Effects of Injection Parameters and EHN Mixing on the Combustion Characteristics of Fueling Pure Methanol in a Compression Ignition Engine
Abstract
:1. Introduction
2. Numerical Model Establishment and Validation
2.1. Model Setup
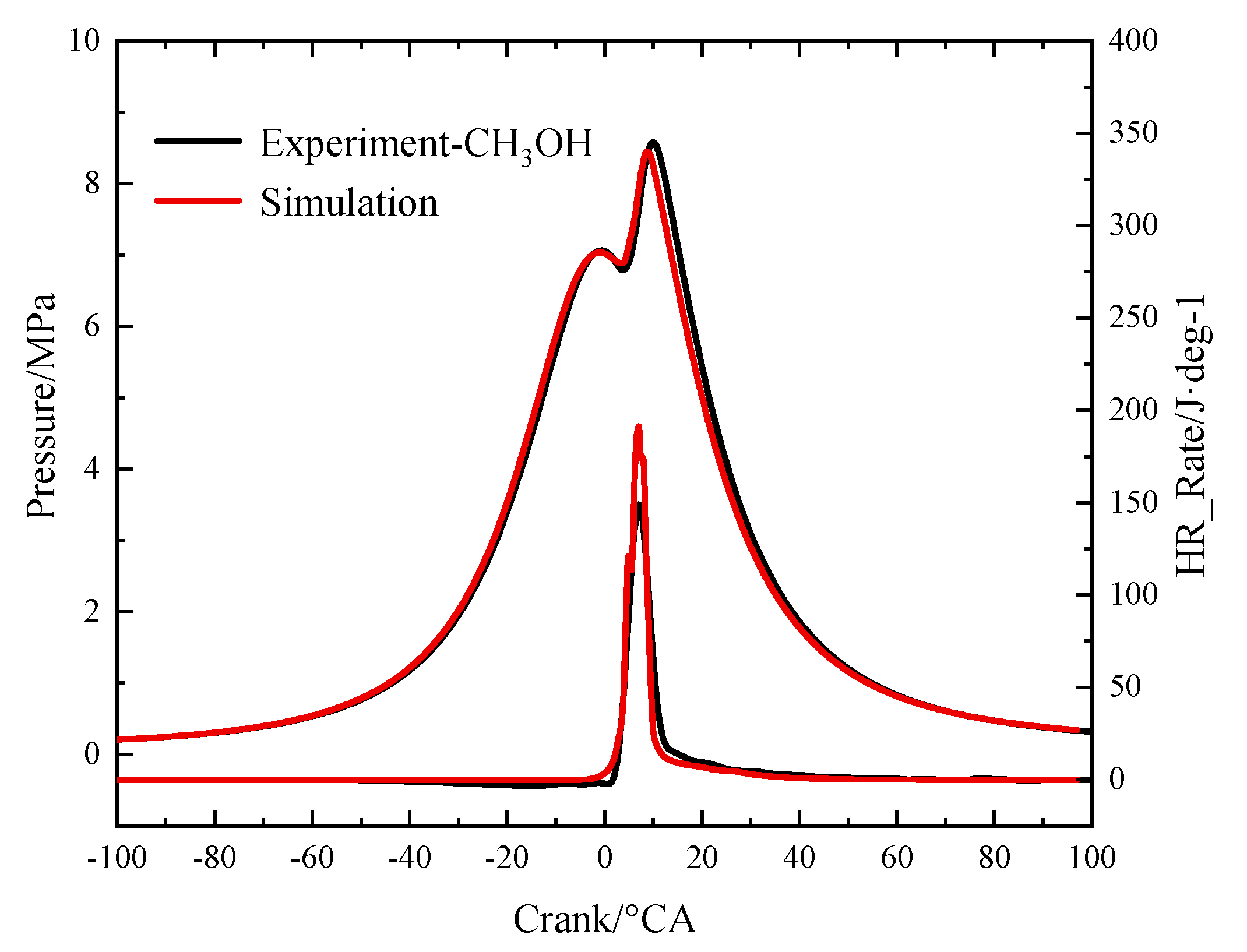
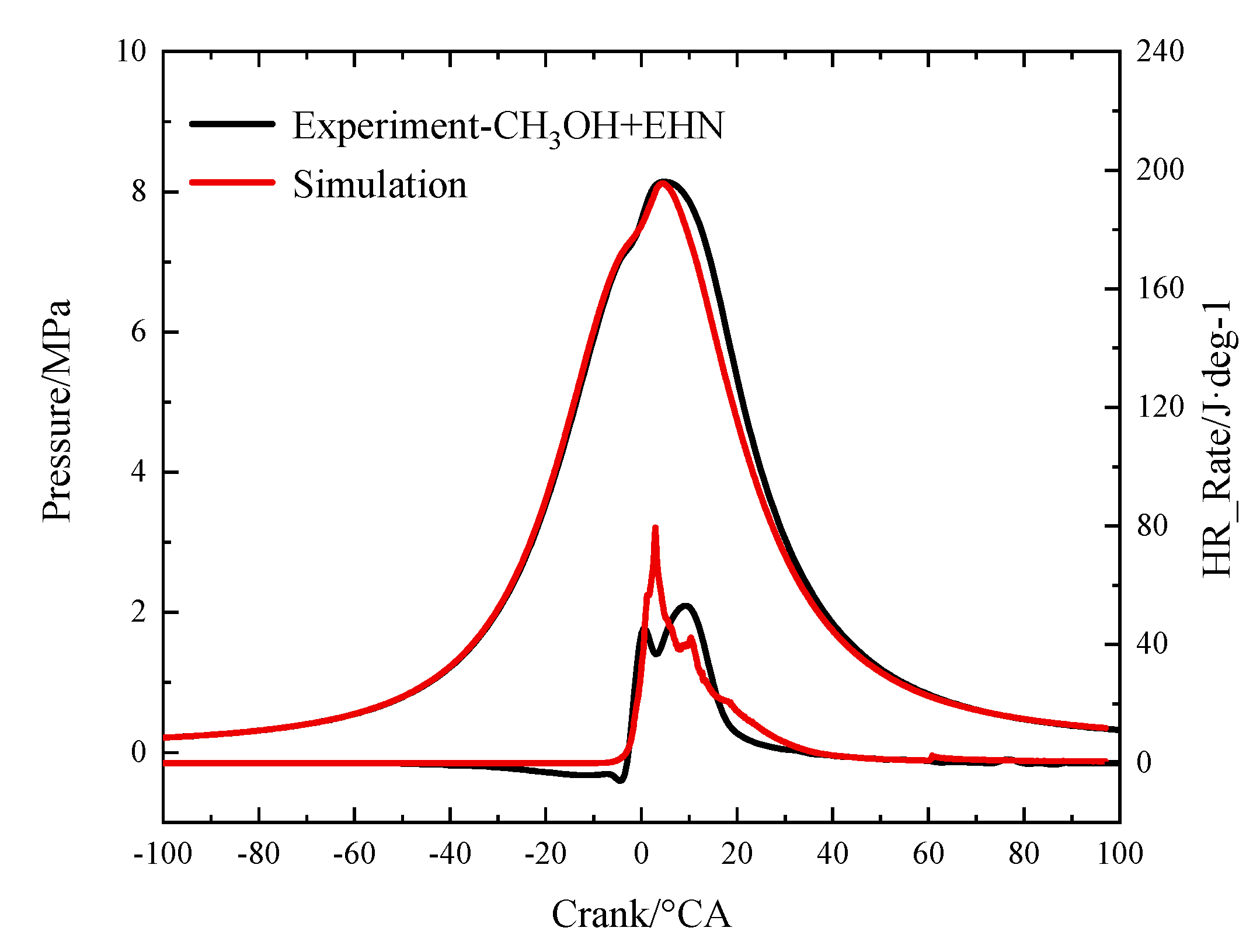
2.2. Model Validation
3. Results and Discussions
3.1. The Influence of Fuel Injection Pressure and EHN Addition on Combustion Characteristics
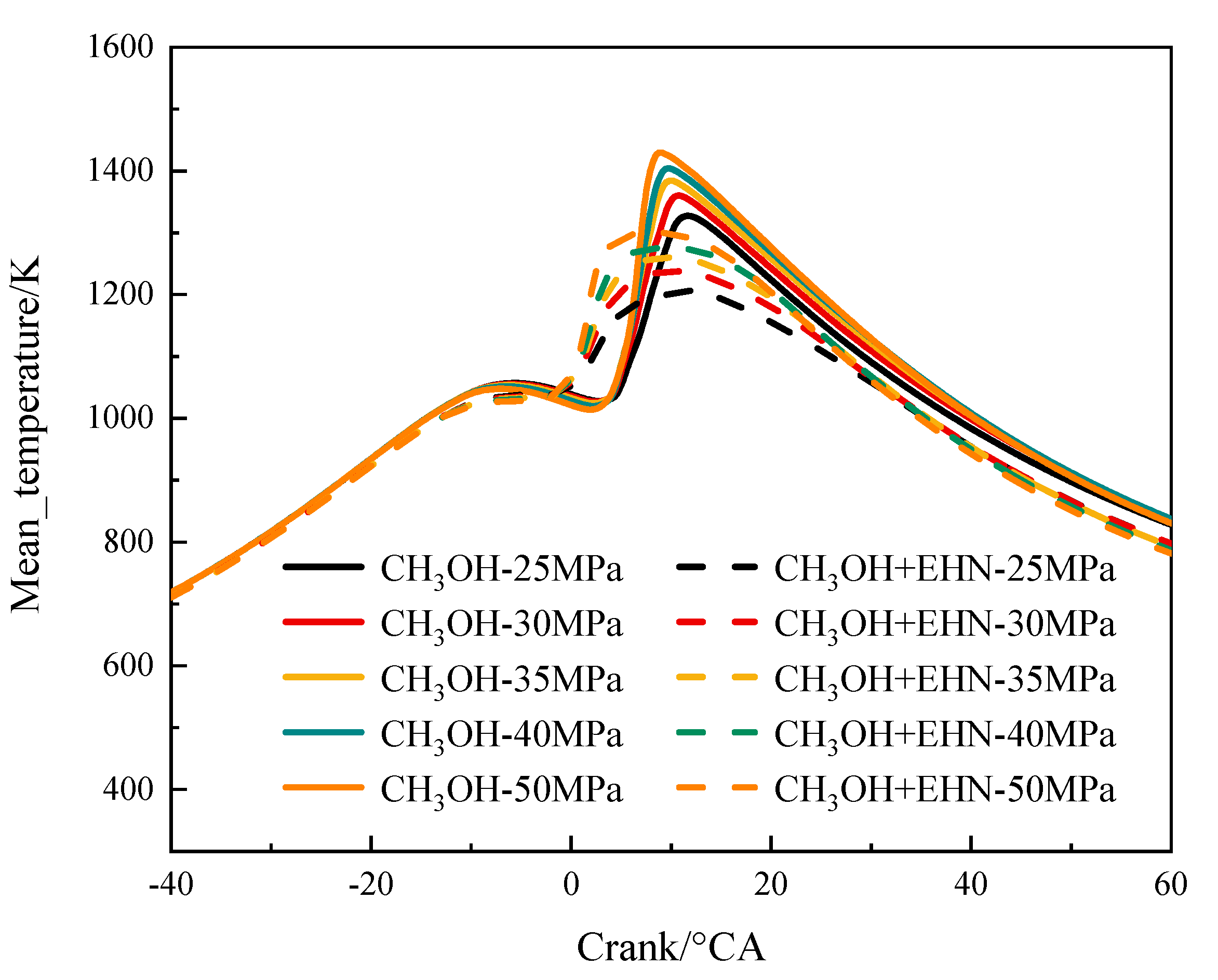
3.1.1. The Effect of Fuel Injection Pressure on Temperature
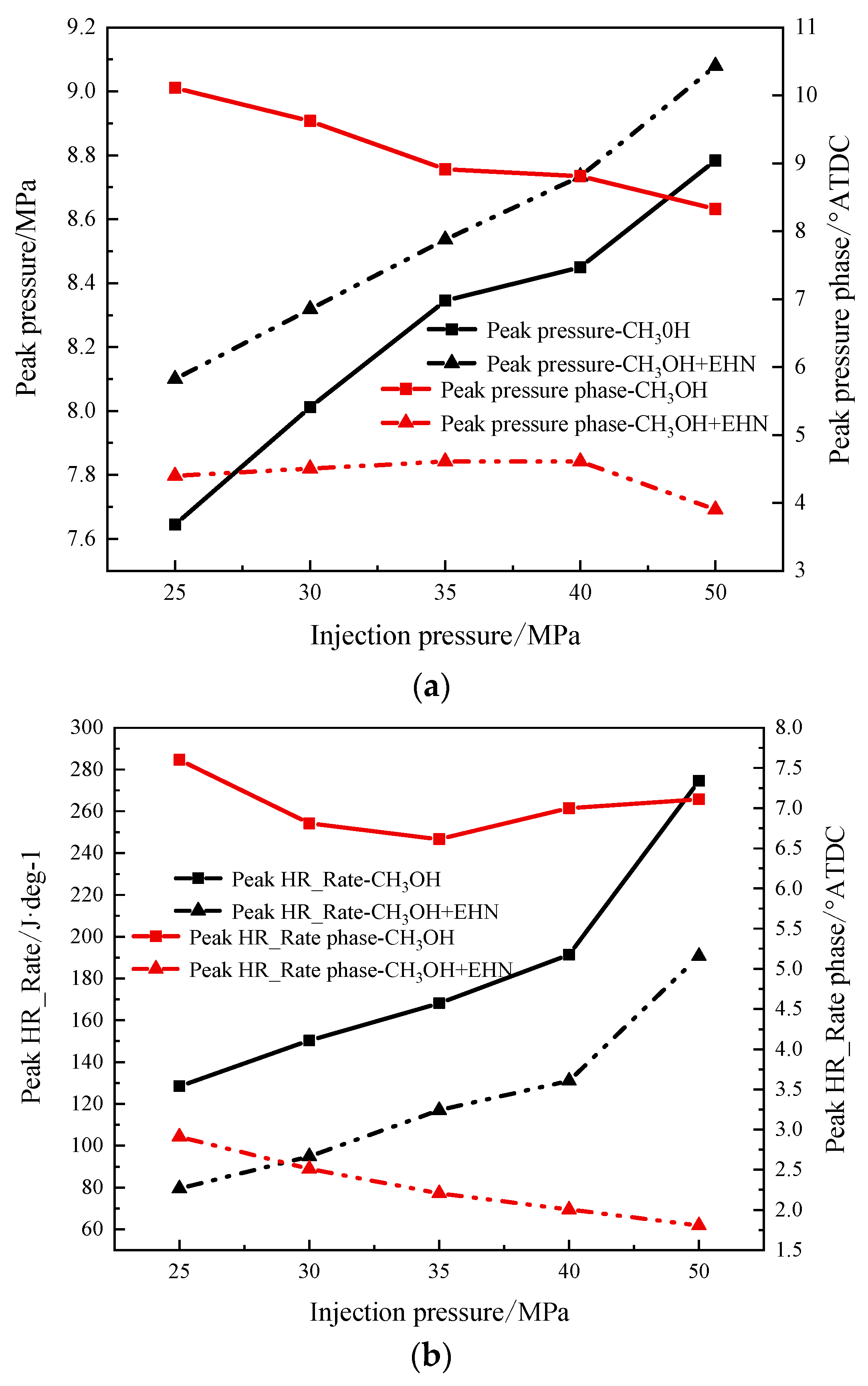
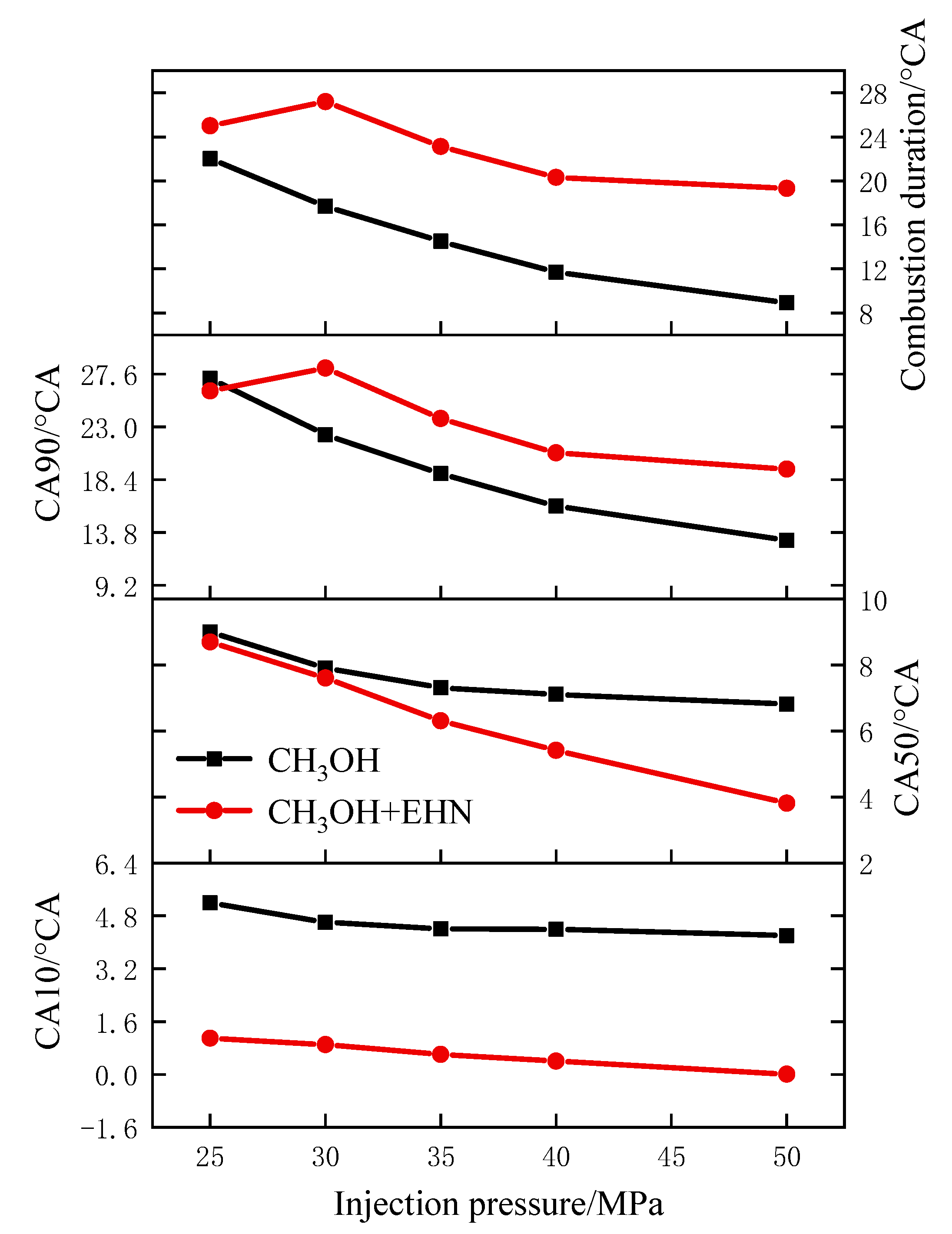
3.1.2. The Effect of Fuel Injection Pressure on the Combustion Process
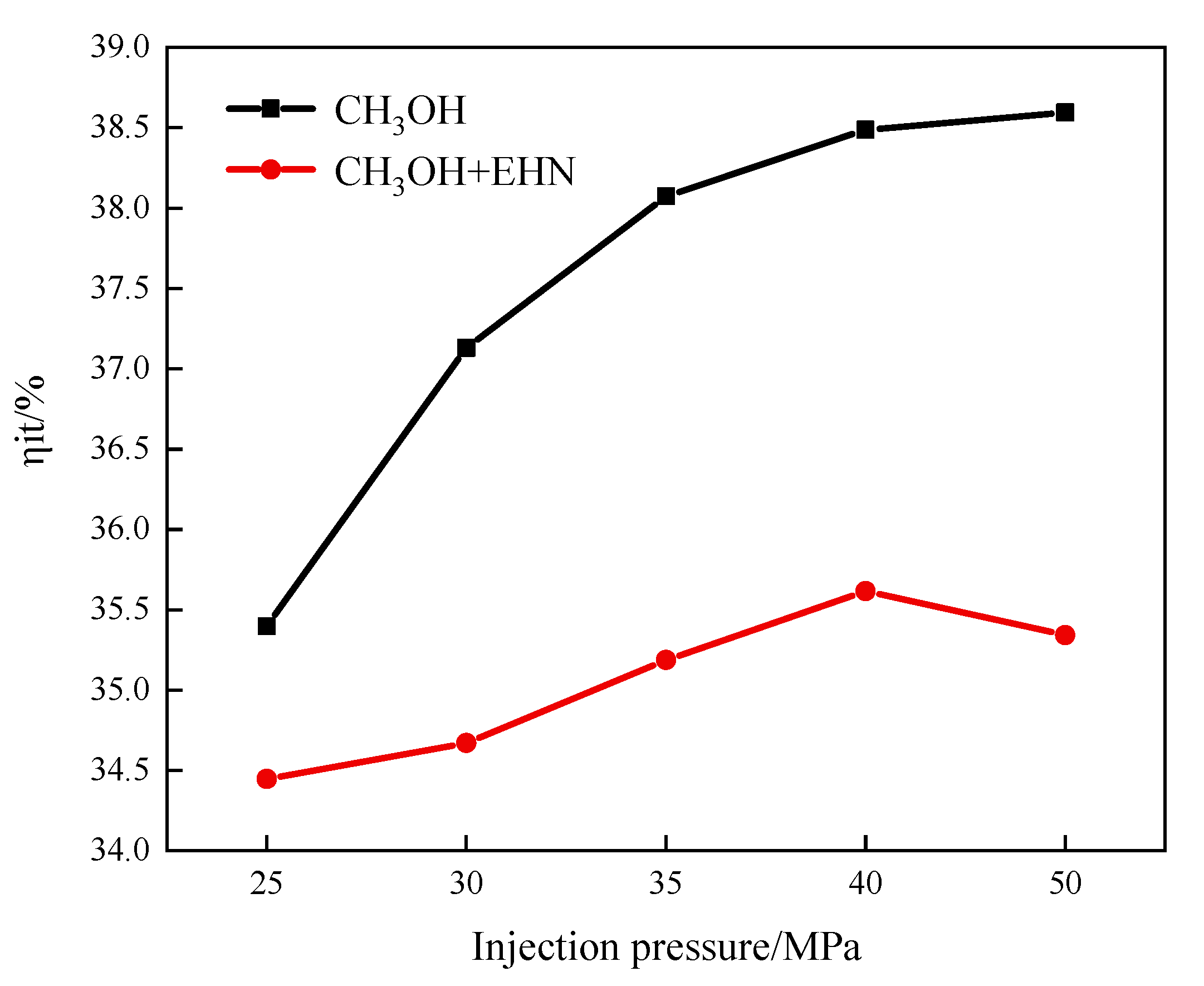
3.1.3. The Effects of Fuel Injection Pressure and EHN on Indicated Thermal Efficiency
3.2. The Influence of Fuel Injection Timing and EHN on Combustion
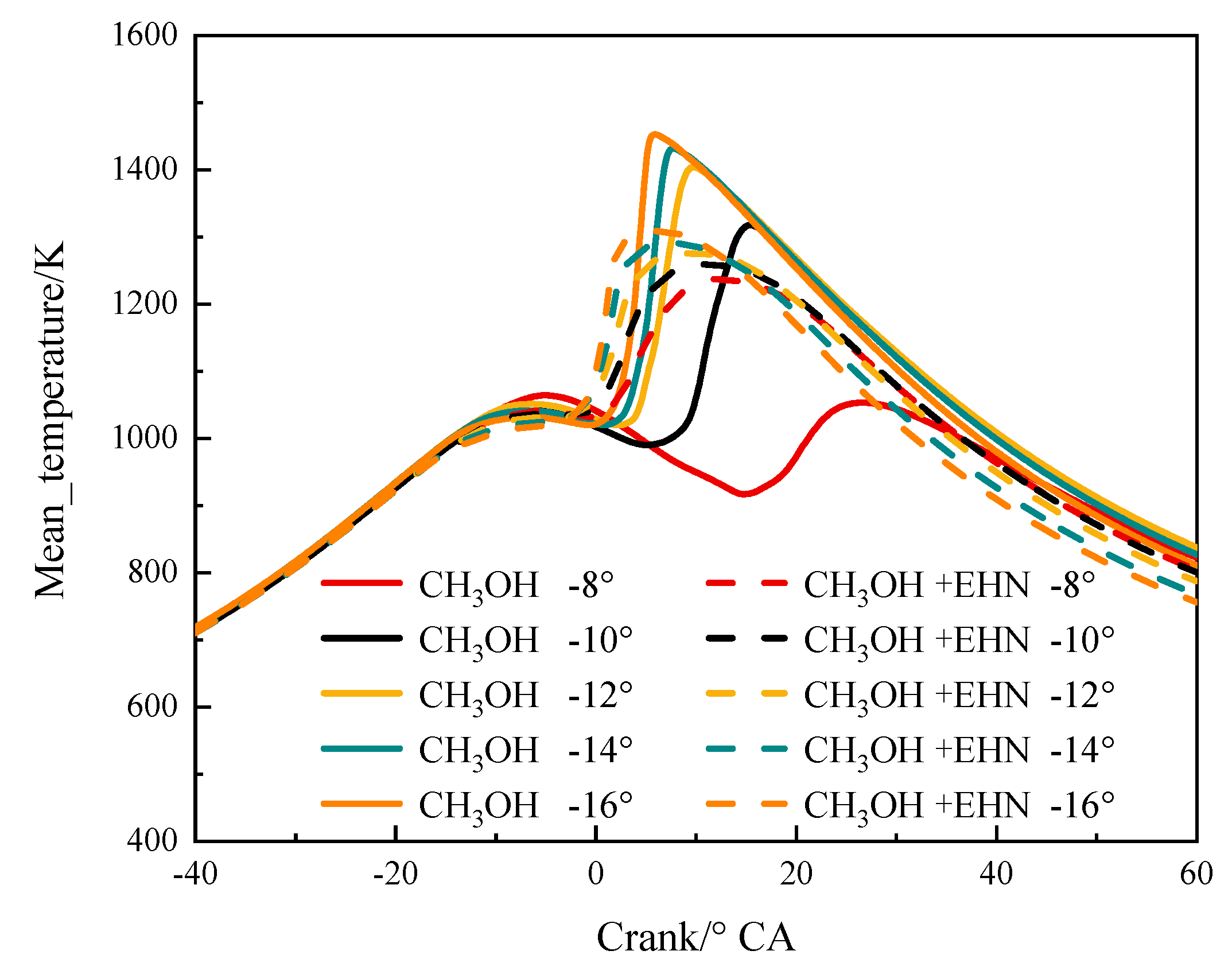
3.2.1. The Effect of Fuel Injection Timing and EHN on Temperature
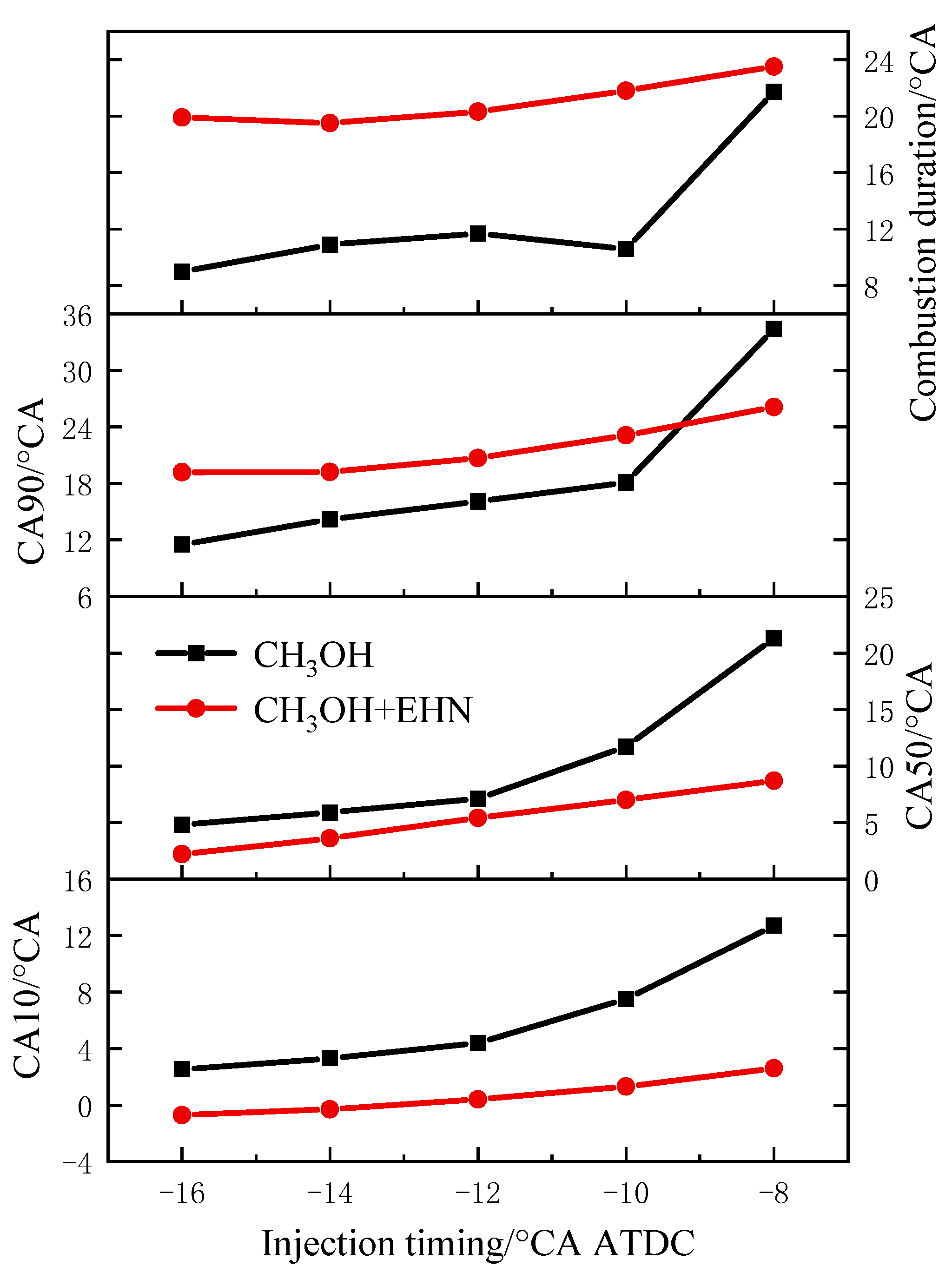
3.2.2. The Impact of Fuel Injection Timing and EHN on the Combustion Process
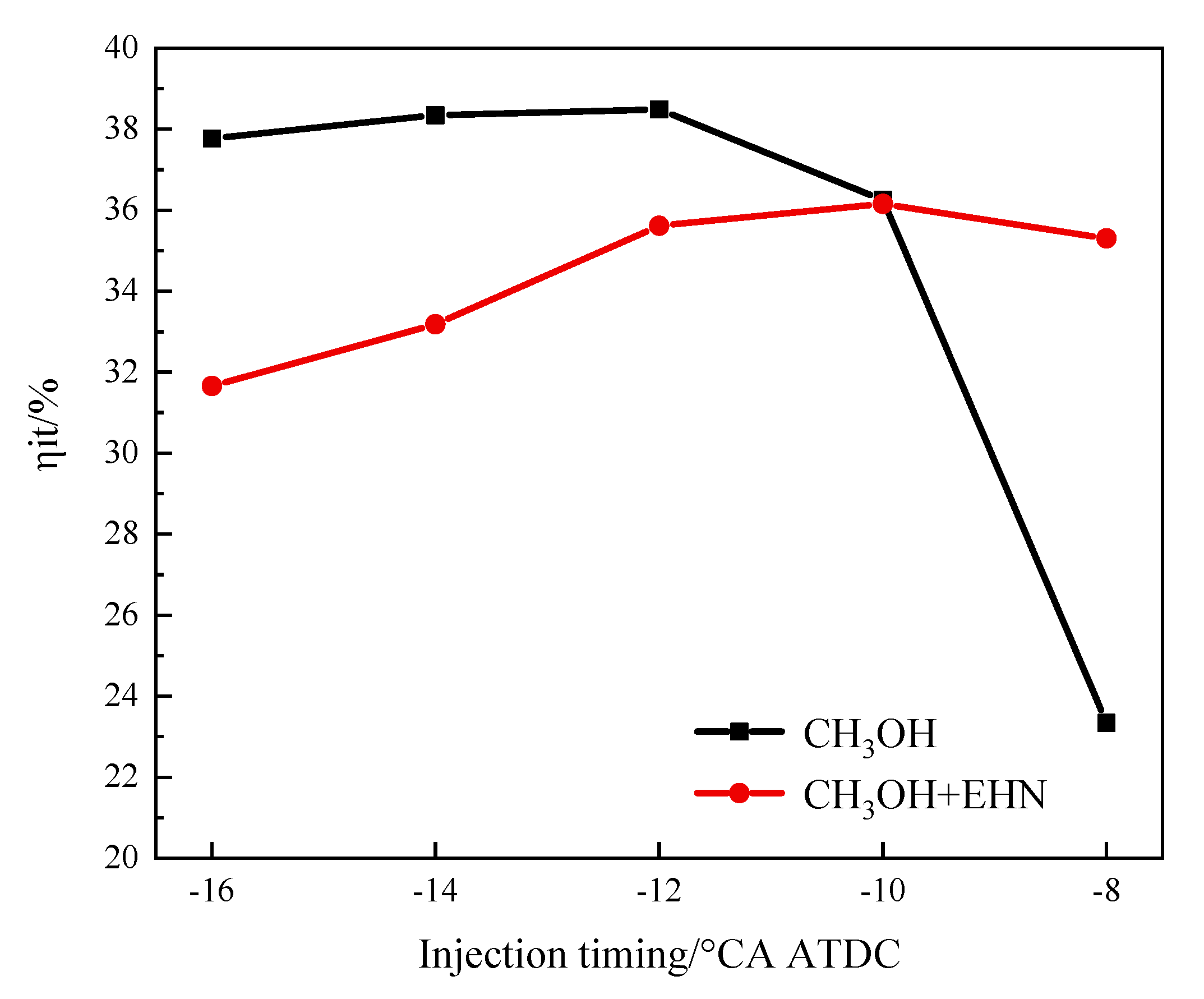
3.2.3. The Effects of Fuel Injection Timing and EHN on Indicated Thermal Efficiency
4. Conclusions
- (1)
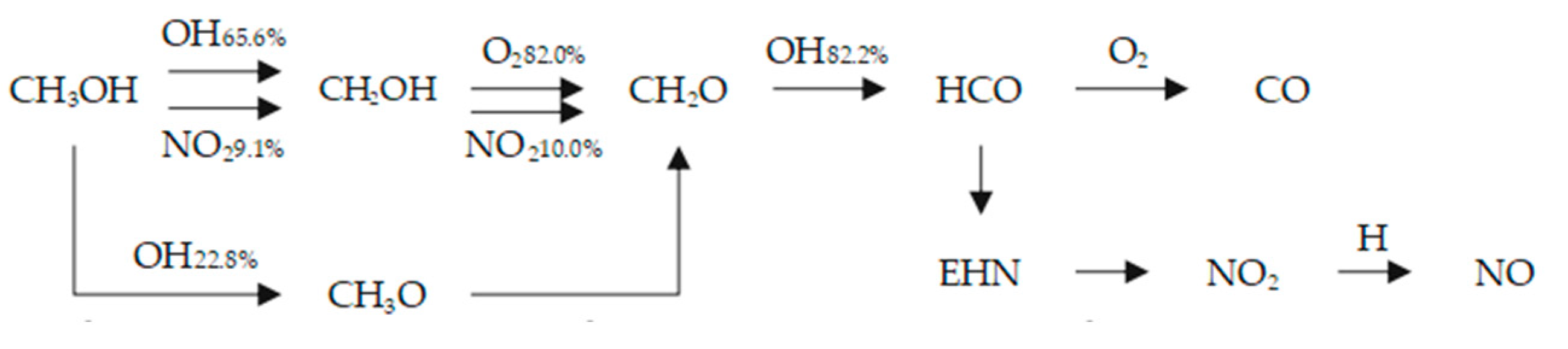
- After adding EHN, the ignition condition requirement of methanol is reduced, the ignition delay period is shortened, and the shortening effect of EHN on the combustion delay period is more significant at low temperatures than at high temperatures. The peak temperature in the cylinder decreases, the high temperature region of the temperature distribution in the cylinder at the top dead center increases, the peak pressure in the cylinder and the instantaneous heat release rate decrease, and the combustion duration is extended, which indicates a decrease in thermal efficiency. This is because NO2 produced by EHN decomposition can directly promote the dehydrogenation of methanol, and the active free radicals C7H15-3 and CH2O produced by EHN decomposition also improve the reaction rate. When pure methanol is burned, the temperature at which methanol starts to burn is higher due to the long ignition delay period, and the sensitivity of high-temperature reactions