic approach. Appl. Catal. A Gen. 2006, 297, 198–219. [Google Scholar] [CrossRef]
- Huang, Z.; Ho, T.C. Effect of thermolysis on resid droplet vaporization in fluid catalytic cracking. Chem. Eng. J. 2003, 91, 45–58. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Safaei, M.R.; Estelle, P.; Jafarnia, S.H. Heat transfer of water-based carbon nanotube nanofluids in the shell and tube cooling heat exchangers of the gasoline product of the residue fluid catalytic cracking unit. J. Therm. Anal. Calorim. 2020, 140, 351–362. [Google Scholar] [CrossRef]
- Stratiev, D.; Ivanov, M.; Chavdarov, I.; Argirov, G.; Strovegli, G. Revamping fluid catalytic cracking unit, and optimizing catalyst to process heavier feeds. Appl. Sci. 2023, 13, 2017. [Google Scholar] [CrossRef]
- Stratiev, D.; Shishkova, I.; Ivanov, M.; Dinkov, R.; Georgiev, B.; Argirov, G.; Atanassova, V.; Vassilev, P.; Atanassov, K.; Yordanov, D.; et al. Role of catalyst in optimizing fluid catalytic cracking performance during cracking of H-oil-derived gas oils. Acs Omega 2021, 6, 7626–7637. [Google Scholar] [CrossRef]
- Nazarova, G.Y.; Ivashkina, E.N.; Ivanchina, E.D.; Mezhova, M.Y. A model of catalytic cracking: Catalyst deactivation induced by feedstock and process variables. Catalysts 2022, 12, 98. [Google Scholar] [CrossRef]
- Farshi, A.; Abri, H.R. The addition of ZSM-5 to a fluid catalytic cracking catalyst for increasing olefins in fluid catalytic cracking light gas. Petrol. Sci. Technol. 2012, 30, 1285–1295. [Google Scholar] [CrossRef]
- Trujillo, W.R.; De Wilde, J. Computational fluid dynamics simulation of fluid catalytic cracking in a rotating fluidized bed in a static geometry. Ind. Eng. Chem. Res. 2010, 49, 5288–5298. [Google Scholar] [CrossRef]
- Stratiev, D.; Shishkova, I.; Ivanov, M.; Chavdarov, I.; Yordanov, D. Dependence of fluid catalytic cracking unit performance on H-oil severity, catalyst activity, and coke selectivity. Chem. Eng. Technol. 2020, 43, 2266–2276. [Google Scholar] [CrossRef]
- Hiramatsu, Y.; Aita, Y.; Umeki, T. Effect of acid properties of catalysts on fluid catalytic cracking of residual oil. J. Jpn. Petrol. Inst. 2012, 55, 319–325. [Google Scholar] [CrossRef]
- Alabdullah, M.A.; Shoinkhorova, T.; Dikhtiarenko, A.; Ould-Chikh, S.; Rodriguez-Gomez, A.; Chung, S.H.; Alahmadi, A.O.; Hita, I.; Pairis, S.; Hazemann, J.L.; et al. Understanding catalyst deactivation during the direct cracking of crude oil. Catal. Sci. Technol. 2022, 12, 5657–5670. [Google Scholar] [CrossRef]
- Liu, L.M. A SoC Controller for Energy Saving and Environmental Protection. Mater. Sci. Environ. Prot. Appl. Res. 2014, 908, 477–480. [Google Scholar] [CrossRef]
- Wei, Q.F.; Guo, A.N.; Wei, L.X.; Ni, W.Y. Analysis of the mechanism of renewable energy on energy-saving and environmental protection industry: Empirical evidence from four countries. Energy Rep. 2022, 8, 205–217. [Google Scholar] [CrossRef]
- Zivkovic, S.; Todorovic, M.; Pejcic, D. Improvements in environmental protection through optimization of production processes a case study. Fresen. Environ. Bull. 2016, 25, 2725–2735. [Google Scholar]
- de Gracia, A.; Cabeza, L.F. Phase change materials and thermal energy storage for buildings. Energy Build. 2015, 103, 414–419. [Google Scholar] [CrossRef]
- Kibria, M.A.; Anisur, M.R.; Mahfuz, M.H.; Saidur, R.; Metselaar, I.H.S.C. A review on thermophysical properties of nanoparticle dispersed phase change materials. Energy Convers. Manag. 2015, 95, 69–89. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Shi, J.M.; Qin, M.L.; Aftab, W.; Zou, R.Q. Flexible phase change materials for thermal energy storage. Energy Storage Mater. 2021, 41, 321–342. [Google Scholar] [CrossRef]
