Hydrothermal Carbonization of Waste Biomass: A Review of Hydrochar Preparation and Environmental Application
Abstract
1. Introduction
2. Influence of Process Parameters
2.1. Temperature
2.2. Pressure
2.3. Residence Time
2.4. Catalyst
3. Environmental Applications of Hydrochar
3.1. Solid Fuel
3.2. Adsorbent of Pollutants from Aqueous Solutions
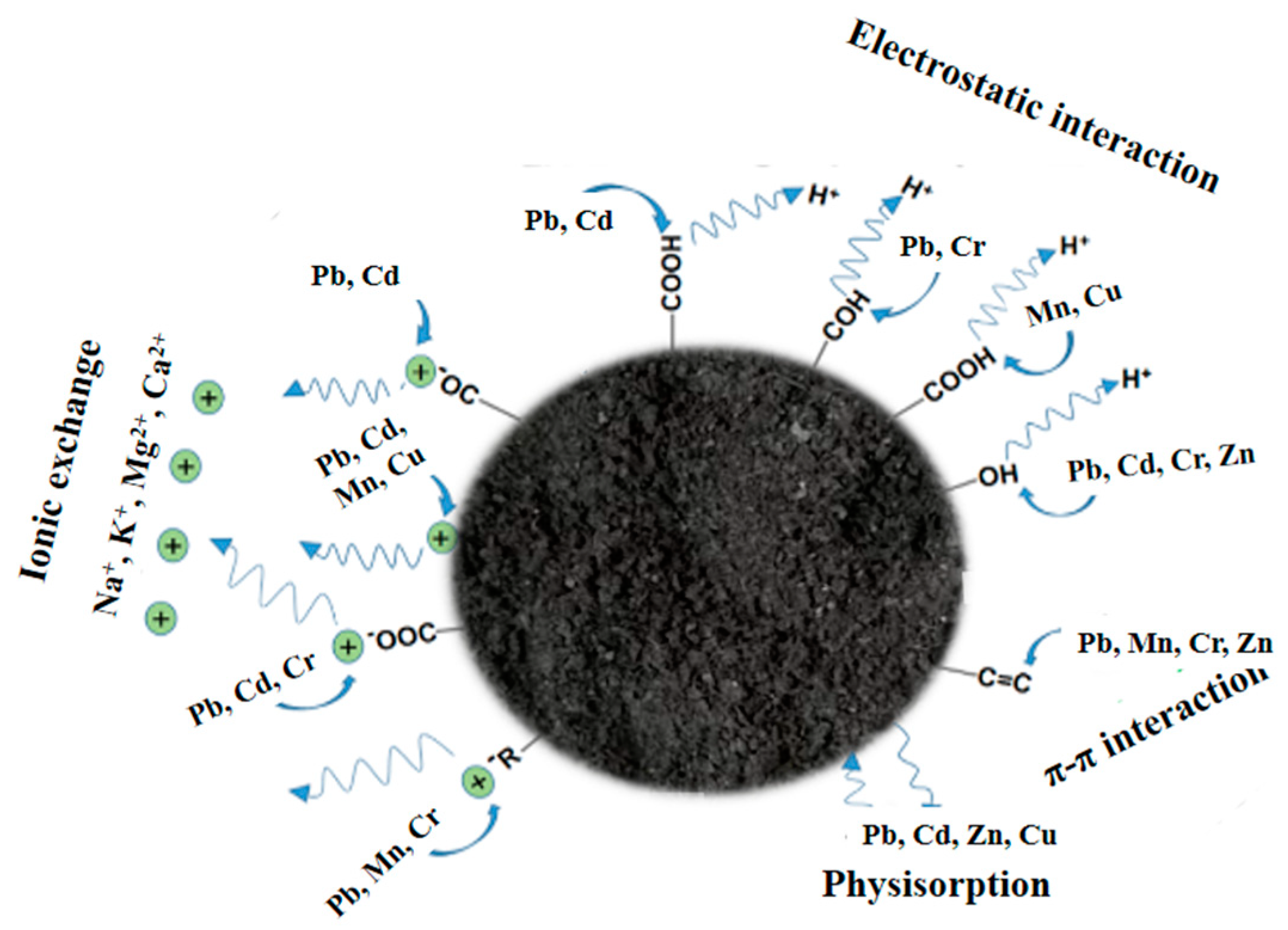
3.2.1. Heavy Metals
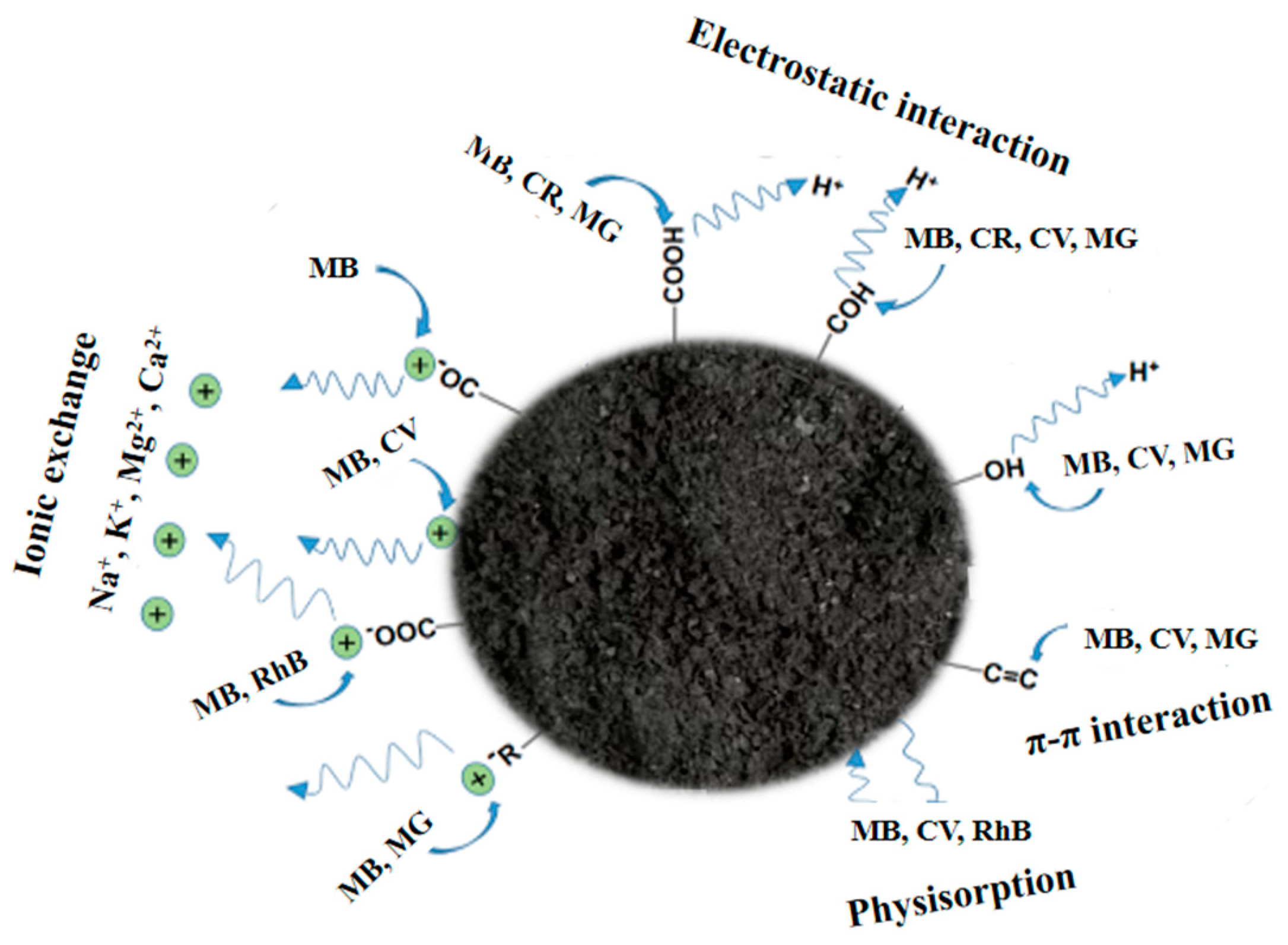
3.2.2. Dyes
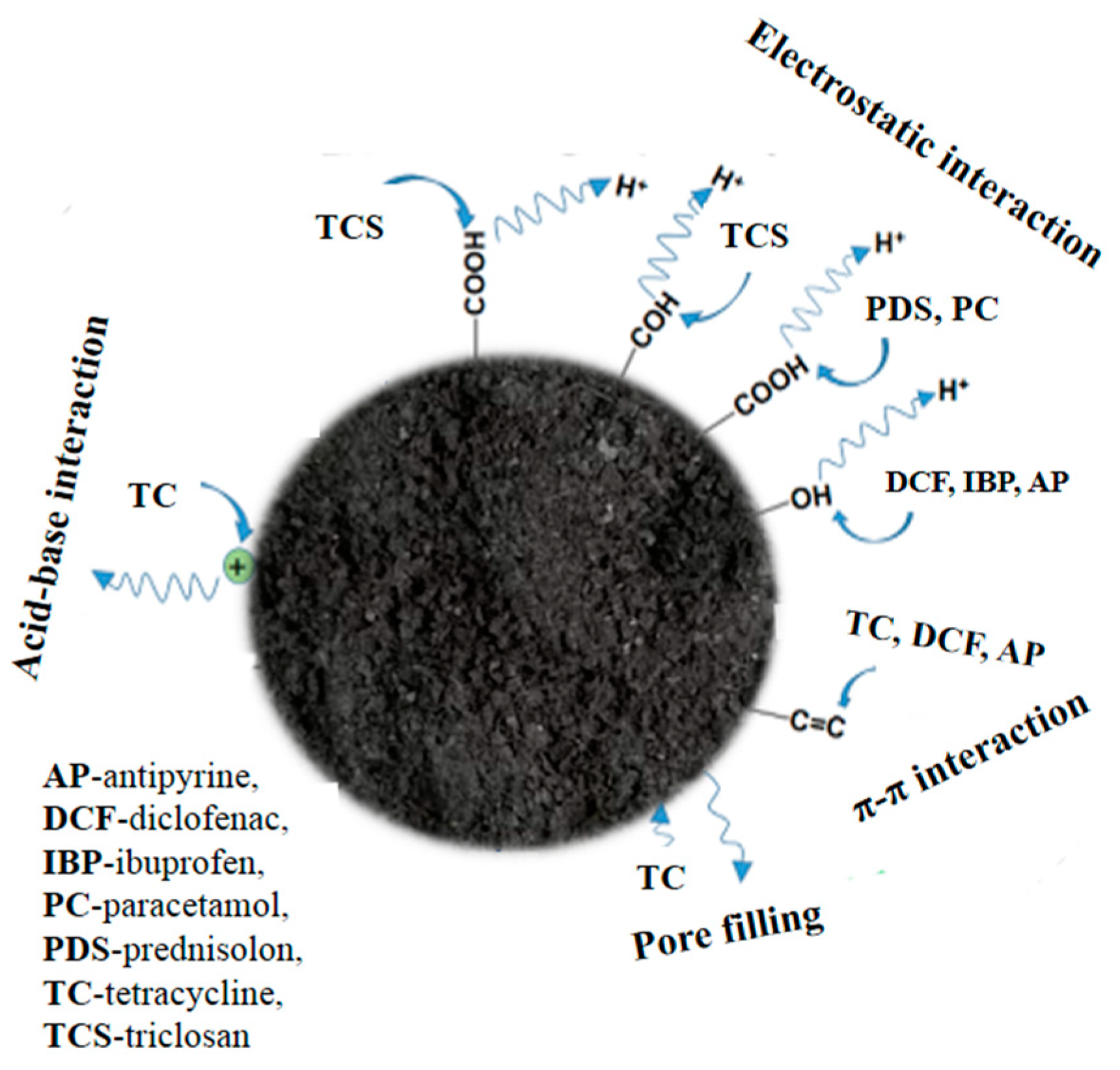
3.2.3. Pharmaceuticals
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cavali, M.; Libardi Junior, N.; Dutra de Sena, J.; Lorenci Woiciechowski, A.; Soccol, C.R.; Belli Filho, P.; Bayard, R.; Benbelkacem, H.; de Castilhos Junior, A.B. A review on hydrothermal carbonization of potential biomass wastes, characterization and environmental applications of hydrochar, and biorefinery perspectives of the process. Sci. Total Environ. 2023, 857, 159627. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sust. Energ. Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Masoumi, S.; Borugadda, V.B.; Nanda, S.; Dalai, A.K. Hydrochar: A Review on Its Production Technologies and Applications. Catalysts 2021, 11, 939. [Google Scholar] [CrossRef]
- Li, Q.; Han, Y.; Liu, X.; Ansari, U.; Cheng, Y.; Yan, C. Hydrate as a by-product in CO2 leakage during the long-term sub-seabed sequestration and its role in preventing further leakage. Environ. Sci. Pollut. Res. 2022, 29, 77737–77754. [Google Scholar] [CrossRef]
- Li, Q.; Wang, F.; Wang, Y.; Forson, K.; Cao, L.; Zhang, C.; Zhou, C.; Zhao, B.; Chen, J. Experimental investigation on the high-pressure sand suspension and adsorption capacity of guar gum fracturing fluid in low-permeability shale reservoirs: Factor analysis and mechanism disclosure. Environ. Sci. Pollut. Res. 2022, 29, 53050–53062. [Google Scholar] [CrossRef] [PubMed]
- Hillier, J.; Whittaker, C.; Dailey, G.; Aylott, M.; Casella, E.; Richter, G.M.; Riche, A.; Murphy, R.; Taylor, G.; Smith, P. Greenhouse gas emissions from four bioenergy crops in England and Wales: Integrating spatial estimates of yield and soil carbon balance in life cycle analyses. GCB Bioenergy 2009, 1, 267–281. [Google Scholar] [CrossRef]
- Petrović, J.; Simić, M.; Mihajlović, M.; Koprivica, M.; Kojić, M.; Nuić, I. Upgrading fuel potentials of waste biomass via hydrothermal carbonization. Hem. Ind. 2021, 75, 297–305. [Google Scholar] [CrossRef]
- Satira, A.; Paone, E.; Bressi, V.; Innazzo, D.; Marra, F.; Calabro, P.S.; Mauriello, F.; Espro, C. Hydrothermal Carbonization as Sustainable Process for the Complete Upgrading of Orange Peel Waste into Value-Added Chemicals and Bio-Carbon Materials. Appl. Sci. 2021, 11, 10983. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Wang, J.; Zhao, X.; Zhao, Y.; Qian, J.; Wang, T. Pyrolysis and hydrothermal carbonization of biowaste: A comparative review on the conversion pathways and potential applications of char product. Sustain. Chem. Pharm. 2023, 33, 101106. [Google Scholar] [CrossRef]
- Zhai, Y.; Peng, C.; Xu, B.; Wang, T.; Li, C.; Zeng, G.; Zhu, Y. Hydrothermal carbonisation of sewage sludge for char production with different waste biomass: Effects of reaction temperature and energy recycling. Energy 2017, 127, 167–174. [Google Scholar] [CrossRef]
