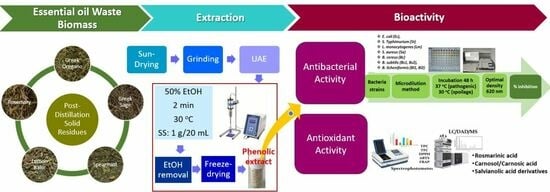
Assessment of Antioxidant and Antibacterial Potential of Phenolic Extracts from Post-Distillation Solid Residues of Oregano, Rosemary, Sage, Lemon Balm, and Spearmint
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Reagents
2.3. Ultrasound-Assisted Extraction (UAE) of Phenolics from Post-Distillation SRs
2.4. Bacterial Strains and Cultures
2.5. Assessement of Antibacterial Activity
2.6. Determination of Total Phenolic (TPC) and Flavonoid Content (TFC)
2.7. Determination of Antioxidant Activity of Phenolic Extracts
2.7.1. ABTS Radical Scavenging Assay
2.7.2. DPPH Radical Scavenging Assay
2.7.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.8. HPLC-DAD-MS Quantification of Phenolics from Solid Residues Extracts
2.9. Statistical Analysis
3. Results and Discussion
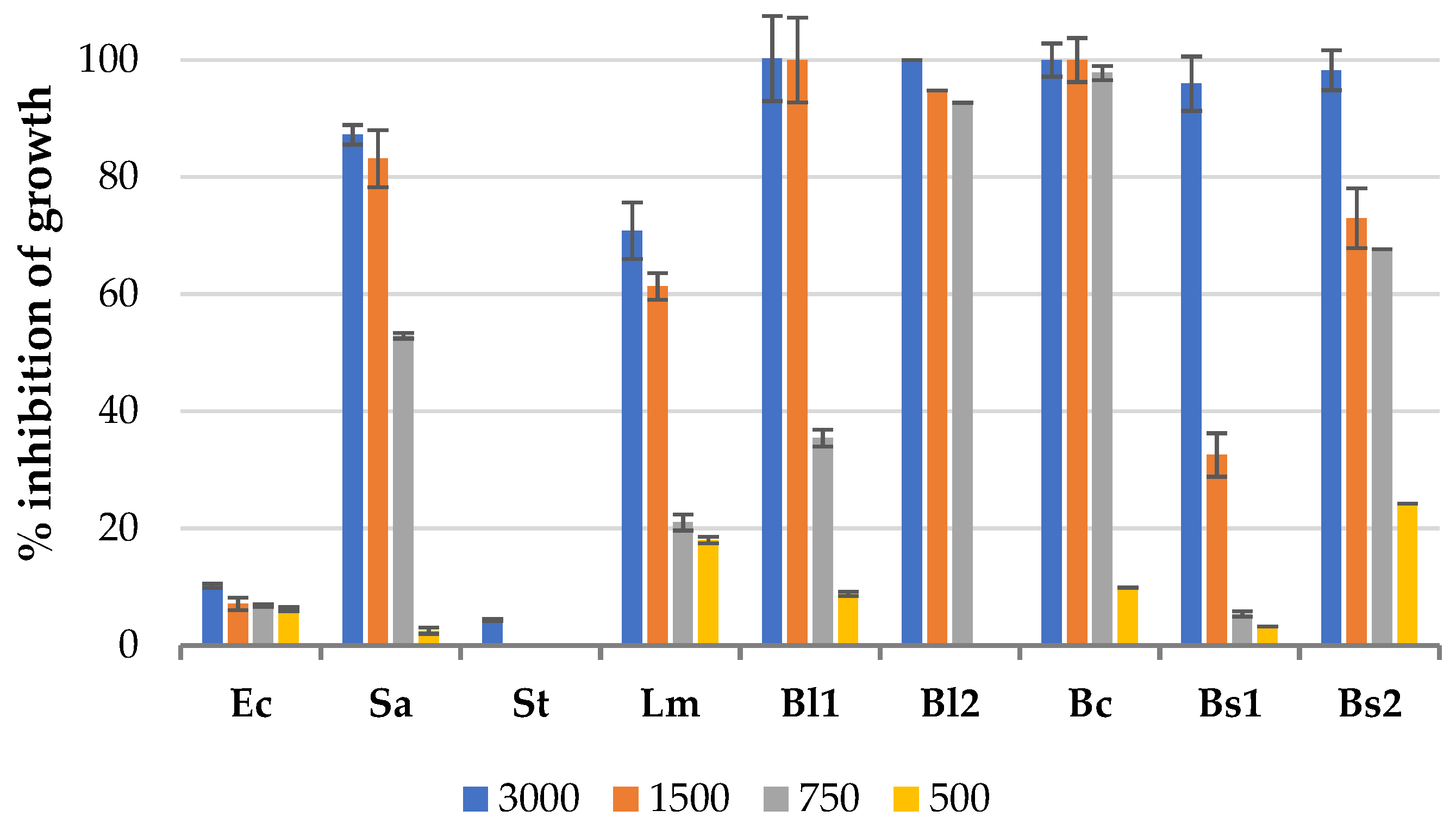
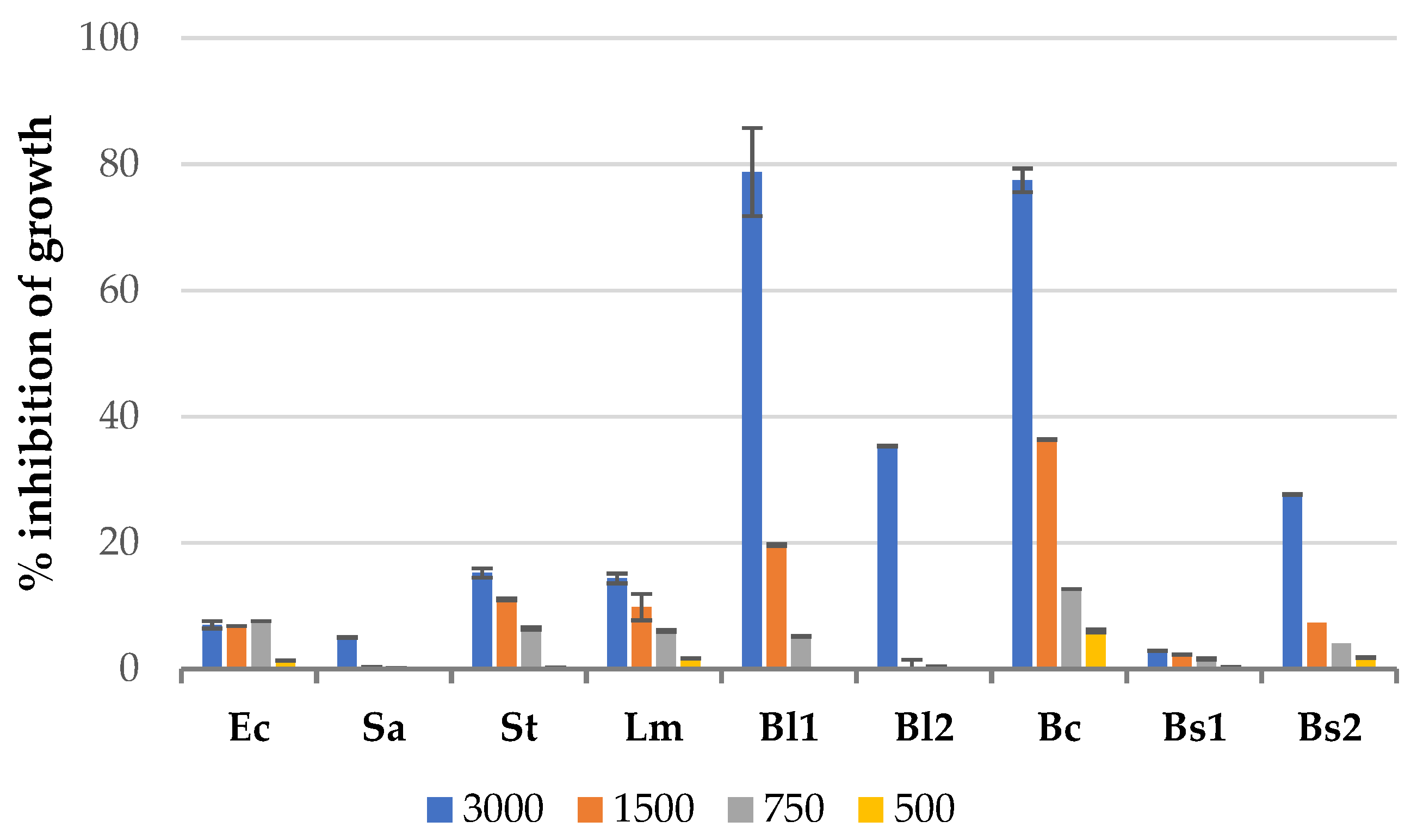
3.1. Antibacterial Effect of Solid Residues Extracts in the Microplate Assay
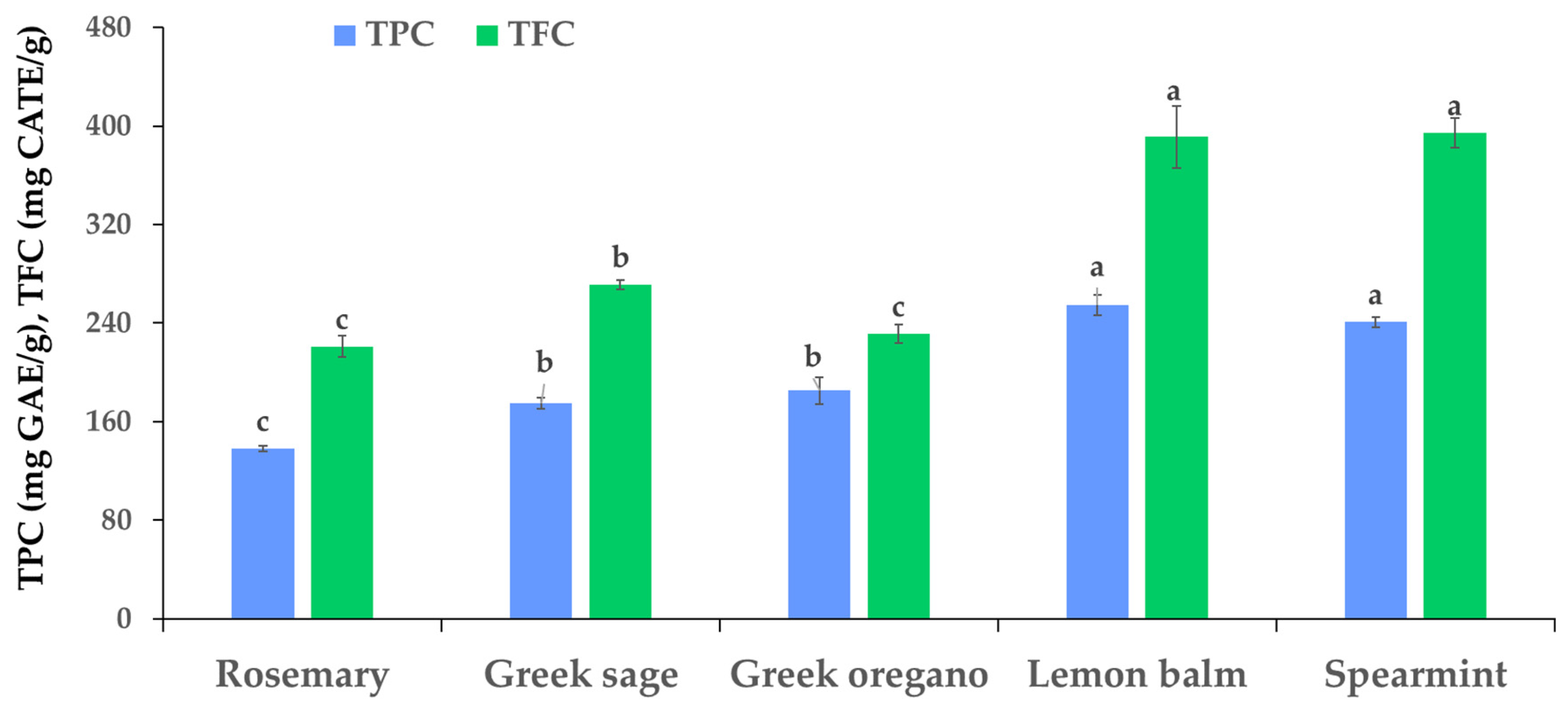
3.2. Total Phenolic Content, Total Flavonoid Content, and Antioxidant Activity of SR Extracts
3.3. Phenolic Composition of SR Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, P.; Mahato, D.K.; Gupta, A.; Pandhi, S.; Mishra, S.; Barua, S.; Tyagi, V.; Kumar, A.; Kumar, M.; Kamle, M. Use of essential oils and phytochemicals against the mycotoxins producing fungi for shelf-life enhancement and food preservation. Int. J. Food Sci. Technol. 2022, 57, 2171–2184. [Google Scholar] [CrossRef]
- Santos, M.I.S.; Marques, C.; Mota, J.; Pedroso, L.; Lima, A. Applications of essential oils as antibacterial agents in minimally processed fruits and vegetables—A review. Microorganisms 2022, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Tashani, F.; Karami, A.; Tahmasebi, A.; Maggi, F. Variability in chemical composition and antibacterial activity of Salvia majdae essential oil under various extraction techniques. J. Essent. Oil Res. 2022, 34, 279–289. [Google Scholar] [CrossRef]
- Peng, X.; Liu, N.; Wang, M.; Liang, B.; Feng, C.; Zhang, R.; Wang, X.; Hu, X.; Gu, H.; Xing, D. Recent advances of kinetic model in the separation of essential oils by microwave-assisted hydrodistillation. Ind. Crop. Prod. 2022, 187, 115418. [Google Scholar] [CrossRef]
- Marrelli, M.; Statti, G.A.; Conforti, F. Origanum spp.: An update of their chemical and biological profiles. Phytochem. Rev. 2018, 17, 873–888. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Albuquerque, T.G.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A novel insight on an ancient aromatic plant: The rosemary (Rosmarinus officinalis L.). Trends Food Sci. Technol. 2015, 45, 355–368. [Google Scholar] [CrossRef]