such as H2O2 (+M) = 2OH (+M) is higher. After adding EHN, the temperature of the methanol reaction is reduced, and the sensitivity of some high-temperature reactions becomes smaller. At the same time, the sensitivity of reactions related to EHN decomposition and early reactions involving decomposition products increased. In the CH3OH→CH2OH→CH2O→HCO reaction path, the proportion of OH and NO2 participating in the reaction increases.
- (2)
- The increase in injection pressure can improve the combustion condition of the in-cylinder mixture, resulting in an increase in the peak in-cylinder temperature and an advanced crankshaft angle to reach the peak. The temperature distribution in the cylinder decreases in the corresponding high-temperature region at the TDC. The peak pressure and heat release rate in the cylinder increase with the increase in injection pressure. The variation of the CA10 is not obvious, while the CA50 and CA90 are advanced, and the combustion duration is shortened, indicating an increase in the indicated thermal efficiency.
- (3)
- The advance of injection timing can make the starting point of combustion near the TDC, and the peak average temperature in the cylinder increases with the advance of the injection timing, and the corresponding crankshaft angle to reach the peak value advances accordingly. The temperature distribution in the cylinder decreases in the corresponding high-temperature region at the TDC. The peak pressure and heat release rate in the cylinder increase with the advance of injection timing. The CA10, CA50, and CA90 are advanced, the combustion duration is shortened, and the thermal efficiency is improved.
- (4)
- In summary, the addition of EHN resulted in a decrease in the ignition conditions and the temperature in the cylinder. Combined with the change in injection time and injection pressure after adding EHN, it can be seen that the indicated thermal efficiency performance of a pure methanol engine is the best under the conditions of an injection pressure of 40 MPa and an injection time of −12°. After adding EHN, the indicated thermal efficiency is best when the injection pressure is 40 MPa and the injection time is −10°. Too high injection pressure or too early injection time will lead to a decrease in its indicated thermal efficiency or no obvious improvement.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bae, C.; Kim, J. Alternative fuels for internal combustion engines. Proc. Combust. Inst. 2017, 36, 3389–3413. [Google Scholar] [CrossRef]