- Jung, K.W.; Lee, S.Y.; Choi, J.W.; Lee, Y.J. A facile one-pot hydrothermal synthesis of hydroxyapatite/biochar nanocomposites: Adsorption behavior and mechanisms for the removal of copper(II) from aqueous media. Chem. Eng. J. 2019, 369, 529–541. [Google Scholar] [CrossRef]
- Petrović, J.; Perišić, N.; Dragišić-Maksimović, J.; Maksimović, V.; Kragović, M.; Stojanović, M.; Laušević, M.; Mihajlović, M. Hydrothermal conversion of grape pomace: Detailed characterization of obtained hydrochar and liquid phase. J. Anal. Appl. Pyrol. 2016, 118, 267–277. [Google Scholar] [CrossRef]
- Xu, Q.; Qian, Q.; Quek, A.; Ai, N.; Zeng, G.; Wang, J. Hydrothermal carbonization of macroalgae and the effects of experimental parameters on the properties of hydrochars. ACS Sustain. Chem. Eng. 2013, 1, 1092–1101. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Antero, R.V.P.; Alves, A.C.F.; de Oliveira, S.B.; Ojala, S.A.; Brum, S.S. Challenges and alternatives for the adequacy of hydrothermal carbonization of lignocellulosic biomass in cleaner production systems: A review. J. Clean. Prod. 2020, 252, 119899. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, X.; Zhou, S.; Shang, H.; Luo, J.; Tsang, D.C.W. Hydrothermal carbonization for hydrochar production and its application. In Biochar from Biomass and Waste: Fundamentals and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 275–294. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Tuning hydrochar properties for enhanced mesopore development in activated carbon by hydrothermal carbonization. Microporous Mesoporous Mater. 2015, 203, 178–185. [Google Scholar] [CrossRef]
- Knez, Ž.; Markočič, E.; Hrnčič, M.K.; Ravber, M.; Škerget, M. High pressure water reforming of biomass for energy and chemicals: A short review. J. Supercrit. Fluids 2015, 96, 46–52. [Google Scholar] [CrossRef]
- Mihajlović, M.; Petrović, J.; MaletiĆ, S.; Kragulj Isakovski, M.; StojanoviĆ, M.; Lopičić, Z.; Trifunović, S. Hydrothermal carbonization of Miscanthus × giganteus: Structural and fuel properties of hydrochars and organic profile with the ecotoxicological assessment of the liquid phase. Energy Convers. Manag. 2018, 159, 254–263. [Google Scholar] [CrossRef]
- Nobre, C.; Alves, O.; Durao, L.; Sen, A.; Vilarinho, C.; Goncalves, M. Characterization of hydrochar and process water from the hydrothermal carbonization of Refuse Derived Fuel. Waste Manag. 2021, 120, 303–313. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.V.; Fregolente, L.G.; Laranja, M.J.; Moreira, A.B.; Ferreira, O.P.; Bisinoti, M.C. Hydrothermal carbonization of sugarcane industry by-products and process water reuse: Structural, morphological, and fuel properties of hydrochars. Biomass Convers. Biorefin. 2022, 12, 153–161. [Google Scholar] [CrossRef]
- Wilk, M.; Sliz, M.; Lubieniecki, B. Hydrothermal co-carbonization of sewage sludge and fuel additives: Combustion performance of hydrochar. Renew. Energy 2021, 178, 1046–1056. [Google Scholar] [CrossRef]
- Ro, K.S.; Libra, J.A.; Alvarez-Murillo, A. Comparative Studies on Water- and Vapor-Based Hydrothermal Carbonization: Process Analysis. Energies 2020, 13, 5733. [Google Scholar] [CrossRef]
- Wang, G.; Li, D.; Xiong, L.; Dan, J.; Xu, K.; Yuan, X.; Kan, G.; Ning, X.; Wang, C. Application of catalysts in biomass hydrothermal carbonization for the preparation of high-quality blast furnace injection fuel. Energy 2023, 283, 129147. [Google Scholar] [CrossRef]
