CO2 Compression and Liquefaction Processes Using a Distillation Column for the Flexible Operation of Transportation
Abstract
1. Introduction
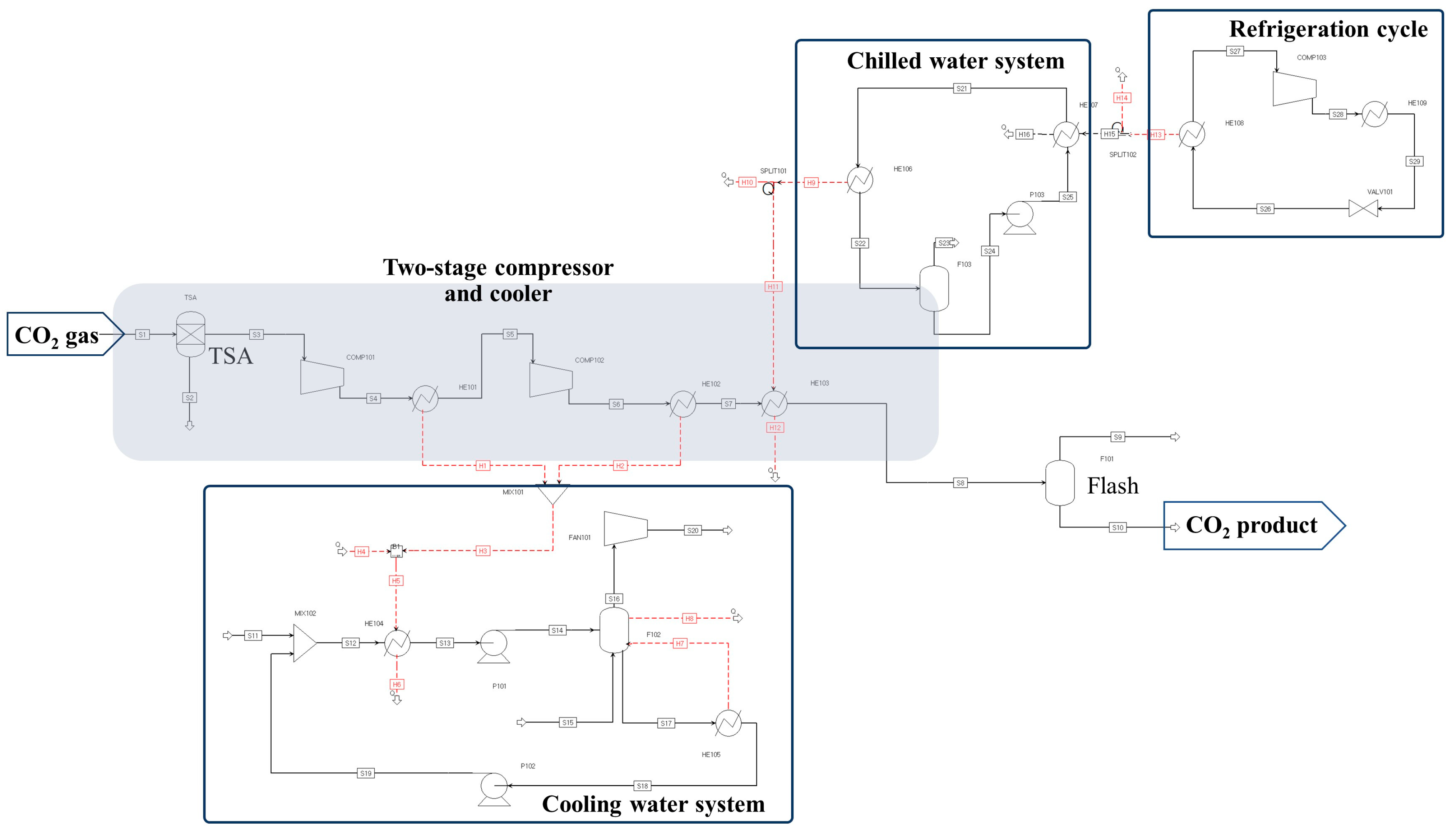
2. Process Description of CO2 Compression and Liquefaction
3. Methodology of Techno-Economic Analysis (TEA)
4. Results and Discussion
4.1. Process Performance of CCL with Flash or Distillation Column
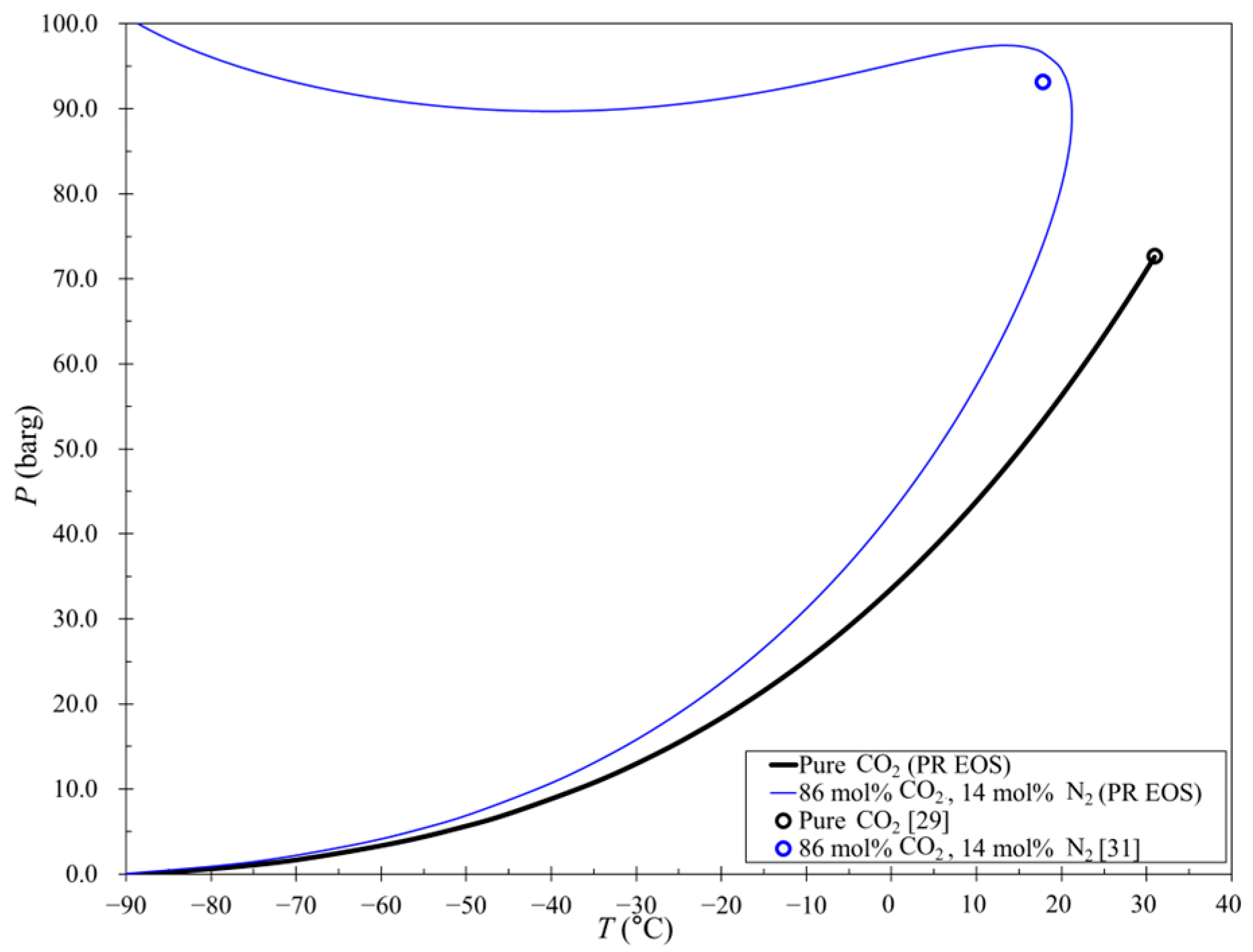
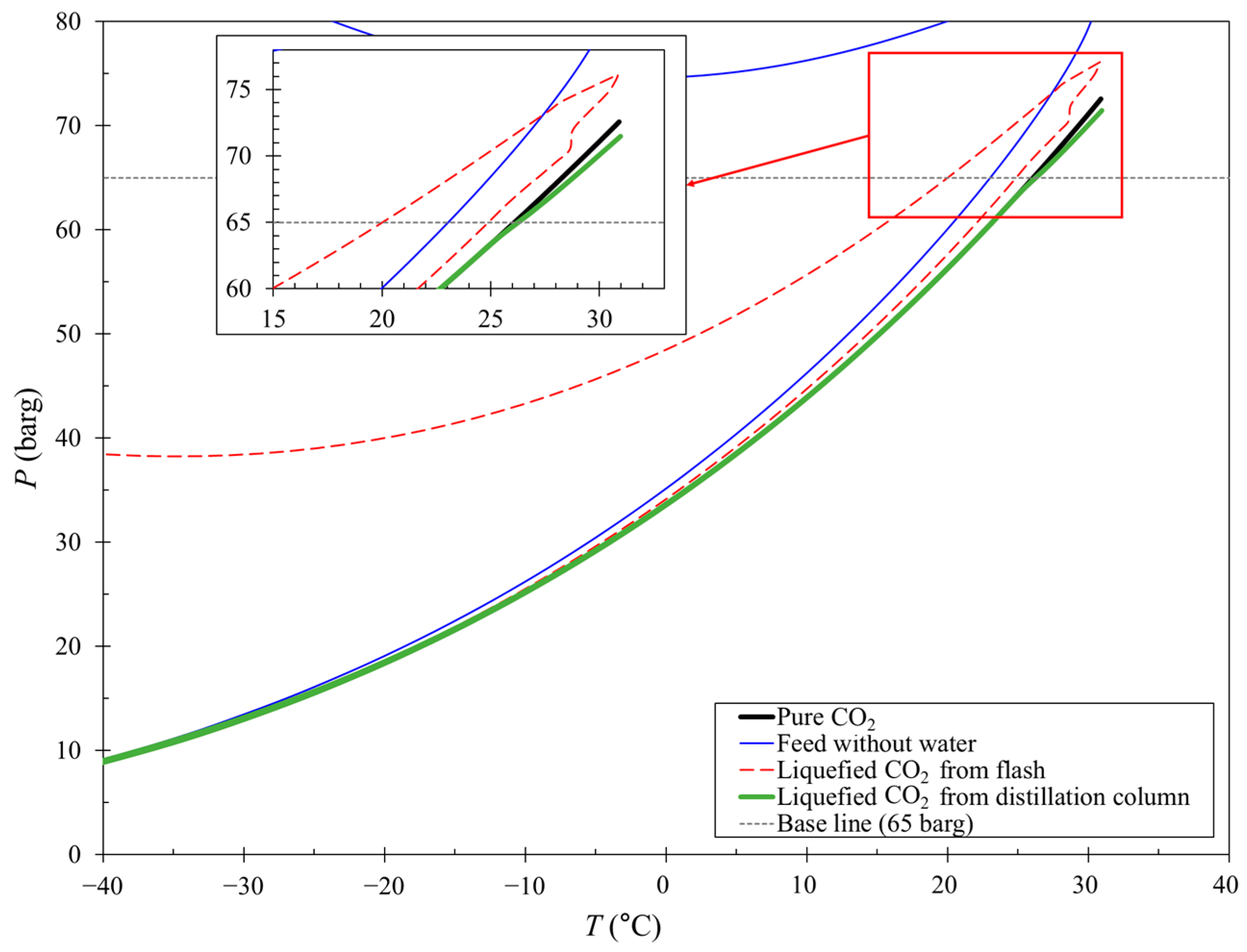
4.2. Phase Envelope for CCL with a Flash or Distillation Column
4.3. Economic Evaluation of CCL with a Flash or Distillation Column
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IEA. World Energy Outlook 2020; International Energy Agencey (IEA): Paris, France, 2020. [Google Scholar]
- Sun, X.; Shang, A.; Wu, P.; Liu, T.; Li, Y. A review of CO2 marine geological sequestration. Processes 2023, 11, 2206. [Google Scholar] [CrossRef]
- Lu, H.; Xin, X.; Ye, J.; Yu, G. Analysis of factors impacting CO2 assisted gravity drainage in oil reservoirs with bottom water. Processes 2023, 11, 3290. [Google Scholar] [CrossRef]