- Dong, S.J.; Cheng, X.B.; Ou, B.; Liu, T.; Wang, Z. Experimental and numerical investigations on the cyclic variability of an ethanol/diesel dual-fuel engine. Fuel 2016, 186, 665–673. [Google Scholar] [CrossRef]
- Kalghatgi, G.T. Developments in internal combustion engines and implications for combustion science and future transport fuels. Proc. Combust. Inst. 2015, 35, 101–115. [Google Scholar] [CrossRef]
- Kalghatgi, G.T. Is it really the end of internal combustion engines and petroleum in transport? Appl. Energy 2018, 225, 965–974. [Google Scholar] [CrossRef]
- Conti, J.; Holtberg, P.; Diefenderfer, J.; LaRose, A.; Turnure, J.T.; Westfall, L. International Energy Outlook 2016 with Projections to 2040; United States Energy Information Administration: Washington, DC, USA, 2016. [Google Scholar]
- BP. BP Statistical Review of World Energy 2019; BP p.l.c.: London, UK, 2019. [Google Scholar]
- Yamada, H.; Hayashi, R.; Tonokura, K. Simultaneous measurements of on-road/in-vehicle nanoparticles and NOx while driving: Actual situations, passenger exposure and secondary formations. Sci. Total Environ. 2016, 563–564, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Zheng, R.; Qin, Y.H.; Peng, J.; Li, M.; Lei, J.; Wu, Y.; Hu, M.; Shuai, S. The impact of fuel compositions on the particulate emissions of direct injection gasoline engine. Fuel 2016, 166, 543–552. [Google Scholar]
- Abdellatief, T.M.M.; Ershov, M.; Kapustin, V.; Abdelkareem, M.; Kamil, M.; Olabi, A. Recent trends for introducing promising fuel components to enhance the anti-knock quality of gasoline: A systematic review. Fuel 2021, 291, 120112. [Google Scholar] [CrossRef]
- Goyal, H.; Kook, S.; Lkeda, Y. The influence of fuel ignition quality and first injection proportion on gasoline compression ignition (GCI) combustion in a small-bore engine. Fuel 2019, 235, 1207–1215. [Google Scholar] [CrossRef]
- Liu, J.; Gong, C.; Peng, L.; Liu, F.; Yu, X.; Li, Y. Numerical study of formaldehyde and unburned methanol emissions of direct injection spark ignition methanol engine under cold start and steady state operating conditions. Fuel 2017, 202, 405–413. [Google Scholar] [CrossRef]
