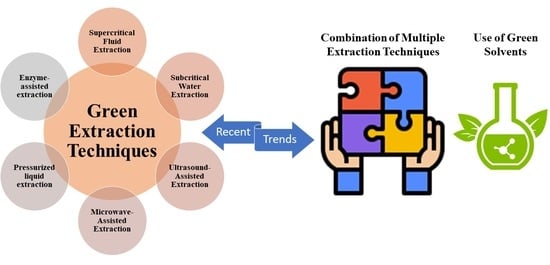
Emerging Trends in Green Extraction Techniques for Bioactive Natural Products
Abstract
1. Introduction
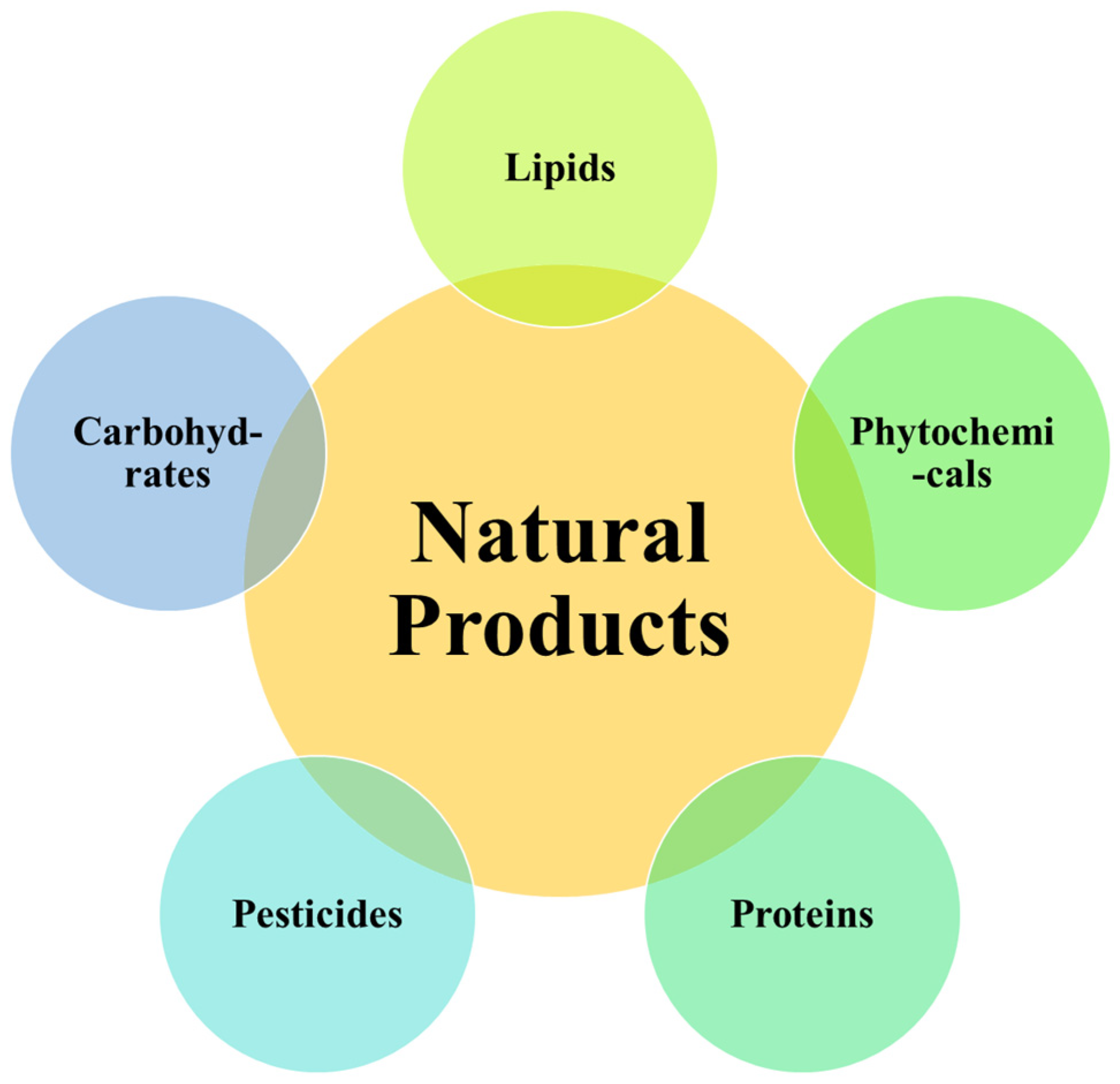
2. Bioactive Natural Products and Sources
3. Green Extraction Techniques
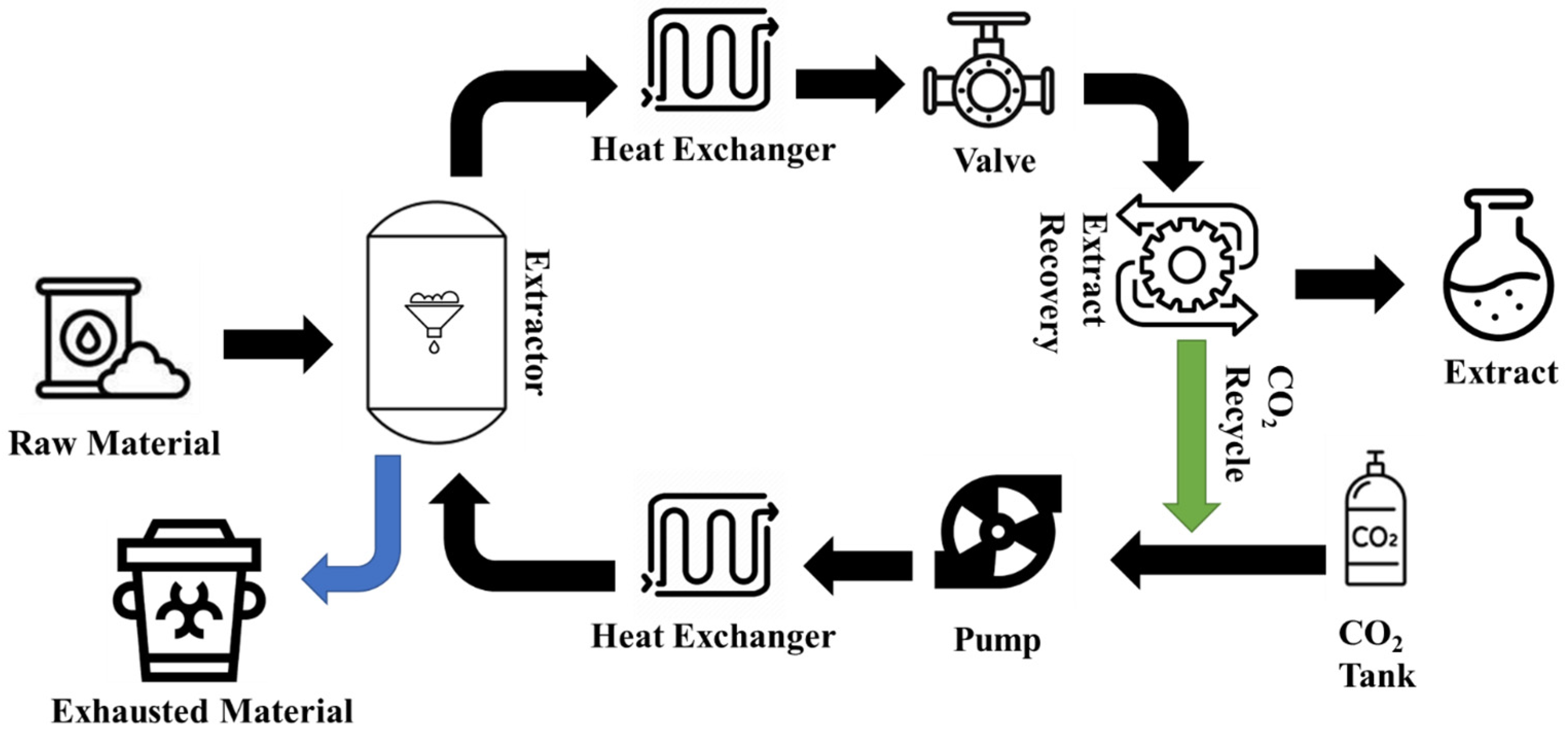
3.1. Supercritical Fluid Extraction (SFE)
3.2. Subcritical Water Extraction (SWE)
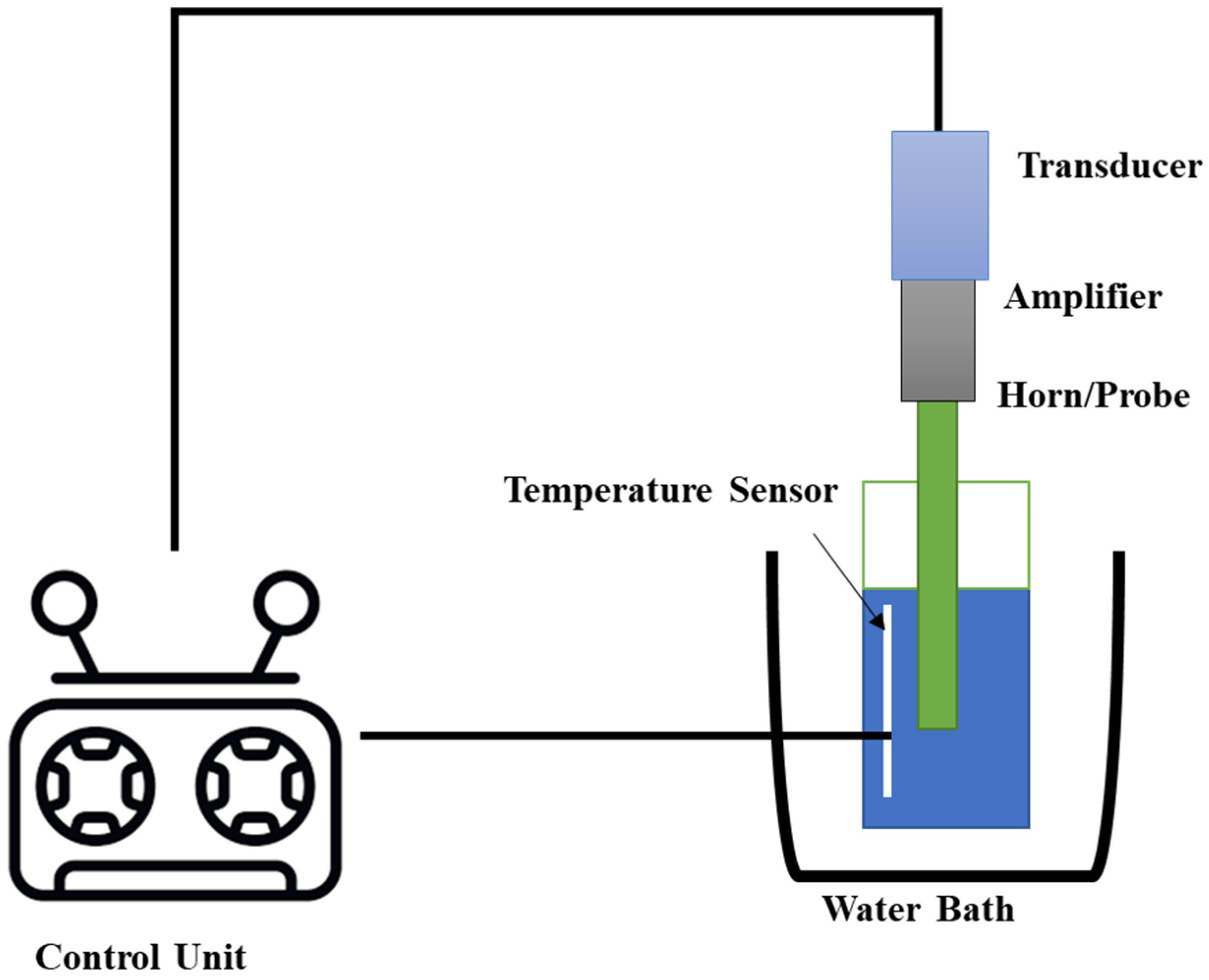
3.3. Ultrasound-Assisted Extraction (UAE)
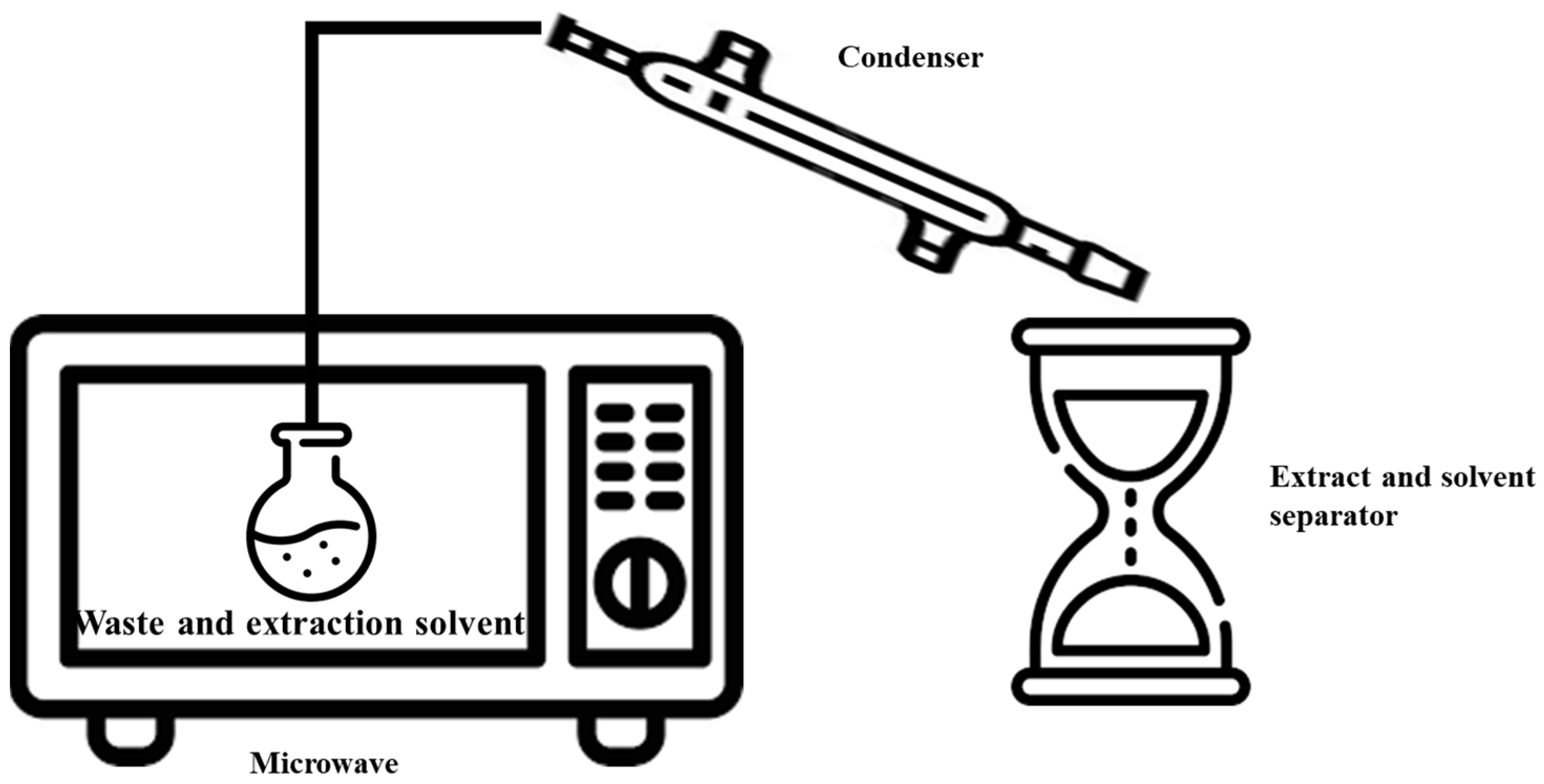
3.4. Microwave-Assisted Extraction (MAE)
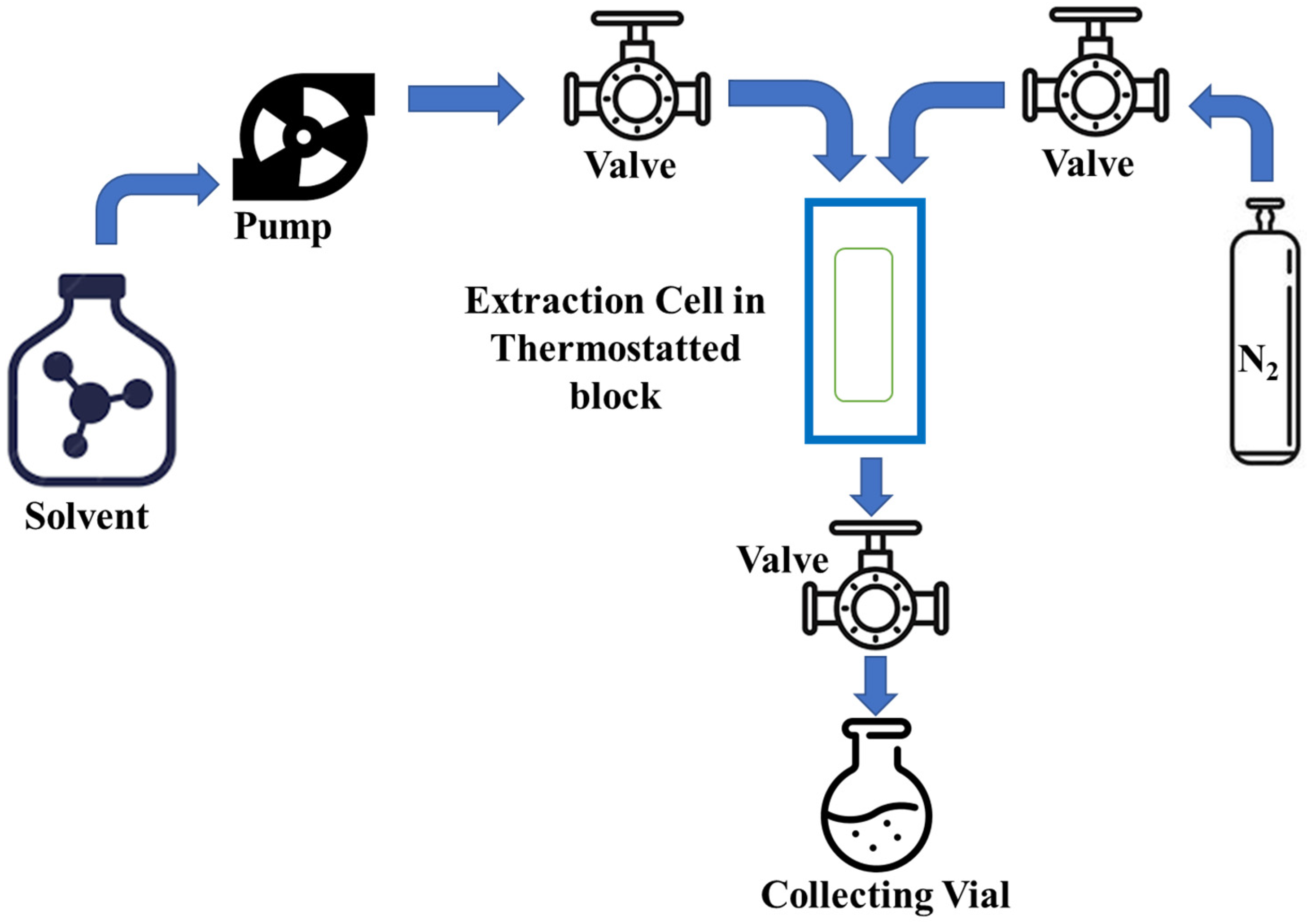
3.5. Pressurized Liquid Extraction (PLE)
3.6. Enzyme-Assisted Extraction (EAE)
4. Recent Trends and Developments in Green Extraction
4.1. Combination of Extraction Techniques
4.1.1. Ultrasound-Microwave-Assisted Extraction (UMAE)
4.1.2. Microwave-Assisted Enzymatic Extraction (MAEE)
4.1.3. Ultrasound-Assisted Enzymatic Extraction (UAEE)
4.1.4. Supercritical Fluid Extraction and Pressurized Fluid Extraction (SFE-PLE)
4.1.5. Supercritical Fluid Extraction Assisted with Ultrasound (SFE–UAE)
4.1.6. Ultrasonic Assisted Extraction and Pressurized Liquid Extraction (UAE-PLE)
4.2. Green Extraction Solvents
4.2.1. Deep Eutectic Solvents (DESs)
4.2.2. Bio-Based Solvents
