Multi-Criteria Optimization Conditions for the Recovery of Bioactive Compounds from Levisticum officinale WDJ Koch Roots Using Green and Sustainable Ultrasound-Assisted Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material—Levisticum Officinale
2.2. Ultrasound-Assisted Extraction (UAE) Procedure
2.3. Quantification of Bioactive Compounds in the Produced Extracts
2.4. Mathematical Model
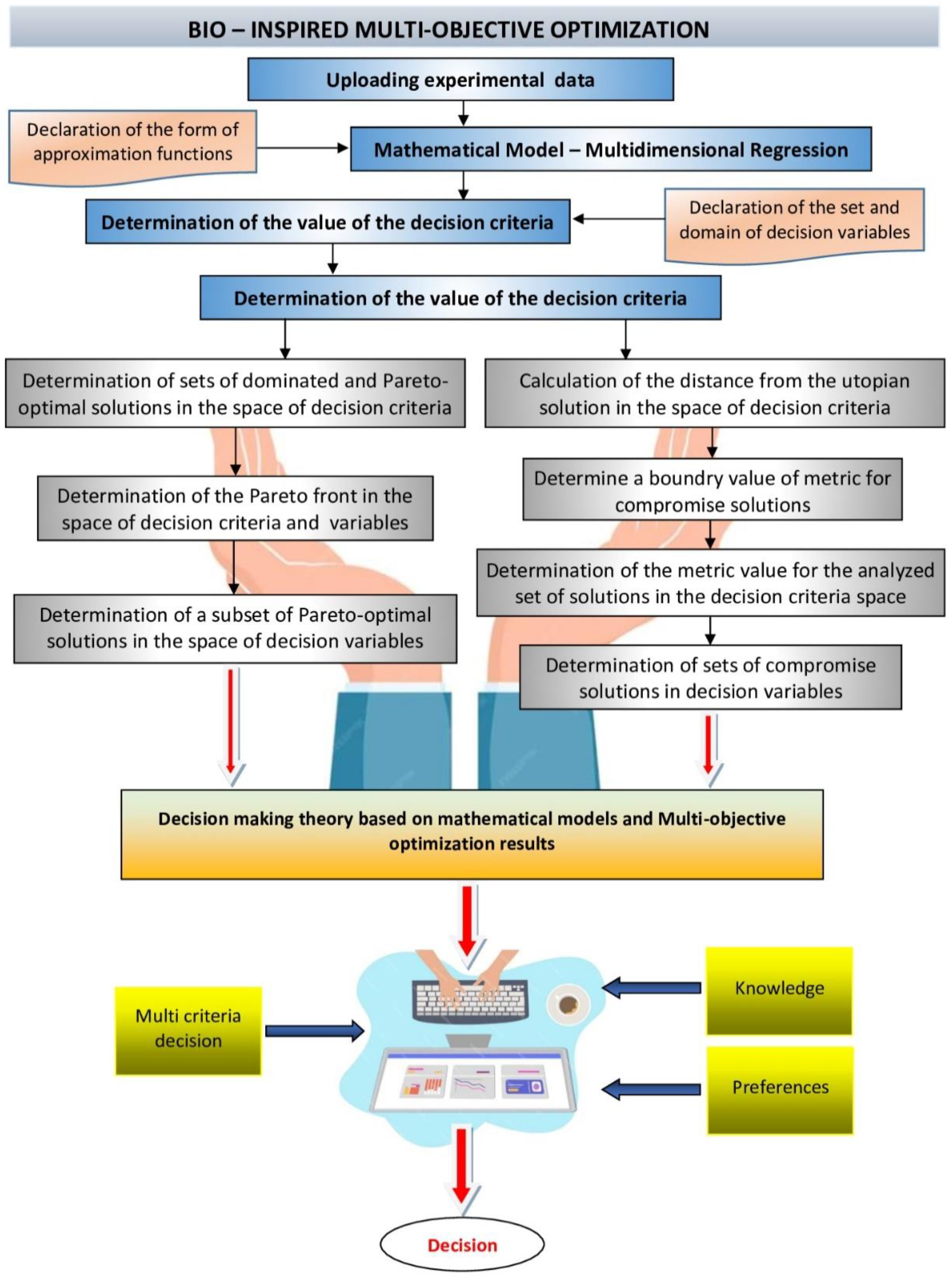
2.5. Multi-Criteria Optimization
2.6. Quantification of Bioactive Compounds in the Produced Extracts
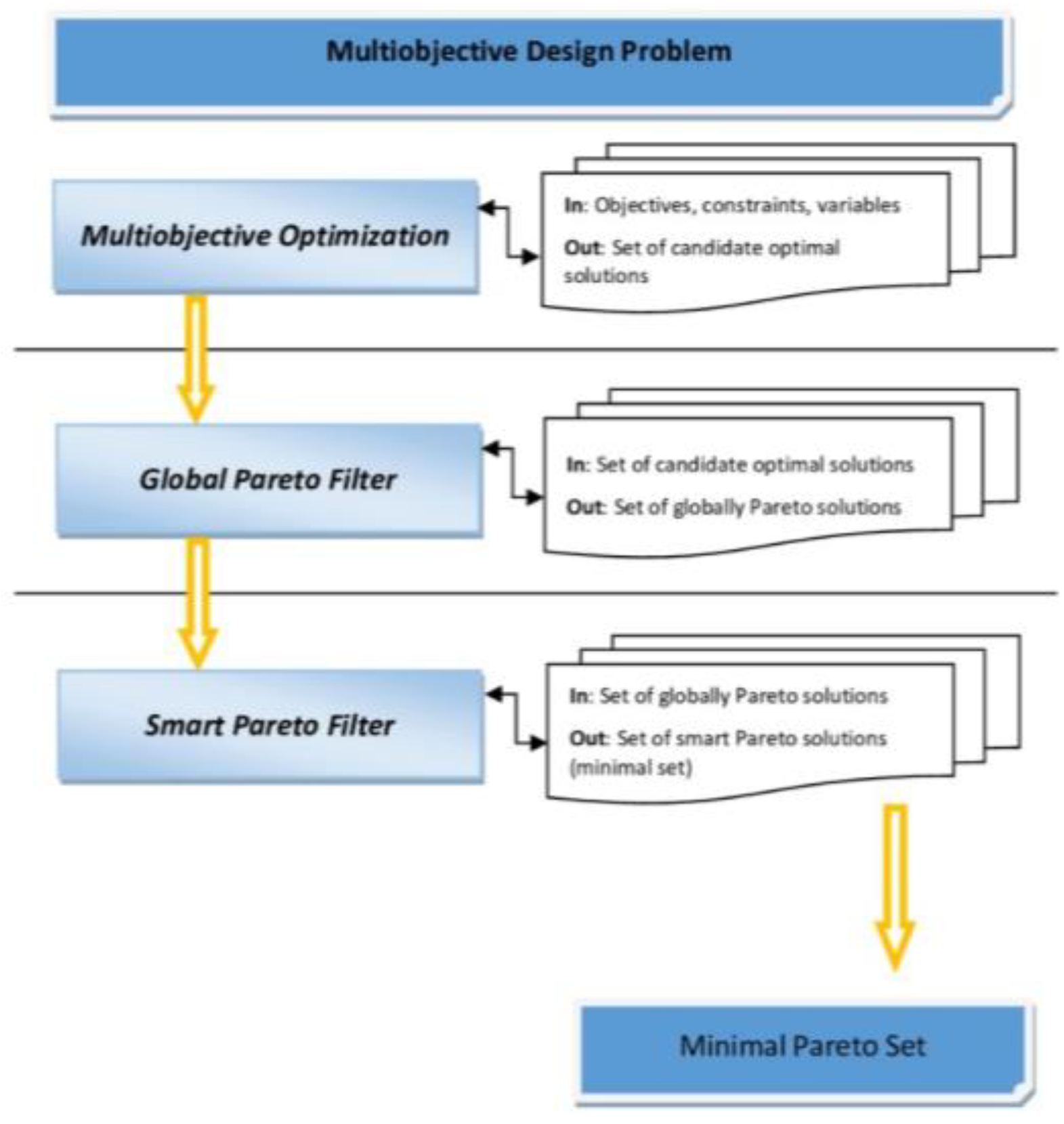
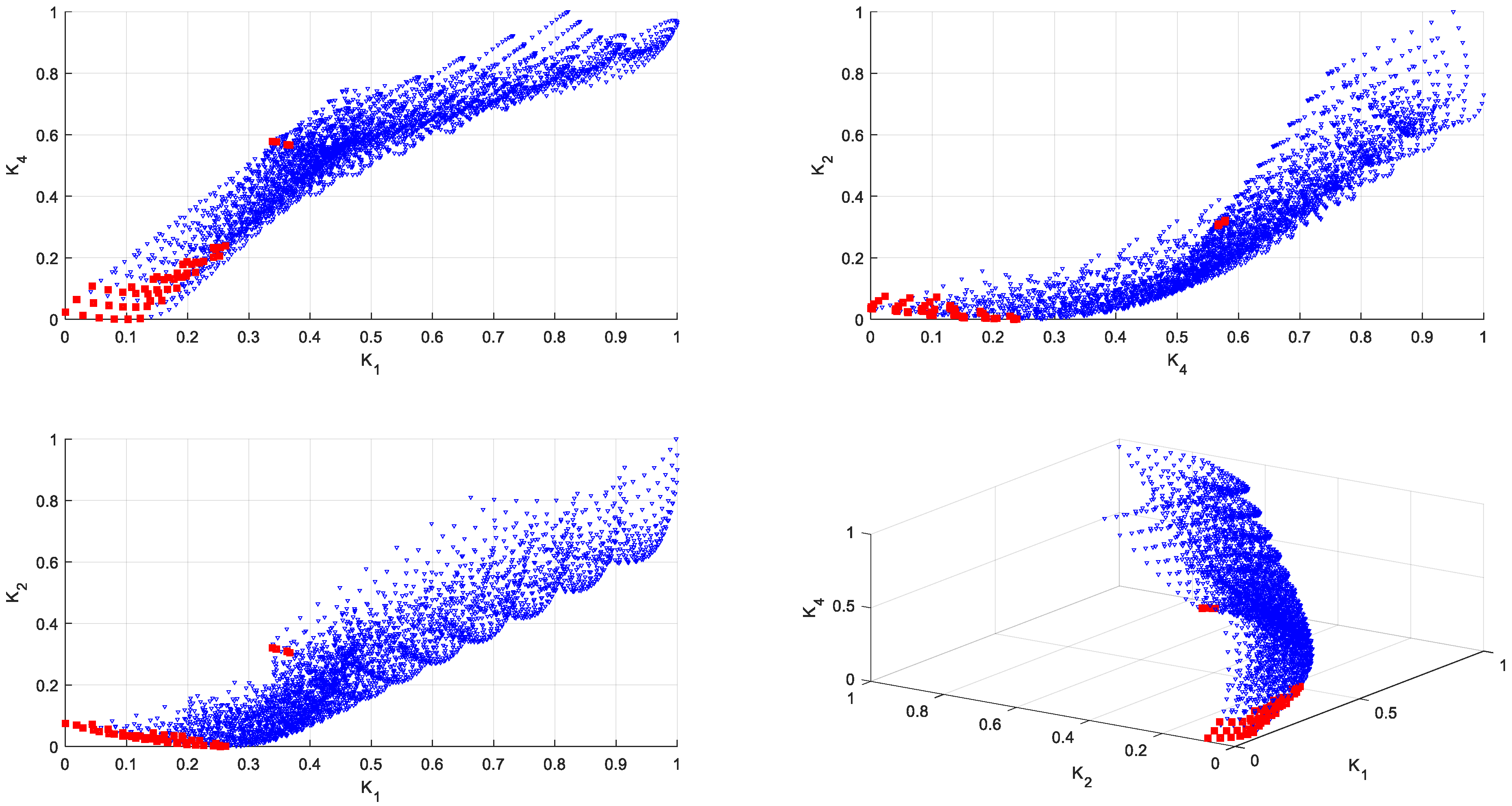
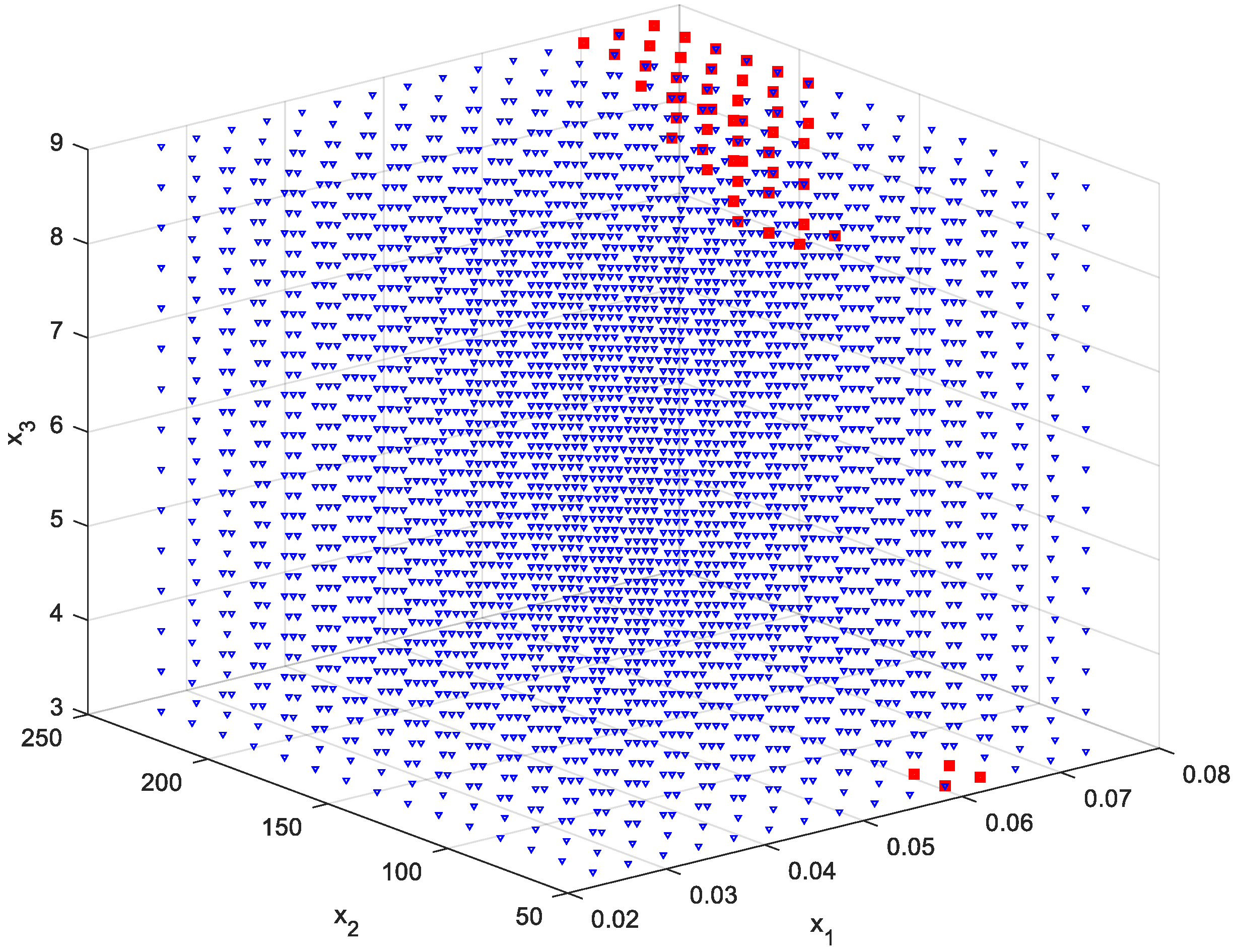
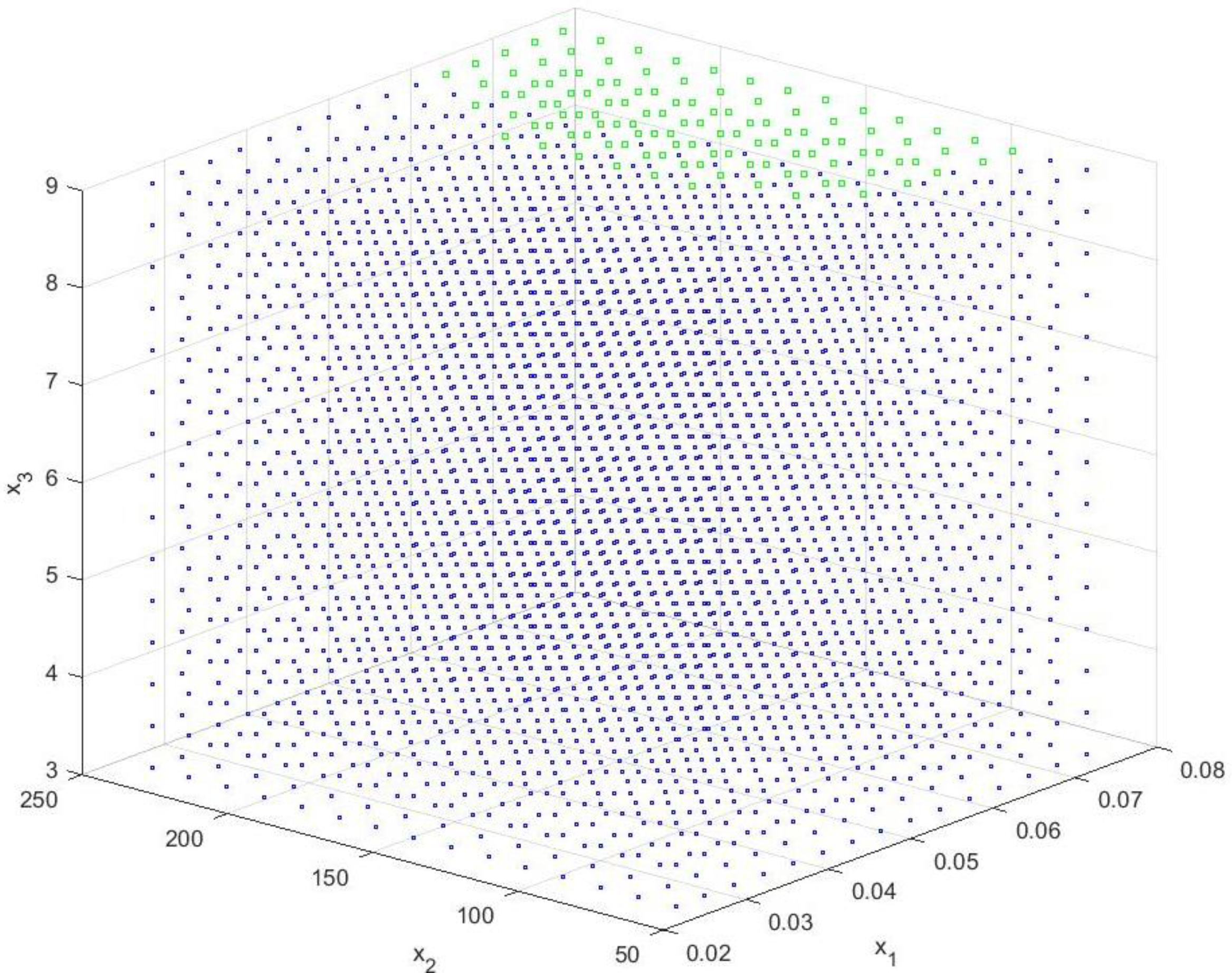
2.7. Reducing the Set of Pareto Optimal Solutions—The Compromise Solutions
3. Results and Discussion
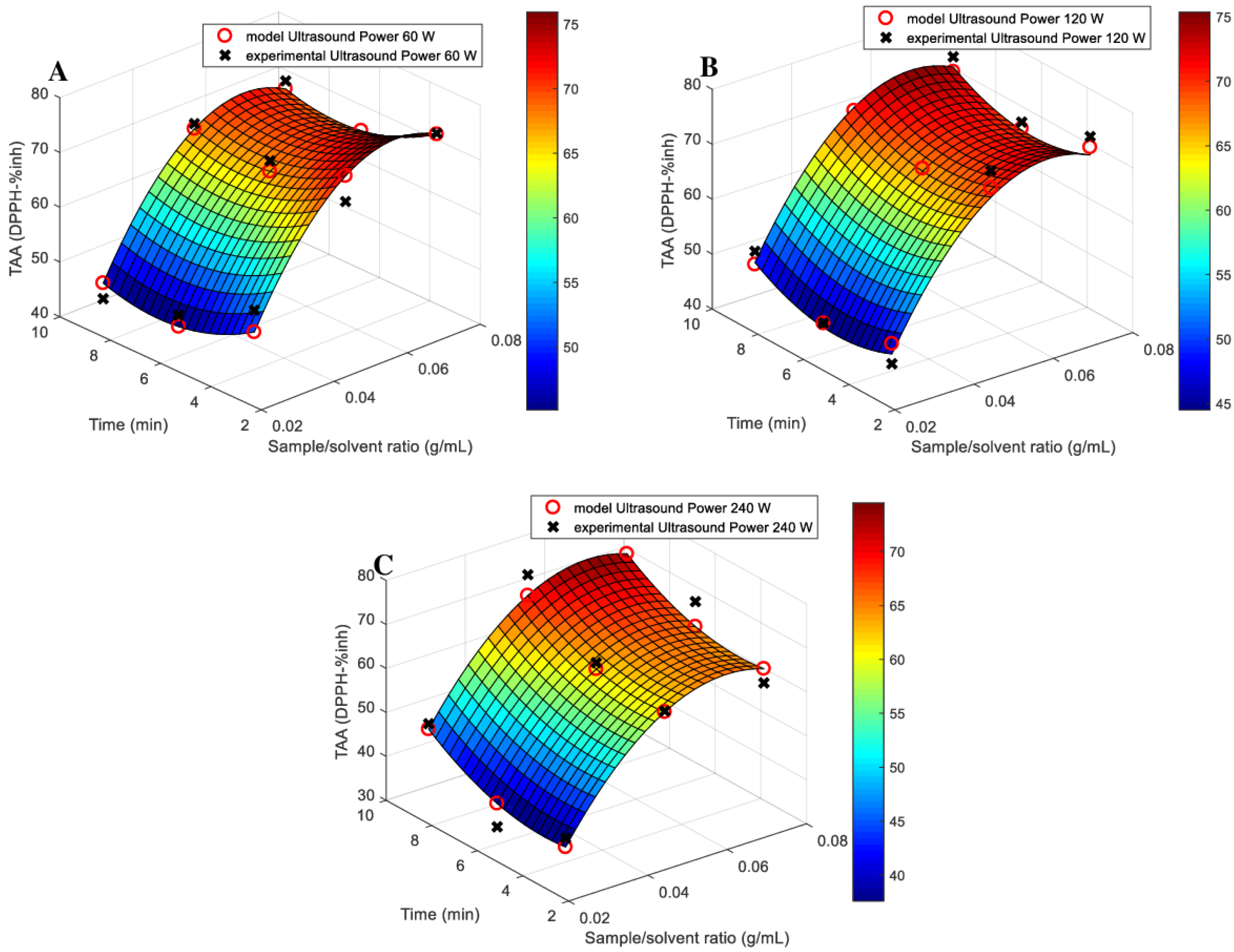
3.1. Models Fitting
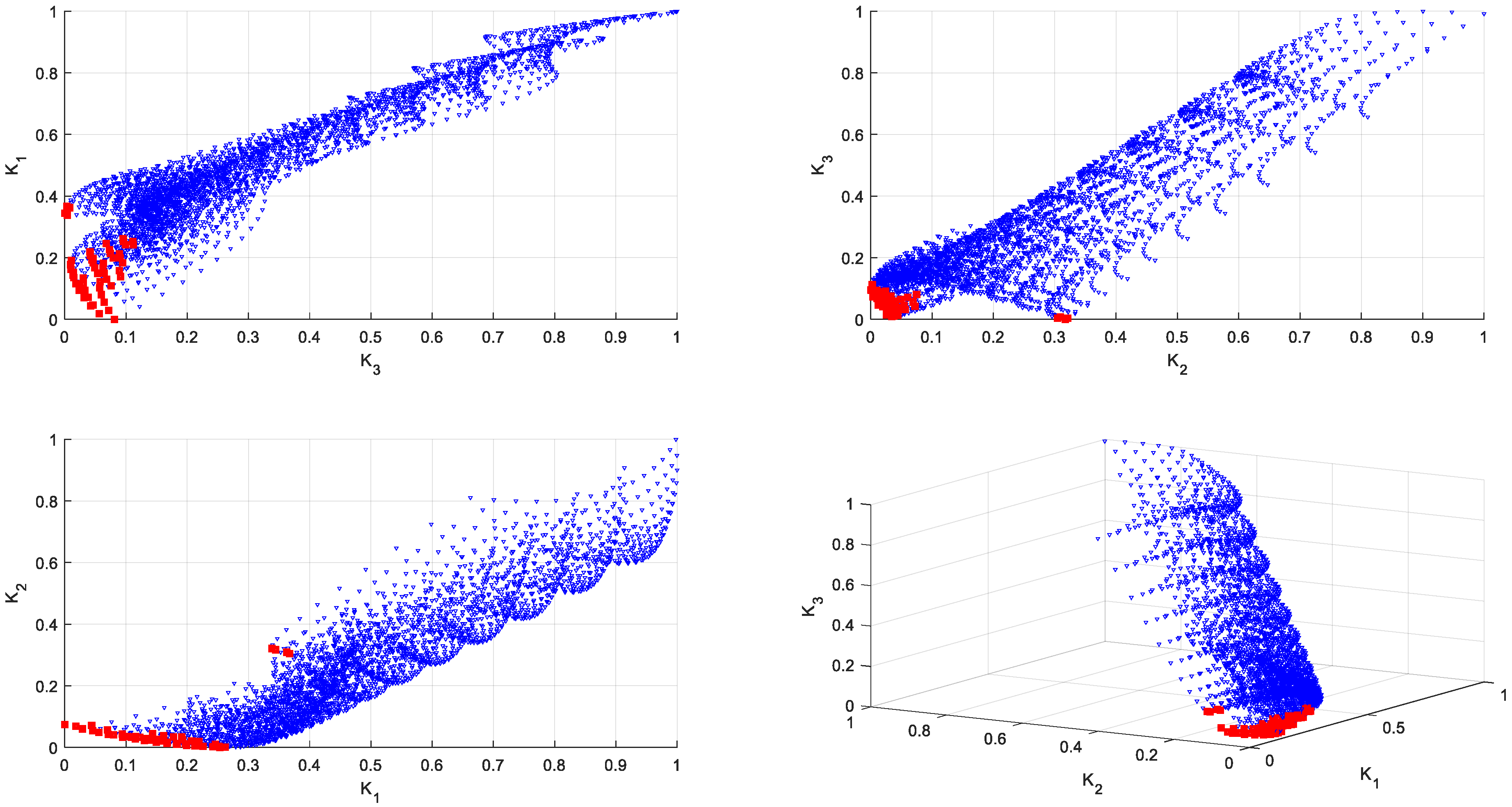
3.2. Multi-Criteria Optimization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, D.C.; Jiang, J.G. Optimization of the microwave-assisted extraction conditions of tea polyphenols from green tea. Int. J. Food Sci. Nutr. 2010, 61, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.S.; Lee, N.; Kim, Y.H.; Lee, C.H.; Hong, S.P.; Jeon, Y.W.; Kim, Y.E. Optimization of ultrasonic extraction of phenolic antioxidants from green tea using response surface methodology. Molecules 2013, 18, 13530–13545. [Google Scholar] [CrossRef]
- Chang, C.J.; Chiu, K.L.; Chen, Y.L.; Chang, C.Y. Separation of catechins from green tea using carbon dioxide extraction. Food Chem. 2000, 68, 109–113. [Google Scholar] [CrossRef]