- Gong, C.; Li, J.; Peng, L.; Chen, Y.; Liu, Z.; Wei, F.; Liu, F. Numerical investigation of intake oxygen enrichment effects on radicals, combustion and unregulated emissions during cold start in a DISI methanol engine. Fuel 2019, 253, 1406–1413. [Google Scholar] [CrossRef]
- Liu, H.F.; Wang, X.C.; Zhang, D.P.; Dong, F.; Liu, X.; Yang, Y.; Huang, H.; Wang, Y.; Wang, Q.; Zheng, Z. Investigation on Blending Effects of Gasoline Fuel with N-Butanol, DMF, and Ethanol on the Fuel Consumption and Harmful Emissions in a GDI Vehicle. Energies 2019, 12, 1845. [Google Scholar] [CrossRef]
- Wen, M.S.; Zhang, C.Q.; Yue, Z.Y.; Liu, X.; Yang, Y.; Dong, F.; Liu, H. Effects of Gasoline Octane Number on Fuel Consumption and Emissions in Two Vehicles Equipped with GDI and PFI Spark-Ignition Engine. J. Energy Eng. 2020, 146, 04020069. [Google Scholar] [CrossRef]
- Alexander, P.; Cristina, I.; Annik, V.B. An effective force field to reproduce the solubility of MTBE in water. Fuel 2020, 264, 116761. [Google Scholar]
- Yang, Q.Y.; Shao, S.; Zhang, Y.; Hou, H.; Qin, C.; Sun, D.; Liu, Y. Comparative study on life cycle assessment of gasoline with methyl tertiary-butyl ether and ethanol as additives. Sci. Total Environ. 2020, 724, 138130. [Google Scholar] [CrossRef] [PubMed]
- Poulopoulos, S.; Philippopoulos, C. Influence of MTBE addition into gasoline on automotive exhaust emissions. Atmos. Environ. 2000, 34, 4781–4786. [Google Scholar] [CrossRef]
- Franklin, P.; Koshland, C.; Lucas, D.; Sawyer, R.F. Evaluation of combustion by-products of MTBE as a component of reformulated gasoline. Chemosphere 2001, 42, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Facetti, J.F.; Nunez, R.; Gomez, C.; Ojeda, J.; Bernal, C.; Leon-Ovelar, R.; Carvallo, F. Methyl tert-butyl ether (MtBE) in deep wells of the Patiño Aquifer, Paraguay: A preliminary characterization. Sci. Total Environ. 2019, 647, 1640–1650. [Google Scholar] [CrossRef]
- Rosell, M.; Lacorte, S.; Barceló, D. Simultaneous determination of methyl tert-butyl ether, its degradation products and other gasoline additives in soil samples by closed-system purge-and-trap gas chromatography–mass spectrometry. J. Chromatogr. A 2006, 1132, 28–38. [Google Scholar] [CrossRef]