- Kim, S.; Lim, Y.I.; Lee, D.; Seo, M.W.; Mun, T.Y.; Lee, J.G. Effects of flue gas recirculation on energy, exergy, environment, and economics in oxy-coal circulating fluidized-bed power plants with CO2 capture. Int. J. Energy Res. 2021, 45, 5852–5865. [Google Scholar] [CrossRef]
- Li, H.; Jakobsen, J.P.; Wilhelmsen, Ø.; Yan, J. PVTxy properties of CO2 mixtures relevant for CO2 capture, transport and storage: Review of available experimental data and theoretical models. Appl. Energy 2011, 88, 3567–3579. [Google Scholar] [CrossRef]
- Pal, M.; Karaliūtė, V.; Malik, S. Exploring the potential of carbon capture, utilization, and storage in Baltic sea region countries: A review of CCUS patents from 2000 to 2022. Processes 2023, 11, 605. [Google Scholar] [CrossRef]
- Abbas, Z.; Mezher, T.; Abu-Zahra, M.R. CO2 purification. Part I: Purification requirement review and the selection of impurities deep removal technologies. Int. J. Greenh. Gas Control 2013, 16, 324–334. [Google Scholar] [CrossRef]
- Peletiri, S.P.; Mujtaba, I.M.; Rahmanian, N. Process simulation of impurity impacts on CO2 fluids flowing in pipelines. J. Clean. Prod. 2019, 240, 118145. [Google Scholar] [CrossRef]
- Morland, B.H.; Svenningsen, G.; Dugstad, A. The challenge of monitoring impurity content of CO2 streams. Processes 2021, 9, 570. [Google Scholar] [CrossRef]