- Jun, X.; Deji, S.; Ye, L.; Rui, Z. Comparison of in vitro antioxidant activities and bioactive components of green tea extracts by different extraction methods. Int. J. Pharm. 2011, 408, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Banerjee, S.; Chatterjee, J. Efficient extraction strategies of tea (Camellia sinensis) biomolecules. J. Food Sci. Technol. 2015, 52, 3158–3168. [Google Scholar] [CrossRef] [PubMed]
- Ayyildiz, S.S.; Karadeniz, B.; Sagcan, N.; Bahar, B.; Us, A.A.; Alasalvar, C. Optimizing the extraction parameters of epigallocatechin gallate using conventional hot water and ultrasound assisted methods from green tea. Food Bioprod. Process. 2018, 111, 37–44. [Google Scholar] [CrossRef]
- Rocha, J.C.G.; Procopio, F.R.; Mendonça, A.C.; Vieira, L.M.; Perrone, I.T.; Barros, A.R.; Stringheta, P.C. Optimization of ultrasound-assisted extraction of phenolic compounds from jussara (Euterpe edulis M.) and blueberry (Vaccinium myrtillus) fruits. Food Sci. Technol. 2018, 38, 45–53. [Google Scholar] [CrossRef]
- Irakli, M.; Chatzopoulou, P.; Ekateriniadou, L. Optimization of ultrasound-assisted extraction of phenolic compounds: Oleuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind. Crops Prod. 2018, 124, 382–388. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Optimization of ultrasound-assisted extraction of oil from olive pomace using response surface technology: Oil recovery, unsaponifiable matter, total phenol content and antioxidant activity. LWT—Food Sci. Technol. 2017, 79, 178–189. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Ivanović, M.; Albreht, A.; Krajnc, P.; Vovk, I.; Razboršek, M.I. Sustainable ultrasound-assisted extraction of valuable phenolics from inflorescences of Helichrysum arenarium L. using natural deep eutectic solvents. Ind. Crops Prod. 2021, 160, 113102. [Google Scholar] [CrossRef]