- Qiu, L.; Ouyang, Y.X.; Feng, Y.H.; Zhang, X.X. Review on micro/nano phase change materials for solar thermal applications. Renew. Energy 2019, 140, 513–538. [Google Scholar] [CrossRef]
- Su, W.G.; Darkwa, J.; Kokogiannakis, G. Review of solid-liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Fukahori, R.; Nomura, T.; Zhu, C.; Sheng, N.; Okinaka, N.; Akiyama, T. Thermal analysis of Al-Si alloys as high-temperature phase-change material and their corrosion properties with ceramic materials. Appl. Energy 2016, 163, 1–8. [Google Scholar] [CrossRef]
- Kageyama, Y.; Morita, K. Compositional and thermophysical study of Al-Si- and Zn-Al-Mg-based eutectic alloys for latent heat storage. High Temp. Mat. Pract.-ISR 2023, 42, 20220269. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, P. Investigation on the performance of a high-temperature packed bed latent heat thermal energy storage system using Al-Si alloy. Energy Convers. Manag. 2017, 150, 500–514. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Wang, H.; Yang, M.; Sun, W.W.; Yin, G.F.; Zhang, Q.Y.; Ren, Z.F. Thermal reliability of Al-Si eutectic alloy for thermal energy storage. Mater. Res. Bull. 2017, 95, 300–306. [Google Scholar] [CrossRef]
- He, F.; Chao, S.; He, X.D.; Li, M.W. Inorganic microencapsulated core/shell structure of Al-Si alloy micro-particles with silane coupling agent. Ceram. Int. 2014, 40, 6865–6874. [Google Scholar] [CrossRef]
- Nomura, T.; Yoolerd, J.; Sheng, N.; Sakai, H.; Hasegawa, Y.; Haga, M.; Saito, G.; Akiyama, T. Microencapsulation of eutectic and hyper-eutectic Al-Si alloy as phase change materials for high-temperature thermal energy storage. Sol. Energy Mat. Sol. C 2018, 187, 255–262. [Google Scholar] [CrossRef]
- Nomura, T.; Zhu, C.; Sheng, N.; Saito, G.; Akiyama, T. Microencapsulation of metal-based phase change material for high-temperature thermal energy storage. Sci. Rep. 2015, 5, 9117. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.P.; Huang, Y.; Wei, H.T.; Yu, F.Z.; Wang, M.S.; Guo, Y.H.; Zhang, J.B.; Deng, R.; Yang, S.Y.; Liu, X.J. A brief strategy for designing self-encapsulated Al-Si base phase change materials with high thermal energy storage performance. J. Energy Storage 2023, 62, 106957. [Google Scholar] [CrossRef]
- Zhu, S.L.; Nguyen, M.T.; Yonezawa, T. Micro- and nano-encapsulated metal and alloy-based phase-change materials for thermal energy storage. Nanoscale Adv. 2021, 3, 4626–4645. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.C.; Zhang, Z.X.; Dong, Z.H.; Zhang, J.J.; Dong, B.W.; Fu, H.T.; An, X.Z. Electromagnetic self-encapsulation of carbon fiber reinforced Al matrix composite phase change material for high-temperature thermal energy storage. J. Alloys Compd. 2022, 901, 163560. [Google Scholar] [CrossRef]
- Han, C.J.; Gu, H.Z.; Zhang, M.J.; Huang, A.; Zhang, Y.; Wang, Y. Al-Si@Al2O3@mullite microcapsules for thermal energy storage: Preparation and thermal properties. Sol. Energy Mat. Sol. C 2020, 217, 110697. [Google Scholar] [CrossRef]
- Han, C.J.; Gu, H.Z.; Zhang, M.J.; Huang, A.; Zhang, Y.; Wang, Y. Thermal properties of Al-Si/Al2O3 core-shell particles prepared by using steam hydration method. J. Alloys Compd. 2020, 817, 152801. [Google Scholar] [CrossRef]
- Nomura, T.; Sheng, N.; Zhu, C.; Saito, G.; Hanzaki, D.; Hiraki, T.; Akiyama, T. Microencapsulated phase change materials with high heat capacity and high cyclic durability for high-temperature thermal energy storage and transportation. Appl. Energy 2017, 188, 9–18. [Google Scholar] [CrossRef]