- Lešnik, S.; Furlan, V.; Bren, U. Rosemary (Rosmarinus Officinalis L.): Extraction Techniques, analytical methods and health-promoting biological effects. Phytochem. Rev. 2021, 20, 1273–1328. [Google Scholar] [CrossRef]
- Jahanian, H.; Kahkeshani, N.; Sanei-Dehkordi, A.; Isman, M.B.; Saeedi, M.; Khanavi, M. Rosmarinus officinalis as a natural insecticide: A Review. Int. J. Pest Manag. 2022, 1–46. [Google Scholar] [CrossRef]
- Amaral, G.P.; Mizdal, C.R.; Stefanello, S.T.; Mendez, A.S.L.; Puntel, R.L.; de Campos, M.M.A.; Soares, F.A.A.; Fachinetto, R. Antibacterial and antioxidant effects of Rosmarinus officinalis L. extract and its fractions. J. Tradit. Complement. Med. 2019, 9, 383–392. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Chen, Y.; Li, Z.-J.; Li, X.; Fan, G. Bioactive properties of the aromatic molecules of spearmint (Mentha spicata L.) essential oil: A review. Food Funct. 2022, 13, 3110–3132. [Google Scholar] [CrossRef]
- Charles, D.J. Antioxidant Properties of Spices, Herbs and Other Sources; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-4309-4. [Google Scholar]
- Karousou, R.; Hanlidou, E.; Kokkini-Gkouzkouni, S. The sage plants in Greece, distribution and infraspecific variation. In Sage, the Genus Salvia; Kintzios, S., Hardman, R., Eds.; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000; Volume 14, pp. 27–46. [Google Scholar]
- Ulubelen, A. Terpenoids in the genus salvia. In Sage, the Genus Salvia; Kintzios, S., Hardman, R., Eds.; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000; Volume 14, pp. 55–69. [Google Scholar]
- Cvetkovikj, I.; Stefkov, G.; Karapandzova, M.; Kulevanova, S. Essential oil composition of Salvia fruticosa Mill. populations from Balkan Peninsula. Maced. Pharm. Bull. 2015, 61, 19–26. [Google Scholar] [CrossRef]
- Giweli, A.A.; Džamić, A.M.; Soković, M.; Ristić, M.S.; Janaćković, P.; Marin, P.D. The chemical composition, antimicrobial and antioxidant activities of the essential oil of Salvia fruticosa growing wild in Libya. Arch. Biol. Sci. 2013, 65, 321–329. [Google Scholar] [CrossRef]
- Leontaritou, P.; Lamari, F.N.; Papasotiropoulos, V.; Iatrou, G. Morphological, genetic and essential oil variation of Greek sage (Salvia fruticosa Mill.) populations from Greece. Ind. Crop. Prod. 2020, 150, 112346. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Herrera-Bravo, J.; Akram, M.; Abbaass, W.; Semwal, P.; Painuli, S.; Konovalov, D.A.; Alfred, M.A.; Kumar, N.V.A.; et al. Phytochemical constituents, biological activities, and health-promoting effects of the Melissa officinalis. Oxid. Med. Cell. Longev. 2021, 2021, 6584693. [Google Scholar] [CrossRef]