- He, B.; Zhang, L.L.; Yue, X.Y.; Liang, J.; Jiang, J.; Gao, X.L.; Yue, P.X. Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Koh, E. Optimization of ultrasound-assisted extraction of anthocyanins and phenolic compounds from black soybeans (Glycine max L.). Food Anal. Methods 2019, 12, 1382–1389. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Martins, N.; Barros, L. Phenolic compounds and its bioavailability: In vitro bioactive compounds or health promoters? In Advances in Food and Nutrition Research; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 82, pp. 1–44. [Google Scholar] [CrossRef]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef] [PubMed]
- Batinić, P.; Čutović, N.; Mrđan, S.; Jovanović, A.A.; Čirić, K.; Marinković, A.; Bugarski, B. The comparison of Ocimum basilicum and Levisticum officinale extracts obtained using different extraction solvents and techniques. Lek. Sirovine 2022, 42, 43. [Google Scholar] [CrossRef]
- Chen, C.; You, L.J.; Abbasi, A.M.; Fu, X.; Liu, R.H. Optimization for ultrasound extraction of polysaccharides from mulberry fruits with antioxidant and hyperglycemic activity in vitro. Carbohydr. Polym. 2015, 130, 122–132. [Google Scholar] [CrossRef]
- Rodrigues, S.; Pinto, G.A.; Fernandes, F.A. Optimization of ultrasound extraction of phenolic compounds from coconut (Cocos nucifera) shell powder by response surface methodology. Ultrason. Sonochem. 2008, 15, 95–100. [Google Scholar] [CrossRef]
- Mozaffarian, V. Flora of Iran; No. 54: Umbelliferae; Publication of Research Institute of Forests and Rangelands; Research Institute of Forests and Rangelands: Tehran, Iran, 2007. [Google Scholar]
- Ghaedi, N.; Pouraboli, I.; Askari, N. Antidiabetic properties of hydroalcoholic leaf and stem extract of Levisticum officinale: An implication for α-amylase inhibitory activity of extract ingredients through molecular docking. Iran. J. Pharm. Res. 2020, 19, 231. [Google Scholar] [CrossRef]