- Katoh, M.; Kimura, M.; Sugino, M.; Horikawa, T.; Nakagawa, K.; Sugiyama, S. Modification of commercial NaY zeolite to give high water diffusivity and adsorb a large amount of water. J. Colloid Interf. Sci. 2015, 455, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, H.; Furuta, S.; Komarneni, S. Microwave versus conventional-hydrothermal synthesis of NaY zeolite. J. Porous Mat. 2001, 8, 5–12. [Google Scholar] [CrossRef]
- Hasanudin, H.; Asri, W.R.; Fanani, Z.; Adisti, S.J.; Hadiah, F.; Maryana, R.; Al Muttaqii, M.; Zhu, Z.Y.; Machado, N.T. Facile fabrication of SiO2/Zr assisted with EDTA complexed-impregnation and templated methods for crude palm oil to biofuels conversion via catalytic hydrocracking. Catalysts 2022, 12, 1522. [Google Scholar] [CrossRef]
- Seo, S.; Chaikittisilp, W.; Koike, N.; Yokoi, T.; Okubo, T. Porous inorganic-organic hybrid polymers derived from cyclic siloxane building blocks: Effects of substituting groups on mesoporous structures. Micropor. Mesopor. Mat. 2019, 278, 212–218. [Google Scholar] [CrossRef]
- Deng, C.S.; Zhang, J.J.; Dong, L.H.; Huang, M.N.; Bin, L.; Jin, G.Z.; Gao, J.B.; Zhang, F.Y.; Fan, M.G.; Zhang, L.M.; et al. The effect of positioning cations on acidity and stability of the framework structure of Y zeolite. Sci. Rep. 2016, 6, 23382. [Google Scholar] [CrossRef]
- An, G.Q.; Gao, H.Y.; Luo, Y.B.; Ouyang, Y.; Wang, G.; Liu, S.Q.; Wang, C.A.; Liu, Z.Y.; Cheng, Z.X.; Shu, X.T. Optimization of MOFs-induced strategy for preparing anatase-free hierarchical TS-1 towards 1-hexene epoxidation: TPAOH dosage regulation, silanization and stabilizing acidic sites. Micropor. Mesopor. Mat. 2023, 360, 112704. [Google Scholar] [CrossRef]
- Shi, J.J.; Guan, J.Y.; Guo, D.W.; Zhang, J.S.; France, L.J.; Wang, L.F.; Li, X.H. Nitrogen chemistry and coke transformation of FCC coked catalyst during the regeneration process. Sci. Rep. 2016, 6, 27309. [Google Scholar] [CrossRef]
- Cumming, K.A.; Wojciechowski, B.W. Hydrogen transfer, coke formation, and catalyst decay and their role in the chain mechanism of catalytic cracking. Catal. Rev. 1996, 38, 101–157. [Google Scholar] [CrossRef]
- Zhao, J.; Yin, Y.C.; Li, Y.; Chen, W.Y.; Liu, B.J. Synthesis and characterization of mesoporous zeolite Y by using block copolymers as templates. Chem. Eng. J. 2016, 284, 405–411. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, G.G.; Qin, L.H.; Li, H.Y.; Chen, Y.; Liu, B.J. Synthesis and catalytic cracking performance of mesoporous zeolite Y. Catal. Commun. 2016, 73, 98–102. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, G.G.; Hu, J.; Liu, B.J.; Li, D.F. Preparation of mesoporous Y zeolite by template method and its application in catalytic cracking. Pet. Process. Petrochem. 2019, 50, 46–51. [Google Scholar]
- Liu, W.R.; Liu, X.M.; Gu, Y.; Liu, Y.X.; Yu, Z.M.; Lyu, Y.C.; Tian, Y.P. A new composite consisting of Y zeolite and ZrO2 for fluid catalytic cracking reaction. Compos. Part B-Eng. 2020, 200, 108317. [Google Scholar] [CrossRef]
- de Souza, E.C.; Pereira, M.M.; Lam, Y.L.; Morgado, E.; Chinelatto, L.S. Aluminum phosphate as active matrix of fluid catalytic cracking catalysts: Y zeolite stabilization. Appl. Catal. A-Gen. 2021, 619, 118156. [Google Scholar] [CrossRef]
- Cui, W.H.; Zhu, D.L.; Tan, J.; Chen, N.; Fan, D.; Wang, J.; Han, J.F.; Wang, L.Y.; Tian, P.; Liu, Z.M. Synthesis of mesoporous high-silica zeolite Y and their catalytic cracking performance. Chin. J. Catal. 2022, 43, 1945–1954. [Google Scholar] [CrossRef]
- Chen, H.L.; Shen, B.J.; Pan, H.F. Acidity and catalytic performance of ZSM-5/Y composite zeolite for heavy oil cracking. Chin. J. Catal. 2004, 25, 715–720. [Google Scholar]