- Saha, A.; Basak, B.B. Scope of Value Addition and utilization of residual biomass from medicinal and aromatic plants. Ind. Crop. Prod. 2020, 145, 111979. [Google Scholar] [CrossRef]
- Olofsson, J.; Börjesson, P. Residual biomass as resource—Life-cycle environmental impact of wastes in circular resource systems. J. Clean. Prod. 2018, 196, 997–1006. [Google Scholar] [CrossRef]
- Miljanović, A.; Dent, M.; Grbin, D.; Pedisić, S.; Zorić, Z.; Marijanović, Z.; Jerković, I.; Bielen, A. Sage, rosemary, and bay laurel hydrodistillation by-products as a source of bioactive compounds. Plants 2023, 12, 2394. [Google Scholar] [CrossRef]
- Gonzalez-Rivera, J.; Campanella, B.; Pulidori, E.; Bramanti, E.; Tiné, M.R.; Bernazzani, L.; Onor, M.; Bàrberi, P.; Duce, C.; Ferrari, C. From Volatiles to solid wastes: Towards the full valorization of lavender and rosemary by simultaneous in situ microwaves and ultrasounds irradiation extraction. Ind. Crop. Prod. 2023, 194, 116362. [Google Scholar] [CrossRef]
- Irakli, M.; Bouloumpasi, E.; Christaki, S.; Skendi, A.; Chatzopoulou, P. Modeling and optimization of phenolic compounds from sage (Salvia fruticosa L.) post-distillation residues: Ultrasound- versus microwave-assisted extraction. Antioxidants 2023, 12, 549. [Google Scholar] [CrossRef]
- Christaki, S.; Bouloumpasi, E.; Lalidou, E.; Chatzopoulou, P.; Irakli, M. Bioactive profile of distilled solid by-products of rosemary, greek sage and spearmint as affected by distillation methods. Molecules 2022, 27, 9058. [Google Scholar] [CrossRef]
- Irakli, M.; Skendi, A.; Bouloumpasi, E.; Chatzopoulou, P.; Biliaderis, C.G. LC-MS Identification and quantification of phenolic compounds in solid residues from the essential oil industry. Antioxidants 2021, 10, 2016. [Google Scholar] [CrossRef] [PubMed]
- Skendi, A.; Irakli, M.; Chatzopoulou, P.; Bouloumpasi, E.; Biliaderis, C.G. Phenolic extracts from solid wastes of the aromatic plant essential oil industry: Potential uses in food applications. Food Chem. Adv. 2022, 1, 100065. [Google Scholar] [CrossRef]
- de Elguea-Culebras, G.O.; Bravo, E.M.; Sánchez-Vioque, R. Potential sources and methodologies for the recovery of phenolic compounds from distillation residues of mediterranean aromatic plants. An approach to the valuation of by-products of the essential oil market—A review. Ind. Crop. Prod. 2022, 175, 114261. [Google Scholar] [CrossRef]
- Ziani, I.; Bouakline, H.; Yahyaoui, M.I.; Belbachir, Y.; Fauconnier, M.L.; Asehraou, A.; Tahani, A.; Talhaoui, A.; El Bachiri, A. The effect of ethanol/water concentration on phenolic composition, antioxidant, and antimicrobial activities of Rosmarinus tournefortii de Noé hydrodistillation solid residues. J. Food Meas. Charact. 2023, 17, 1602–1615. [Google Scholar] [CrossRef]
