Effect of Slaked Lime on the Properties of Sodium Sulfate-Activated Alkali-Activated Slag Cement
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Experimental Works
2.2.1. Mix Proportions
2.2.2. Experimental Methods
3. Results and Analysis
3.1. Setting Time
3.2. Mechanical Strength
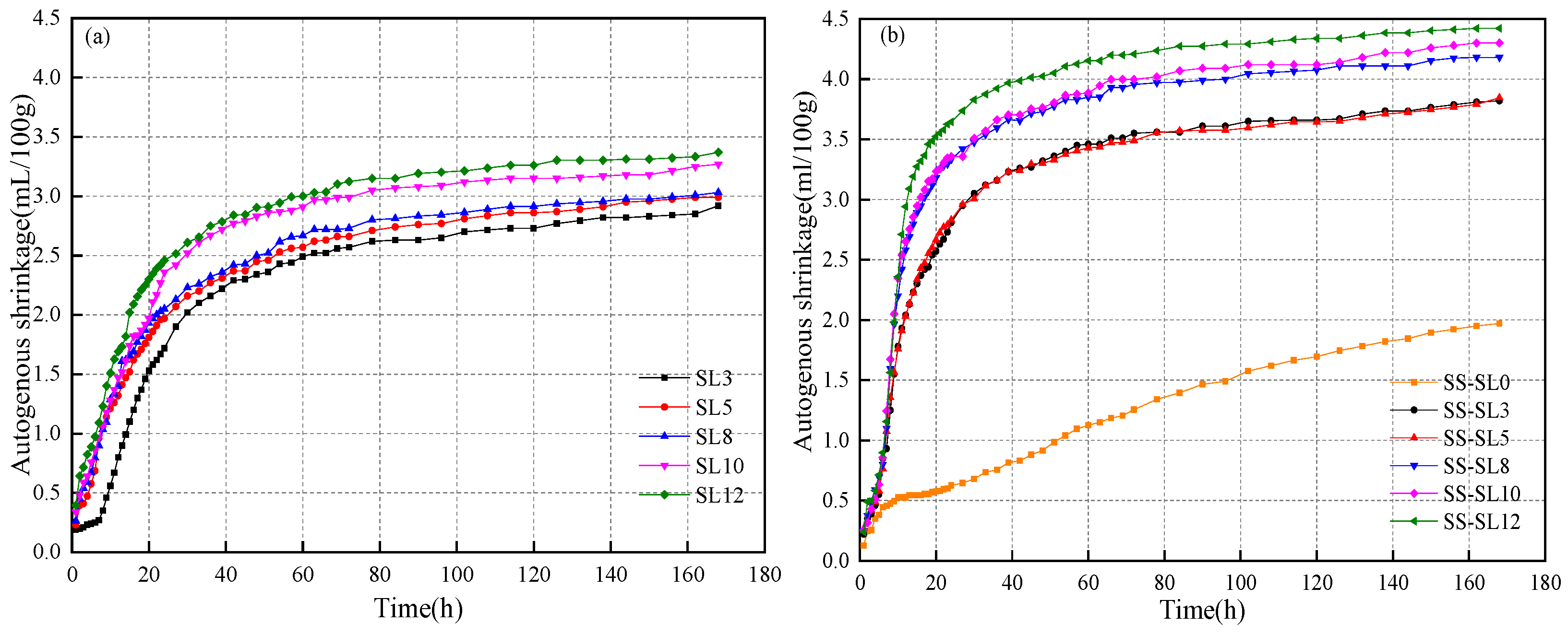
3.3. Autogenous Shrinkage
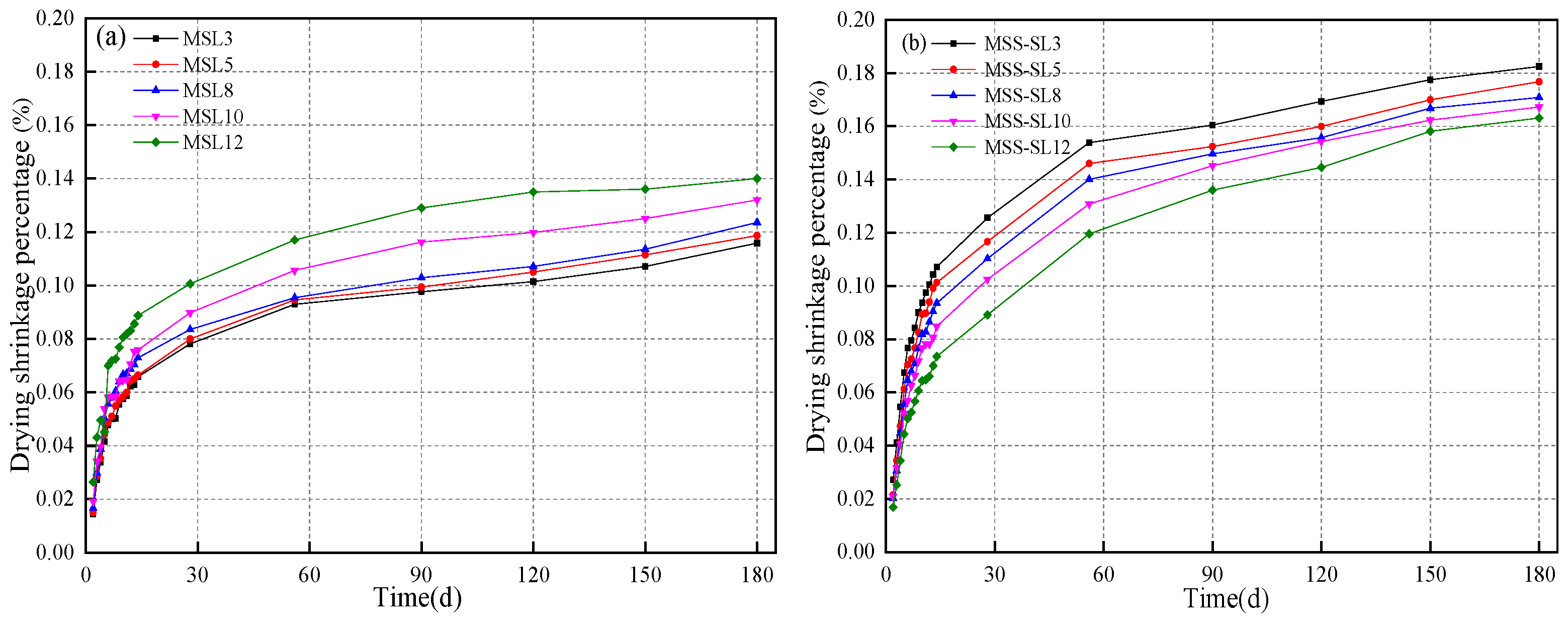
3.4. Drying Shrinkage
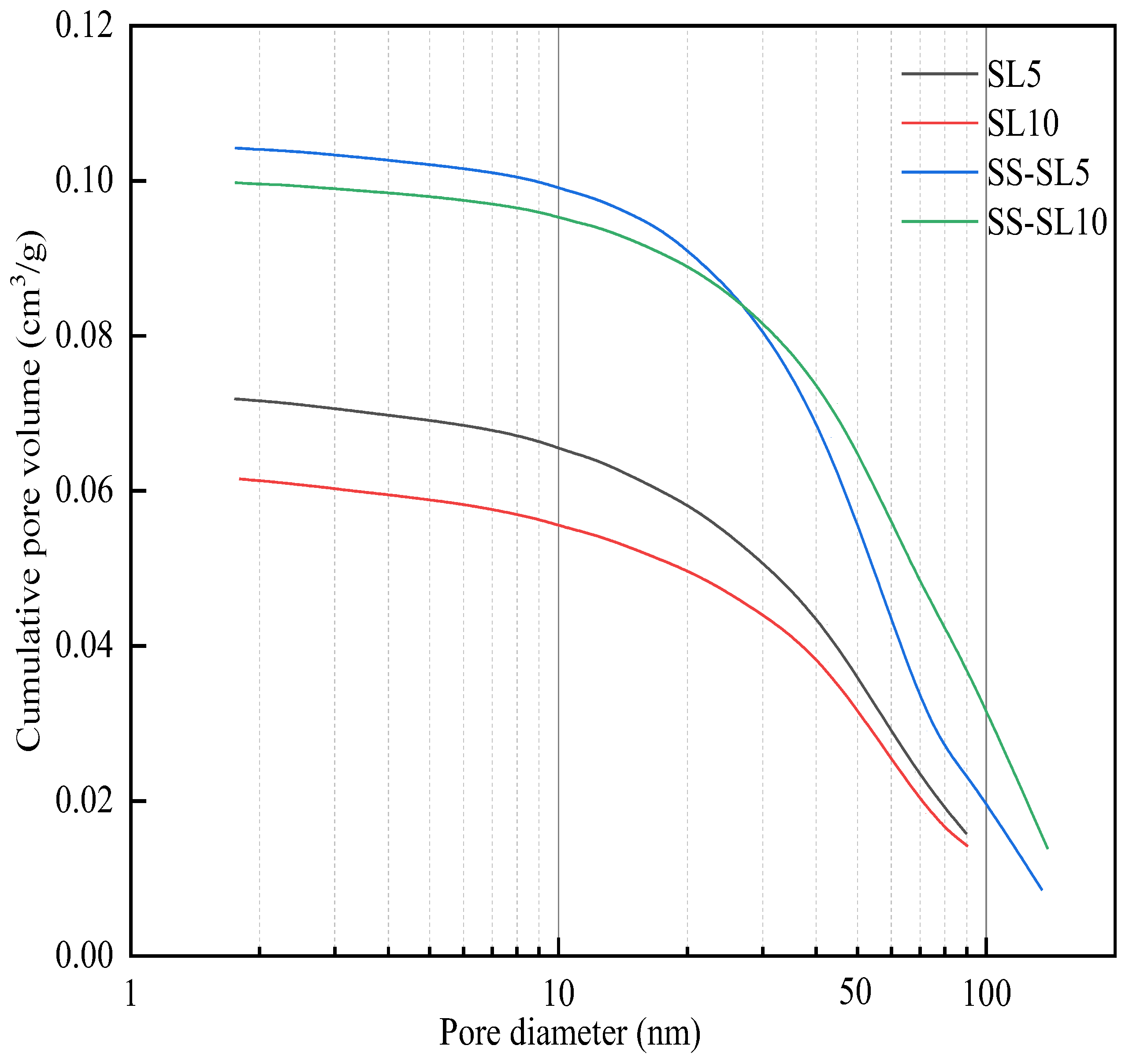
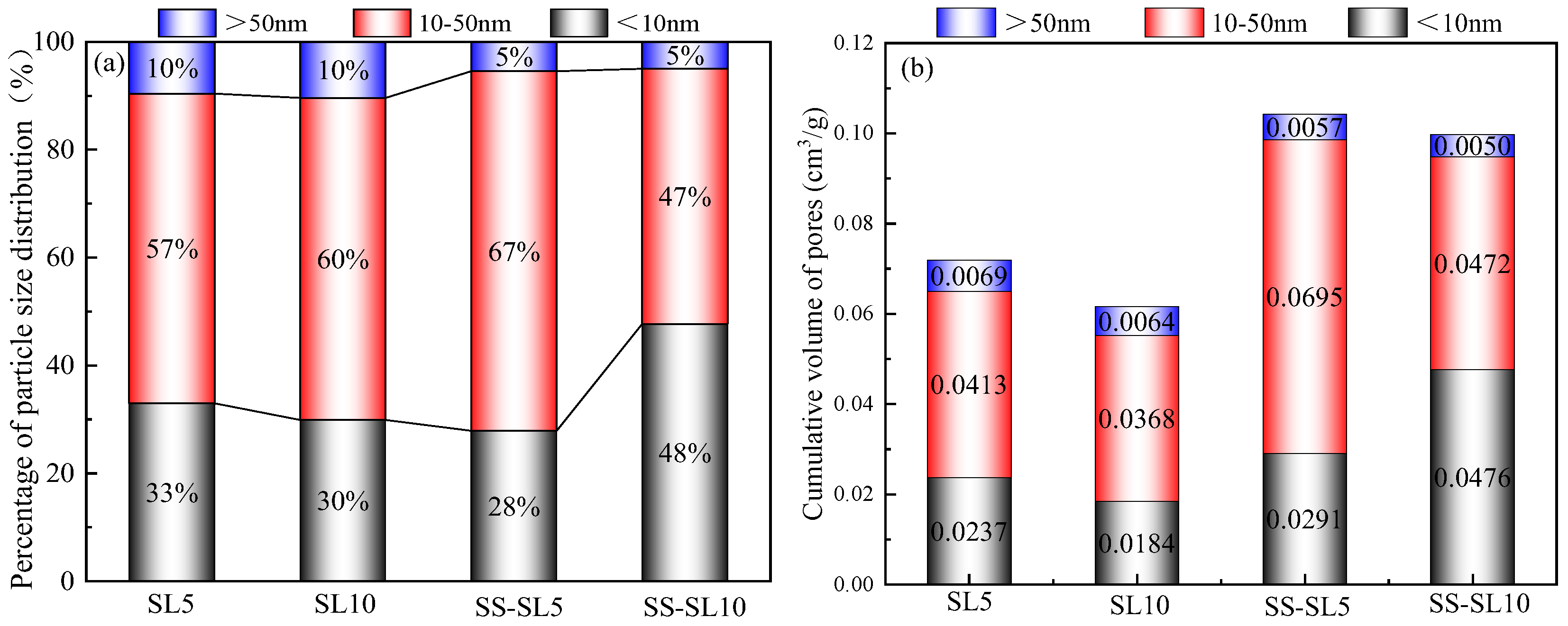
3.5. Effect of SL on the Microstructure of AASC
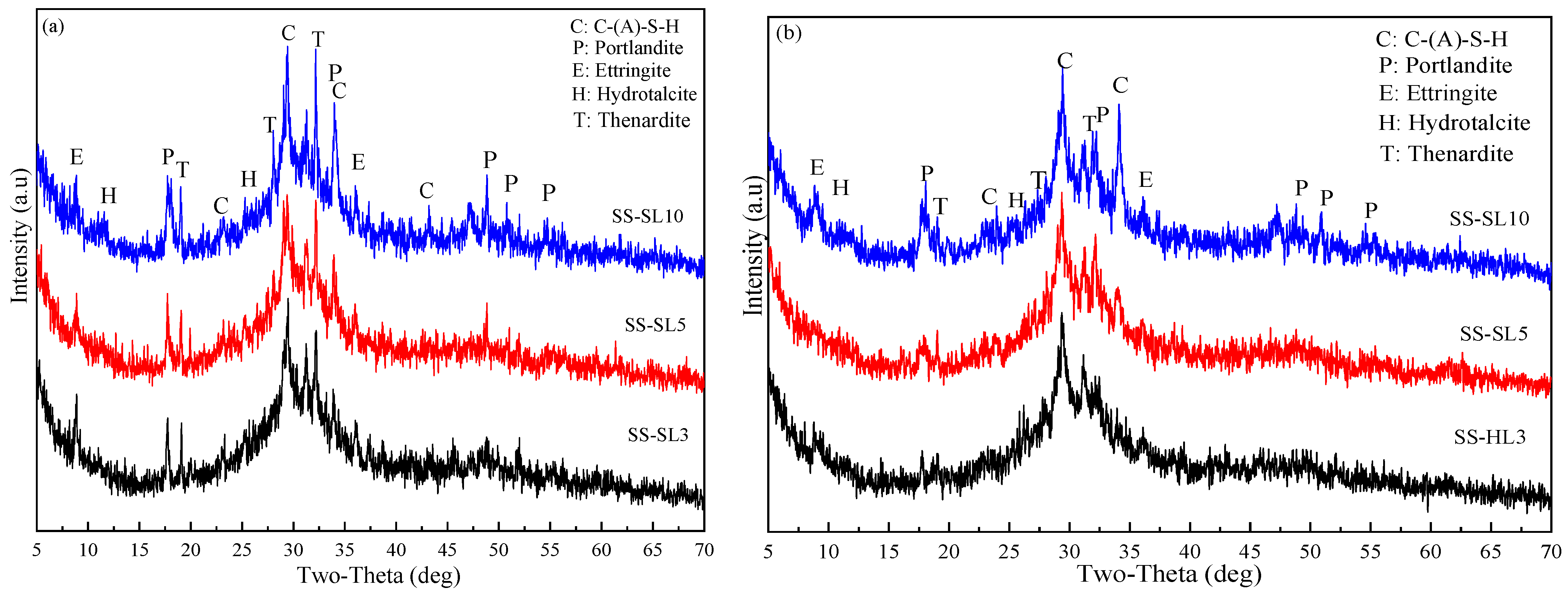