- Li, H.; Wilhelmsen, Ø.; Yan, J. Properties of CO2 mixtures and impacts on carbon capture and storage. In Handbook of Clean Energy Systems; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1–17. [Google Scholar]
- Woods, M.; Matuszewski, M. Quality Guideline for Energy System Studies: CO2 Impurity Design Parameters; DOE/NETL-341/011212; National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA; Morgantown, WV, USA, 2013. [Google Scholar]
- Hussain, B.Y. Dynamic Simulations of Carbon Dioxide Pipeline Transportation for the Purpose of Carbon Capture and Storage. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2017. [Google Scholar]
- Aspelund, A.; Jordal, K. Gas conditioning-The interface between CO2 capture and transport. Int. J. Greenh. Gas Control 2007, 1, 343–354. [Google Scholar] [CrossRef]
- Porter, R.T.; Fairweather, M.; Pourkashanian, M.; Woolley, R.M. The range and level of impurities in CO2 streams from different carbon capture sources. Int. J. Greenh. Gas Control 2015, 36, 161–174. [Google Scholar] [CrossRef]
- De Visser, E.; Hendriks, C.; Barrio, M.; Mølnvik, M.J.; De Koeijer, G.; Liljemark, S.; Le Gallo, Y. Dynamis CO2 quality recommendations. Int. J. Greenh. Gas Control 2008, 2, 478–484. [Google Scholar] [CrossRef]