- Szparaga, A.; Kocira, S.; Kapusta, I.; Zaguła, G. Exploring the agro-potential of extract from Levisticum officinale WDJ Koch in soybean cultivation. Ind. Crops Prod. 2023, 203, 117235. [Google Scholar] [CrossRef]
- Szparaga, A. Biostimulating Extracts from Arctium lappa L. As Ecological Additives in Soybean Seed Coating Applications. Agric. Eng. 2023, 27, 1–10. [Google Scholar] [CrossRef]
- Szparaga, A.; Kocira, S.; Kapusta, I.; Zaguła, G. Solid–liquid extraction of bioactive compounds as a green alternative for developing novel biostimulant from Linum usitatissimum L. Chem. Biol. Technol. Agric. 2023, 10, 108. [Google Scholar] [CrossRef]
- Spréa, R.M.; Fernandes, Â.; Finimundy, T.C.; Pereira, C.; Alves, M.J.; Calhelha, R.C.; Canan, C.; Barros, L.; Amaral, J.S.; Ferreira, I.C. Lovage (Levisticum officinale WDJ Koch) roots: A source of bioactive compounds towards a circular economy. Resources 2020, 9, 81. [Google Scholar] [CrossRef]
- Spréa, R.M.; Fernandes, Â.; Calhelha, R.C.; Pereira, C.; Pires, T.C.S.P.; Alves, M.J.; Canan, C.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical and bioactive characterization of the aromatic plant Levisticum officinale W.D.J. Koch: A comprehensive study. Food Funct. 2020, 11, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Birch, J.; Pei, J.; Mohamed Ahmed, I.A.; Yang, H.; Dias, G.; Abd El-Aty, A.M.; Bekhit, A.E.-D. Identification of Six Phytochemical Compounds from Asparagus officinalis L. Root Cultivars from New Zealand and China Using UAE-SPE-UPLC-MS/MS: Effects of Extracts on H2O2-Induced Oxidative Stress. Nutrients 2019, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Szparaga, A.; Kocira, S.; Findura, P.; Kapusta, I.; Zaguła, G.; Świeca, M. Uncovering the Multi-Level Response of Glycine max L. to the Application of Allelopathic Biostimulant from Levisticum officinale Koch. Sci. Rep. 2021, 11, 15360. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Tamreihao, K.; Devi, L.J.; Khunjamayum, R.; Mukherjee, S.; Ashem, R.S.; Ningthoujam, D.S. Biofertilizing potential of feather hydrolysate produced by indigenous keratinolytic Amycolatopsis sp. MBRL 40 for rice cultivation under field conditions. Biocatal. Agric. Biotechnol. 2017, 10, 317–320. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Kaur, M.; Bhari, R.; Singh, R.S. Chicken feather waste-derived protein hydrolysate as a potential biostimulant for cultivation of mung beans. Biologia 2021, 76, 1807–1815. [Google Scholar] [CrossRef]
- Mugwagwa, L.R.; Chimphango, A.F.A. Box-Behnken design based multi-objective optimisation of sequential extraction of pectin and anthocyanins from mango peels. Carbohydr. Polym. 2019, 219, 29–38. [Google Scholar] [CrossRef]
- Iqbal, S.; Younas, U.; Sirajuddin; Chan, K.W.; Sarfraz, R.A.; Uddin, M.K. Proximate Composition and Antioxidant Potential of Leaves from Three Varieties of Mulberry (Morus sp.): A Comparative Study. Int. J. Mol. Sci. 2012, 13, 6651–6664. [Google Scholar] [CrossRef]
- Krivorotova, T.; Sereikaite, J. Determination of fructan exohydrolase activity in the crude extracts of plants. Electron. J. Biotechnol. 2014, 17, 329–333. [Google Scholar] [CrossRef]
- Rocha, M.S.; Rocha, L.C.S.; Feijó, M.B.D.S.; Marotta, P.L.L.D.S.; Mourão, S.C. Multiobjective optimization of the flaxseed mucilage extraction process using normal-boundary intersection approach. Br. Food J. 2021, 123, 3805–3823. [Google Scholar] [CrossRef]
- Curve Fitting Toolbox for Use with Matlab. The MathWorks Inc., Natick. Available online: http://cda.psych.uiuc.edu/matlab_pdf/curvefit.pdf (accessed on 14 November 2024).