- Trifan, A.; Zengin, G.; Korona-Glowniak, I.; Skalicka-Woźniak, K.; Luca, S.V. Essential oils and sustainability: In Vitro bioactivity screening of Myristica fragrans Houtt. post-distillation by-products. Plants 2023, 12, 1741. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Zengin, G.; Sinan, K.I.; Korona-Glowniak, I.; Minceva, M.; Skalicka-Woźniak, K.; Trifan, A. Value-added compounds with antimicrobial, antioxidant, and enzyme-inhibitory effects from post-distillation and post-supercritical CO2 extraction by-products of rosemary. Antioxidants 2023, 12, 244. [Google Scholar] [CrossRef] [PubMed]
- Bouloumpasi, E.; Hatzikamari, M.; Lazaridou, A.; Chatzopoulou, P.; Biliaderis, C.G.; Irakli, M. Antibacterial and antioxidant properties of oregano and rosemary essential oil distillation by-products. Biol. Life Sci. Forum 2021, 6, 47. [Google Scholar]
- de Elguea-Culebras, G.O.; Sánchez-Vioque, R.; Santana-Méridas, O.; Herraiz-Peñalver, D.; Carmona, M.; Berruga, M.I. In Vitro antifungal activity of residues from essential oil industry against Penicillium Verrucosum, a common contaminant of ripening cheeses. LWT 2016, 73, 226–232. [Google Scholar] [CrossRef]
- Valerio, F.; Mezzapesa, G.N.; Ghannouchi, A.; Mondelli, D.; Logrieco, A.F.; Perrino, E.V. Characterization and antimicrobial properties of essential oils from four wild taxa of Lamiaceae family growing in Apulia. Agronomy 2021, 11, 1431. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1998, 299, 152–178. [Google Scholar]
- Bao, J.S.; Cai, Y.; Sun, M.; Wang, G.Y.; Corke, H. Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability. J. Agric. Food Chem. 2005, 53, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C.A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Benzie, F.; Strain, J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–23. [Google Scholar] [PubMed]
- Thompson, J.M.; Dodd, C.E.; Waites, W.M. Spoilage of bread by Bacillus. Int. Biodeterior. Biodegrad. 1993, 32, 55–66. [Google Scholar] [CrossRef]
- Tsitlakidou, P.; Papachristoforou, A.; Tasopoulos, N.; Matzara, A.; Hatzikamari, M.; Karamanoli, K.; Mourtzinos, I. Sensory analysis, volatile profiles and antimicrobial properties of Origanum vulgare L. essential oils. Flavour Fragr. J. 2021, 37, 43–51. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Singh, R.G.; Negi, P.S.; Radha, C. Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringa oleifera seed flour. J. Funct. Foods 2013, 5, 1883–1891. [Google Scholar] [CrossRef]
- Bhalodia, N.R.; Shukla, V.J. Antibacterial and antifungal activities from leaf extracts of Cassia fistula l.: An ethnomedicinal plant. J. Adv. Pharm. Technol. Res. 2011, 2, 104–109. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef]