| Feedstock | Green Extraction | Final Products and Classifications | Remarks | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Source | Type | Technique | Solvent | Target Bioactive NPs | Class | Yield (%, w/w) | ||
| Tea | Seeds | Solid Liquid Extraction (SLE) | ChCl-Gly | Phenolic compounds | Phytochemicals | 0.01 | 93.2% higher than those extracted with methanol/water | [191] |
| Morus alba L. | Leaves | UAE | ChCl-Ca | Phenolic compounds | Phytochemicals | 2.26 | 48.20% higher than those extracted with conventional extraction | [192] |
| Allium cepa L. | Onion peel | MAE | ChCl:U | Phenolic compounds | Phytochemicals | 22.29 | Similar results with the methanol extraction solvent | [193] |
| Grape | Pomace | UMAE | ChCl-Ca | Anthocyanins | Phytochemicals | 0.177 | --- | [194] |
| Actinidia deliciosa | Fruit peel | MAE | GVL | Phenolic compounds | Phytochemicals | 2.97 | The extraction yield followed by GVL:ethanol > acetone > ethanol:water. | [190] |
| Aqueous matrices | -- | Liquid -Liquid Extraction (LLE) | 2-MeTFH | Phenolic compounds | Phytochemicals | 100 | ---- | [195] |
5. Challenges and Future Perspectives
- Standardization and reproducibility: Achieving consistent and reproducible results across different studies and laboratories remains a challenge. The standardization of extraction protocols is essential to ensure the reliability and comparability of results.