3.5.1. XRD of AASC
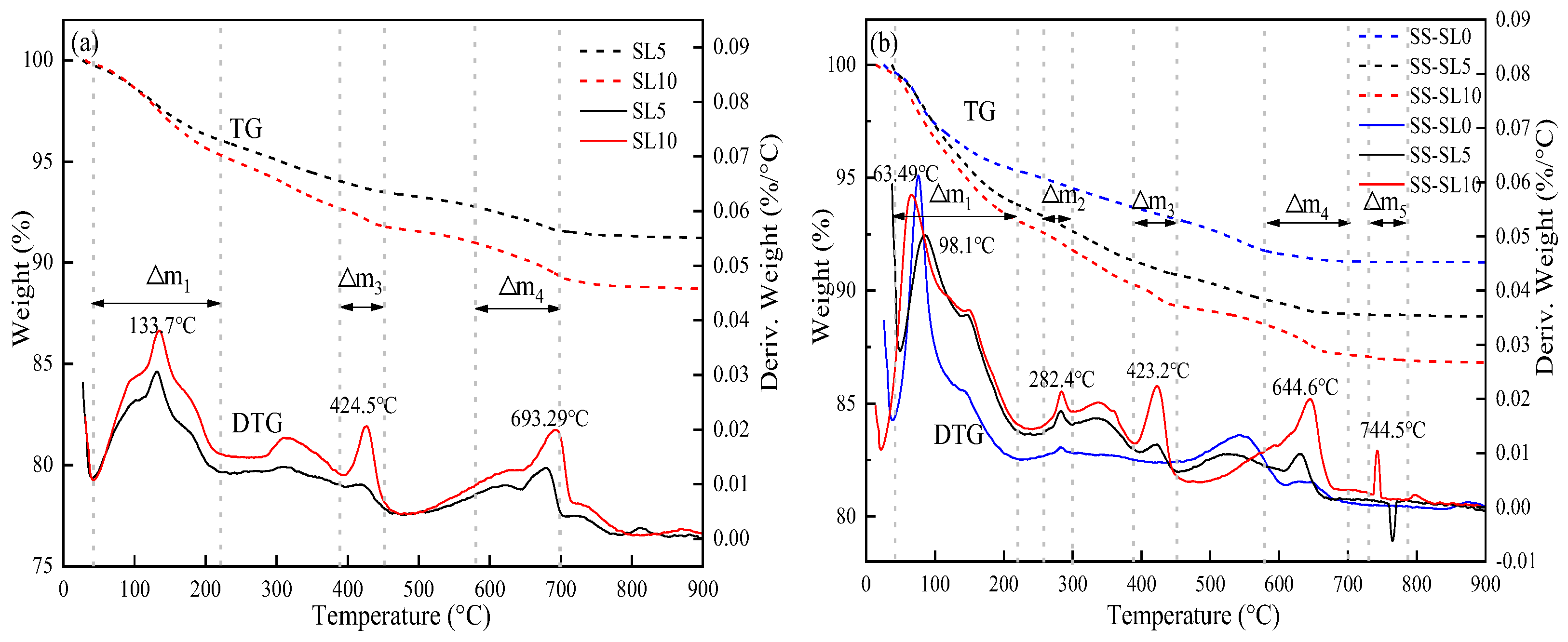
3.5.2. TG-DTG of AASC
3.5.3. SEM of AASC
4. Conclusions
- (1)
- When SL was mixed alone or SL was mixed with SS, when SL increased, the setting time decreased, although the decrease was small. The mechanical strength of the SL system was poor. In the SS-SL system, when SL increased, the setting time decreased, but the mechanical properties did not increase. When the content of SL was 3%, its mechanical properties were optimal.
- (2)
- The autogenous shrinkage of the SS-SL system was obviously greater and developed more rapidly than that with SL alone. When SL increased, the autogenous shrinkage of both systems increased.
- (3)
- When SL was added alone, the increase in SL led to an increase in drying shrinkage. When SL and SS were mixed, the increase in SL led to a reduction in drying shrinkage. The influence of SL on the dry shrinkage in these two systems was different. From the perspective of the hydration product, when SL was used alone, the increase in SL led to an increase in alkalinity, and the introduction of Ca2+ was conducive to the formation of more gels. When SS-SL was mixed, the crystalline phase in the product was mainly hydrotalcite and AFt, which can inhibit the development of dry shrinkage. From the analysis in Section 3.5, the increase in SL had little effect on the crystalline phase product. The greater amounts of AFt, hydrotalcite and portlandite in SS-SL10 compared with SS-SL5 all contributed to reducing drying shrinkage.
- (4)
- SL as an activator can effectively promote the hydration of AASC. However, the addition of SS promoted the formation of AFt with micro-expansion properties, which helped to reduce drying shrinkage.