- Al-Hashimi, A.G. Antioxidant and antibacterial activities of Hibiscus sabdariffa L. extracts. Afr. J. Food Sci. 2012, 6, 506–511. [Google Scholar]
- Shene, C.; Reyes, A.K.; Villarroel, M.; Sineiro, J.; Pinelo, M.; Rubilar, M. Plant location and extraction procedure strongly alter the antimicrobial activity of murta extracts. Eur. Food Res. Technol. 2009, 228, 467–475. [Google Scholar] [CrossRef]
- Falleh, H.; Ksouri, R.; Chaieb, K.; Karray-Bouraoui, N.; Trabelsi, N.; Boulaaba, M.; Abdelly, C. Phenolic composition of Cynara cardunculus L. organs, and their biological activities. C. R. Biol. 2008, 331, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Marino, S.; Vivot, E.; Cruanes, M.C.; Munoz, J.D.; Cruanes, J.; Ferraro, G.; Gutkind, G.; Martino, V. Antimicrobial activity of argentine plants used in the treatment of infectious diseases: Isolation of 15 active compounds from Sebastiania brasiliensis. J. Ethnopharmacol. 2001, 77, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Mith, H.; Dure, R.; Delcenserie, V.; Zhiri, A.; Daube, G.; Clinquart, A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci. Nutr. 2014, 2, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Rota, C.; Carraminana, J.J.; Burillo, J.; Herrera, A. In Vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens. J. Food Prot. 2004, 67, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, H.; Papadopoulou, C. Antimicrobial activity of basil, oregano, and thyme essential oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef]
- Peter, Y.Y.; Wong, D.D. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006, 97, 505–515. [Google Scholar]
- Celano, R.; Piccinelli, A.L.; Pagano, I.; Roscigno, G.; Campone, L.; De Falco, E.; Russo, M.; Rastrelli, L. Oil distillation wastewaters from aromatic herbs as new natural source of antioxidant compounds. Food Res. Int. 2017, 99, 298–307. [Google Scholar] [CrossRef]
- Cirlini, M.; Mena, P.; Tassotti, M.; Herrlinger, K.A.; Nieman, K.M.; Dall’Asta, C.; Del Rio, D. Phenolic and volatile composition of a dry spearmint (Mentha spicata L.) extract. Molecules 2016, 21, 1007. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Ponce-Alquicira, E. Environmentally friendly techniques and their comparison in the extraction of natural antioxidants from green tea, rosemary, clove, and oregano. Molecules 2021, 26, 1869. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Velamuri, R.; Fagan, J.; Schaefer, J. Full-spectrum analysis of bioactive compounds in rosemary (Rosmarinus of-ficinalis L.) as influenced by different extraction methods. Molecules 2020, 25, 4599. [Google Scholar] [CrossRef]