- Gómez-Salazar, J.A.; Patlán-González, J.; Sosa-Morales, M.E.; Segovia-Hernandez, J.G.; Sánchez-Ramírez, E.; Ramírez-Márquez, C. Multi-objective optimization of sustainable red prickly pear (Opuntia streptacantha) peel drying and biocompounds extraction using a hybrid stochastic algorithm. Food Bioprod. Process. 2022, 132, 155–166. [Google Scholar] [CrossRef]
- Infantes, M.; Naranjo-Pérez, J.; Sáez, A.; Jiménez-Alonso, J.F. Determining the best Pareto-solution in a multi-objective approach for model updating. In Proceedings of the IABSE Symposium, Towards a Resilient Built Environment–Risk and Asset Management, Guimarães, Portugal, 27–29 March 2019; pp. 523–530. [Google Scholar]
- Das, I. A preference ordering among various Pareto optimal alternatives. Struct. Optim. 1999, 18, 30–35. [Google Scholar] [CrossRef]
- Foroughi, A.H.; Razavi, M.J. Multi-objective shape optimization of bone scaffolds: Enhancement of mechanical properties and permeability. Acta Biomater. 2022, 146, 317–340. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Rana, M.R.; Maisha, M.R.; Sayem, A.S.M.; Rahman, M.; Ara, R. Optimization of ultrasound-assisted extraction of phenolic content & antioxidant activity of hog plum (Spondias pinnata L. f. kurz) pulp by response surface methodology. Heliyon 2022, 8, e11109. [Google Scholar] [CrossRef]
- Bouloumpasi, E.; Skendi, A.; Christaki, S.; Biliaderis, C.C.; Irakli, M. Optimizing conditions for the recovery of lignans from sesame cake using three green extraction methods: Microwave-, ultrasound-and accelerated-assisted solvent extraction. Ind. Crops Prod. 2024, 207, 117770. [Google Scholar] [CrossRef]
- Nikolić, V.G.; Troter, D.Z.; Savić, I.M.; Gajić, I.M.S.; Zvezdanović, J.B.; Konstantinović, I.B.; Konstantinović, S.S. Design and optimization of “greener” and sustainable ultrasound-assisted extraction of valuable bioactive compounds from common centaury (Centaurium erythraea Rafn) aerial parts: A comparative study using aqueous propylene glycol and ethanol. Ind. Crops Prod. 2023, 192, 116070. [Google Scholar] [CrossRef]
- Brahmi, F.; Blando, F.; Sellami, R.; Mehdi, S.; De Bellis, L.; Negro, C.; Haddadi-Guemghar, H.; Madani, K.; Makhlouf-Boulekbache, L. Optimization of the conditions for ultrasound-assisted extraction of phenolic compounds from Opuntia ficus-indica [L.] Mill. flowers and comparison with conventional procedures. Ind. Crops Prod. 2022, 184, 114977. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Kutlu, N.; Kamiloglu, A.; Elbir, T. Optimization of Ultrasound Extraction of Phenolic Compounds from Tarragon (Artemisia dracunculus L.) Using Box–Behnken Design. Biomass Convers. Biorefin. 2022, 12, 5397–5408. [Google Scholar] [CrossRef]
- Garcia-Mendoza, M.D.P.; Espinosa-Pardo, F.A.; Savoire, R.; Etchegoyen, C.; Harscoat-Schiavo, C.; Subra-Paternault, P. Recovery and antioxidant activity of phenolic compounds extracted from walnut press-cake using various methods and conditions. Ind. Crops Prod. 2021, 167, 113546. [Google Scholar] [CrossRef]
- Assefa, A.D.; Saini, R.K.; Keum, Y.S. Extraction of antioxidants and flavonoids from yuzu (Citrus junos Sieb ex Tanaka) peels: A response surface methodology study. J. Food Meas. Charact. 2017, 11, 364–379. [Google Scholar] [CrossRef]
- Babotă, M.; Frumuzachi, O.; Gâvan, A.; Iacoviță, C.; Pinela, J.; Barros, L.; Ferreira, I.C.; Zhang, L.; Lucini, L.; Rocchetti, G.; et al. Optimized ultrasound-assisted extraction of phenolic compounds from Thymus comosus Heuff. ex Griseb. et Schenk (wild thyme) and their bioactive potential. Ultrason. Sonochem. 2022, 84, 105954. [Google Scholar] [CrossRef]
- Dawidowicz, A.; Wianowska, D.; Olszowy, M. On practical problems in estimation of antioxidant activity of compounds by DPPH method (Problems in estimation of antioxidant activity). Food Chem. 2002, 131, 1037–1043. [Google Scholar] [CrossRef]
- Pekal, A.; Pyrzynska, K. Effect of pH and metal ions on DPPH radical scavenging activity of tea. Int. J. Food Sci. Nutr. 2015, 66, 58–62. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy, M. Mechanism change in estimating of antioxidant activity of phenolic compounds. Talanta 2012, 97, 312–317. [Google Scholar] [CrossRef]
- Bolling, B.W.; Chen, Y.-Y.; Kamil, A.G.; Chen, C.Y.O. Assay dilution factors confound measures of total antioxidant capacity in polyphenol-rich juices. J. Food Sci. 2012, 77, H69–H75. [Google Scholar] [CrossRef]
- Ferri, M.; Gianotti, A.; Tassoni, A. Optimisation of assay conditions for the determination of antioxidant capacity and polyphenols in cereal food components. J. Food Compost. Anal. 2013, 30, 94–101. [Google Scholar] [CrossRef]
- Mondal, S.C.; Lee, W.H.; Eun, J.B. Ultrasonic extraction of reducing sugar and polyphenols from burdock (Arctium lappa L.) root waste and evaluation of antioxidants and α-glucosidase inhibition activity. Biomass Convers. Biorefin. 2023, 1, 1–18. [Google Scholar] [CrossRef]
- AlYammahi, J.; Hai, A.; Krishnamoorthy, R.; Arumugham, T.; Hasan, S.W.; Banat, F. Ultrasound-assisted extraction of highly nutritious date sugar from date palm (Phoenix dactylifera) fruit powder: Parametric optimization and kinetic modeling. Ultrason. Sonochem. 2022, 88, 106107. [Google Scholar] [CrossRef]
- Nuerxiati, R.; Abuduwaili, A.; Mutailifu, P.; Wubulikasimu, A.; Rustamova, N.; Jingxue, C.; Aisa, H.A.; Yili, A. Optimization of ultrasonic-assisted extraction, characterization and biological activities of polysaccharides from Orchis chusua D. Don (Salep). Int. J. Biol. Macromol. 2019, 141, 431–443. [Google Scholar] [CrossRef] [PubMed]