- Onyebuchi, V.E.; Kolios, A.; Hanak, D.P.; Biliyok, C.; Manovic, V. A systematic review of key challenges of CO2 transport via pipelines. Renew. Sust. Energ. Rev. 2018, 81, 2563–2583. [Google Scholar] [CrossRef]
- Kahlke, S.-L.; Pumpa, M.; Schütz, S.; Kather, A.; Rütters, H. Potential dynamics of CO2 stream composition and mass flow rates in CCS clusters. Processes 2020, 8, 1188. [Google Scholar] [CrossRef]
- Raimondi, L. CO2 transportation with pipelines-model analysis for steady, dynamic and relief simulation. Chem. Eng. Trans. 2014, 36, 619–624. [Google Scholar]
- Verma, S.; Oakes, C.; Chugunov, N.; Ramakrishnan, T. Effect of contaminants on the thermodynamic properties of CO2-rich fluids and ramifications in the design of surface and injection facilities for geologic CO2 sequestration. Energy Procedia 2011, 4, 2340–2347. [Google Scholar] [CrossRef]
- Brownsort, P.A. Briefing on Carbon Dioxide Specifications for Transport. CCUS Projects Network, Norway, EU. 2019. Available online: https://www.ccusnetwork.eu/sites/default/files/TG3_Briefing-CO2-Specifications-for-Transport.pdf (accessed on 29 December 2023).