- Geo, V.E.; Godwin, D.J.; Thiyagarajana, S.; Saravanan, C.; Aloui, F. Effect of higher and lower order alcohol blending with gasoline on performance, emission and combustion characteristics of SI engine. Fuel 2019, 256, 115806. [Google Scholar]
- Wang, C.M.; Li, Y.F.; Xu, C.S.; Badawy, T.; Sahu, A.; Jiang, C. Methanol as an octane booster for gasoline fuels. Fuel 2019, 248, 76–84. [Google Scholar] [CrossRef]
- Waluyo, B.; Setiyo, M.; Saifudin; Wardana, I. Fuel performance for stable homogeneous gasoline-methanol-ethanol blends. Fuel 2021, 294, 120565. [Google Scholar] [CrossRef]
- Bilgin, A.; Sezer, I. Effects of methanol addition to gasoline on the performance and fuel cost of a spark ignition engine. Energy Fuels 2008, 22, 131–142. [Google Scholar] [CrossRef]
- Abu-Zaid, M.; Badran, O.; Yamin, J. Effect of Methanol Addition on the Performance of Spark Ignition Engines. Energy Fuels 2004, 18, 312–315. [Google Scholar] [CrossRef]
- Hao, H.; Liu, Z.W.; Zhao, F.Q.; Du, J.; Chen, Y. Coal-derived alternative fuels for vehicle use in China: A review. J. Clean. Prod. 2017, 141, 774–790. [Google Scholar] [CrossRef]
- Karavalakis, G.; Short, D.; Vu, D.; Russell, R.L.; Asa-Awuku, A.; Jung, H.; Johnson, K.C.; Durbin, T.D. The impact of ethanol and iso-butanol blends on gaseous and particulate emissions from two passenger cars equipped with spray-guided and wall-guided direct injection SI (spark ignition) engines. Energy 2015, 82, 168–179. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, P.; Du, Y.D.; Yu, X.; Dong, W.; Zhou, J. Improvement of combustion and emission by combined combustion of ethanol premix and gasoline direct injection in SI engine. Fuel 2021, 292, 120403. [Google Scholar] [CrossRef]
- Silva, A.; Hauber, J.; Cancino, L.R.; Huber, K. The research octane numbers of ethanol-containing gasoline surrogates. Fuel 2019, 243, 306–313. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ma, Y.F.; Qian, Y.J.; Teng, Q.; Wang, C. Effects of ethanol injection strategies on mixture formation and combustion process in an ethanol direct injection (EDI) plus gasoline port injection (GPI) spark-ignition engine. Fuel 2020, 268, 117346. [Google Scholar] [CrossRef]