- Zhang, L.; Hu, Q.X.; Qin, Y.C.; Liu, H.H.; Liu, H.F.; Cao, G.Z.; Gao, X.H.; Song, L.J.; Sun, Z.L. Optimizing the accessibility of zeolite Y on FCC catalyst to improve heavy oil conversion capacity. Micropor. Mesopor. Mat. 2023, 359, 112627. [Google Scholar] [CrossRef]





| Sample | Crystallizing Process | Melting Process | Encapsulation Parameters | ||||
|---|---|---|---|---|---|---|---|
| Tc (°C) | (J/g) | Tm (°C) | (J/g) | Een (%) | Ees (%) | Ces (%) | |
| Al-12Si pure alloy | 560.57 | 433.63 | 581.72 | 382.37 | / | / | / |
| Al-12Si@Al2O3 | 524.87 | 251.65 | 580.01 | 283.73 | 74.20 | 65.61 | 88.42 |
| Al-12Si@Al2O3 (30 cycles) | 526.19 | 299.62 | 580.94 | 280.34 | 73.32 | 71.07 | 96.93 |
| Samples | SBET a (m2 g−1) | SMic b (m2 g−1) | Micropore Volume b (cm3 g−1) | Total Pore Volume c (cm3 g−1) | Mesopore Volume d (cm3 g−1) |
|---|---|---|---|---|---|
| Al-12Si@Al2O3/Y zeolite | 471.30 | 422.25 | 0.163 | 0.256 | 0.091 |
| Composite FCC blank sample | 479.61 | 434.00 | 0.168 | 0.260 | 0.088 |
| Samples | NH3-TPD (μmol/g) | ||
|---|---|---|---|
| Weak Acid Sites | Medium Acid Sites | Strong Acid Sites | |
| Al-12Si@Al2O3/Y zeolite | 787.62 | 405.44 | 0.00 |
| Composite FCC blank sample | 789.41 | 410.21 | 0.00 |
| Entry | Sample | Conv. (%) | Feedstock Type | Reaction Conditions |
|---|---|---|---|---|
| 1 | Meso-CAT | 63.0 | light diesel oil | reaction temperature: 460 °C, catalyst loading: 5 g, total oil-feed mass: 1.56 g, reaction duration: 70 s) [42] |
| 2 | Meso-CAT-3 | 63.3 | light diesel oil | reaction temperature: 460 °C, catalyst loading: 5 g, total oil-feed mass: 1.56 g, reaction duration: 70 s) [43] |
| 3 | F-MCAT | 69.9 | light diesel oil | reaction temperature: 460 °C, catalyst loading: 5 g, total oil-feed mass: 1.56 g [44] |
| 4 | H–Y/ZrO2-50 | 67.81 | high-basic nitrogen vacuum gas oil | reaction temperature: 500 °C, catalyst loading: 5 g, total oil-feed mass: 1.67 g, reaction duration: 70 s) [45] |
| 5 | CAT-A-d | 68.7 ± 0.6 | Brazilian vacuum gas oil | reaction temperature: 535 °C, catalyst loading: 9 g, total oil-feed mass: 1.50 g, reaction duration: 75 s) [46] |
| 6 | MSY10.7-based catalyst | 93.1 | heavy oil | reaction temperature: 482 °C, catalyst loading: 4 g, total oil-feed mass: 1.33 g, reaction duration: 75 s) [47] |
| 7 | MM03-2P | 69.9 | Xinjiang heavy oil - | reaction temperature: 500 °C, reaction duration: 60 s, catalyst to oil ratio: 3.75) [48] |
| 8 | Base catalyst +15%cat-24 | 86.3 | heavy oil | reaction temperature: 500 °C, reaction duration: 70 s, catalyst to oil ratio: 3.20) [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, G.; Cheng, Z.; Ouyang, Y.; Liu, S.; Gao, H. Construction of a Multifunctional PCM@Catalyst Composite and Its Application in the Fluid Catalytic Cracking Process. Processes 2023, 11, 2659. https://doi.org/10.3390/pr11092659
An G, Cheng Z, Ouyang Y, Liu S, Gao H. Construction of a Multifunctional PCM@Catalyst Composite and Its Application in the Fluid Catalytic Cracking Process. Processes. 2023; 11(9):2659. https://doi.org/10.3390/pr11092659
Chicago/Turabian StyleAn, Guoqing, Zhixiang Cheng, Ying Ouyang, Siqi Liu, and Hongyi Gao. 2023. "Construction of a Multifunctional PCM@Catalyst Composite and Its Application in the Fluid Catalytic Cracking Process" Processes 11, no. 9: 2659. https://doi.org/10.3390/pr11092659
APA StyleAn, G., Cheng, Z., Ouyang, Y., Liu, S., & Gao, H. (2023). Construction of a Multifunctional PCM@Catalyst Composite and Its Application in the Fluid Catalytic Cracking Process. Processes, 11(9), 2659. https://doi.org/10.3390/pr11092659