- Goos, E.; Riedel, U.; Zhao, L.; Blum, L. Phase diagrams of CO2 and CO2-N2 gas mixtures and their application in compression processes. Energy Procedia 2011, 4, 3778–3785. [Google Scholar] [CrossRef]
- Xu, G.; Liang, F.; Yang, Y.; Hu, Y.; Zhang, K.; Liu, W. An improved CO2 separation and purification system based on cryogenic separation and distillation theory. Energies 2014, 7, 3484–3502. [Google Scholar] [CrossRef]
- Seo, Y.; Huh, C.; Lee, S.; Chang, D. Comparison of CO2 liquefaction pressures for ship-based carbon capture and storage (CCS) chain. Int. J. Greenh. Gas Control 2016, 52, 1–12. [Google Scholar] [CrossRef]
- Adu, E.; Zhang, Y.; Liu, D.; Tontiwachwuthikul, P. Parametric process design and economic analysis of post-combustion CO2 capture and compression for coal-and natural gas-fired power plants. Energies 2020, 13, 2519. [Google Scholar] [CrossRef]
- Deng, H.; Roussanaly, S.; Skaugen, G. Techno-economic analyses of CO2 liquefaction: Impact of product pressure and impurities. Int. J. Refrig. 2019, 103, 301–315. [Google Scholar] [CrossRef]
- Gong, W.; Remiezowicz, E.; Fosbøl, P.L.; Dlamini, G.M.; von Solms, N. Techno-economic analysis of novel CO2 liquefaction processes. In Proceedings of the 16th Greenhouse Gas Control Technologies Conference (GHGT-16), Lyon, France, 23–24 October 2022. [Google Scholar]
- Øi, L.E.; Eldrup, N.; Adhikari, U.; Bentsen, M.H.; Badalge, J.L.; Yang, S. Simulation and cost comparison of CO2 liquefaction. Energy Procedia 2016, 86, 500–510. [Google Scholar] [CrossRef]
- Porter, R.T.; Mahgerefteh, H.; Brown, S.; Martynov, S.; Collard, A.; Woolley, R.M.; Fairweather, M.; Falle, S.A.E.G.; Wareing, C.J.; Nikolaidis, I.K.; et al. Techno-economic assessment of CO2 quality effect on its storage and transport: CO2QUEST: An overview of aims, objectives and main findings. Int. J. Greenh. Gas Control 2016, 54, 662–681. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Y.-X. The influence of impurities on the transportation safety of an anthropogenic CO2 pipeline. Process Saf. Environ. Prot. 2014, 92, 80–92. [Google Scholar] [CrossRef]
- Martynov, S.; Daud, N.; Mahgerefteh, H.; Brown, S.; Porter, R. Impact of stream impurities on compressor power requirements for CO2 pipeline transportation. Int. J. Greenh. Gas Control 2016, 54, 652–661. [Google Scholar] [CrossRef]
- Ke, J.; Oag, R.M.; King, P.; George, M.W.; Poliakoff, M. Sensing the critical point of high-pressure mixtures. Angew. Chem. Int. Ed. 2004, 43, 5192–5195. [Google Scholar] [CrossRef]
- Venkatarathnam, G. Density marching method for calculating phase envelopes. Ind. Eng. Chem. Res. 2014, 53, 3723–3730. [Google Scholar] [CrossRef]
- Babar, M.; Bustam, M.; Ali, A.; Maulud, A. Identification and quantification of CO2 solidification in cryogenic CO2 capture from natural gas. Int. J. Automot. Mech. Eng. 2018, 15, 5367–5376. [Google Scholar] [CrossRef]