- Wilk, M.; Śliz, M.; Czerwińska, K.; Śledź, M. The effect of an acid catalyst on the hydrothermal carbonization of sewage sludge. J. Environ. Manag. 2023, 345, 118820. [Google Scholar] [CrossRef]
- Fallah, S.; Alavi, N.; Tavakoli, O.; Shahsavani, A.; Sadani, M. Optimization of hydrothermal carbonization of food waste as sustainable energy conversion approach: Enhancing the properties of hydrochar by landfill leachate substitution as reaction medium and acetic acid catalyst addition. Energy Convers. Manag. 2023, 297, 117647. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Q.; Li, X.; Kong, G.; Cao, T.; Cheng, Q.; Zhang, Z.; Zhang, X.; Han, L. Catalytic hydrothermal co-liquefaction of sewage sludge and agricultural biomass for promoting advanced biocrude production. J. Clean. Prod. 2023, 428, 139470. [Google Scholar] [CrossRef]
- Kojić, M.; Petrović, J.; Petrović, M.; Stanković, S.; Porobić, S.; Marinović-Cincović, M.; Mihajlović, M. Hydrothermal carbonization of spent mushroom substrate: Physicochemical characterization, combustion behavior, kinetic and thermodynamic study. J. Anal. Appl. Pyrol. 2021, 155, 105028. [Google Scholar] [CrossRef]
- Ahmad, S.; Zhu, X.; Wei, X.; Zhang, S. Influence of process parameters on hydrothermal modification of soybean residue: Insight into the nutrient, solid biofuel, and thermal properties of hydrochars. J. Environ. Manag. 2021, 283, 111981. [Google Scholar] [CrossRef]
- Xiong, J.B.; Pan, Z.Q.; Xiao, Z.F.; Huang, H.; Lai, F.Y.; Wang, J.X.; Chen, S.W. Study on the hydrothermal carbonization of swine manure: The effect of process parameters on the yield/properties of hydrochar and process water. J. Anal. Appl. Pyrol. 2019, 144, 104692. [Google Scholar] [CrossRef]
- Islam, M.A.; Akber, M.A.; Limon, S.H.; Akbor, A.; Islam, A. Characterization of solid biofuel produced from banana stalk via hydrothermal carbonization. Biomass Conv. Bioref. 2019, 9, 651–658. [Google Scholar] [CrossRef]
- Cai, J.; Li, B.; Chen, C.; Wang, J.; Zhao, M.; Zhang, K. Hydrothermal carbonization of tobacco stalk for fuel application. Bioresour. Technol. 2016, 220, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuel. Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sust. Energ. Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Hoekman, K.S.; Balasubramanian, R. Chemical, structural and combustion characteristics of carbonaceous products obtained by hydrothermal carbonization of palm empty fruit bunches. Bioresour. Technol. 2013, 135, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Nakason, K.; Panyapinyopol, B.; Kanokkantapong, V.; Viriya-empikul, N.; Kraithong, W.; Pavasan, P. Hydrothermal carbonization of unwanted biomass materials: Effect of process temperature and retention time on hydrochar and liquid fraction. J. Energy Inst. 2018, 91, 786–796. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. Strength, storage, and combustion characteristics of densified lignocellulosic biomass produced via torrefaction and hydrothermal carbonization. Appl. Energy 2014, 135, 182–191. [Google Scholar] [CrossRef]
- Djandja, O.S.; Liew, R.K.; Liu, C.; Liang, J.; Yuan, H.; He, W.; Feng, Y.; Lougou, B.G.; Duan, P.G.; Lu, X.; et al. Catalytic hydrothermal carbonization of wet organic solid waste: A review. Sci. Total Environ. 2023, 873, 162119. [Google Scholar] [CrossRef]