- de Torre, M.P.; Cavero, R.Y.; Calvo, M.I. Anticholinesterase activity of selected medicinal plants from navarra region of Spain and a detailed phytochemical investigation of Origanum vulgare L. ssp. Vulgare. Molecules 2022, 27, 710. [Google Scholar] [CrossRef] [PubMed]
- Miron, T.L.; Herrero, M.; Ibánez, E. Enrichment of antioxidant compounds from lemon balm (Melissa officinalis) by pressurized liquid extraction and enzyme-assisted extraction. J. Chrom. A 2013, 1288, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profiles of cultivated, in vitro cultured and commercial samples of Melissa officinalis L. infusions. Food Chem. 2013, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Fernández-Gutiérrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015, 26, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, B.F.; Walch, S.G.; Tinzoh, L.N.; Stühlinger, W.; Lachenmeier, D.W. Rapid UHPLC determination of polyphenols in aqueous infusions of Salvia officinalis L. (sage tea). J. Chrom. B 2011, 879, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Shojaeifard, Z.; Hemmateenejad, B.; Jassbi, A.R. Chemometrics-based LC-UV-ESIMS analyses of 50 Salvia species for detecting their antioxidant constituents. J. Pharm. Biomed. 2021, 193, 113745–113758. [Google Scholar] [CrossRef]
- Gavarić, N.; Kladar, N.; Mišan, A.; Nikolić, A.; Samojlik, I.; Mimica-Dukić, N.; Božin, B. Postdistillation waste material of thyme (Thymus vulgaris L., Lamiaceae) as a potential source of biologically active compounds. Ind. Crop. Prod. 2015, 74, 457–464. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Mani, J.S.; Johnson, J.B.; Hosking, H.; Ashwath, N.; Walsh, K.B.; Neilsen, P.M.; Broszczak, D.A.; Naiker, M. Antioxidative and therapeutic potential of selected Australian plants: A review. J. Ethnopharmacol. 2021, 268, 113580. [Google Scholar] [CrossRef] [PubMed]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178. [Google Scholar] [CrossRef] [PubMed]
- Ecevit, K.; Barros, A.A.; Silva, J.M.; Reis, R.L. Preventing microbial infections with natural phenolic compounds. Future Pharmacol. 2022, 2, 460–498. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and antimicrobial properties of rosemary (Rosmarinus officinalis, L.): A review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, 1231–1249. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, A.; Asgarpanah, J.; Sadaghiyani, P.N.; Faizi, M. Total phenolic and flavonoid content and antibacterial activity of Punica granatum L. var. Pleniflora flowers (Golnar) against bacterial strains causing foodborne diseases. BMC Complement. Altern. Med. 2015, 15, 366. [Google Scholar] [CrossRef]
- Tang, Q.L.; Kang, A.R.; Lu, C.X. Phytochemical analysis, antibacterial activity and mode of action of the methanolic extract of Scutellaria barbata against various clinically important bacterial pathogens. Int. J. Pharmacol. 2016, 12, 116–125. [Google Scholar] [CrossRef]