- Peletiri, S.P.; Rahmanian, N.; Mujtaba, I.M. Effects of impurities on CO2 pipeline performance. Chem. Eng. Trans. 2017, 57, 355–360. [Google Scholar]
- Forbes, S.M.; Verma, P.; Curry, T.E.; Friedmann, S.J.; Wade, S.M. Guidelines for Carbon Dioxide Capture, Transport and Storage; World Resources Institute: Washington, DC, USA, 2008. [Google Scholar]
- Wilson, D.; Basu, R. Thermodynamic properties of a new stratospherically safe working fluid-refrigerant 134a. ASHRAE Trans. 1988, 94, 2095–2118. [Google Scholar]
- Kim, S.; Lim, Y.-I. Heat integration and economic analysis of dry flue gas recirculation in a 500 MWe oxy-coal circulating fluidized-bed (CFB) power plant with ultra-supercritical steam cycle. Korean Chem. Eng. Res. 2021, 59, 60–67. [Google Scholar]
- Kim, S.; Lim, Y.-I.; Lee, D.; Cho, W.; Seo, M.W.; Lee, J.G.; Ok, Y.S. Perspectives of oxy-coal power plants equipped with CO2 capture, utilization, and storage in terms of energy, economic, and environmental impacts. Energy Convers. Manag. 2022, 273, 116361. [Google Scholar] [CrossRef]
- Do, T.X.; Mujahid, R.; Lim, H.S.; Kim, J.-K.; Lim, Y.-I.; Kim, J. Techno-economic analysis of bio heavy-oil production from sewage sludge using supercritical and subcritical water. Renew. Energy 2020, 151, 30–42. [Google Scholar] [CrossRef]
- Vu, T.T.; Lim, Y.-I.; Song, D.; Mun, T.-Y.; Moon, J.-H.; Sun, D.; Hwang, Y.-T.; Lee, J.-G.; Park, Y.C. Techno-economic analysis of ultra-supercritical power plants using air-and oxy-combustion circulating fluidized bed with and without CO2 capture. Energy 2020, 194, 116855. [Google Scholar] [CrossRef]
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A. Analysis, Synthesis, and Design of Chemical Processes; Pearson Education: London, UK, 2018. [Google Scholar]
- Kemper, J.; Sutherland, L.; Watt, J.; Santos, S. Evaluation and analysis of the performance of dehydration units for CO2 capture. Energy Procedia 2014, 63, 7568–7584. [Google Scholar] [CrossRef]
- Botticella, F.; De Rossi, F.; Mauro, A.; Vanoli, G.; Viscito, L. Multi-criteria (thermodynamic, economic and environmental) analysis of possible design options for residential heating split systems working with low GWP refrigerants. Int. J. Refrig. 2018, 87, 131–153. [Google Scholar] [CrossRef]



| Component | Purity Limits for Pipeline | Purity Limits for Geological Storage | |
|---|---|---|---|
| DYNAMIS | NETL | ||
| CO2 | >95.5 vol% | 95 vol% | >90 vol% |
| H2O | 500 ppmv | 300 ppmwt | <500 ppmv |
| H2S | 200 ppmv | 0.01 vol% | <1.5% |
| CO | 2000 ppmv | 35 ppmv | <4 vol% (all non-condensable gasses) |
| CH4 | Aquifer < 4 vol% | 4 vol% | |
| N2 | <4 vol% (all non-condensable gasses) | 4 vol% | |
| Ar | 4 vol% | ||
| H2 | 4 vol% | ||