- Selectivity: Green extraction techniques may not always provide sufficient selectivity, leading to the co-extraction of unwanted compounds. Enhancing the selectivity of these techniques for specific bioactive NPs is an ongoing challenge.
- Optimization: There is a need for further optimization of extraction parameters, including temperature, pressure, time, and solvent composition. Fine-tuning these parameters is essential for maximizing yield and maintaining the integrity of bioactive NPs.
- Scalability: While these techniques show promise at the laboratory scale, translating them to larger industrial scales may pose challenges. Scaling up without compromising efficiency and sustainability is a critical consideration.
- Solvent compatibility: The compatibility of green solvents with specific bioactive compounds needs careful assessment. Some bio-based and deep eutectic solvents may not be suitable for the extraction of certain classes of NPs.
- Economic viability: The cost-effectiveness of green extraction methods compared with traditional techniques is a significant consideration. Developing economically viable and sustainable processes is crucial for widespread adoption.
- Understanding the mechanisms: A deeper understanding of the mechanisms involved in green extraction processes is needed. This includes elucidating the interactions between solvents and bioactive NPs to optimize extraction efficiency.
- Waste management: Addressing the issue of waste generated during the extraction process is vital. Ensuring that the by-products or waste are environmentally friendly and can be appropriately managed is essential for the overall sustainability of the process.
Future Research
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, Y.H.; Verpoorte, R. Green Solvents for the Extraction of Bioactive Compounds from Natural Products Using Ionic Liquids and Deep Eutectic Solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Bitwell, C.; Sen, S.I.; Chimuka, L.; Kakoma, M.K. A Review of Modern and Conventional Extraction Techniques and Their Applications for Extracting Phytochemicals from Plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Jadimurthy, R.; Jagadish, S.; Nayak, S.C.; Kumar, S.; Mohan, C.D.; Rangappa, K.S. Phytochemicals as Invaluable Sources of Potent Antimicrobial Agents to Combat Antibiotic Resistance. Life 2023, 13, 948. [Google Scholar] [CrossRef] [PubMed]
- MartíN, J.F.; García-Estrada, C.; Liras, P. Insights into the Molecular Mechanisms of β-Lactam Antibiotic Synthesizing and Modifying Enzymes in Fungi. In Biotechnology of Microbial Enzymes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 199–228. [Google Scholar]
- Chen, Z.-H.; Guo, Y. Recent Advances on Marine Mollusk-Derived Natural Products: Chemistry, Chemical Ecology and Therapeutical Potential. Nat. Prod. Rep. 2023, 40, 509–556. [Google Scholar] [CrossRef] [PubMed]
- CITES. Convention on International Trade in Endangered Species of Wild Fauna and Flora. 2019. Available online: https://www.cites.org/ (accessed on 12 October 2023).
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H.V.; Bock, N.A.; Busta, L.; Pearce, M.; Roston, R.; Shomo, Z.D.; Terrell, C.R. Introducing Climate Change into the Biochemistry and Molecular Biology Curriculum. Biochem. Mol. Biol. Educ. 2020, 49, 167–188. [Google Scholar] [CrossRef]
- Liu, Z.; De Souza, T.S.P.; Holland, B.J.; Dunshea, F.R.; Barrow, C.J.; Suleria, H.A.R. Valorization of Food Waste to Produce Value-Added Products Based on Its Bioactive Compounds. Processes 2023, 11, 840. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Sridhar, K.; Inbaraj, B.S.; Singh, R.; Kamma, S.; Tripathi, M.; Sharma, M. Transformation of Agro-Waste into Value-Added Bioproducts and Bioactive Compounds: Micro/Nano Formulations and Application in the Agri-Food-Pharma Sector. Bioengineering 2023, 10, 152. [Google Scholar] [CrossRef]
- Rasool, K.; Hussain, S.; Shahzad, A.; Miran, W.; Mahmoud, K.A.; Ali, N.; Almomani, F. Comprehensive Insights into Sustainable Conversion of Agricultural and Food Waste into Microbial Protein for Animal Feed Production. Rev. Environ. Sci. Bio/Technol. 2023, 22, 527–562. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Publications: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- World Bank. Trends in Solid Waste Management. Available online: https://datatopics.worldbank.org/what-a-waste/trends_in_solid_waste_management.html (accessed on 12 October 2023).
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food Waste: A Potential Bioresource for Extraction of Nutraceuticals and Bioactive Compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Bhawani, S.A.; Khan, A.; Ahmad, F.B. Extraction of Natural Products from Agro-Industrial Wastes: A Green and Sustainable Approach; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Chémat, F.; Vian, M.A.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjević, V.; Cravotto, G. Green Extraction of Natural Products. Origins, Current Status, and Future Challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Chémat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.-S. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [PubMed]
- Putra, N.R.; Yustisia, Y.; Heryanto, B.; Asmaliyah, A.; Miswarti, M.; Rizkiyah, D.N.; Yunus, M.A.C.; Irianto, I.; Qomariyah, L.; Rohman, G.A.N. Advancements and Challenges in Green Extraction Techniques for Indonesian Natural Products: A Review. S. Afr. J. Chem. Eng. 2023, 46, 88–98. [Google Scholar] [CrossRef]
- Othman, S.B.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.K. A Review on Potential of Bioactive Compounds Obtained from Processing Waste of Various Fruits and Vegetables. Int. J. Pure Appl. Biosci. 2018, 6, 680–686. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, A.; Shevkani, K.; Singh, N. Composition, Bioactive Compounds and Antioxidant Activity of Common Indian Fruits and Vegetables. J. Food Sci. Technol. 2016, 53, 4056–4066. [Google Scholar] [CrossRef]
- FAO. Crops and Livestock Products. Food and Agriculture Organization of the United States. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 19 November 2023).
- Pan, M.; Liu, K.; Yang, J.; Liu, S.; Wang, S.; Wang, S. Advances on Food-Derived Peptidic Antioxidants—A Review. Antioxidants 2020, 9, 799. [Google Scholar] [CrossRef]
- Corrêa, A.P.F.; Daroit, D.J.; Fontoura, R.; Meira, S.M.M.; Segalin, J.; Brandelli, A. Hydrolysates of Sheep Cheese Whey as a Source of Bioactive Peptides with Antioxidant and Angiotensin-Converting Enzyme Inhibitory Activities. Peptides 2014, 61, 48–55. [Google Scholar] [CrossRef]
- Okino-Delgado, C.H.; Fleuri, L.F. Orange and Mango By-Products: Agro-Industrial Waste as Source of Bioactive Compounds and Botanical versus Commercial Description—A Review. Food Rev. Int. 2015, 32, 1–14. [Google Scholar] [CrossRef]
- Kasapidou, E.; Sossidou, E.; Mitlianga, P. Fruit and Vegetable Co-Products as Functional Feed Ingredients in Farm Animal Nutrition for Improved Product Quality. Agriculture 2015, 5, 1020–1034. [Google Scholar] [CrossRef]
- Gołębiewska, E.; Kalinowska, M. Agricultural Residues as a Source of Bioactive Substances—Waste Management with the Idea of Circular Economy. Environ. Sci. Proc. 2021, 9, 2. [Google Scholar] [CrossRef]
- Oleszek, M.; Kowalska, I.; Bertuzzi, T.; Oleszek, W. Phytochemicals Derived from Agricultural Residues and Their Valuable Properties and Applications. Molecules 2023, 28, 342. [Google Scholar] [CrossRef] [PubMed]
- Casas-Godoy, L.; Campos-Valdez, A.R.; Alcázar-Valle, M.; Barrera-Martínez, I. Comparison of Extraction Techniques for the Recovery of Sugars, Antioxidant and Antimicrobial Compounds from Agro-Industrial Wastes. Sustainability 2022, 14, 5956. [Google Scholar] [CrossRef]
- Ngwasiri, P.N.; Ambindei, W.A.; Adanmengwi, V.A.; Ngwi, P.; Mah, A.T.; Ngangmou, N.T.; Fonmboh, D.J.; Ngwabie, N.M.; Ngassoum, M.B.; Richard, E. A Review Paper on Agro-Food Waste and Food by-Product Valorization into Value Added Products for Application in the Food Industry: Opportunities and Challenges for Cameroon Bioeconomy. Asian J. Biotechnol. Bioresour. Techonol. 2022, 8, 32–61. [Google Scholar] [CrossRef]
- Rodrigues, F.; Nunes, M.A.; Alves, R.C.; Oliveira, M.B.P. Applications of recovered bioactive compounds in cosmetics and other products. In Handbook of Coffee Processing By-Products; Academic Press: London, UK, 2017; pp. 195–220. [Google Scholar]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A. Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.-H.; Al-Sayed, H.M.A.; Yasin, N.M.N.; Afifi, E. Effect of Different Extraction Methods on Stablity of Anthocyanins Extracted from Red Onion Peels (Allium cepa) and Its Uses as Food Colorants. Egypt. J. Nutr. 2016, 47, 1–24. [Google Scholar] [CrossRef][Green Version]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Phenolic Compounds within Banana Peel and Their Potential Uses: A Review. J. Funct. Foods 2018, 40, 238–248. [Google Scholar] [CrossRef]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 1000196. [Google Scholar] [CrossRef]
- Tambun, R.; Alexander, V.; Ginting, Y. Performance Comparison of Maceration Method, Soxhletation Method, and Microwave-Assisted Extraction in Extracting Active Compounds from Soursop Leaves (Annona muricata): A Review. IOP Conf. Ser. 2021, 1122, 012095. [Google Scholar] [CrossRef]