- Park, C.; Choi, Y.; Kim, C.; Oh, S.; Lim, G.; Moriyoshi, Y. Performance and exhaust emission characteristics of a spark ignition engine using ethanol and ethanol-reformed gas. Fuel 2010, 89, 2118–2125. [Google Scholar] [CrossRef]
- Turner, D.; Xu, H.; Cracknell, R.F.; Natarajan, V.; Chen, X. Combustion performance of bio-ethanol at various blend ratios in a gasoline direct injection engine. Fuel 2011, 90, 1999–2006. [Google Scholar] [CrossRef]
- Ceviz, M.A.; Yüksel, F. Effects of ethanol–unleaded gasoline blends on cyclic variability and emissions in an SI engine. Appl. Therm. Eng. 2005, 25, 917–925. [Google Scholar] [CrossRef]
- He, B.Q.; Wang, J.X.; Hao, J.M.; Yan, X.G.; Xiao, J.H. A study on emission characteristics of an EFI engine with ethanol blended gasoline fuels. Atmos. Environ. 2003, 37, 949–957. [Google Scholar] [CrossRef]
- Balki, M.K.; Sayin, C.; Canakci, M. The effect of different alcohol fuels on the performance, emission and combustion characteristics of a gasoline engine. Fuel 2014, 115, 901–906. [Google Scholar] [CrossRef]
- Esan, A.O.; Adeyemi, A.D.; Ganesan, S. A review on the recent application of dimethyl carbonate in sustainable biodiesel production. J. Clean. Prod. 2020, 257, 120561. [Google Scholar] [CrossRef]
- Kartikeya, S.; Vimal, C.S. Synthesis of organic carbonates from alcoholysis of urea: A review. Catal. Rev. 2017, 59, 1–43. [Google Scholar]
- Maier, T.; Härtl, H.; Jacob, E.; Wachtmeister, G. Dimethyl carbonate (DMC) and Methyl Formate (MeFo): Emission characteristics of novel, clean and potentially CO2-neutral fuels including PMP and sub-23 nm nanoparticle-emission characteristics on a spark-ignition DI-engine. Fuel 2019, 256, 115925. [Google Scholar] [CrossRef]
- Labeckas, G.; Slavinskas, S.; Vilutiene, V. Effect of the cetane number improving additive on combustion, performance, and emissions of a DI diesel engine operating on JP-8 fuel. J. Energy Eng. 2015, 141, C4014005. [Google Scholar] [CrossRef]
- Lü, X.; Yang, J.; Zhang, W.; Huang, Z. Improving the combustion and emissions of direct injection compression ignition engines using oxygenated fuel additives combined with a cetane number improver. Energy Fuels 2005, 19, 1879–1888. [Google Scholar] [CrossRef]