- (5)
- SS could not be used as an activator alone, while SL could be used as an activator alone. SS could be combined with SL to prepare AASC. Although SL could be used as an activator alone, it was detrimental to the development of mechanical strength. The AASC prepared by mixing SS and SL had better mechanical strength: when the content of SL was 3%, the highest mechanical strength was obtained, but the drying shrinkage was the largest. Therefore, when SS and SL were used as compound activators, their effects on mechanical strength and drying shrinkage should be considered comprehensively.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Malhotra, V.M. Introduction: Sustainable Development and Concrete Technology. Concr. Int. 2002, 24, 22. [Google Scholar]
- Gartner, E. Industrially interesting approaches to “low-CO2” cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Haha, M.B.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics. hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Aydın, S.; Baradan, B. Effect of activator type and content on properties of alkali-activated slag mortars. Compos. Part B—Eng. 2014, 57, 166–172. [Google Scholar] [CrossRef]
- He, J.; Bai, W.; Zheng, W.; He, J.; Sang, G. Influence of hydrated lime on mechanical and shrinkage properties of alkali-activated slag cement. Constr. Build. Mater. 2021, 289, 123201. [Google Scholar] [CrossRef]
- Yuan, B.; Yu, Q.L.; Brouwers, H.J.H. Reaction kinetics. Reaction products and compressive strength of ternary activators activated slag designed by Taguchi method. Mater. Des. 2015, 86, 878–886. [Google Scholar] [CrossRef]
- He, J.; Zheng, W.; Bai, W.; Hu, T.; He, J.; Song, X. Effect of reactive MgO on hydration and properties of alkali-activated slag pastes with different activators. Constr. Build. Mater. 2021, 271, 121608. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.-B. Alkali activation of Australian slag cements. Cem. Concr. Res. 1999, 29, 113–120. [Google Scholar] [CrossRef]
- Fang, S.; Lam, E.S.S.; Li, B.; Wu, B. Effect of alkali contents, moduli and curing time on engineering properties of alkali activated slag. Constr. Build. Mater. 2020, 249, 118799. [Google Scholar] [CrossRef]
- Song, J.-K.; Yang, K.-H.; Kim, G.-W.K.B. Properties of sodium alkali-activated ground granulated blast-furnace slag (GGBS) mortar. J. Archit. Inst. Korea 2010, 26, 61–68. [Google Scholar]
- Neto, A.A.M.; Cincotto, M.A.; Repette, W. Drying and autogenous shrinkage of pastes and mortars with activated slag cement. Cem. Concr. Res. 2008, 38, 565–574. [Google Scholar] [CrossRef]
- Chen, W.; Li, B.; Guo, M.-Z.; Wang, J.; Chen, Y.-T. Impact of heat curing regime on the compressive strength and drying shrinkage of alkali-activated slag mortar. Dev. Built. Environ. 2023, 14, 100123. [Google Scholar] [CrossRef]
- Atiş, C.D.; Bilim, C.; Çelik, Ö.; Karahan, O. Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Effect of pore size distribution on drying shrinkage of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1401–1406. [Google Scholar] [CrossRef]
- Taghvayi, H.; Behfarnia, K.; Khalili, M. The Effect of Alkali Concentration and Sodium Silicate Modulus on the Properties of Alkali-Activated Slag Concrete. J. Adv. Concr. Technol. 2018, 16, 293–305. [Google Scholar] [CrossRef]
- Shi, C.; Roy, D.; Krivenko, P. Alkali-Activated Cements and Concretes; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Habert, G.; de Lacaillerie, J.B.D.; Roussel, N. An environmental evaluation of geopolymer based concrete production: Reviewing current research trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Bernal, S.A.; Nicolas, R.S.; Deventer, J.S.J.V.; Provis, J.L. Alkali-activated slag cements produced with a blended sodium carbonate/sodium silicate activator. Adv. Cem. Res. 2016, 28, 262–273. [Google Scholar] [CrossRef]
- Yuan, B.; Yu, Q.L.; Brouwers, H.J.H. Evaluation of slag characteristics on the reaction kinetics and mechanical properties of Na2CO3 activated slag. Constr. Build. Mater. 2017, 131, 334–346. [Google Scholar] [CrossRef]
- Rashad, A.M.; Bai, Y.; Basheer, P.A.M.; Milestone, N.B.; Collier, N.C. Hydration and properties of sodium sulfate activated slag. Cem. Concr. Compos. 2013, 37, 20–29. [Google Scholar] [CrossRef]
- Tan, H.; Deng, X.; He, X.; Zhang, J.; Zhang, X.; Su, Y.; Yang, J. Compressive strength and hydration process of wet-grinded granulated blast-furnace slag activated by sodium sulfate and sodium carbonate. Cem. Concr. Compos. 2019, 97, 387–398. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, B.; Wang, L.; Long, G.; Xie, Y. Clean and low-alkalinity one-part geopolymeric cement: Effects of sodium sulfate on microstructure and properties. J. Clean. Prod. 2020, 252, 119279. [Google Scholar] [CrossRef]
- Nguyen, H.-A.; Chang, T.-P.; Thymotie, A. Enhancement of early engineering characteristics of modified slag cement paste with alkali silicate and sulfate. Constr. Build. Mater. 2020, 230, 117013. [Google Scholar] [CrossRef]
- Yang, K.-H.; Cho, A.-R.; Song, J.-K.; Nam, S.-H. Hydration products and strength development of calcium hydroxide-based alkali-activated slag mortars. Constr. Build. Mater. 2012, 29, 410–419. [Google Scholar] [CrossRef]
- Yang, K.-H.; Sim, J.-I.; Nam, S.-H. Enhancement of reactivity of calcium hydroxide-activated slag mortars by the addition of barium hydroxide. Constr. Build. Mater. 2010, 24, 241–251. [Google Scholar] [CrossRef]
- Neto, A.A.M.; Cincotto, M.A.; Repette, W. Mechanical properties, drying and autogenous shrinkage of blast furnace slag activated with hydrated lime and gypsum. Cem. Concr. Compos. 2010, 32, 312–318. [Google Scholar] [CrossRef]
- GB/T 1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. China Standards Press: Beijing, China, 2011.