- Rifna, E.J.; Misra, N.N.; Dwivedi, M. Recent Advances in Extraction Technologies for Recovery of Bioactive Compounds Derived from Fruit and Vegetable Waste Peels: A Review. Crit. Rev. Food Sci. Nutr. 2021, 63, 719–752. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, S.; Zuleta, M.; Osorio-Tobón, J.F. Techno-Economic Evaluation of the Extraction of Anthocyanins from Purple Yam (Dioscorea alata) Using Ultrasound-Assisted Extraction and Conventional Extraction Processes. Food Bioprod. Process. 2020, 122, 111–123. [Google Scholar] [CrossRef]
- Phong, W.N.; Gibberd, M.; Payne, A.D.; Dykes, G.A.; Coorey, R. Methods Used for Extraction of Plant Volatiles Have Potential to Preserve Truffle Aroma: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1677–1701. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.; Yu, K.; Mahlia, T.M.I. Sustainability of Direct Biodiesel Synthesis from Microalgae Biomass: A Critical Review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Easmin, S.; Sarker, Z.I.; Ferdosh, S.; Shamsudin, S.H.; Yunus, K.; Uddin, A.; Sarker, M.R.; Jahurul, M.H.A.; Hossain, S.; Khalil, H.A. Bioactive Compounds and Advanced Processing Technology: Phaleria macrocarpa (Sheff.) Boerl, a Review. J. Chem. Technol. Biotechnol. 2014, 90, 981–991. [Google Scholar] [CrossRef]
- Aziz, A.H.A.; Idrus, N.F.M.; Putra, N.R.; Awang, M.A.; Idham, Z.; Mamat, H.; Yunus, M.A.C. Solubility of Rosmarinic Acid in Supercritical Carbon Dioxide Extraction from Orthosiphon stamineus Leaves. ChemEngineering 2022, 6, 59. [Google Scholar] [CrossRef]
- Argun, M.E.; Argun, M.Ş.; Arslan, F.N.; Nas, B.; Ateş, H.; Tongur, S.; Çakmakcı, Ö. Recovery of Valuable Compounds from Orange Processing Wastes Using Supercritical Carbon Dioxide Extraction. J. Clean. Prod. 2022, 375, 134169. [Google Scholar] [CrossRef]
- King, J.W. Modern Supercritical Fluid Technology for Food Applications. Annu. Rev. Food Sci. Technol. 2014, 5, 215–238. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Khan, M.S.; Mohamed, A.A.A.; Ferdosh, S.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Vági, E.; Balázs, M.; Komóczi, A.; Mihalovits, M.; Székely, E. Fractionation of Phytocannabinoids from Industrial Hemp Residues with High-Pressure Technologies. J. Supercrit. Fluids 2020, 164, 104898. [Google Scholar] [CrossRef]
- Goyeneche, R.; Fanovich, A.; Rodrígues, C.R.; Nicolao, M.C.; Di Scala, K. Supercritical CO2 Extraction of Bioactive Compounds from Radish Leaves: Yield, Antioxidant Capacity and Cytotoxicity. J. Supercrit. Fluids 2018, 135, 78–83. [Google Scholar] [CrossRef]
- Uquiche, E.; Campos, C.J.R.; Marillán, C. Assessment of the Bioactive Capacity of Extracts from Leptocarpha Rivularis Stalks Using Ethanol-Modified Supercritical CO2. J. Supercrit. Fluids 2019, 147, 1–8. [Google Scholar] [CrossRef]
- Hassim, N.; Markom, M.; Rosli, M.I.; Harun, S. Scale-up Approach for Supercritical Fluid Extraction with Ethanol–Water Modified Carbon Dioxide on Phyllanthus Niruri for Safe Enriched Herbal Extracts. Sci. Rep. 2021, 11, 15818. [Google Scholar] [CrossRef] [PubMed]
- Nuapia, Y.; Al-Hamimi, S.; Chimuka, L.; Turner, C. Ultrahigh-Pressure Supercritical Fluid Extraction and Chromatography of Moringa oleifera and Moringa peregrina Seed Lipids. Anal. Bioanal. Chem. 2019, 411, 3685–3693. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Q.; Qiao, G.; Qiu, Z.; Zhuang, W.; Wen, X. Optimizing the Supercritical Carbon Dioxide Extraction of Sweet Cherry (Prunus avium L.) Leaves and UPLC-MS/MS Analysis. Anal. Methods 2020, 12, 3004–3013. [Google Scholar] [CrossRef] [PubMed]
- De Andrade Lima, M.; Andreou, R.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Carbon Dioxide Extraction of Phenolic Compounds from Potato (Solanum tuberosum) Peels. Appl. Sci. 2021, 11, 3410. [Google Scholar] [CrossRef]
- Trentini, C.P.; Cuco, R.P.; Cardozo-Filho, L.; Da Silva, C. Extraction of Macauba Kernel Oil Using Supercritical Carbon Dioxide and Compressed Propane. Can. J. Chem. Eng. 2018, 97, 785–792. [Google Scholar] [CrossRef]
- Ferrentino, G.; Giampiccolo, S.; Morozova, K.; Haman, N.; Spilimbergo, S.; Scampicchio, M. Supercritical Fluid Extraction of Oils from Apple Seeds: Process Optimization, Chemical Characterization and Comparison with a Conventional Solvent Extraction. Innov. Food Sci. Emerg. Technol. 2020, 64, 102428. [Google Scholar] [CrossRef]
- De Andrade Lima, M.; Charalampopoulos, D.; Chatzifragkou, A. Optimisation and Modelling of Supercritical CO2 Extraction Process of Carotenoids from Carrot Peels. J. Supercrit. Fluids 2018, 133, 94–102. [Google Scholar] [CrossRef]
- Rosas-Quina, Y.E.; Mejía-Nova, F.C. Supercritical Fluid Extraction with Cosolvent of Alkaloids from Lupinus mutabilis Sweet and Comparison with Conventional Method. J. Food Process Eng. 2021, 44, e13657. [Google Scholar] [CrossRef]
- Di Sanzo, G.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical Carbon Dioxide Extraction of Astaxanthin, Lutein, and Fatty Acids from Haematococcus Pluvialis Microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef] [PubMed]
- Leone, G.P.; Balducchi, R.; Mehariya, S.; Martino, M.; Larocca, V.; Di Sanzo, G.; Iovine, A.; Casella, P.; Marino, T.; Karatza, D.; et al. Selective Extraction of ω-3 Fatty Acids from Nannochloropsis sp. Using Supercritical CO2 Extraction. Molecules 2019, 24, 2406. [Google Scholar] [CrossRef] [PubMed]
- Jaime, L.; Vázquez, E.S.; Fornari, T.; Del Carmen López-Hazas, M.; García-Risco, M.R.; Santoyo, S.; Reglero, G. Extraction of Functional Ingredients from Spinach (Spinacia oleracea L.) Using Liquid Solvent and Supercritical CO2 Extraction. J. Sci. Food Agric. 2014, 95, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Porcedda, S.; Falconieri, D.; Fais, A.; Era, B.; Carta, G.; Rosa, A. Supercritical Extraction of Volatile and Fixed Oils from Petroselinum crispum L. Seeds: Chemical Composition and Biological Activity. Nat. Prod. Res. 2020, 36, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Topiař, M.; Sajfrtová, M.; Karban, J. Fractionation of Turmerones from Turmeric SFE Isolate Using Semi-Preparative Supercritical Chromatography Technique. J. Ind. Eng. Chem. 2019, 77, 223–229. [Google Scholar] [CrossRef]
- Pellicanò, T.M.; Sicari, V.; Loizzo, M.R.; Leporini, M.; Falco, T.; Poiana, M. Optimizing the Supercritical Fluid Extraction Process of Bioactive Compounds from Processed Tomato Skin By-Products. Food Sci. Technol. 2020, 40, 692–697. [Google Scholar] [CrossRef]
- Todd, R.I.; Baroutian, S. A Techno-Economic Comparison of Subcritical Water, Supercritical CO2 and Organic Solvent Extraction of Bioactives from Grape Marc. J. Clean. Prod. 2017, 158, 349–358. [Google Scholar] [CrossRef]
- Hassas-Roudsari, M.; Chang, P.R.; Pegg, R.B.; Tyler, R.T. Antioxidant Capacity of Bioactives Extracted from Canola Meal by Subcritical Water, Ethanolic and Hot Water Extraction. Food Chem. 2009, 114, 717–726. [Google Scholar] [CrossRef]
- Kim, W.J.; Kim, J.-H.; Veriansyah, B.; Kim, J.D.; Lee, Y.W.; Oh, S.; Tjandrawinata, R.R. Extraction of Bioactive Components from Centella asiatica Using Subcritical Water. J. Supercrit. Fluids 2009, 48, 211–216. [Google Scholar] [CrossRef]
- Zaibunnisa, A.H.; Saim, N.; Said, M.; Osman, H. An Experimental Design Approach for the Extraction of Volatile Compounds from Turmeric Leaves (Curcuma domestica) Using Pressurised Liquid Extraction (PLE). LWT 2009, 42, 233–238. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Kamal, S.M.M. Subcritical Water Extraction of Bioactive Compounds from Plants and Algae: Applications in Pharmaceutical and Food Ingredients. Food Eng. Rev. 2015, 8, 23–34. [Google Scholar] [CrossRef]
- Duba, K.S.; Casazza, A.A.; Mohamed, H.B.; Perego, P.; Fiori, L. Extraction of Polyphenols from Grape Skins and Defatted Grape Seeds Using Subcritical Water: Experiments and Modeling. Food Bioprod. Process. 2015, 94, 29–38. [Google Scholar] [CrossRef]
- Ong, E.S.; Cheong, J.S.H.; Goh, D. Pressurized Hot Water Extraction of Bioactive or Marker Compounds in Botanicals and Medicinal Plant Materials. J. Chromatogr. A 2006, 1112, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, D.B.; Barba, F.J.; Granato, D.; Galanakis, C.M.; Herceg, Z.; Dragović–Uzelac, V. Pressurized Hot Water Extraction (PHWE) for the Green Recovery of Bioactive Compounds and Steviol Glycosides from Stevia Rebaudiana Bertoni Leaves. Food Chem. 2018, 254, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Essien, S.; Young, B.R.; Baroutian, S. Subcritical Water Extraction for Selective Recovery of Phenolic Bioactives from Kānuka Leaves. J. Supercrit. Fluids 2020, 158, 104721. [Google Scholar] [CrossRef]
- Nuapia, Y.; Maraba, K.; Tutu, H.; Chimuka, L.; Cukrowska, E. In Situ Decarboxylation-Pressurized Hot Water Extraction for Selective Extraction of Cannabinoids from Cannabis sativa. Chemometric Approach. Molecules 2021, 26, 3343. [Google Scholar] [CrossRef] [PubMed]
- Nastić, N.; Švarc-Gajić, J.; Delerue-Matos, C.; Morais, S.; Barroso, M.F.; Moreira, M.M. Subcritical Water Extraction of Antioxidants from Mountain Germander (Teucrium montanum L.). J. Supercrit. Fluids 2018, 138, 200–206. [Google Scholar] [CrossRef]
- Essien, S.; Young, B.R.; Baroutian, S. The Antibacterial and Antiproliferative Ability of Kānuka, Kunzea Ericoides, Leaf Extracts Obtained by Subcritical Water Extraction. J. Chem. Technol. Biotechnol. 2020, 96, 1308–1315. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Baseggio, A.M.; Mayanga-Torres, P.C.; Maróstica, M.R.; Rostagno, M.A.; Forster-Carneiro, T. Subcritical Water Extraction of Flavanones from Defatted Orange Peel. J. Supercrit. Fluids 2018, 138, 7–16. [Google Scholar] [CrossRef]