- Wu, T.; Zang, X.; He, M.; Pan, S.; Xu, X. Structure–activity relationship of flavonoids on their anti-Escherichia coli activity and inhibition of DNA gyrase. J. Agric. Food Chem. 2013, 61, 8185–8190. [Google Scholar] [CrossRef]
- Konaté, K.; Hilou, A.; Mavoungou, J.; Lepengue, A.; Souza, A.; Barro, N.; Datte, J.Y.; M’Batchi, B.; Nacoulma, O. Antimicrobial activity of polyphenol-rich fractions from Sida alba L. (Malvaceae) against co-trimoxazol-resistant bacteria strains. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 5–6. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
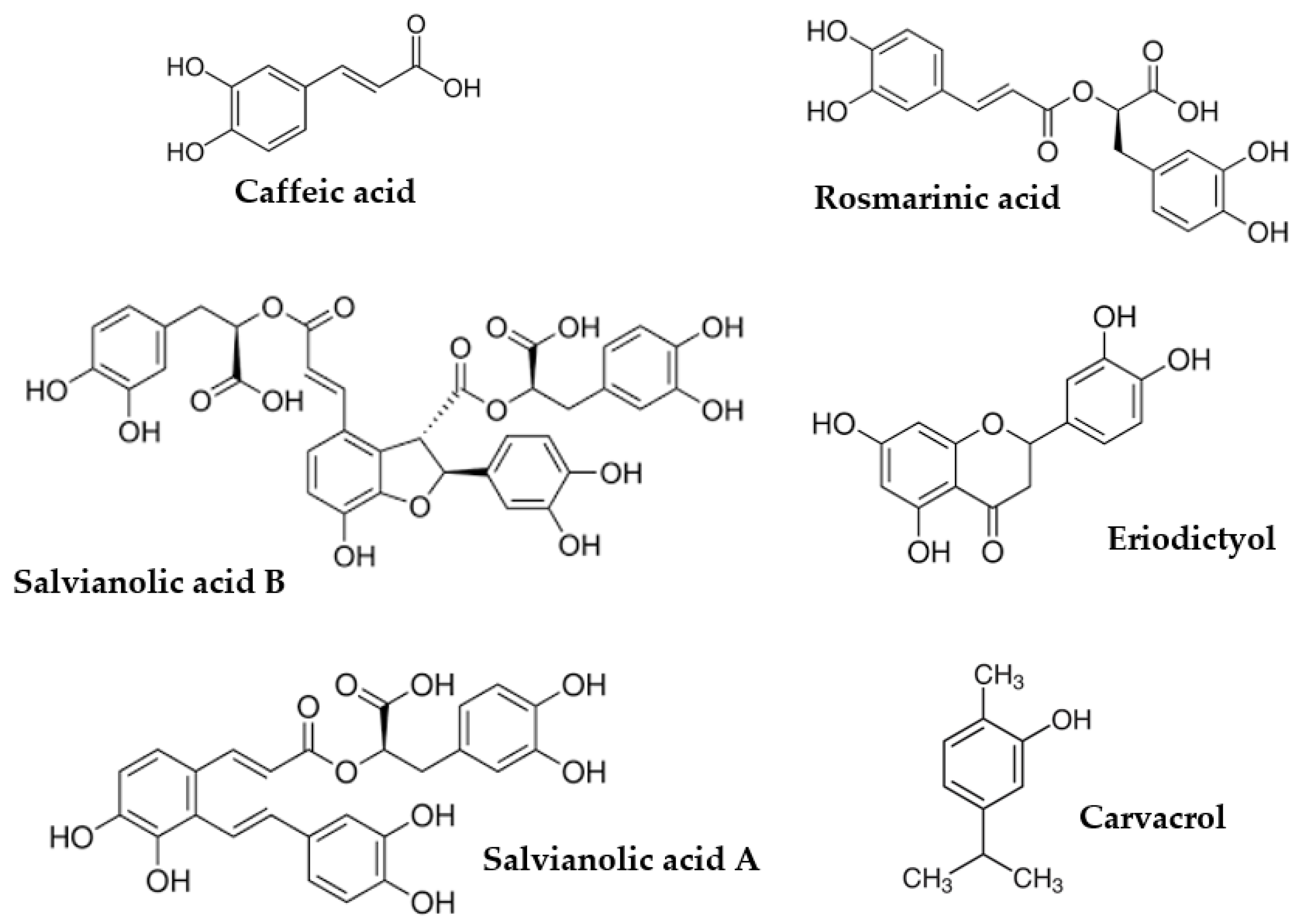
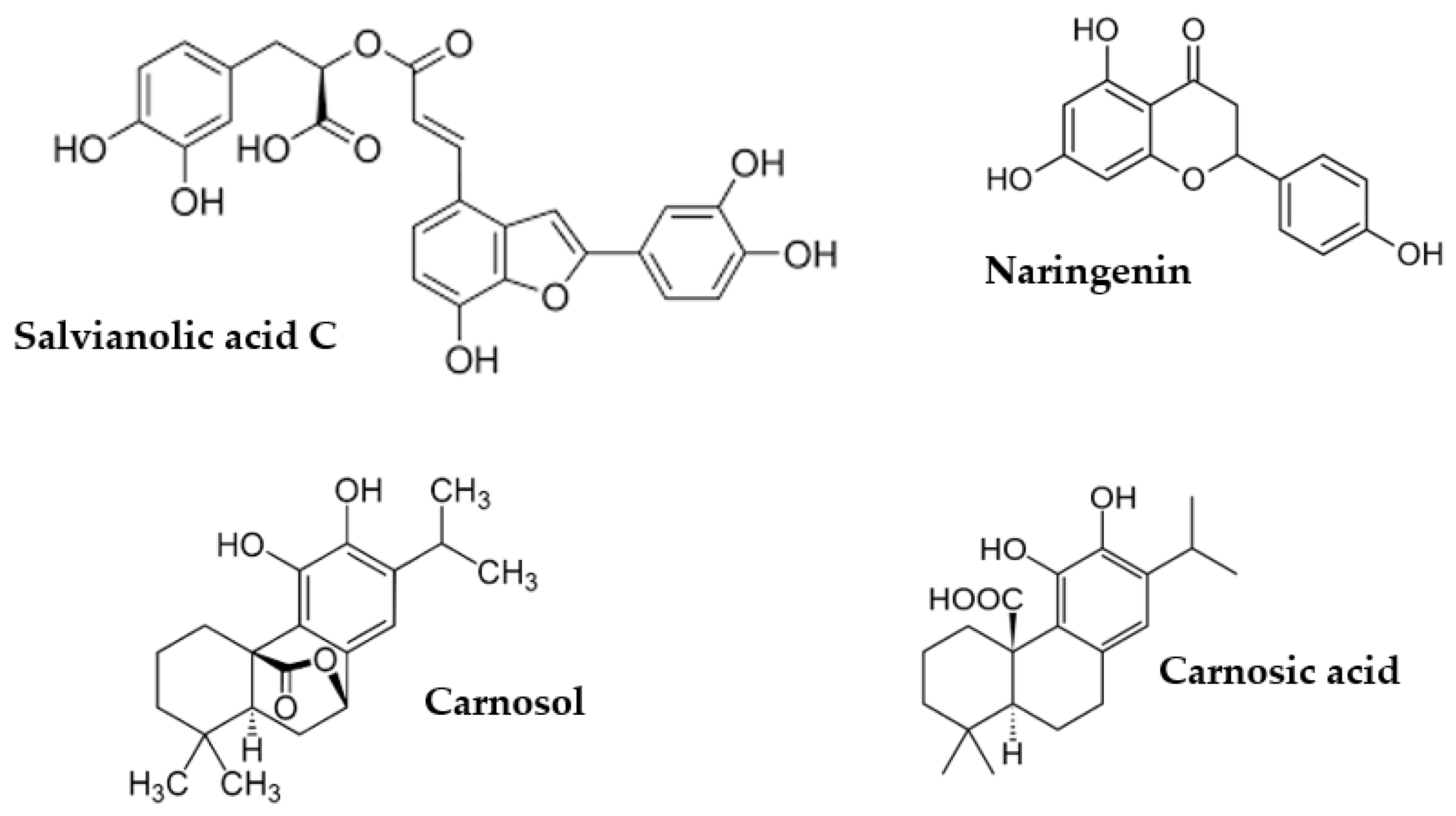

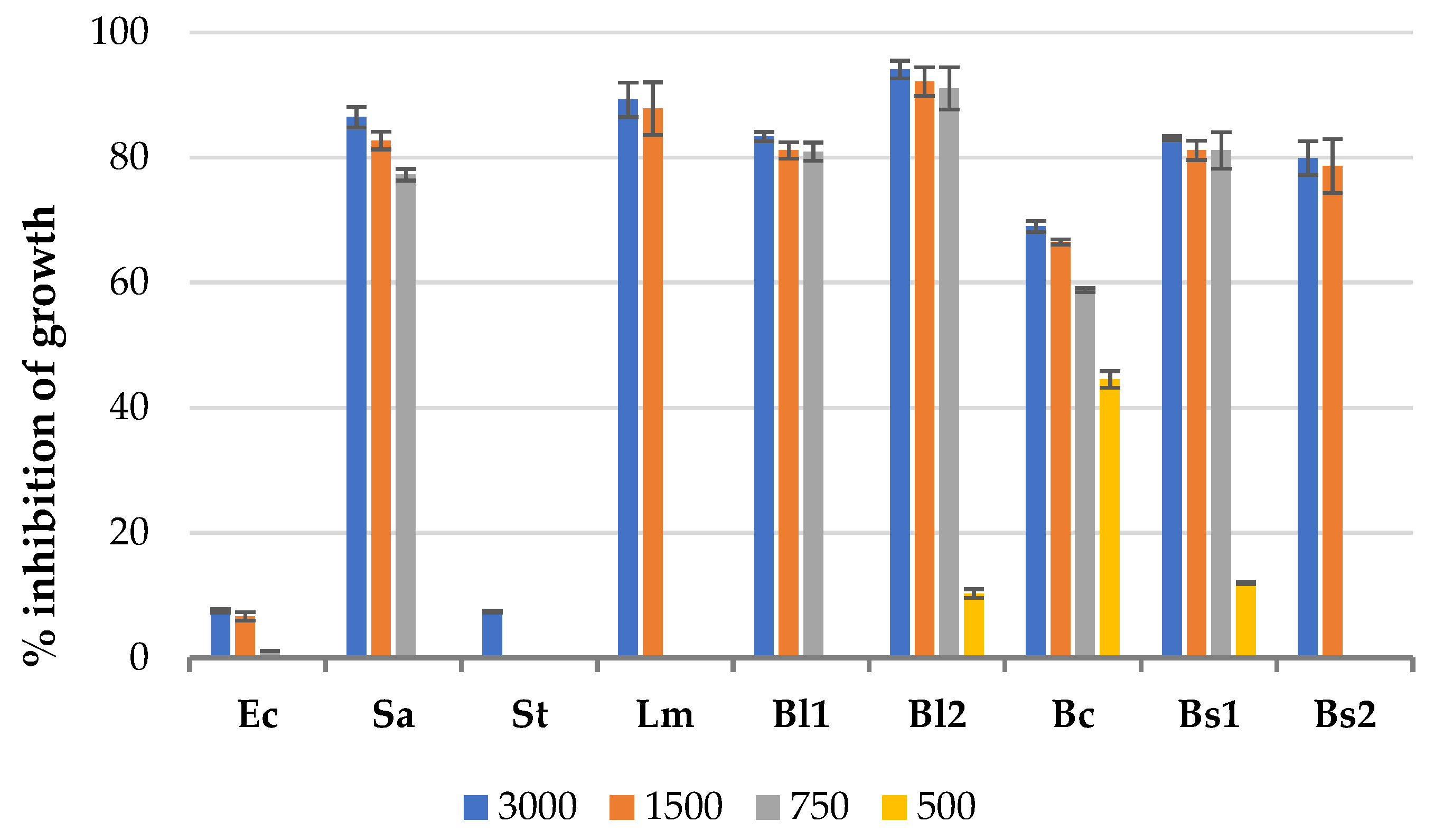




| Peak | RT (min) | UV λmax (nm) | [M-H]-(m/z) | Tentative Identification | Ref. | Extract |
|---|---|---|---|---|---|---|
| 1 | 2.48 | - | 289 | unknown | - | O, L, SP, R, S |
| 2 | 2.79 | 256 | 387 | unknown | - | O, L, SP, S |
| 3 | 2.89 | 277 | 191 | quinic acid | st | O, L, SP, R, S |
| 4 | 3.79 | 279 | 191 | citric acid | st | O, L, SP, R, S |
| 5 | 4.17 | 281 | 197 | danshensu | [52,53] | O, L, SP, R, S |
| 6 | 6.39 | 270, 335 | 593 | vicenin-2 | st | O, S |
| 7 | 6.93 | 239, 284, 314 | 305 | gallocatechin isomer | [54,55] | O, L, SP, R, S |
| 8 | 7.49 | 320 | 179 | caffeic acid | st | O, L, SP, R, S |
| 9 | 9.21 | 252, 285, 344 | 537(493) | salvianolic acid isomer | [56,57] | O, L |
| 10 | 9.28 | 260, 345 | 593 | luteolin-7-O-rutinoside | st | O, SP, R, S |
| 11 | 9.63 | 239, 275 | 597 | yunnaneic acid F | [52,58] | L, R |
| 12 | 9.93 | 253, 366 | 447 | luteolin-7-O-glucoside | st | L |
| 13 | 9.95 | 260, 345 | 461 | luteolin-7-O-glucuronide | st | SP, R, S |
| 14 | 10.36 | 272, 345 | 477 | isorhamnetin-3-O-D-glucoside | st | R, S |
| 15 | 10.62 | 254, 283, 341 | 717(579) | salvianolic acid L | [53,59] | SP |
| 16 | 10.91 | 239, 284, 333 | 578(303) | unkown | - | O |
| 17 | 10.98 | 232, 285, 334 | 717(537) | salvianolic acid isomer | [53,59] | SP |
| 18 | 11.42 | 329 | 439 | sulphated rosmarinic acid | [58] | L |
| 19 | 11.45 | 283 | 609 | hesperidin | st | SP, R |
| 20 | 11.62 | 241, 286, 321 | 555 | salvianolic acid K | [60] | S |
| 21 | 11.72 | 331 | 461 | hispidulin-7-O-glucoside | [61] | R, S |
| 22 | 12.22 | 329, 285sh | 359 | rosmarinic acid | st | O, L, SP, R, S |
| 23 | 12.88 | 287, 325 | 717(537) | salvianolic acid B | st | O, L, SP, R, S |
| 24 | 13.47 | 243, 269, 337 | 503 | caffeoyl-hexosyl-hexose | [52] | R |
| 25 | 13.53 | 239, 293, 338 | 537(493) | lithospermic acid A | [53,58] | L, SP |