| O2 | - | 4 vol% | |
| NOx | - | 100 ppmv | <200 ppmv |
| SOx | - | 100 ppmv | <200 ppmv |
| NH3 | - | 50 ppmv | |
| Feed 1 | Feed 2 | Feed 3 | |
|---|---|---|---|
| Temperature (T, °C) | 25 | 25 | 25 |
| Pressure (P, barg) | 15 | 15 | 15 |
| Mass flow rate (F, kg/hr) | 50,068 | 50,068 | 50,068 |
| Mole fraction (yi) | |||
| CO2 | 0.9913 | 0.9700 | 0.9700 |
| CO | 0.0002 | 0.0000 | 0.0000 |
| H2 | 0.0061 | 0.0000 | 0.0285 |
| CH4 | 0.0009 | 0.0000 | 0.0000 |
| H2O | 0.0015 | 0.0015 | 0.0015 |
| N2 | 0.0000 | 0.0285 | 0.0000 |
| Total | 1.0000 | 1.0000 | 1.0000 |
| Process Performance | Feed 1 | Feed 2 | Feed 3 | ||||
|---|---|---|---|---|---|---|---|
| Flash | Distil. | Flash | Distil. | Flash | Distil. | ||
| Feed | Flow rate (kg/h) CO2 purity (mol%) | 50,068 99.13 | 50,068 99.13 | 50,068 97.00 | 50,068 97.00 | 50,068 97.00 | 50,068 97.00 |
| Product | Flow rate (kg/h) CO2 purity (mol%) | 50,038 99.28 | 46,508 99.95 | 42,892 97.79 | 45,735 99.81 | 33,084 98.94 | 46,472 99.99 |
| CO2 recovery (%) | 100.0 | 93.0 | 86.1 | 93.0 | 66.2 | 93.0 | |
| Electricity consumption (kWe) | CO2 compression | 1747 | 1747 | 1830 | 1830 | 1867 | 1867 |
| Cooling utilities | 707 | 1139 | 648 | 1204 | 564 | 1396 | |
| Cooling duty after compressor (kW) | 2425 | 3964 | 2060 | 4125 | 1775 | 4886 | |
| Steam consumption in reboiler (kWth) | - | 1699 | - | 1871 | - | 2608 | |
| Economic Values | Feed 1 | Feed 2 | Feed 3 | |||
|---|---|---|---|---|---|---|
| Flash | Distil. | Flash | Distil. | Flash | Distil. | |
| Total capital investment (TCI, M$) | 17.55 | 20.56 | 16.00 | 17.60 | 15.57 | 19.44 |
| Total production cost (TPC, M$/y) | 5.96 | 7.17 | 5.80 | 7.10 | 5.68 | 7.69 |
| Levelized cost of CO2 liquefaction (LCCL, $/t-CO2) | 13.24 | 17.07 | 14.90 | 16.90 | 18.90 | 18.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Jung, P.-G.; Lim, Y.-I.; Kim, H.; Moon, H.-M. CO2 Compression and Liquefaction Processes Using a Distillation Column for the Flexible Operation of Transportation. Processes 2024, 12, 115. https://doi.org/10.3390/pr12010115
Kim S, Jung P-G, Lim Y-I, Kim H, Moon H-M. CO2 Compression and Liquefaction Processes Using a Distillation Column for the Flexible Operation of Transportation. Processes. 2024; 12(1):115. https://doi.org/10.3390/pr12010115
Chicago/Turabian StyleKim, Semie, Pyeong-Gon Jung, Young-Il Lim, Hyojoon Kim, and Hung-Man Moon. 2024. "CO2 Compression and Liquefaction Processes Using a Distillation Column for the Flexible Operation of Transportation" Processes 12, no. 1: 115. https://doi.org/10.3390/pr12010115
APA StyleKim, S., Jung, P.-G., Lim, Y.-I., Kim, H., & Moon, H.-M. (2024). CO2 Compression and Liquefaction Processes Using a Distillation Column for the Flexible Operation of Transportation. Processes, 12(1), 115. https://doi.org/10.3390/pr12010115