- GB/T 17671-2021; Method of Testing Cements-Determination of Strength. China Standards Press: Beijing, China, 2021.
- JGJ/T 70-2009; Standard for Test Method of Performance on Building Mortar. China Architecture and Building Press: Beijing, China, 2009.
- Lee, N.K.; Lee, H.K. Reactivity and reaction products of alkali-activated. fly ash/slag paste. Constr. Build. Mater. 2015, 81, 303–312. [Google Scholar] [CrossRef]
- Puligilla, S.; Monda, P. Role of slag in microstructural development and hardening of fly ash-slag geopolymer. Cem. Concr. Res. 2013, 43, 70–80. [Google Scholar] [CrossRef]
- Harutyunyan, V.S.; Kirchheim, A.P.; Monteiro, P.J.M.; Aivazyan, A.P.; Fischer, P. Investigation of early growth of calcium hydroxide crystals in cement solution by soft X-ray transmission microscopy. J. Mater. Sci. 2009, 44, 962–969. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, M.; Shen, X. Growth Habit of Portlandite Crystal in Cement Paste. J. Chin. Ceram. Soc. 2016, 44, 232–238. [Google Scholar] [CrossRef]
- Zhu, X.-H.; Tang, D.-S.; Yang, K.; Zhang, Z.-L.; Li, Q.; Pan, Q.; Yang, C.-H. Effect of Ca(OH)2 on shrinkage characteristics and microstructures of alkali-activated slag concrete. Constr. Build. Mater. 2018, 175, 467–482. [Google Scholar] [CrossRef]
- Yang, K.-H.; Cho, A.-R.; Song, J.-K. Effect of water–binder ratio on the mechanical properties of calcium hydroxide-based alkali-activated slag concrete. Constr. Build. Mater. 2012, 29, 504–511. [Google Scholar] [CrossRef]
- Bernal, S.A.; Krivenko, P.V.; Provis, J.L.; Puertas, F.; Rickard, W.D.A.; Shi, C.; van Riessen, A. Other Potential Applications for Alkali-Activated Materials. In Alkali Activated Materials: State-of-the-Art Report, RILEM TC 224-AAM; Provis, J.L., van Deventer, J.S.J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 339–379. [Google Scholar]
- Li, Z.; Lu, T.; Liang, X.; Dong, H.; Ye, G. Mechanisms of autogenous shrinkage of alkali-activated slag and fly ash pastes. Cem. Concr. Res. 2020, 135, 10610. [Google Scholar] [CrossRef]
- Li, Z.; Liang, X.; Chen, Y.; Ye, G. Effect of metakaolin on the autogenous shrinkage of alkali-activated slag-fly ash paste. Constr. Build. Mater. 2021, 278, 122397. [Google Scholar] [CrossRef]
- Liao, Y.; Gui, Y.; Ke, F.; Liao, G. Effect of Temperature on Compressive Strength, Electrical Resistivity and Chemical Shrinkage of Calcium Sulphoaluminate. Cem. J. Build. Mater. 2018, 21, 478–489. [Google Scholar]
- Fang, G.; Bahrami, H.; Zhang, M. Mechanisms of autogenous shrinkage of alkali-activated fly ash-slag pastes cured at ambient temperature within 24 h. Constr. Build. Mater. 2018, 171, 377–387. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Shrinkage mitigation strategies in alkali-activated slag. Cem. Concr. Res. 2017, 101, 131–143. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Shrinkage mechanisms of alkali-activated slag. Cem. Concr. Res. 2016, 88, 126–135. [Google Scholar] [CrossRef]
- Hojati, M.; Radlińska, A. Shrinkage and strength development of alkali-activated fly ash-slag binary cements. Constr. Build. Mater. 2017, 150, 808–816. [Google Scholar] [CrossRef]