| 26 | 14.08 | 239, 299 (240) | 493(137) | salvianolic acid A (IS) | [53,58] | O, L, SP, R, S |
| 27 | 14.45 | 243, 269, 337 | 503 | caffeoyl-hexosyl-hexose | [52] | R |
| 28 | 14.56 | 245, 286, 334 | 715(537) | salvianolic acid isomer | [53] | SP |
| 29 | 15.26 | 287 | 287 | eriodictyol | st | O |
| 30 | 15.27 | 282 | 285 | luteolin | st | SP, R, S |
| 31 | 15.35 | 243, 286, 318 | 715(493) | salvianolic acid C | [57] | L |
| 32 | 15.88 | 241, 286, 321 | 717(519) | salvianolic acid E | [53,59] | SP |
| 33 | 17.13 | - | 583 | unknown | - | L |
| 34 | 17.49 | 259, 294, 334 | 329 | unknown | - | O, SP |
| 35 | 17.94 | 288 | 271 | naringenin | st | O, SP, R, S |
| 36 | 18.20 | 289, 345 | 359 | rosmarinic acid derivative | [53] | SP |
| 37 | 22.53 | 280 | 345 | rosmanol isomer | [52,60] | L, R, S |
| 38 | 23.87 | 280 | 345 | rosmanol isomer | [52,60] | L, R, S |
| 39 | 25.48 | 280 | 345 | rosmanol isomer | [52,60] | S |
| 40 | 33.79 | 280 | 329(285) | carnosol | st | O, L, R, S |
| 41 | 34.76 | 280 | 329(285) | carnosol | st | R, S |
| 42 | 37.02 | 280 | 331(287) | carnosic acid | st | L, SP, R, S |
| 43 | 38.28 | 279 | 345 | rosmanol isomer | [52,53,60] | O, L, SP, R, S |
| 44 | 39.22 | 263, 286 | 317 | unkown | - | R, S |
| Extracts | Rosmarinic Acid | Phenolic Diterpenoids | Salvianolic AcidIsomers |
|---|---|---|---|
| Rosemary | 53.31 ± 3.81 E | 393.09 ± 29.51 A | 8.03 ± 0.26 D |
| Greek sage | 79.57 ± 7.91 C | 155.42 ± 11.27 B | 3.23 ± 0.15 D |
| Greek oregano | 66.38 ± 1.78 D | - | 41.78 ± 1.88 A |
| Lemon balm | 95.42 ± 4.02 B | - | 32.78 ± 1.28 B |
| Spearmint | 109.90 ± 6.32 A | - | 17.36 ± 4.27 C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouloumpasi, E.; Hatzikamari, M.; Christaki, S.; Lazaridou, A.; Chatzopoulou, P.; Biliaderis, C.G.; Irakli, M. Assessment of Antioxidant and Antibacterial Potential of Phenolic Extracts from Post-Distillation Solid Residues of Oregano, Rosemary, Sage, Lemon Balm, and Spearmint. Processes 2024, 12, 140. https://doi.org/10.3390/pr12010140
Bouloumpasi E, Hatzikamari M, Christaki S, Lazaridou A, Chatzopoulou P, Biliaderis CG, Irakli M. Assessment of Antioxidant and Antibacterial Potential of Phenolic Extracts from Post-Distillation Solid Residues of Oregano, Rosemary, Sage, Lemon Balm, and Spearmint. Processes. 2024; 12(1):140. https://doi.org/10.3390/pr12010140
Chicago/Turabian StyleBouloumpasi, Elisavet, Magdalini Hatzikamari, Stamatia Christaki, Athina Lazaridou, Paschalina Chatzopoulou, Costas G. Biliaderis, and Maria Irakli. 2024. "Assessment of Antioxidant and Antibacterial Potential of Phenolic Extracts from Post-Distillation Solid Residues of Oregano, Rosemary, Sage, Lemon Balm, and Spearmint" Processes 12, no. 1: 140. https://doi.org/10.3390/pr12010140