- Kim, S.W.; Ko, M.J.; Chung, M.S. Extraction of the Flavonol Quercetin from Onion Waste by Combined Treatment with Intense Pulsed Light and Subcritical Water Extraction. J. Clean. Prod. 2019, 231, 1192–1199. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Correa, J.R.P. Glycerol as Alternative Co-Solvent for Water Extraction of Polyphenols from Carménère Pomace: Hot Pressurized Liquid Extraction and Computational Chemistry Calculations. Biomolecules 2020, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-N.; Saravana, P.S.; Nkurunziza, D.; Chun, B. Biofunctional Properties of Wild Cultivated and Cultivated Ginseng (Panax ginseng Meyer) Extracts Obtained Using Subcritical Water Extraction. Sep. Sci. Technol. 2020, 56, 1370–1382. [Google Scholar] [CrossRef]
- Wu, H.; Li, C.; Li, Z.; Liu, R.; Zhang, A.; Xiao, Z.; Ma, L.; Li, J.; Deng, S. Simultaneous Extraction of Oil and Tea Saponin from Camellia Oleifera Abel. Seeds under Subcritical Water Conditions. Fuel Process. Technol. 2018, 174, 88–94. [Google Scholar] [CrossRef]
- Gagić, T.; Knez, Ž.; ŠKerget, M. Hydrothermal Hydrolysis of Sweet Chestnut (Castanea sativa) Tannins. J. Serbian Chem. Soc. 2020, 85, 869–883. [Google Scholar] [CrossRef]
- Cha, J.; Kim, C.-T.; Kim, T.; Cho, Y.-J. Optimization of Subcritical Extraction Process for Cinnamon (Cinnamomum cassia Blume) Using Response Surface Methodology. Food Sci. Biotechnol. 2019, 28, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Dorosh, O.; Moreira, M.M.; Pinto, D.; Peixoto, A.F.; Freire, C.; Costa, P.; Rodrigues, F.; Delerue-Matos, C. Evaluation of the Extraction Temperature Influence on Polyphenolic Profiles of Vine-Canes (Vitis vinifera) Subcritical Water Extracts. Foods 2020, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.G.G.; Mazzutti, S.; Vitali, L.; Micke, G.A.; Ferreira, S.R.S. Recovery of Bioactive Phenolic Compounds from Papaya Seeds Agroindustrial Residue Using Subcritical Water Extraction. Biocatal. Agric. Biotechnol. 2019, 22, 101367. [Google Scholar] [CrossRef]
- Koyu, H.; Kazan, A.; Öztürk, T.K.; Yeşil-Çeliktaş, Ö.; Haznedaroğlu, M.Z. Optimizing Subcritical Water Extraction of Morus nigra L. Fruits for Maximization of Tyrosinase Inhibitory Activity. J. Supercrit. Fluids 2017, 127, 15–22. [Google Scholar] [CrossRef]
- Chikari, F.; Han, J.; Wang, Y.; Ao, W. Synergized Subcritical-Ultrasound-Assisted Aqueous Two-Phase Extraction, Purification, and Characterization of Lentinus Edodes Polysaccharides. Process Biochem. 2020, 95, 297–306. [Google Scholar] [CrossRef]
- Nkurunziza, D.; Pendleton, P.; Chun, B.S. Optimization and Kinetics Modeling of Okara Isoflavones Extraction Using Subcritical Water. Food Chem. 2019, 295, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, L.; Wu, W.; Zhang, G.; Zheng, Y.; Ma, C.; Li, W.; Yan, Y.; Xu, Z. Green Extraction of Active Ingredients from Finger Citron Using Subcritical Water and Assessment of Antioxidant Activity. Ind. Crops Prod. 2023, 200, 116821. [Google Scholar] [CrossRef]
- Zeković, Z.; Cvetanović, A.; Švarc-Gajić, J.; Gorjanović, S.; Sužnjević, D.Ž.; Mašković, P.; Savić, S.; Radojković, M.; Đurović, S. Chemical and Biological Screening of Stinging Nettle Leaves Extracts Obtained by Modern Extraction Techniques. Ind. Crops Prod. 2017, 108, 423–430. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Tomšik, A.; Pavlić, B.; Vladić, J.; Cindrić, M.; Jovanov, P.; Sakač, M.; Mandić, A.; Vidović, S. Subcritical Water Extraction of Wild Garlic (Allium ursinum L.) and Process Optimization by Response Surface Methodology. J. Supercrit. Fluids 2017, 128, 79–88. [Google Scholar] [CrossRef]
- Vladić, J.; Nastić, N.; Stanojkoivć, T.; Žižak, Ž.; Čakarević, J.; Popović, L.; Vidović, S. Subcritical Water for Recovery of Polyphenols from Comfrey Root and Biological Activities of Extracts. Acta Chim. Slov. 2019, 66, 473–783. [Google Scholar] [CrossRef] [PubMed]
- Vladić, J.; Janković, T.; Živković, J.; Tomić, M.; Zdunić, G.; Šavikin, K.; Vidović, S. Comparative Study of Subcritical Water and Microwave-Assisted Extraction Techniques Impact on the Phenolic Compounds and 5-Hydroxymethylfurfural Content in Pomegranate Peel. Plant Foods Hum. Nutr. 2020, 75, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Riaño, P.; Ramos, C.; Trigueros, E.; Beltrán, S.; Sanz, M.T. Study of Subcritical Water Scale-up from Laboratory to Pilot System for Brewer’s Spent Grain Valorization. Ind. Crops Prod. 2023, 191, 115927. [Google Scholar] [CrossRef]
- Khoza, B.S.; Dubery, I.A.; Byth-Illing, H.-A.; Steenkamp, P.A.; Chimuka, L.; Madala, N.E. Optimization of Pressurized Hot Water Extraction of Flavonoids from Momordica Foetida Using UHPLC-qTOF-MS and Multivariate Chemometric Approaches. Food Anal. Methods 2015, 9, 1480–1489. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Cerdà, V.; Clavijo, S.; Suárez, R.; Mašković, P.; Cvetanović, A.; Delerue-Matos, C.; Carvalho, A.P.; Novakov, V. Bioactive Compounds of Sweet and Sour Cherry Stems Obtained by Subcritical Water Extraction. J. Chem. Technol. Biotechnol. 2018, 93, 1627–1635. [Google Scholar] [CrossRef]
- Chémat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Périno, S.; Fabiano-Tixier, A.; Vian, M.A. Review of Green Food Processing Techniques. Preservation, Transformation, and Extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Li, X. Advances in Ultrasound Assisted Extraction of Bioactive Compounds from Cash Crops—A Review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K. Ultrasound: A Clean, Green Extraction Technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing by-Products: A Review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.F.; Giordani, D.; Lutckemier, R.; Gurak, P.D.; Cladera-Olivera, F.; Marczak, L.D.F. Extraction of Pectin from Passion Fruit Peel Assisted by Ultrasound. LWT 2016, 71, 110–115. [Google Scholar] [CrossRef]
- Sengar, A.S.; Rawson, A.; Muthiah, M.; Kalakandan, S.K. Comparison of Different Ultrasound Assisted Extraction Techniques for Pectin from Tomato Processing Waste. Ultrason. Sonochem. 2020, 61, 104812. [Google Scholar] [CrossRef] [PubMed]
- Maran, J.P.; Priya, B.; Al-Dhabi, N.A.; Karuppiah, P.; Moorthy, I.G.; Sivarajasekar, N. Ultrasound Assisted Citric Acid Mediated Pectin Extraction from Industrial Waste of Musa Balbisiana. Ultrason. Sonochem. 2017, 35, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Patience, N.A.; Schieppati, D.; Boffito, D.C. Continuous and Pulsed Ultrasound Pectin Extraction from Navel Orange Peels. Ultrason. Sonochem. 2021, 73, 105480. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Karuppiah, P.; Maran, J.P. Development and Validation of Ultrasound-Assisted Solid-Liquid Extraction of Phenolic Compounds from Waste Spent Coffee Grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef]
- Samaram, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M.; Bordbar, S.; Serjouie, A. Optimisation of Ultrasound-Assisted Extraction of Oil from Papaya Seed by Response Surface Methodology: Oil Recovery, Radical Scavenging Antioxidant Activity, and Oxidation Stability. Food Chem. 2015, 172, 7–17. [Google Scholar] [CrossRef]
- Moorthy, I.G.; Maran, J.P.; Surya, S.; Naganyashree, S.; Shivamathi, C.S. Response Surface Optimization of Ultrasound Assisted Extraction of Pectin from Pomegranate Peel. Int. J. Biol. Macromol. 2015, 72, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Guandalini, B.B.V.; Rodrigues, N.P.; Marczak, L.D.F. Sequential Extraction of Phenolics and Pectin from Mango Peel Assisted by Ultrasound. Food Res. Int. 2019, 119, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.S.; Khodaiyan, F.; Kazemi, M.; Khodaiyan, F. Optimization and Characterization of Pectin Extracted from Sour Orange Peel by Ultrasound Assisted Method. Int. J. Biol. Macromol. 2019, 125, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Khodaiyan, F. Eggplant Peel as a High Potential Source of High Methylated Pectin: Ultrasonic Extraction Optimization and Characterization. LWT 2019, 105, 182–189. [Google Scholar] [CrossRef]
- Murador, D.C.; Braga, A.R.C.; Martins, P.L.G.; Mercadante, A.Z.; De Rosso, V.V. Ionic Liquid Associated with Ultrasonic-Assisted Extraction: A New Approach to Obtain Carotenoids from Orange Peel. Food Res. Int. 2019, 126, 108653. [Google Scholar] [CrossRef]
- Thakker, M.R.; Parikh, J.; Desai, M.A. Ultrasound Assisted Hydrotropic Extraction: A Greener Approach for the Isolation of Geraniol from the Leaves of Cymbopogon Martinii. ACS Sustain. Chem. Eng. 2018, 6, 3215–3224. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Rai, D.K.; Sun, D.; Tiwari, B.K. Ultrasound-assisted Extraction (UAE) of Bioactive Compounds from Coffee Silverskin: Impact on Phenolic Content, Antioxidant Activity, and Morphological Characteristics. J. Food Process Eng. 2019, 42, e13191. [Google Scholar] [CrossRef]
- Da Rosa, G.S.; Vanga, S.K.; Garièpy, Y.; Raghavan, V. Comparison of Microwave, Ultrasonic and Conventional Techniques for Extraction of Bioactive Compounds from Olive Leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- García-Vaquero, M.; Ummat, V.; Tiwari, B.K.; Rajauria, G. Exploring Ultrasound, Microwave and Ultrasound–Microwave Assisted Extraction Technologies to Increase the Extraction of Bioactive Compounds and Antioxidants from Brown Macroalgae. Mar. Drugs 2020, 18, 172. [Google Scholar] [CrossRef]
- Nishad, J.; Saha, S.; Kaur, C. Enzyme- and Ultrasound-assisted Extractions of Polyphenols from Citrus sinensis (Cv. Malta) Peel: A Comparative Study. J. Food Process. Preserv. 2019, 43, e14046. [Google Scholar] [CrossRef]
- Petigny, L.; Périno-Issartier, S.; Wajsman, J.; Chémat, F. Batch and Continuous Ultrasound Assisted Extraction of Boldo Leaves (Peumus boldus Mol.). Int. J. Mol. Sci. 2013, 14, 5750–5764. [Google Scholar] [CrossRef] [PubMed]