- Lee, N.K.; Jang, J.G.; Lee, H.K. Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem. Concr. Compos. 2014, 53, 239–248. [Google Scholar] [CrossRef]
- Kumarappa, D.B.; Peethamparan, S.; Ngami, M. Autogenous shrinkage of alkali activated slag mortars: Basic mechanisms and mitigation methods. Cem. Concr. Res. 2018, 109, 1–9. [Google Scholar] [CrossRef]
- Aydın, S.; Baradan, B. Mechanical and microstructural properties of heat cured alkali-activated slag mortars. Mater. Des. 2012, 35, 374–383. [Google Scholar] [CrossRef]
- Shi, C.; He, F.; Fernández-Jiménez, A.; Krivenko, V.P.; Palomo, A. Classification and Characteristics of Alkali-Activated Cements. J. Chin. Ceram. Soc. 2012, 40, 69–75. [Google Scholar] [CrossRef]
- Alcamand, H.A.; Borges, P.H.R.; Silva, F.A.; Trindade, A.C.C. The effect of matrix composition and calcium content on the sulfate durability of metakaolin and metakaolin/slag alkali-activated mortars. Ceram. Int. 2018, 44, 5037–5044. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J. Workability and mechanical properties of alkali activated slag concrete. Cem. Concr. Res. 1999, 29, 455–458. [Google Scholar] [CrossRef]
- Jambunathan, N.; Sanjayan, J.G.; Pan, Z.; Li, G.; Liu, Y.; Korayem, A.H.; Duan, W.H.; Collins, F. The role of alumina on performance of alkali-activated slag paste exposed to 50 °C. Cem. Concr. Res. 2013, 54, 143–150. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Strength and shrinkage properties of alkali-activated slag concrete containing porous coarse aggregate. Cem. Concr. Res. 1999, 29, 607–610. [Google Scholar] [CrossRef]
- Jin, F.; Al-Tabbaa, A. Strength and drying shrinkage of slag paste activated by sodium carbonate and reactive MgO. Constr. Build. Mater. 2015, 81, 58–65. [Google Scholar] [CrossRef]
- Frost, R.L.; Martens, W.; Ding, Z.; Kloprogge, J.T. DSC and high-resolution TG of synthesized hydrotalcites of Mg and Zn. J. Therm. Anal. Calorim. 2003, 71, 429–438. [Google Scholar] [CrossRef]
- Lachowski, E.E.; Diamond, S. Investigation of Composition and Morphology of Individual Particles of Portland Cement Pastes: 1, C-S-H Gel and Ca (OH)2 Particles. Cem. Concr. Res. 1983, 13, 177–185. [Google Scholar] [CrossRef]
- Qaidi, S.; Najm, H.M.; Abed, S.M.; Ahmed, H.U.; Al Dughaishi, H.; Al Lawati, J.; Sabri, M.M.; Alkhatib, F.; Milad, A. Fly Ash-Based Geopolymer Composites: A Review of the Compressive Strength and Microstructure Analysis. Materials 2022, 15, 7098. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.U.M.; Mohammed, A.S.; Qaidi, S.M.A.; Faraj, R.H.; Sor, N.H.; Mohammed, A.A. Compressive strength of geopolymer concrete composites: A systematic comprehensive review, analysis and modeling. Eur. J. Environ. Civ. Eng. 2022, 27, 1383–1428. [Google Scholar] [CrossRef]
- Wang, S.-D.; Pu, X.-C.; Scrivener, K.L.; Pratt, P.L. Alkali-activated slag cement and concrete: A review of properties and problems. Adv. Cem. Res. 1995, 7, 93–102. [Google Scholar] [CrossRef]
- Song, S.; Sohn, D.; Jennings, H.M.; Mason, T.O. Hydration of alkali-activated ground granulated blast furnace slag. J. Mater. Sci. 2000, 35, 249–257. [Google Scholar] [CrossRef]