- Ewald, J.; Peters, N. A level set based flamelet model for the prediction of combustion in spark ignition engines. In Proceedings of the 15th International Multidimensional Engine Modeling User’s Group Meeting, Detroit, MI, USA, 13 April 2005. [Google Scholar]
- Senecal, P.K.; Pomraning, E.; Richards, K.J.; Briggs, T.E.; Choi, C.Y.; Mcdavid, R.M.; Patterson, M.A. Multi-dimensional modeling of direct-injection diesel spray liquid length and flame lift-off length using CFD and parallel detailed chemistry. SAE Trans. 2003, 112, 1331–1351. [Google Scholar]
- Heywood, J.B. Combustion engine fundamentals. 1ª Edição. Estados Unidos 1988, 25, 1117–1128. [Google Scholar]
- Chang, W.; Wang, C.; Wu, Y.; Jin, C.; Zhang, Z.; Liu, H. Study on the mechanism of influence of cetane improver on methanol ignition. Fuel 2023, 354, 129383. [Google Scholar] [CrossRef]




| Parameter Name (Unit) | Value |
|---|---|
| Engine | Six cylinders in line, water cooling, four strokes |
| Displacement (L) | 7.7 |
| Bore (mm) × stroke (mm) | 110 × 135 |
| Geometric compression ratio | 21.5:1 |
| Number of valves per cylinder | 4 |
| Theoretical calibration power (kw/rpm) | 235/2200 |
| Theoretical maximum torque (N·m/rpm) | 1350/(1100–1600) |
| Number of spray holes | 8 |
| Spray hole diameter | 0.153 |
| Jet hole angle (°) | 147 |
| Booster system | Exhaust gas turbocharger |
| Test Parameter | Range | Sensibility | Test Error |
|---|---|---|---|
| Engine torque | 0~2100 N·m | ±2.8 N·m | ±0.2% |
| Engine speed | 0~7000 rpm | ±1 rpm | ±0.01% |
| Pressure | 0~250 bar | 16 pC/bar | ±0.4% |
| Air mass flow | 0~2500 kg/h | ±1.75 kg/h | ±0.5% |
| Fuel mass flow | 0~150 kg/h | ±0.01 kg/h | ±1% |
| Intake pressure | 0~1000 kPa | ±0.5% kPa | ±0.1% |
| Intake Temperature | 223.15~573.15 K | ±0.05% K | ±0.35% |
| Item | Model |
|---|---|
| Turbulence flow modeling | RNG |
| Spray model | KH-RT |
| Collisional polymerization model | NTC |
| Droplet collision model | Wall film-O’Rourk |
| Fuel evaporation model | Frossling |
| Combustion model | SAGE |
| Nitrogen oxide generation model | Zeldovich |
| Carbon smoke generation model | Hiroyasu Soot |
| Item (Unit) | Value | |
|---|---|---|
| Fuel | Pure Methanol | Methanol + 3%v EHN |
| Intake temperature (K) | 394.5 | 394.5 |
| Intake pressure (MPa) | 0.135 | 0.135 |
| Turbulent kinetic energy (m2/s2) | 20.0 | 20.0 |