- Lang, Q.; Guo, X.; Wang, C.; Li, L.; Li, Y.; Xu, J.; Zhao, X.; Li, J.; Liu, B.; Sun, Q.; et al. Characteristics and phytotoxicity of hydrochar-derived dissolved organic matter: Effects of feedstock type and hydrothermal temperature. J. Environ. Sci. 2023, in press. [Google Scholar] [CrossRef]
- Alvarez-Murillo, A.; Libra, J.A.; Ro, K.S. Theoretical framework for estimating design reactor pressure for water-based hydrothermal carbonization (HTC) systems. Therm. Sci. Eng. Prog. 2022, 30, 101241. [Google Scholar] [CrossRef]
- Güleç, F.; Garsia Riesco, L.M.; Williams, O.; Kostas, E.; Samson, A.; Lester, E. Hydrothermal conversion of different lignocellulosic biomass feedstocks—Effect of the process conditions on hydrochar structures. Fuel 2021, 302, 121166. [Google Scholar] [CrossRef]
- Minaret, J.; Dutta, A. Comparison of liquid and vapor hydrothermal carbonization of corn husk for the use as a solid fuel. Bioresour. Technol. 2016, 200, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; He, L.; Prabowo, B.; Fang, Z.; Lin, J.; Xu, Z.; Hu, Y. Effect of pressure and atmosphere during hydrothermal treatment on the properties of sewage sludge-derived solid fuel. J. Mater. Cycles Waste Manag. 2018, 20, 1594–1604. [Google Scholar] [CrossRef]
- Khan, N.; Mohan, S.; Dinesha, P. Regimes of hydrochar yield from hydrothermal degradation of various lignocellulosic biomass: A review. J. Clean. Prod. 2021, 288, 125629. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Khiari, B.; Jellali, S.; Ghimbeu, C.M.; Jeguirim, M. Hydrochars production, characterization and application for wastewater treatment: A review. Renew. Sust. Energ. Rev. 2020, 127, 109882. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Wang, J.; Li, X.; Cheng, J.; Yang, H. Effect of residence time on chemical and structural properties of hydrochar obtained by hydrothermal carbonization of water hyacinth. Energy 2013, 58, 376–383. [Google Scholar] [CrossRef]
- Zhang, T.; Kumar, R.; Tsai, Y.D.; Elander, R.T.; Wyman, C.E. Xylose yields and relationship to combined severity for dilute acid post-hydrolysis of xylooligomers from hydrothermal pretreatment of corn stover. Green Chem. 2015, 17, 394–403. [Google Scholar] [CrossRef]
- He, M.; Cao, Y.; Xu, Z.; You, S.; Ruan, R.; Gao, B.; Wong, K.H.; Tsang, D.C.W. Process water recirculation for catalytic hydrothermal carbonization of anaerobic digestate: Water-Energy-Nutrient Nexus. Bioresour. Technol. 2022, 361, 127694. [Google Scholar] [CrossRef]
- Sarrion, A.; de la Rubia, A.; Coronella, C.; Mohedano, A.F.; Diaz, E. Acid-mediated hydrothermal treatment of sewage sludge for nutrient recovery. Sci. Total Environ. 2022, 838, 156494. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Y.; Zheng, M.; Xiao, Y.; Hu, H.; Liang, Y.; Liu, Y.; Dong, L. Preparation of High-Performance Porous Carbon Materials by Citric Acid-Assisted Hydrothermal Carbonization of Bamboo and Their Application in Electrode Materials. Energ. Fuel. 2022, 36, 9303–9312. Available online: https://pubs.acs.org/doi/10.1021/acs.energyfuels.2c01828 (accessed on 30 November 2023). [CrossRef]
- Ma, R.; Fakudze, S.; Shang, Q.; Wei, Y.; Chen, J.; Liu, C.; Han, J.; Chu, Q. Catalytic hydrothermal carbonization of pomelo peel for enhanced combustibility of coal/hydrochar blends and reduced CO2 emission. Fuel 2021, 304, 121422. [Google Scholar] [CrossRef]