- da Silva Donadone, D.B.; Giombelli, C.; Silva, D.L.G.; Stevanato, N.; da Silva, C.; Bolanho Barros, B.C. Ultrasound-assisted extraction of phenolic compounds and soluble sugars from the stem portion of peach palm. J. Food Process. Preserv. 2020, 44, e14636. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, Q.; Pang, Z.; Xie, J.; Lin, L.; Yao, Q. Optimization extraction, characterization and anticancer activities of polysaccharides from mango pomace. Int. J. Biol. Macromol. 2018, 117, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.P.; Lai, P.L.; Jen, H.W.K. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- Othman, A.; Mukhtar, N.J.; Ismail, N.S.; Chang, S.K. Phenolics, flavonoids content and antioxidant activities of 4 Malaysian herbal plants. Int. Food Res. J. 2014, 21, 759–766. [Google Scholar]
- Paixao, N.; Perestrelo, R.; Marques, J.; Camara, J. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- Thoo, Y.Y.; Ho, S.K.; Liang, J.Y.; Ho, C.W.; Tan, C.P. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia). Food Chem. 2010, 120, 290–295. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; Van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.-C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef]
- Woinaroschy, A.; Damşa, F. Multiobjective Optimization of Total Monomeric Anthocyanins and Total Flavonoids Contents in Ultrasound-Assisted Extraction from Purple Potato Tubers. J. Food Process Eng. 2017, 40, e12422. [Google Scholar] [CrossRef]
- Rangaiah, G.P. Multi-Objective Optimization: Techniques and Applications in Chemical Engineering; World Scientific: Singapore, 2009. [Google Scholar]
- Kao, G.K.; Jacobson, S.H. Finding preferred subsets of Pareto optimal solutions. Comput. Optim. Appl. 2008, 40, 73–95. [Google Scholar] [CrossRef]
- Kasprzak, E.M.; Lewis, K.E. An approach to facilitate decision tradeoffs in Pareto solution sets. J. Eng. Valuat. Cost Anal. 2000, 3, 173–187. [Google Scholar]
- Mattson, C.A.; Mullur, A.A.; Messac, A. Smart Pareto filter: Obtaining a minimal representation of multiobjective design space. Eng. Optim. 2004, 36, 721–740. [Google Scholar] [CrossRef]
- Kasprzak, E.M.; Lewis, K.E. Pareto analysis in multiobjective optimization using the collinearity theorem and scaling method. Struct. Multidiscip. Optim. 2001, 22, 208–218. [Google Scholar] [CrossRef]
- Messac, A.; Ismail-Yahaya, A.; Mattson, C.A. The normalized normal constraint method for generating the Pareto frontier. Struct. Multidiscip. Optim. 2003, 25, 86–98. [Google Scholar] [CrossRef]
- Messac, A.; Mattson, C.A. Normal constraint method with guarantee of even representation of complete Pareto frontier. AIAA J. 2004, 42, 2101–2111. [Google Scholar] [CrossRef]
- Gebreel, A.Y. The Best Compromise Solution for Multi-objective Programming Problems. Int. J. Glob. Oper. Res. 2023, 4, 189–204. [Google Scholar] [CrossRef]
- Gebreel, A.Y. Solving the multi-objective convex programming problems to get the best compromise solution. Aust. J. Basic Appl. Sci. 2021, 15, 17–29. [Google Scholar]
- Hancock, B.J.; Mattson, C.A. The smart normal constraint method for directly generating a smart Pareto set. Struct. Multidiscip. Optim. 2013, 48, 763–775. [Google Scholar] [CrossRef]
- Machuca, E.; Mandow, L.; Galand, L. An evaluation of best compromise search in graphs. In Conference of the Spanish Association for Artificial Intelligence, Proceedings of the 15th Conference of the Spanish Association for Artificial Intelligence, CAEPIA 2013, Madrid, Spain, 17–20 September 2013; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–11. [Google Scholar] [CrossRef]
- Wang, H.; Olhofer, M.; Jin, Y. A mini-review on preference modeling and articulation in multi-objective optimization: Current status and challenges. Complex Intell. Syst. 2017, 3, 233–245. [Google Scholar] [CrossRef]
- Reynoso-Meza, G.; Sanchis, J.; Blasco, X.; Garcia-Nieto, S. Physical programming for preference driven evolutionary multi-objective optimization. Appl. Soft Comput. 2014, 24, 341–362. [Google Scholar] [CrossRef]







| Source | Total Phenolic Content (TPC) (mg GAE/g) | Total Flavonoids Content (TFC) (μmol CAT/L) | Total Antioxidant Activity (TAA) (DPPH—%inh) | Reducing Sugar Content (RSC) (g GE/L) |
|---|---|---|---|---|
| Absolute term, a0 | 166.92 | −126 | 22.9 | 4.02 |
| Linear | ||||
| a1 | 4852.1 | 10,707.8 | 2122.25 | 87.53 |
| a2 | −0.956 | 0.29 | −0.33 | 0.02 |
| a3 | −20.533 | 44.8 | −5.97 | −1.79 |
| Interaction | ||||
| a4 | 5.58 | 2.89 | 0.32 | 0.035 |
| a5 | 213.7 | 105.3 | −2.22 | 21.12 |
| a6 | 0.043 | 0.045 | 0.012 | 0.003 |
| Quadratic | ||||
| a7 | −49,195.96 | −87,147.45 | −16,726.9 | −1197.48 |
| a8 | 0.0012 | −0.0026 | −0.00029 | −0.0001 |
| a9 | 0.66 | −3.87 | 0.387 | 0.059 |
| Indicators | ||||
| MSE | 373.84 | 1308.28 | 16.84 | 1.11 |
| R-square | 0.91 | 0.88 | 0.93 | 0.91 |
| Adjusted R square | 0.86 | 0.804 | 0.889 | 0.854 |
| F-statistic | ||||
| sse | 6.36 × 103 | 2.22 × 104 | 286.1482 | 18.8592 |
| F-value | 18.21 | 12.796 | 24.0004 | 17.9322 |
| ssr | 6.13 × 104 | 1.51 × 105 | 3.64 × 103 | 1.79 × 102 |
| p-value | 4.74 × 10−7 | 6.05 × 10−6 | 5.91 × 10−8 | 5.31 × 10−7 |
| x1opt (Sample/Solvent Ratio) | x3opt (Time) | x2opt (Ultrasound Power) | x1opt (Sample/Solvent Ratio) | x3opt (Time) | x2opt (Ultrasound Power) |
|---|---|---|---|---|---|
| 0.0607 | 3.0000 | 60.0000 | 0.0679 | 8.5714 | 188.5714 |
| 0.0607 | 3.0000 | 72.8571 | 0.0679 | 9.0000 | 188.5714 |
| 0.0643 | 3.0000 | 60.0000 | 0.0679 | 8.5714 | 201.4286 |
| 0.0643 | 3.0000 | 72.8571 | 0.0679 | 9.0000 | 201.4286 |
| 0.0643 | 8.1429 | 162.8571 | 0.0679 | 9.0000 | 214.2857 |
| 0.0643 | 8.5714 | 162.8571 | 0.0679 | 9.0000 | 227.1429 |
| 0.0643 | 9.0000 | 162.8571 | 0.0679 | 9.0000 | 240.0000 |
| 0.0643 | 8.5714 | 175.7143 | 0.0714 | 7.7143 | 150.0000 |