| Turbulent dissipation number | 17,183.4 | 17,183.4 |
| Cylinder head temperature (K) | 450 | 450 |
| Wall temperature (K) | 450 | 450 |
| Piston top temperature (K) | 500 | 500 |
| Injection timing (°CA ATDC) | −12 | −12 |
| Fuel injection quantity (mg) | 49 | 49 |
| Injection pressure (MPa) | 40 | 25 |
| Injection duration (°CA) | 16.7 | 21.2 |
| Item (Unit) | Experiment | Simulation | Experiment | Simulation |
|---|---|---|---|---|
| Fuel | Pure Methanol | Pure Methanol | Methanol + 3%v EHN | Methanol + 3%v EHN |
| Peak cylinder pressure (MPa) | 8.58 | 8.45 | 8.14 | 8.10 |
| Crank angle at peak cylinder pressure (°CA ATDC) | 10 | 8.81 | 4.5 | 4.4 |
| Peak heat release rate (J) | 149.127 | 191.34 | 53.13 | 79.45 |
| Crank angle at peak heat release rate (°CA ATDC) | 7 | 7 | 9 | 3.0 |
| Total heat release (J) | 942.12 | 1008.32 | 1033.14 | 966.12 |
| CA10 (°CA ATDC) | 5 | 4.4 | 3 | 1.4 |
| CA50 (°CA ATDC) | 8 | 7.11 | 10.5 | 9.6 |
| CA90 (°CA ATDC) | 25.5 | 16.1 | 27 | 29.6 |
| NOX (g/kWh) | 12.69 | 14.44 | 5.67 | 6.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Li, M.; Wei, H.; Wang, C.; Song, T.; Huang, Z.; Zhang, Z.; Cui, Y.; Jin, C. Effects of Injection Parameters and EHN Mixing on the Combustion Characteristics of Fueling Pure Methanol in a Compression Ignition Engine. Processes 2024, 12, 48. https://doi.org/10.3390/pr12010048
Liu H, Li M, Wei H, Wang C, Song T, Huang Z, Zhang Z, Cui Y, Jin C. Effects of Injection Parameters and EHN Mixing on the Combustion Characteristics of Fueling Pure Methanol in a Compression Ignition Engine. Processes. 2024; 12(1):48. https://doi.org/10.3390/pr12010048
Chicago/Turabian StyleLiu, Haifeng, Mengjia Li, Hongyuan Wei, Can Wang, Tengda Song, Zhixiong Huang, Zhao Zhang, Yanqing Cui, and Chao Jin. 2024. "Effects of Injection Parameters and EHN Mixing on the Combustion Characteristics of Fueling Pure Methanol in a Compression Ignition Engine" Processes 12, no. 1: 48. https://doi.org/10.3390/pr12010048
APA StyleLiu, H., Li, M., Wei, H., Wang, C., Song, T., Huang, Z., Zhang, Z., Cui, Y., & Jin, C. (2024). Effects of Injection Parameters and EHN Mixing on the Combustion Characteristics of Fueling Pure Methanol in a Compression Ignition Engine. Processes, 12(1), 48. https://doi.org/10.3390/pr12010048