- Faradilla, R.H.F.; Lucia, L.; Hakovirta, M. Remarkable physical and thermal properties of hydrothermal carbonized nanoscale cellulose observed from citric acid catalysis and acetone rinsing. Nanomaterials 2020, 10, 1049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, X.; Chen, X.; Tian, Y.; Zhou, Y.; Lu, X.; Huang, T. Conversion of water hyacinth to value-added fuel via hydrothermal carbonization. Energy 2020, 197, 117193. [Google Scholar] [CrossRef]
- Qi, R.; Xu, Z.; Zhou, Y.; Zhang, D.; Sun, Z.; Chen, W.; Xiong, M. Clean solid fuel produced from cotton textiles waste through hydrothermal carbonization with FeCl3: Upgrading the fuel quality and combustion characteristics. Energy 2021, 214, 118926. [Google Scholar] [CrossRef]
- Koprivica, M.; Petrović, J.; Ercegović, M.; Simić, M.; Milojković, J.; Šoštarić, T.; Dimitrijević, J. Improvement of combustible characteristics of Paulownia leaves via hydrothermal carbonization. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Ischia, G.; Fiori, L.; Gao, L.; Goldfarb, J.L. Valorizing municipal solid waste via integrating hydrothermal carbonization and downstream extraction for biofuel production. J. Clean. Prod. 2021, 289, 125781. [Google Scholar] [CrossRef]
- Santos Santana, M.; Pereira Alves, R.; da Silva Borges, W.M.; Francisquini, E.; Guerreiro, M.C. Hydrochar production from defective coffee beans by hydrothermal carbonization. Bioresour. Technol. 2020, 300, 122653. [Google Scholar] [CrossRef]
- Wang, L.; Li, A.; Chang, Y. Hydrothermal treatment coupled with mechanical expression at increased temperature for excess sludge dewatering: Heavy metals, volatile organic compounds and combustion characteristics of hydrochar. Chem. Eng. J. 2016, 297, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, S.A.; Yu, K.; Guo, J.Z.; Wang, Y.X.; Li, B. Adsorption of lead ions and methylene blue on acrylate-modified hydrochars. Bioresour. Technol. 2023, 379, 129067. [Google Scholar] [CrossRef]
- Petrović, J.; Ercegović, M.; Simić, M.; Kalderis, D.; Koprivica, M.; Milojković, J.; Radulović, D. Novel Mg-doped pyro-hydrochars as methylene blue adsorbents: Adsorption behavior and mechanism. J. Mol. Liq. 2023, 376, 121424. [Google Scholar] [CrossRef]
- Petrović, J.; Stojanović, M.; Milojković, J.; Petrović, M.; Šoštarić, T.; Laušević, M.; Mihajlović, M. Alkali modified hydrochar of grape pomace as a perspective adsorbent of Pb2+ from aqueous solution. J. Environ. Manag. 2016, 182, 292–300. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Huang, S.A.; Teng, H.J.; Su, Y.T.; Liu, X.M.; Li, B. Trithiocyanurate-functionalized hydrochar for effectively removing methylene blue and Pb (II) cationic pollutants. Environ. Pollut. 2023, 337, 122585. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Meng, W.; Cheng, S.; Xing, B.; Shi, C.; Nie, Y.; Wang, Q.; Xia, H. Efficient removal of heavy metal and antibiotics from wastewater by phosphate-modified hydrochar. Chemosphere 2023, 345, 140484. [Google Scholar] [CrossRef]
- Jiang, L.; Li, K.; Xia, L.; Gao, J.; Tang, L.; Jia, Y. KOH-modified hydrochar produced from Cd/Zn hyperaccumulator Sedum Alfredii Hance for aqueous Cd(Ⅱ) removal: Behavior and mechanism. J. Environ. Chem. Eng. 2023, 11, 110925. [Google Scholar] [CrossRef]