- Puertas, F.; Martnez-Ramrez, S.; Alonso, S.; Vázquez, T. Alkali-activated fly ash/slag cements: Strength behaviour and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Wang, S.D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]

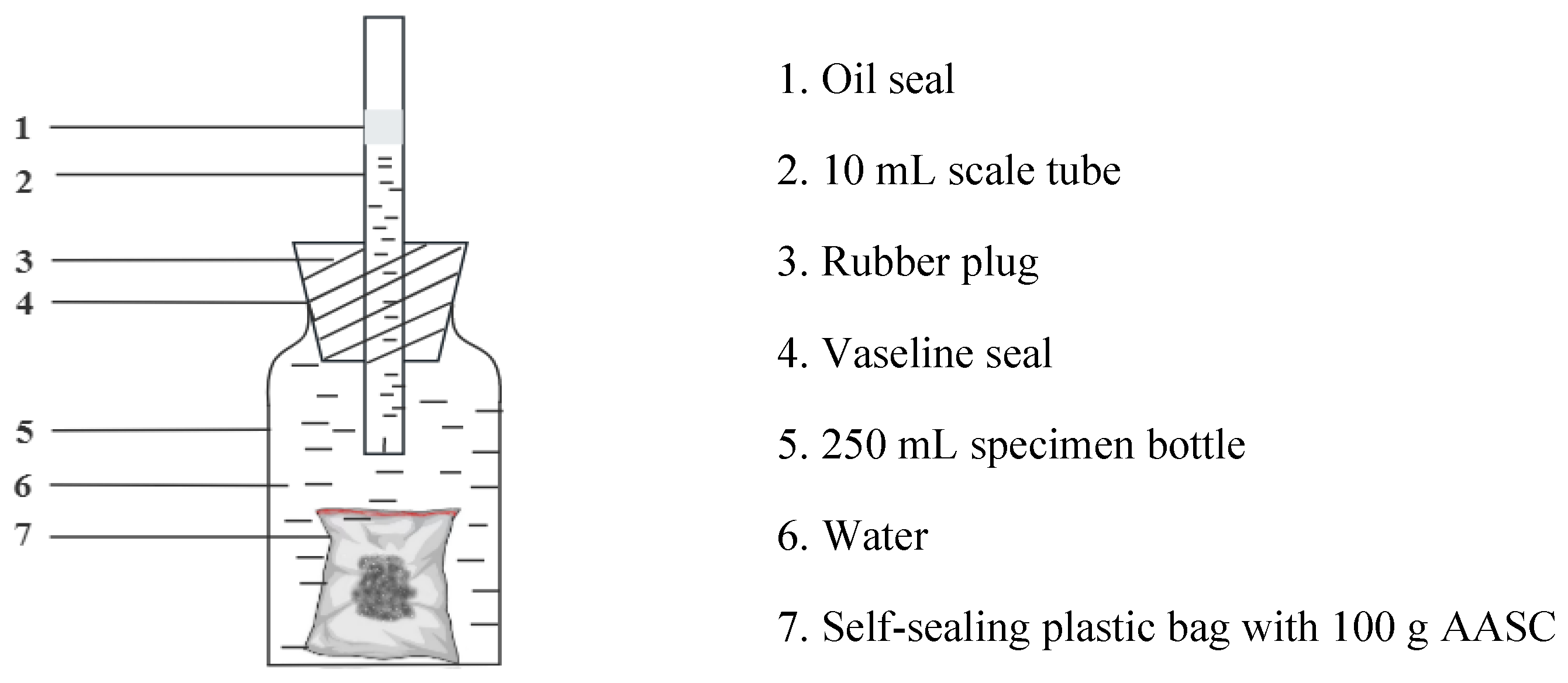





| SiO2 (%) | Al2O3 (%) | CaO (%) | Fe2O3 (%) | MgO (%) | SO3(%) | K2O (%) | Na2O (%) | Ignition Loss (%) |
|---|---|---|---|---|---|---|---|---|
| 31.68 | 13.96 | 41.36 | 0.66 | 8.42 | 1.27 | 0.47 | 0.50 | 1.01 |
| Number | GBFS | SL | SS | Water |
|---|---|---|---|---|
| SL3 | 97 | 3 | -- | 35 |
| SL5 | 95 | 5 | -- | 35 |
| SL8 | 92 | 8 | -- | 35 |
| SL10 | 90 | 10 | -- | 35 |
| SL12 | 88 | 12 | -- | 35 |
| SS-SL0 | 100 | 0 | 9.16 | 35 |
| SS-SL3 | 97 | 3 | 9.16 | 35 |
| SS-SL5 | 95 | 5 | 9.16 | 35 |
| SS-SL8 | 92 | 8 | 9.16 | 35 |
| SS-SL10 | 90 | 10 | 9.16 | 35 |
| SS-SL12 | 88 | 12 | 9.16 | 35 |
| Number | GBFS | SL | SS | Water | Sand |
|---|---|---|---|---|---|
| MSL3 | 97 | 3 | -- | 40 | 200 |
| MSL5 | 95 | 5 | -- | 40 | 200 |
| MSL8 | 92 | 8 | -- | 40 | 200 |
| MSL10 | 90 | 10 | -- | 40 | 200 |
| MSL12 | 88 | 12 | -- | 40 | 200 |
| MSS-SL0 | 100 | 0 | 9.16 | 40 | 200 |
| MSS-SL3 | 97 | 3 | 9.16 | 40 | 200 |
| MSS-SL5 | 95 | 5 | 9.16 | 40 | 200 |
| MSS-SL8 | 92 | 8 | 9.16 | 40 | 200 |
| MSS-SL10 | 90 | 10 | 9.16 | 40 | 200 |
| MSS-SL12 | 88 | 12 | 9.16 | 40 | 200 |
| Number | Initial Setting Time (h:min) | Final Setting Time (h:min) |
|---|---|---|
| SL3 | 4:49 | 6:08 |
| SL5 | 4:42 | 5:53 |
| SL8 | 4:38 | 5:41 |
| SL10 | 4:28 | 5:27 |
| SL12 | 4:18 | 5:17 |
| SS-SL0 | -- | -- |
| SS-SL3 | 4:42 | 5:12 |
| SS-SL5 | 4:23 | 5:02 |
| SS-SL8 | 4:12 | 4:57 |
| SS-SL10 | 4:03 | 4:43 |
| SS-SL12 | 3:48 | 4:38 |
| Specimen | Temperature (°C) | ||||
|---|---|---|---|---|---|
| 40–220 °C | 260–300 °C | 390–450 °C | 580–700 °C | 730–780 °C | |
| Δm1 | Δm2 | Δm3 | Δm4 | Δm5 | |
| SL5 | 3.69 | - | 0.54 | 1.19 | 0.11 |
| SL10 | 4.51 | - | 0.86 | 1.67 | 0.21 |
| SS-SL0 | 4.37 | 0.41 | 0.50 | 0.46 | 0.01 |
| SS-SL5 | 5.99 | 0.63 | 0.60 | 0.63 | 0.01 |
| SS-SL10 | 6.83 | 0.73 | 0.91 | 1.42 | 0.15 |
| Serial Number | C | O | Na | Mg | Al | Si | S | K | Ca | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.16 | 45.19 | 32.58 | -- | -- | -- | 20.01 | -- | 0.56 | 0.14 |
| 2 | 8.20 | 53.66 | 1.55 | 0.48 | 5.31 | 2.55 | 7.98 | 0.12 | 20.14 | -- |
| 3 | 4.78 | 40.91 | 4.97 | -- | 4.86 | 3.51 | 2.85 | 0.23 | 37.89 | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Li, M.; Bai, W.; Sang, G.; Song, X. Effect of Slaked Lime on the Properties of Sodium Sulfate-Activated Alkali-Activated Slag Cement. Processes 2024, 12, 184. https://doi.org/10.3390/pr12010184
He J, Li M, Bai W, Sang G, Song X. Effect of Slaked Lime on the Properties of Sodium Sulfate-Activated Alkali-Activated Slag Cement. Processes. 2024; 12(1):184. https://doi.org/10.3390/pr12010184
Chicago/Turabian StyleHe, Juan, Mengmeng Li, Wenbin Bai, Guochen Sang, and Xuefeng Song. 2024. "Effect of Slaked Lime on the Properties of Sodium Sulfate-Activated Alkali-Activated Slag Cement" Processes 12, no. 1: 184. https://doi.org/10.3390/pr12010184