- Del Hierro, J.N.; Herrera, T.; García-Risco, M.R.; Fornari, T.; Reglero, G.; Martín, D. Ultrasound-Assisted Extraction and Bioaccessibility of Saponins from Edible Seeds: Quinoa, Lentil, Fenugreek, Soybean and Lupin. Food Res. Int. 2018, 109, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, A.; Podgórski, R. Influence of Different Extraction Methods on the Quantification of Selected Flavonoids and Phenolic Acids from Tilia Cordata Inflorescence. Ind. Crops Prod. 2015, 76, 509–514. [Google Scholar] [CrossRef]
- Da Fonseca Machado, A.P.; Sumere, B.R.; Mekaru, C.; Martínez, J.; Bezerra, R.M.N.; Rostagno, M.A. Extraction of Polyphenols and Antioxidants from Pomegranate Peel Using Ultrasound: Influence of Temperature, Frequency and Operation Mode. Int. J. Food Sci. Technol. 2019, 54, 2792–2801. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of Acoustic Frequency and Power Density on the Aqueous Ultrasonic-Assisted Extraction of Grape Pomace (Vitis vinifera L.)—A Response Surface Approach. Ultrason. Sonochem. 2014, 21, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2019, 51, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Tram, N.N. Optimizing the Extraction Conditions of Phenolic Compounds from Fresh Tea Shoot. J. Food Nutr. Sci. 2015, 3, 106. [Google Scholar] [CrossRef][Green Version]
- Taşkın, B.; Özbek, Z. Optimisation of Microwave Effect on Bioactives Contents and Colour Attributes of Aqueous Green Tea Extracts by Central Composite Design. J. Food Meas. Charact. 2020, 14, 2240–2252. [Google Scholar] [CrossRef]
- Hu, B.; Xi, X.; Li, H.; Qin, Y.; Li, C.; Zhang, Z.; Liu, Y.; Zhang, Q.; Liu, A.; Liu, S.; et al. A Comparison of Extraction Yield, Quality and Thermal Properties from Sapindus mukorossi Seed Oil between Microwave Assisted Extraction and Soxhlet Extraction. Ind. Crops Prod. 2021, 161, 113185. [Google Scholar] [CrossRef]
- Elik, A.; Yanık, D.K.; Göğüş, F. Microwave-Assisted Extraction of Carotenoids from Carrot Juice Processing Waste Using Flaxseed Oil as a Solvent. LWT 2020, 123, 109100. [Google Scholar] [CrossRef]
- Fernández-Marín, R.; Fernandes, S.C.M.; Andrés, M.Á.; Labidi, J. Microwave-Assisted Extraction of Curcuma longa L. Oil: Optimization, Chemical Structure and Composition, Antioxidant Activity and Comparison with Conventional Soxhlet Extraction. Molecules 2021, 26, 1516. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Gao, X.; Chi, M.; Chen, K.; Zhang, Y.; Kong, W.; Zi-Ying, L.; Huang, S.; Kunming, Q. Microwave-Assisted Extraction Coupled with Mass Spectrometry for Determining Five Volatile Compounds from Soy Sauces. J. Anal. Methods Chem. 2021, 2021, 6625929. [Google Scholar] [CrossRef] [PubMed]
- Rahmawati, A.; Fachri, B.A.; Oktavia, S.; Abrori, F. Extraction Bioactive Compound of Pegagan (Centella asiatica L.) Using Solvent-Free Microwave-Assisted Extraction. IOP Conf. Ser. 2021, 1053, 012125. [Google Scholar] [CrossRef]
- Doulabi, M.; Golmakani, M.; Ansari, S. Evaluation and Optimization of Microwave-assisted Extraction of Bioactive Compounds from Eggplant Peel By-product. J. Food Process. Preserv. 2020, 44, e14853. [Google Scholar] [CrossRef]
- Jiménez-Amezcua, I.; González-Prada, A.; Díez-Municio, M.; Soria, A.C.; Ruiz-Matute, A.I.; Sanz, M.L. Simultaneous Microwave-Assisted Extraction of Bioactive Compounds from Aged Garlic. J. Chromatogr. A 2023, 1704, 464128. [Google Scholar] [CrossRef] [PubMed]
- Nisoa, M.; Plodkaew, A.; Sirisathitkul, C.; Wattanasit, K.; Somjit, B.; Pacdeepin, P.; Sirisathitkul, Y. Simulation and Experimentation on Parameters Influencing Microwave-Assisted Extraction of Bioactive Compounds from Kaempferia parviflora Rhizomes. Alex. Eng. J. 2023, 65, 357–366. [Google Scholar] [CrossRef]
- Mali, P.S.; Kumar, P. Simulation and Experimentation on Parameters Influencing Microwave-Assisted Extraction of Bioactive Compounds from Punica granatum Waste and Its Preliminary Analysis. Food Chem. Adv. 2023, 3, 100344. [Google Scholar] [CrossRef]
- Wong, J.C.J.; Nillian, E. Microwave-assisted Extraction of Bioactive Compounds from Sarawak liberica sp. Coffee Pulp: Statistical Optimization and Comparison with Conventional Methods. Food Sci. Nutr. 2023, 11, 5364–5378. [Google Scholar] [CrossRef]
- Bansod, S.P.; Parikh, J.; Sarangi, P.K. Pineapple Peel Waste Valorization for Extraction of Bio-Active Compounds and Protein: Microwave Assisted Method and Box Behnken Design Optimization. Environ. Res. 2023, 221, 115237. [Google Scholar] [CrossRef]
- Feng, T.; Zhang, M.; Sun, Q.; Mujumdar, A.S.; Yu, D. Extraction of Functional Extracts from Berries and Their High Quality Processing: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2022, 63, 7108–7125. [Google Scholar] [CrossRef]
- Belwal, T.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Microwave-Assisted Extraction (MAE) Conditions Using Polynomial Design for Improving Antioxidant Phytochemicals in Berberis asiatica Roxb. Ex DC. Leaves. Ind. Crops Prod. 2017, 95, 393–403. [Google Scholar] [CrossRef]
- Heleno, S.A.; Prieto, M.A.; Barros, L.; Rodrigues, A.E.; Barreiro, M.F.; Ferreira, I.C.F.R. Optimization of Microwave-Assisted Extraction of Ergosterol from Agaricus bisporus L. by-Products Using Response Surface Methodology. Food Bioprod. Process. 2016, 100, 25–35. [Google Scholar] [CrossRef]
- Ismail-Suhaimy, N.W.; Gani, S.S.A.; Zaidan, U.H.; Halmi, M.I.E.; Bawon, P. Optimizing Conditions for Microwave-Assisted Extraction of Polyphenolic Content and Antioxidant Activity of Barleria lupulina Lindl. Plants 2021, 10, 682. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Rizwan, D.; Bakshi, R.A.; Wani, S.M.; Masoodi, F.A. Extraction of Carotenoids from Agro-Industrial Waste. In Elsevier eBooks; Elsevier: Amsterdam, The Netherlands, 2023; pp. 157–178. [Google Scholar]
- Vînătoru, M.; Mason, T.J.; Călinescu, I. Ultrasonically Assisted Extraction (UAE) and Microwave Assisted Extraction (MAE) of Functional Compounds from Plant Materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2011, 5, 409–424. [Google Scholar] [CrossRef]
- Álvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A. Pressurized Liquid Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–398. [Google Scholar]
- Fraguela-Meissimilly, H.; Bastías-Montes, J.M.; Vergara, C.E.; Ortiz-Viedma, J.; Lemus-Mondaca, R.; Flores, M.; Toledo-Merma, P.R.; Alcázar-Alay, S.C.; Bedoya, M.G. New Trends in Supercritical Fluid Technology and Pressurized Liquids for the Extraction and Recovery of Bioactive Compounds from Agro-Industrial and Marine Food Waste. Molecules 2023, 28, 4421. [Google Scholar] [CrossRef]
- De Moraes, M.R.; Ryan, S.M.; Godoy, H.T.; Thomas, A.L.; Maia, J.G.S.; Richards, K.M.; Tran, K.; Smith, R.E. Phenolic Compounds and Metals in Some Edible Annonaceae Fruits. Biol. Trace Elem. Res. 2020, 197, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Khaleghipour, L.; Linares-Pastén, J.A.; Rashedi, H.; Siadat, S.O.R.; Jasilionis, A.; Al-Hamimi, S.; Sardari, R.R.R.; Karlsson, E.N. Extraction of Sugarcane Bagasse Arabinoxylan, Integrated with Enzymatic Production of Xylo-Oligosaccharides and Separation of Cellulose. Biotechnol. Biofuels 2021, 14, 153. [Google Scholar] [CrossRef]
- Tacchini, M.; Burlini, I.; Bernardi, T.; De Risi, C.; Massi, A.; Guerrini, A.; Sacchetti, G. Chemical Characterisation, Antioxidant and Antimicrobial Screening for the Revaluation of Wine Supply Chain by-Products Oriented to Circular Economy. Plant Biosyst. 2018, 153, 809–816. [Google Scholar] [CrossRef]
- Ortiz-Viedma, J.; Bastías-Montes, J.M.; Char, C.; Vega, C.; Quintriqueo, A.; Bedoya, M.G.; Flores, M.; Aguilera, J.M.; Miranda, J.M.; Barros-Velázquez, J. Sequential Biorefining of Bioactive Compounds of High Functional Value from Calafate Pomace (Berberis microphylla) Using Supercritical CO2 and Pressurized Liquids. Antioxidants 2023, 12, 323. [Google Scholar] [CrossRef]
- Constantin, O.E.; Milea, A.Ș.; Bolea, C.A.; Mihalcea, L.; Enachi, E.; Copolovici, D.M.; Copolovici, L.; Munteanu, F.; Bahrim, G.; Râpeanu, G. Onion (Allium cepa L.) Peel Extracts Characterization by Conventional and Modern Methods. Int. J. Food Eng. 2020, 17, 485–493. [Google Scholar] [CrossRef]
- Barrales, F.M.; Silveira, P.F.; De Paula Menezes Barbosa, P.; Ruviaro, A.R.; Paulino, B.N.; Pastore, G.M.; Macedo, G.A.; Martínez, J. Recovery of Phenolic Compounds from Citrus By-Products Using Pressurized Liquids—An Application to Orange Peel. Food Bioprod. Process. 2018, 112, 9–21. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Soares, I.D.; Cuevas, M.S.; Crevelin, E.J.; De Moraes, L.A.B.; De Melo, M.P.; De Oliveira, A.L.; Da Costa Rodrigues, C.E. Pressurized Liquid Extraction of Flavanols and Alkaloids from Cocoa Bean Shell Using Ethanol as Solvent. Food Res. Int. 2018, 114, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; Quintana, S.E.; Reglero, G.; Fornari, T.; García-Risco, M.R. Pressurized Liquid Extraction (PLE) as an Innovative Green Technology for the Effective Enrichment of Galician Algae Extracts with High Quality Fatty Acids and Antimicrobial and Antioxidant Properties. Mar. Drugs 2018, 16, 156. [Google Scholar] [CrossRef] [PubMed]
- Khanum, F. Therapeutic Foods: An Overview; CRC Press: London, UK, 2021; pp. 31–70. [Google Scholar]