- Algethami, J.S.; Alhamami, M.A.M.; Alqadami, A.A.; Melhi, S.; Seliem, A.F. Adsorptive performance of a new magnetic hydrochar nanocomposite for highly efficient removal of cadmium ions from water: Mechanism, modeling, and reusability studies. Environ. Technol. Innov. 2023, 32, 103404. [Google Scholar] [CrossRef]
- Fouda-Mbanga, B.G.; Velempini, T.; Pillay, K.; Tywabi-Ngeva, Z. Hydrothermal development of magnetic-hydrochar nanocomposite from pineapple leaves and its performance as an adsorbent for the uptake of Mn2+ and reuse of the metal loaded adsorbent in latent fingerprint. J. Saudi Chem. Soc. 2023, 27, 101624. [Google Scholar] [CrossRef]
- Koprivica, M.; Simić, M.; Petrović, J.; Ercegović, M.; Dimitrijević, J. Evaluation of Adsorption Efficiency on Pb(II) Ions Removal Using Alkali-Modified Hydrochar from Paulownia Leaves. Processes 2023, 11, 1327. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.Y.; Choi, J.W.; Jung, K.W. Synergistic effect in simultaneous removal of cationic and anionic heavy metals by nitrogen heteroatom doped hydrochar from aqueous solutions. Chemosphere 2023, 323, 138269. [Google Scholar] [CrossRef]
- Chen, Z.L.; Xu, H.; Bai, L.Q.; Feng, Y.L.; Li, B. Protonated-amino-functionalized bamboo hydrochar for efficient removal of hexavalent chromium and methyl orange. Prog. Nat. Sci. Mater. 2023, 33, 501–507. [Google Scholar] [CrossRef]
- Deng, J.; Li, X.; Wei, X.; Liu, Y.; Liang, J.; Song, B.; Shao, Y.; Huang, W. Hybrid silicate-hydrochar composite for highly efficient removal of heavy metal and antibiotics: Coadsorption and mechanism. Chem. Eng. J. 2020, 387, 124097. [Google Scholar] [CrossRef]
- Kojić, M.; Mihajlović, M.; Marinović-Cincović, M.; Petrović, J.; Katanić, Đ.; Krstić, A.; Butulija, S.; Onija, A. Calcium-pyro-hydrochar derived from the spent mushroom substrate as a functional sorbent of Pb2+ and Cd2+ from aqueous solutions. Waste Manag. Res. 2022, 40, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Haris, M.; Khan, M.W.; Paz-Ferreiro, J.; Mahmood, N.; Eshtiaghi, N. Synthesis of functional hydrochar from olive waste for simultaneous removal of azo and non-azo dyes from water. Chem. Eng. J. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Madduri, S.; Elsayed, I.; Hassan, E.B. Novel oxone treated hydrochar for the removal of Pb(II) and methylene blue (MB) dye from aqueous solutions. Chemosphere 2020, 260, 127683. [Google Scholar] [CrossRef] [PubMed]
- Camilo, F.C.; de Araújo, T.P.; Quesada, H.B.; Moura, A.A.; Murilo Pereira, M.; Bergamasco, R.; Faria, S.H.; Simões Dornellas de Barros, M.A. Synthesis of hydrochars derived from industrial laundry sludge and its application in the removal of cationic dye. J. Water Process. Eng. 2021, 40, 101999. [Google Scholar] [CrossRef]
- Kohzadi, S.; Marzban, N.; Libra, J.A.; Bundschuh, M.; Maleki, A. Removal of RhB from water by Fe-modified hydrochar and biochar—An experimental evaluation supported by genetic programming. J. Mol. Liq. 2023, 369, 120971. [Google Scholar] [CrossRef]
- Algethami, J.S.; Alhamami, M.A.M.; Alqadami, A.A.; Melhi, S.; Seliem, A.F. Magnetic hydrochar grafted-chitosan for enhanced efficient adsorption of malachite green dye from aqueous solutions: Modeling, adsorption behavior, and mechanism analysis. Int. J. Biol. Macromol. 2024, 254, 127767. [Google Scholar] [CrossRef]
- Jais, F.J.; Ibrahim, S.; Chee, C.Y.; Ismail, Z. Solvothermal growth of the bimetal organic framework (NiFe-MOF) on sugarcane bagasse hydrochar for the removal of dye and antibiotic. J. Environ. Chem. Eng. 2021, 9, 106367. [Google Scholar] [CrossRef]