- Picot-Allain, M.C.N.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus Green Extraction Techniques—A Comparative Perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Perez-Vazquez, A.; Carpena, M.; Barciela, P.; Cassani, L.; Simal-Gándara, J.; Prieto, M.A. Pressurized Liquid Extraction for the Recovery of Bioactive Compounds from Seaweeds for Food Industry Application: A Review. Antioxidants 2023, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.M.B.; Viganó, J.; Sanches, V.L.; Rostagno, M.A. Extraction of Potential Bioactive Compounds from Industrial Tahiti Lime (Citrus latifólia Tan.) by-Product Using Pressurized Liquids and Ultrasound-Assisted Extraction. Food Res. Int. 2022, 157, 111381. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Oliver, M.; Ponce-Alquicira, E. Environmentally Friendly Techniques and Their Comparison in the Extraction of Natural Antioxidants from Green Tea, Rosemary, Clove, and Oregano. Molecules 2021, 26, 1869. [Google Scholar] [CrossRef]
- Das, S.; Nadar, S.S.; Rathod, V.K. Integrated Strategies for Enzyme Assisted Extraction of Bioactive Molecules: A Review. Int. J. Biol. Macromol. 2021, 191, 899–917. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Enzyme-Assisted Extraction of Bioactive Non-Extractable Polyphenols from Sweet Cherry (Prunus avium L.) Pomace. Food Chem. 2021, 339, 128086. [Google Scholar] [CrossRef]
- Vilet, N.Z.; Gué, E.; Servent, A.; Delalonde, M.; Wisniewski, C. Filtration-Compression Step as Downstream Process for Flavonoids Extraction from Citrus Peels: Performances and Flavonoids Dispersion State in the Filtrate. Food Bioprod. Process. 2020, 120, 104–113. [Google Scholar] [CrossRef]
- Macedo, G.A.; Santana, Á.L.; Crawford, L.M.; Wang, S.C.; Dias, F.F.G.; De Moura Bell, J.M.L.N. Integrated Microwave- and Enzyme-Assisted Extraction of Phenolic Compounds from Olive Pomace. LWT 2021, 138, 110621. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Hu, D.; Xiao, K.; Wu, J. Efficient Extraction of Pectin from Sisal Waste by Combined Enzymatic and Ultrasonic Process. Food Hydrocoll. 2018, 79, 189–196. [Google Scholar] [CrossRef]
- Mir-Cerdà, A.; Núñez, Ó.; Granados, M.; Sentellas, S.; Saurina, J. An Overview of the Extraction and Characterization of Bioactive Phenolic Compounds from Agri-Food Waste within the Framework of Circular Bioeconomy. TrAC Trends Anal. Chem. 2023, 161, 116994. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Wang, T.; Sun, J.; Guo, T.; Zhang, L.; Yu, G.; Xia, X. Antidiabetic Activity of Armillaria Mellea Polysaccharides: Joint Ultrasonic and Enzyme Assisted Extraction. Ultrason. Sonochem. 2023, 95, 106370. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhang, Y.; Lin, S.; Jian, Y.; Miao, S.; Zheng, B. Ultrasonic–Microwave Synergistic Extraction (UMSE) and Molecular Weight Distribution of Polysaccharides from Fortunella margarita (Lour.) Swingle. Sep. Purif. Technol. 2015, 144, 97–106. [Google Scholar] [CrossRef]
- Sun, H.; Li, C.; Ni, Y.; Yao, L.; Jiang, H.; Ren, X.; Fu, Y.; Zhao, C. Ultrasonic/Microwave-Assisted Extraction of Polysaccharides from Camptotheca acuminata Fruits and Its Antitumor Activity. Carbohydr. Polym. 2019, 206, 557–564. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved Extraction of Vegetable Oils under High-Intensity Ultrasound and/or Microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef]
- Milman, B.L.; Журкoвич, И.К. The Chemical Space for Non-Target Analysis. TrAC Trends Anal. Chem. 2017, 97, 179–187. [Google Scholar] [CrossRef]
- Bagherian, H.; Ashtiani, F.Z.; Fouladitajar, A.; Mohtashamy, M. Comparisons between Conventional, Microwave- and Ultrasound-Assisted Methods for Extraction of Pectin from Grapefruit. Chem. Eng. Process.-Process Intensif. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Cheng, Z.; Song, H.; Yang, Y.; Liu, Y.; Liu, Z.; Hu, H.; Zhang, Y. Optimization of Microwave-Assisted Enzymatic Extraction of Polysaccharides from the Fruit of Schisandra chinensis Baill. Int. J. Biol. Macromol. 2015, 76, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Huang, Z.; Yu, Q.; Peng, G.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Microwave Assisted Extraction with Three Modifications on Structural and Functional Properties of Soluble Dietary Fibers from Grapefruit Peel. Food Hydrocoll. 2020, 101, 105549. [Google Scholar] [CrossRef]
- Mehmood, A.; Ishaq, M.; Zhao, L.; Yaqoob, S.; Safdar, B.; Nadeem, M.; Munir, M.; Wang, C. Impact of Ultrasound and Conventional Extraction Techniques on Bioactive Compounds and Biological Activities of Blue Butterfly Pea Flower (Clitoria ternatea L.). Ultrason. Sonochem. 2019, 51, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, S.-S.; Wang, M.; He, L. PEG-Based Ultrasound-Assisted Enzymatic Extraction of Polysaccharides from Ginkgo biloba Leaves. Int. J. Biol. Macromol. 2015, 80, 644–650. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Liu, Y.; Zhang, H. Optimisation of Ultrasound-Assisted Enzymatic Extraction of Arabinoxylan from Wheat Bran. Food Chem. 2014, 150, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, H.; Tian, J.; Wang, J.; Wang, Y.; Xing, L. Enzymolysis-Ultrasonic Assisted Extraction, Chemical Characteristics and Bioactivities of Polysaccharides from Corn Silk. Carbohydr. Polym. 2014, 101, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Fang, X.; Dan, D.; Diao, E.; Lu, Z. Ultrasonic-Assisted Enzymatic Extraction of a Water Soluble Polysaccharide from Dragon Fruit Peel and Its Antioxidant Activity. RSC Adv. 2018, 8, 42145–42152. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F.; Carvalho, P.I.N.; Rostagno, M.A.; Petenate, A.J.; Meireles, M.Â.A. Extraction of Curcuminoids from Deflavored Turmeric (Curcuma longa L.) Using Pressurized Liquids: Process Integration and Economic Evaluation. J. Supercrit. Fluids 2014, 95, 167–174. [Google Scholar] [CrossRef]
- Santos, P.D.; De Aguiar, A.C.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Supercritical Carbon Dioxide Extraction of Capsaicinoids from Malagueta Pepper (Capsicum frutescens L.) Assisted by Ultrasound. Ultrason. Sonochem. 2015, 22, 78–88. [Google Scholar] [CrossRef]
- Sumere, B.R.; Souza, M.C.; Santos, M.P.; Bezerra, R.M.N.; Da Cunha, D.T.; Martínez, J. Combining Pressurized Liquids with Ultrasound to Improve the Extraction of Phenolic Compounds from Pomegranate Peel (Punica granatum L.). Ultrason. Sonochem. 2018, 48, 151–162. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of Bioactive Compounds from Plant Materials Using Combination of Various Novel Methods: A Review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. Evaluating Green Solvents for Bio-Oil Extraction: Advancements, Challenges, and Future Perspectives. Energies 2023, 16, 5852. [Google Scholar] [CrossRef]
- Hashemi, B.; Zohrabi, P.; Dehdashtian, S. Application of Green Solvents as Sorbent Modifiers in Sorptive-Based Extraction Techniques for Extraction of Environmental Pollutants. TrAC Trends Anal. Chem. 2018, 109, 50–61. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESS) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.; Canals, A. Magnetic Deep Eutectic Solvents in Microextraction Techniques. TrAC Trends Anal. Chem. 2022, 146, 116500. [Google Scholar] [CrossRef]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A Green Extraction Process for Polyphenols from Elderberry (Sambucus nigra) Flowers Using Deep Eutectic Solvent and Ultrasound-Assisted Pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Cui, Q.; Wang, L.; Meng, Y.; Yu, L.; Li, Y.; Fu, Y. A Green and Integrated Strategy for Enhanced Phenolic Compounds Extraction from Mulberry (Morus alba L.) Leaves by Deep Eutectic Solvent. Microchem. J. 2020, 154, 104598. [Google Scholar] [CrossRef]
- Tobiszewski, M. Analytical Chemistry with Biosolvents. Anal. Bioanal. Chem. 2019, 411, 4359–4364. [Google Scholar] [CrossRef]
- Janicka, P.; Płotka-Wasylka, J.; Jatkowska, N.; Chabowska, A.; Fares, M.Y.; Andruch, V.; Kaykhaii, M.; Gębicki, J. Trends in the New Generation of Green Solvents in Extraction Processes. Curr. Opin. Green Sustain. Chem. 2022, 37, 100670. [Google Scholar] [CrossRef]
- Silva, S.S.; Justi, M.; Chagnoleau, J.-B.; Papaïconomou, N.; Fernández, X.; Santos, S.A.O.; Passos, H.; Ferreira, A.M.; Coutinho, J. Using Biobased Solvents for the Extraction of Phenolic Compounds from Kiwifruit Industry Waste. Sep. Purif. Technol. 2023, 304, 122344. [Google Scholar] [CrossRef]
- Wang, X.; Jia, W.; Lai, G.; Wang, L.; Del Mar Contreras, M.; Yang, D. Extraction for Profiling Free and Bound Phenolic Compounds in Tea Seed Oil by Deep Eutectic Solvents. J. Food Sci. 2020, 85, 1450–1461. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, X.; Liu, P.; Huang, J.; Wang, C.; Pan, M.; Kuang, Z. Enhanced Phenolic Compounds Extraction from Morus alba L. Leaves by Deep Eutectic Solvents Combined with Ultrasonic-Assisted Extraction. Ind. Crops Prod. 2018, 120, 147–154. [Google Scholar] [CrossRef]
- Pal, C.B.T.; Jadeja, G.C. Deep Eutectic Solvent-based Extraction of Polyphenolic Antioxidants from Onion (Allium cepa L.) Peel. J. Sci. Food Agric. 2018, 99, 1969–1979. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Cravotto, G.; Redovniković, I.R. Enabling Technologies for the Extraction of Grape-Pomace Anthocyanins Using Natural Deep Eutectic Solvents in up-to-Half-Litre Batches Extraction of Grape-Pomace Anthocyanins Using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef]
- Cañadas, R.; González-Miquel, M.; González, E.J.; Díaz, I.; Rodríguez, M. Evaluation of Bio-Based Solvents for Phenolic Acids Extraction from Aqueous Matrices. J. Mol. Liq. 2021, 338, 116930. [Google Scholar] [CrossRef]
- Hashemi, B.; Shiri, F.; Švec, F.; Nováková, L. Green Solvents and Approaches Recently Applied for Extraction of Natural Bioactive Compounds. TrAC Trends Anal. Chem. 2022, 157, 116732. [Google Scholar] [CrossRef]