- Jais, F.M.; Chee, C.Y.; Ismail, Z.; Ibrahim, S. Experimental design via NaOH activation process and statistical analysis for activated sugarcane bagasse hydrochar for removal of dye and antibiotic. J. Environ. Chem. Eng. 2021, 9, 104829. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar]
- Delgado-Moreno, L.; Bazhari, S.; Gasco, G.; Méndez, A.; Azzouzi, M.E.; Romero, E. New insights into the efficient removal of emerging contaminants by biochars and hydrochars derived from olive oil wastes. Sci. Total Environ. 2021, 752, 141838. [Google Scholar] [CrossRef]
- Yudha, S.P.; Tekasakul, S.; Phoungthong, K.; Chuenchom, L. Green synthesis of low-cost and eco-friendly adsorbent for dye and pharmaceutical adsorption: Kinetic, isotherm, thermodynamic and regeneration studies. Mater. Res. Express 2019, 6, 125526. [Google Scholar] [CrossRef]
- Kumar, P.S.; Shanmugapriya, M.; Prasannamedha, G.; Rangasamy, G. Immobilization of hydrochar in cellulose beads for eradicating paracetamol from synthetic and sewage water. Environ. Pollut. 2023, 342, 123035. [Google Scholar] [CrossRef] [PubMed]
- Hayoun, B.; Escudero-Curiel, S.; Bourouina, M.; Bourouina-Bacha, S.; Angeles Sanromán, M.; Pazos, M. Preparation and characterization of high performance hydrochar for efficient adsorption of drugs mixture. J. Mol. Liq. 2022, 353, 118797. [Google Scholar] [CrossRef]
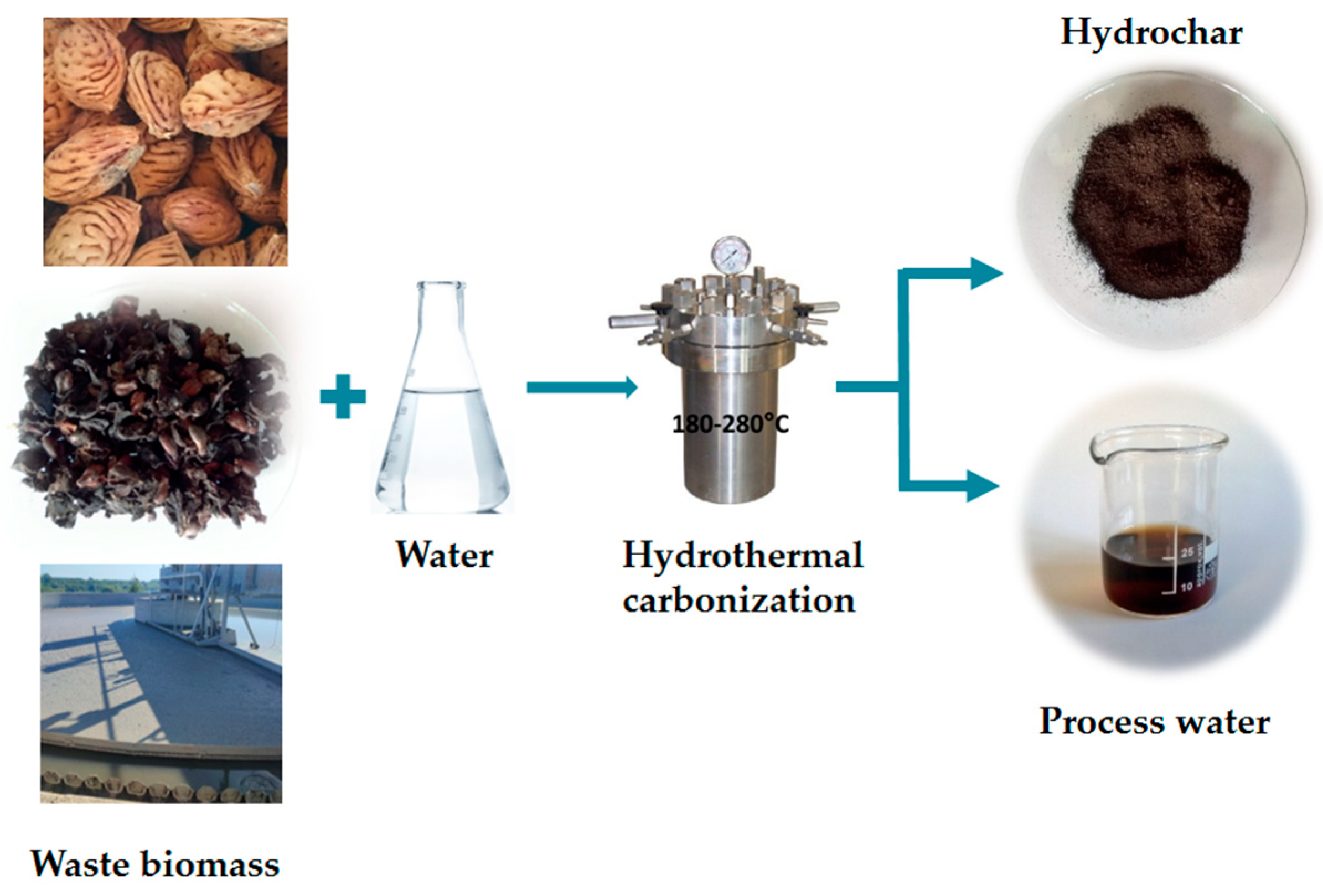
| Biomass | T (°C) | Residence Time (min) | Catalyst | Hydrochar Yield (%) | HHV (MJ/kg) | Fixed Carbon (%) | Reference |
|---|---|---|---|---|---|---|---|
| Wood chip | 240 | 60 | 2% Fe(NO3)3·9H2O | 56.08 | 26.80 | 42.95 | [24] |
| 4% Fe(NO3)3·9H2O | 55.32 | 30.05 | 44.31 | ||||
| Sewage sludge | 200 | 120 | sulfuric acid | 6.20 | 18.96 | 10.04 | [25] |
| Municipal solid waste | 260 | 300 | acetic acid | 45 | 32.56 | 38.81 | [26] |
| Sewage sludge/wheat straw | 300 | 90 | Ni@CSB | 35.27 | 36.96 | ND | [27] |
| Spent mushroom substrate | 180 | 60 | - | 57.2 | 14.9 | 11.6 | [28] |
| 200 | 55.23 | 15.4 | 10.42 | ||||
| 220 | 43.79 | 15.82 | 12.25 | ||||
| 240 | 41.98 | 16.82 | 13.44 | ||||
| 260 | 38.32 | 17.06 | 13.5 | ||||
| Soybean residue | 200 | 120 | - | 42.9 | 19.1 | 42.3 | [29] |
| 250 | 37.4 | 20.9 | 23.7 | ||||
| 300 | 32.3 | 22.0 | 19.2 | ||||
| Swine manure | 200 | 0–60 | - | 58.7 | 12.4 | 9.2 | [30] |
| 220 | 56.3 | 12.8 | 11.8 | ||||
| 240 | 55.7 | 13.7 | 12.6 | ||||
| 260 | 51.7 | 15.9 | 15.2 | ||||
| 280 | 50.2 | 16.0 | 15.7 | ||||
| Banana stalk | 160 | 60 | - | 75.3 | 18.1 | 16.9 | [31] |
| 160 | 120 | 73.6 | 18.4 | 21.0 | |||
| 160 | 180 | 72.8 | 18.4 | 21.1 | |||
| 180 | 60 | 68.2 | 18.5 | 22.5 | |||
| 180 | 120 | 65.2 | 18.6 | 24.9 | |||
| 180 | 180 | 61.5 | 18.7 | 27.5 | |||
| 200 | 60 | 61.8 | 18.8 | 35.0 | |||
| 200 | 120 | 60.9 | 18.8 | 35.3 | |||
| 200 | 180 | 57.8 | 18.9 | 44.3 | |||
| Miscanthus | 180 | 60 | - | 73 | 19.66 | 10.83 | [19] |
| 200 | 54 | 20.0 | 16.82 | ||||
| 220 | 51 | 21.18 | 22.9 | ||||
| Tobacco stalk | 180 | 120 | - | 80 | 18.72 | 15.6 | [32] |
| 200 | 67 | 19.07 | 18.5 | ||||
| 220 | 61 | 19.65 | 20.6 | ||||
| 240 | 63 | 21.12 | 23.02 | ||||
| 260 | 59 | 21.42 | 26.77 | ||||
| Grape pomace | 180 | 60 | - | 86 | 24.49 | 25.84 | [12] |
| 200 | 78 | 25.71 | 25.73 | ||||
| 220 | 66 | 26.13 | 26.64 |
| Hydrochar | Pollutant | Capacity (mg/g) | Reference |
|---|---|---|---|
| phosphate-modified poplar sawdust | Pb(II) | 119.61 | [64] |
| grape pomace | Pb(II) | 27.8 | [61] |
| KOH-modified grape pomace | 137 | ||
| Sedum alfredii Hance | Cd(II) | 1.52 | [65] |
| KOH Sedum alfredii Hance | 25.69 | ||
| magnetic watermelon seed waste | Cd(II) | 347.2 | [66] |
| magnetic pineapple leaves | Mn(II) | 2.99 | [67] |
| Paulownia leaves | Pb(II) | 82.37 | [68] |
| NaOH paulownia leaves | 174.75 | ||
| NH4Cl-modified corncob | Cu(II) | 77.75 | [69] |
| Cr(VI) | 103.82 | ||
| amino-modified bamboo hydrochar | Cr(VI) | 523.57 | [70] |
| MgSi sawdust | Cu(II) | 214.7 | [71] |
| Zn(II) | 227.3 | ||
| Ca-doped spent mushroom substrate | Pb(II) | 297 | [72] |
| Cd(II) | 131 |
| Hydrochar | Pollutant | Capacity (mg/g) | Reference |
|---|---|---|---|
| olive waste | Methylene blue (MB) | 14.26 | [73] |
| Congo red (CR) | 11.58 | ||
| pine wood | Methylene blue (MB) | 86.70 | [74] |
| KHCO3-modified hydrochar from industrial laundry sludge | Methylene blue (MB) | 808.83 | [75] |
| Mg-doped grape pomace | Methylene blue (MB) | 289.65 | [60] |
| Mg-doped corn cob | 262.30 | ||
| Mg-doped Miscanthus × giganteus | 232.48 | ||
| Fe-modified wheat straw | Rhodamin B | 80.00 | [76] |
| magnetic watermelon seed-grafted chitosan | Malachite green (MG) | 420.02 | [77] |
| NiFe-MOF incorporation on sugarcane bagasse | Cristal violet (CV) | 395.90 | [78] |
| NaOH-activated sugarcane bagasse | Cristal violet (CV) | 47.97 | [79] |
| Hydrochar | Pollutant | Capacity (mg/g) | Reference |
|---|---|---|---|
| unmodified biochar from olive oil waste | Diclofenac | 10.00 | [81] |
| Ibuprofen | 2.50 | ||
| hydrochar from olive oil waste | Diclofenac | 11.00 | [82] |
| Ibuprofen | 10.00 | ||
| green tea waste | Ibuprofen | 63.69 | [77] |
| phosphate-modified poplar sawdust | Ciprofloxacin | 98.38 | [64] |
| NaOH-treated sugarcane bagasse | Tetracycline | 22.60 | [79] |
| immobilized bamboo | Paracetamol | 48.12 | [83] |
| loquat cores | Diclofenac | 8.586 | [84] |
| Antipyrine | 11.369 | ||
| Prednisolone | 9.629 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrović, J.; Ercegović, M.; Simić, M.; Koprivica, M.; Dimitrijević, J.; Jovanović, A.; Janković Pantić, J. Hydrothermal Carbonization of Waste Biomass: A Review of Hydrochar Preparation and Environmental Application. Processes 2024, 12, 207. https://doi.org/10.3390/pr12010207
Petrović J, Ercegović M, Simić M, Koprivica M, Dimitrijević J, Jovanović A, Janković Pantić J. Hydrothermal Carbonization of Waste Biomass: A Review of Hydrochar Preparation and Environmental Application. Processes. 2024; 12(1):207. https://doi.org/10.3390/pr12010207
Chicago/Turabian StylePetrović, Jelena, Marija Ercegović, Marija Simić, Marija Koprivica, Jelena Dimitrijević, Aleksandar Jovanović, and Jovana Janković Pantić. 2024. "Hydrothermal Carbonization of Waste Biomass: A Review of Hydrochar Preparation and Environmental Application" Processes 12, no. 1: 207. https://doi.org/10.3390/pr12010207
APA StylePetrović, J., Ercegović, M., Simić, M., Koprivica, M., Dimitrijević, J., Jovanović, A., & Janković Pantić, J. (2024). Hydrothermal Carbonization of Waste Biomass: A Review of Hydrochar Preparation and Environmental Application. Processes, 12(1), 207. https://doi.org/10.3390/pr12010207