| Feedstock | SFE Operating Conditions | Final Products and Classifications | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Type | Pressure (MPa) | Temperature (°C) | Time (min) | Co-Solvent | Target Bioactive NPs | Class | Yield (%, w/w) | |
| Moringa oleifera | Seeds | 80 | 57 | -- | -- | Oil (fatty acids) | Lipids | 39.6 | [52] |
| Moringa peregrine | 52.9 | ||||||||
| Prunus avium L. (sweet cherry) | Leaves | 28 | 40 | 120 | -- | Polyphenols Phenolic compounds Carotenoids | Phytochemicals | 2.47 | [53] |
| Solanum tuberosum | Potato peels | 35 | 80 | -- | 20% Methanol | Phenolics | Phytochemicals | 37 | [54] |
| Acrocomia aculeate | Fruits | 22 | 40 | 200 | -- | Oil | Lipids | 41.55 | [55] |
| 8 | 30 | 80 | Propane | 44.12 | |||||
| Apples | Seeds | 24 | 40 | 140 | -- | Oil | Lipids | 20.5 | [56] |
| Nantes carrots | Carrot peels | 35 | 59 | -- | 15.5% Ethanol | Carotenoid | Phytochemicals | 86.1 | [57] |
| Lupinus mutabilis Sweet | Seeds | 27 | 50 | -- | Ethanol | Alkaloids | Phytochemicals | ~4 | [58] |
| Cannabis sativa L. (Hemp) | Stalks and leaves | 30 | 45 | -- | 10% Ethanol | Phyto cannabinoids | Phytochemicals | 6.6 | [48] |
| L. rivularis | Stalks | 40 | -- | -- | 1% Ethanol | Phenolics | Phytochemicals | 1 | [50] |
| Haematococcus pluvialis | Microalgae | 40 | 65 | 120 | -- | Astaxanthin Lutein Oil (fatty acids) | Phytochemicals Lipids | 27.9 | [59] |
| Nannochloropsis sp. | Microalgae | 55 | 75 | -- | -- | Oil (fatty acids) | Lipids | 9.4 | [60] |
| Spinach | Herbs | 25 | 40 | 360 | -- | Carotenoids Phenolic compounds | Phytochemicals | 21.6 | [61] |
| Petroselinum crispum | Parsley and seeds | 9–30 | 40 | -- | -- | Phenylpropanoids Essential fatty acid | Phytochemicals Lipids | 96.4 0.4–2.6 | [62] |
| Curcuma longa L. | Turmeric | 30 | 40 | -- | -- | Turmerones | Phytochemicals | 3.1 | [63] |
| Tomato | Skin of ripe tomato fruits | 55 | 40 | 80 | -- | Carotenoids | Phytochemicals | 79 | [64] |
| Phyllanthus niruri | Herbal plant | 20 | 60 | 200 | 50/50 Ethanol–water | Oil (fatty acids) | Lipids | ~20 | [51] |
| Feedstock | SWE Operating Conditions | Final Products and Classifications | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Type | Pressure (MPa) | Temperature (°C) | Time (min) | Co-Solvent | Target Bioactive NPs | Class | Yield (%, w/w) | |
| Cannabis sativa | Seeds | 100 | 30 | -- | Cannabinoids | Phytochemicals | 96–100 | [74] | |
| Teucrium montanum L. | Aerial parts | 0.1 | 160 | -- | -- | Phenolics | Phytochemicals | 42.63 | [75] |
| Kānuka | Leaves | -- | 170 | 20 | -- | Phenolic Flavonoid | Phytochemicals | 3.81 | [73,76] |
| Defatted orange | Peel | -- | 150 | -- | -- | Flavanones | Phytochemicals | 21 | [77] |
| Onion waste | Skin | 10 | 145 | 15 | -- | Flavonol quercetin | Phytochemicals | ~1.8 | [78] |
| Carménère | Pomace | 10 | 150 | -- | 50% Glycerol | Polyphenols | Phytochemicals | -- | [79] |
| Panax ginseng Meyer | Root | 9 | 240 | -- | -- | Phenolic Sugar Protein | Phytochemicals Carbohydrates Protein | 12 | [80] |
| Camellia oleifera | Seeds | -- | 133.59 | 32 | -- | Oil (fatty acids) | Lipids | 94.07 | [81] |
| Castanea sativa (sweet) | Nuts | -- | 250 | 5 | -- | Oil (fatty acids) | Lipids | 29.55 | [82] |
| Cinnamomum Cassia Blume | Spice | 2.66 | 130 | 60 | -- | Flavoring compounds (coumarin, cinnamic acid, cinnamaldehyde, cinnamyl alcohol, etc.) | Phytochemicals | 10.95 | [83] |
| Vitis vinifera | Vine canes | -- | 250 | -- | -- | Phenolic content Flavonoids Phenolic acids Flavonols | Phytochemicals | 38.4 | [84] |
| Carica papaya L. | Seeds | 10 | 150 | 5 | -- | Phenolic acids Flavonoids Stilbene | Phytochemicals | 26.3 | [85] |
| Morus nigra L. | Fruit | 15 | 60 | 60 | Phenolic Flavonoids Anthocyanin | Phytochemicals | 3.89 | [86] | |
| Lentinus edodes | Fruit | -- | 30 | 30 | -- | Polysaccharides Xylose Mannose | Carbohydrates | 94–97 | [87] |
| Feedstock | UAE Operating Conditions | Final Products and Classifications | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Type | Temperature (°C) | Frequency (kHz) | Time (min) | Solvent | Target Bioactive NPs | Class | Yield (%, w/w) | |
| Pomegranate | Peel | 61.9 | 20 | 28.31 | Citric acid solution | Pectin | Phytochemicals | 23.87 | [108] |
| Mango | Peel | 85 | 20 | 10 | Nitric acid | Pectin | Phytochemicals | 8.6 | [109] |
| Orange | Peel | 20 | 10 | Citric acid solution | Pectin | Phytochemicals | 28.07 | [110] | |
| Eggplant | Peel | -- | -- | 30 | Acidified water | Pectin | Phytochemicals | 33.64 | [111] |
| Orange | Peel | -- | -- | -- | Ionic liquid | Carotenoids | Phytochemicals | 52–63 * | [112] |
| Cymbopogon martinii | Leaves | -- | 26 | 16 | Sodium cumene sulfonate | Geraniol | Phytochemicals | 1.9 | [113] |
| Coffee silverskin | Flake | -- | 20 | 10 | Deionized water or methanol–water (80/20, v/v), | Phenolic contents | Phytochemicals | 0.89 | [114] |
| Olive (Oleaeuropaea L.) | leaves | 27 | 20 | -- | Distilled water and ethanol | Phenolic compounds | Phytochemicals | 5.7–11.5 | [115] |
| A. nodosum | Macroalgae | -- | 20 | 2–5 | Polysaccharides Carbohydrates Phenolic compounds | Carbohydrates Phytochemicals | 16.54 | [116] | |
| Feedstock | MAE Operating Conditions | Final Products and Classifications | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Type | Temperature (°C) | Power (W) | Time (min) | Solvent | Target Bioactive NPs | Class | Yield (%, w/w) | |
| Green tea | Tea bags | -- | 350.65 | 5 | Distilled water | Phenolic content Flavonoid content Tannin content | Phytochemicals | 17.58 | [125] |
| Sapindus mukorossi | Seed | 72 | 460 | 42 | n-hexane | Oil | Lipids | 40.12 | [126] |
| Carrots | Juice waste | 165 | 9.39 | Flaxseed oil | Carotenoids | Phytochemicals | 77.48 | [127] | |
| Curcuma longa L. (turmeric) | Roots | 160 | 29.99 | Ethanol | Curcuma oil | Lipids | 10.32 | [128] | |
| Soy sauce | Sauce | 70 | -- | 30 | Cyclohexane Toluene Chlorobenzene Styrene | Volatile oils | Lipids | 80.86–105.71 | [129] |
| Centella asiatica L. (Tiger grass) | Leaves | -- | 450 | 60 | --- | Phenolic | Phytochemicals | 45,474 * | [130] |
| Solanum melongena L. | Eggplant peel | -- | 269.82 | 7.98 | Ethanol | Phenolics Flavonoid Anthocyanin | Phytochemicals | 3.27 | [131] |
| Aged garlic | Vegetable | 120 | 1200 | 60 | Water | Organosulfur Carbohydrates Phenolic | Phytochemicals Carbohydrates | 4.05 | [132] |
| Kaempferia parviflora rhizomes | Plant | 83 | -- | 2.5 | Methanol | Phenolics Flavonoid Gallic acid | Phytochemicals Lipids | 379.5+ | [133] |
| Punica granatum | Pomegranate peel | -- | 450 | 4 | Ethanol | Phenolic content | Phytochemicals | 47.3 | [134] |
| Coffea liberica | Coffee | -- | 700 | 3 | Methanol | Phenolics Flavonoid Carbohydrates | Phytochemicals Carbohydrates | 89.87 | [135] |
| Pineapple | Peel | 600 | 40 | Ethanol + distilled Water | Phenolics Flavonoid Tannin Protein | Phytochemicals Protein | -- | [136] | |
| Feedstock | PLE Operating Conditions | Final Products and Classifications | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Type | Temperature (°C) | Pressure (MPa) | Time (min) | Solvent | Target Bioactive NPs | Class | Yield (%, w/w) | |
| Annona muricata | Fruit | 100 | 10 | 3 | Methanol | Phenolic Fatty acids | Phytochemicals Lipids | -- | [146] |
| Saccharum officinarum | Sugarcane Bagasse | 120 | 10 | 60 | Water/NaOH | Arabinoxylan Xylan | Phytochemicals | 33.31 | [147] |
| Red wine grape | Bagasse | 120 | 9 | 90 | 50% Ethanol | Phenolic compounds | Phytochemicals | 50.6 | [148] |
| Spirulina | Microalga | 115 | 6.9 | 15 | Ethanol | Carotenoids Phenolic compounds Chlorophyll | Phytochemicals | 4.1 | [149] |
| Onion | Peel | 165 | 9–13 | 15 | Water | Flavonols | Phytochemicals | 21.2 | [150] |
| Pomegranate | Peel | 65 | 10 | Ethanol + water | Phenolic compounds | Phytochemicals | 1.93 | [151] | |
| Cocoa | Shell | 60–90 | 10.35 | 5–50 | Ethanol | Flavanols Alkaloids | Phytochemicals | 1.339 | [152] |
| Galician Algae | Alga | 160 | 10 | 10 | Ethanol + water | Fatty acids | Lipids | 57.19 | [153] |
| Feedstock | EAE Operating Conditions | Final Products and Classifications | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Type | Temperature (°C) | Time (min) | Enzymes (µg/g of Sample) | Solvent | Target Bioactive NPs | Class | Yield (%, w/w) | |
| Prunus avium L. | Fruit | 55 | 300 | 120 | Sodium phosphate buffer | Non-extractable polyphenols | Phytochemicals | -- | [160] |
| Grapes | Peel | 50 | 120 | 300 | -- | Flavonoids | Phytochemicals | 80 | [161] |
| Olive | Pomace | 60 | 120 | 2% | Ethanol + water | Phenolic compounds | Phytochemicals | 34.1 | [162] |
| Sisal | Waste | 50 | 1200 | 88 | Ethanol | Pectin | Phytochemicals | 62.8 | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usman, M.; Nakagawa, M.; Cheng, S. Emerging Trends in Green Extraction Techniques for Bioactive Natural Products. Processes 2023, 11, 3444. https://doi.org/10.3390/pr11123444
Usman M, Nakagawa M, Cheng S. Emerging Trends in Green Extraction Techniques for Bioactive Natural Products. Processes. 2023; 11(12):3444. https://doi.org/10.3390/pr11123444
Chicago/Turabian StyleUsman, Muhammad, Mayuko Nakagawa, and Shuo Cheng. 2023. "Emerging Trends in Green Extraction Techniques for Bioactive Natural Products" Processes 11, no. 12: 3444. https://doi.org/10.3390/pr11123444
APA StyleUsman, M., Nakagawa, M., & Cheng, S. (2023). Emerging Trends in Green Extraction Techniques for Bioactive Natural Products. Processes, 11(12), 3444. https://doi.org/10.3390/pr11123444