| 0.0643 | 9.0000 | 175.7143 | 0.0714 | 7.7143 | 162.8571 |
| 0.0643 | 8.5714 | 188.5714 | 0.0714 | 8.1429 | 162.8571 |
| 0.0643 | 9.0000 | 188.5714 | 0.0714 | 8.5714 | 162.8571 |
| 0.0643 | 9.0000 | 201.4286 | 0.0714 | 8.1429 | 175.7143 |
| 0.0679 | 7.7143 | 150.0000 | 0.0714 | 8.5714 | 175.7143 |
| 0.0679 | 7.7143 | 162.8571 | 0.0714 | 9.0000 | 175.7143 |
| 0.0679 | 8.1429 | 162.8571 | 0.0714 | 8.1429 | 188.5714 |
| 0.0679 | 8.5714 | 162.8571 | 0.0714 | 8.5714 | 188.5714 |
| 0.0679 | 7.7143 | 175.7143 | 0.0714 | 9.0000 | 188.5714 |
| 0.0679 | 8.1429 | 175.7143 | 0.0714 | 8.5714 | 201.4286 |
| 0.0679 | 8.5714 | 175.7143 | 0.0714 | 9.0000 | 201.4286 |
| 0.0679 | 9.0000 | 175.7143 | 0.0714 | 8.5714 | 214.2857 |
| 0.0679 | 8.1429 | 188.5714 | 0.0714 | 9.0000 | 214.2857 |
| x1 (Sample/Solvent Ratio) | x3 (Time) | x2 (Ultrasound Power) | x1 (Sample/Solvent Ratio) | x3 (Time) | x2 (Ultrasound Power) |
|---|---|---|---|---|---|
| 0.0643 | 9.0000 | 188.5714 | 0.0714 | 9.0000 | 201.4286 |
| 0.0643 | 9.0000 | 201.4286 | 0.0714 | 8.5714 | 214.2857 |
| 0.0679 | 9.0000 | 175.7143 | 0.0714 | 9.0000 | 214.2857 |
| 0.0679 | 9.0000 | 188.5714 | 0.0714 | 9.0000 | 227.1429 |
| 0.0679 | 8.5714 | 201.4286 | 0.0714 | 9.0000 | 240.0000 |
| 0.0679 | 9.0000 | 201.4286 | 0.0750 | 9.0000 | 175.7143 |
| 0.0679 | 9.0000 | 214.2857 | 0.0750 | 8.5714 | 188.5714 |
| 0.0679 | 9.0000 | 227.1429 | 0.0750 | 9.0000 | 188.5714 |
| 0.0679 | 9.0000 | 240.0000 | 0.0750 | 9.0000 | 201.4286 |
| 0.0714 | 9.0000 | 175.7143 | 0.0750 | 9.0000 | 214.2857 |
| 0.0714 | 8.5714 | 188.5714 | 0.0750 | 9.0000 | 227.1429 |
| 0.0714 | 9.0000 | 188.5714 | 0.0750 | 9.0000 | 240.0000 |
| 0.0714 | 8.5714 | 201.4286 |
| x1 (Sample/Solvent Ratio) | x3 (Time) | x2 (Ultrasound Power) | x1 (Sample/Solvent Ratio) | x3 (Time) | x2 (Ultrasound Power) |
|---|---|---|---|---|---|
| 0.0607 | 9.0000 | 162.8571 | 0.0714 | 9.0000 | 137.1429 |
| 0.0607 | 9.0000 | 175.7143 | 0.0714 | 8.1429 | 150.0000 |
| 0.0607 | 9.0000 | 188.5714 | 0.0714 | 8.5714 | 150.0000 |
| 0.0607 | 9.0000 | 201.4286 | 0.0714 | 9.0000 | 150.0000 |
| 0.0607 | 9.0000 | 214.2857 | 0.0714 | 8.1429 | 162.8571 |
| 0.0607 | 9.0000 | 227.1429 | 0.0714 | 8.5714 | 162.8571 |
| 0.0607 | 9.0000 | 240.0000 | 0.0714 | 9.0000 | 162.8571 |
| 0.0643 | 9.0000 | 124.2857 | 0.0714 | 8.1429 | 175.7143 |
| 0.0643 | 9.0000 | 137.1429 | 0.0714 | 8.5714 | 175.7143 |
| 0.0643 | 9.0000 | 150.0000 | 0.0714 | 9.0000 | 175.7143 |
| 0.0643 | 8.5714 | 162.8571 | 0.0714 | 8.1429 | 188.5714 |
| 0.0643 | 9.0000 | 162.8571 | 0.0714 | 8.5714 | 188.5714 |
| 0.0643 | 8.5714 | 175.7143 | 0.0714 | 9.0000 | 188.5714 |
| 0.0643 | 9.0000 | 175.7143 | 0.0714 | 8.1429 | 201.4286 |
| 0.0643 | 8.5714 | 188.5714 | 0.0714 | 8.5714 | 201.4286 |
| 0.0643 | 9.0000 | 188.5714 | 0.0714 | 9.0000 | 201.4286 |
| 0.0643 | 8.5714 | 201.4286 | 0.0714 | 8.1429 | 214.2857 |
| 0.0643 | 9.0000 | 201.4286 | 0.0714 | 8.5714 | 214.2857 |
| 0.0643 | 8.5714 | 214.2857 | 0.0714 | 9.0000 | 214.2857 |
| 0.0643 | 9.0000 | 214.2857 | 0.0714 | 8.1429 | 227.1429 |
| 0.0643 | 8.5714 | 227.1429 | 0.0714 | 8.5714 | 227.1429 |
| 0.0643 | 9.0000 | 227.1429 | 0.0714 | 9.0000 | 227.1429 |
| 0.0643 | 8.5714 | 240.0000 | 0.0714 | 8.1429 | 240.0000 |
| 0.0643 | 9.0000 | 240.0000 | 0.0714 | 8.5714 | 240.0000 |
| 0.0679 | 9.0000 | 98.5714 | 0.0714 | 9.0000 | 240.0000 |
| 0.0679 | 9.0000 | 111.4286 | 0.0750 | 9.0000 | 85.7143 |
| 0.0679 | 8.5714 | 124.2857 | 0.0750 | 9.0000 | 98.5714 |
| 0.0679 | 9.0000 | 124.2857 | 0.0750 | 8.5714 | 111.4286 |
| 0.0679 | 8.5714 | 137.1429 | 0.0750 | 9.0000 | 111.4286 |
| 0.0679 | 9.0000 | 137.1429 | 0.0750 | 8.5714 | 124.2857 |
| 0.0679 | 8.5714 | 150.0000 | 0.0750 | 9.0000 | 124.2857 |
| 0.0679 | 9.0000 | 150.0000 | 0.0750 | 8.1429 | 137.1429 |
| 0.0679 | 8.5714 | 162.8571 | 0.0750 | 8.5714 | 137.1429 |
| 0.0679 | 9.0000 | 162.8571 | 0.0750 | 9.0000 | 137.1429 |
| 0.0679 | 8.1429 | 175.7143 | 0.0750 | 8.1429 | 150.0000 |
| 0.0679 | 8.5714 | 175.7143 | 0.0750 | 8.5714 | 150.0000 |
| 0.0679 | 9.0000 | 175.7143 | 0.0750 | 9.0000 | 150.0000 |
| 0.0679 | 8.1429 | 188.5714 | 0.0750 | 8.1429 | 162.8571 |
| 0.0679 | 8.5714 | 188.5714 | 0.0750 | 8.5714 | 162.8571 |
| 0.0679 | 9.0000 | 188.5714 | 0.0750 | 9.0000 | 162.8571 |
| 0.0679 | 8.1429 | 201.4286 | 0.0750 | 8.1429 | 175.7143 |
| 0.0679 | 8.5714 | 201.4286 | 0.0750 | 8.5714 | 175.7143 |
| 0.0679 | 9.0000 | 201.4286 | 0.0750 | 9.0000 | 175.7143 |
| 0.0679 | 8.1429 | 214.2857 | 0.0750 | 8.1429 | 188.5714 |
| 0.0679 | 8.5714 | 214.2857 | 0.0750 | 8.5714 | 188.5714 |
| 0.0679 | 9.0000 | 214.2857 | 0.0750 | 9.0000 | 188.5714 |
| 0.0679 | 8.1429 | 227.1429 | 0.0750 | 8.1429 | 201.4286 |
| 0.0679 | 8.5714 | 227.1429 | 0.0750 | 8.5714 | 201.4286 |
| 0.0679 | 9.0000 | 227.1429 | 0.0750 | 9.0000 | 201.4286 |
| 0.0679 | 8.1429 | 240.0000 | 0.0750 | 8.1429 | 214.2857 |
| 0.0679 | 8.5714 | 240.0000 | 0.0750 | 8.5714 | 214.2857 |
| 0.0679 | 9.0000 | 240.0000 | 0.0750 | 9.0000 | 214.2857 |
| 0.0714 | 9.0000 | 85.7143 | 0.0750 | 8.1429 | 227.1429 |
| 0.0714 | 9.0000 | 98.5714 | 0.0750 | 8.5714 | 227.1429 |
| 0.0714 | 8.5714 | 111.4286 | 0.0750 | 9.0000 | 227.1429 |
| 0.0714 | 9.0000 | 111.4286 | 0.0750 | 8.1429 | 240.0000 |
| 0.0714 | 8.5714 | 124.2857 | 0.0750 | 8.5714 | 240.0000 |
| 0.0714 | 9.0000 | 124.2857 | 0.0750 | 9.0000 | 240.0000 |
| 0.0714 | 8.5714 | 137.1429 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plawgo, M.; Kocira, S.; Bohata, A. Multi-Criteria Optimization Conditions for the Recovery of Bioactive Compounds from Levisticum officinale WDJ Koch Roots Using Green and Sustainable Ultrasound-Assisted Extraction. Processes 2024, 12, 275. https://doi.org/10.3390/pr12020275
Plawgo M, Kocira S, Bohata A. Multi-Criteria Optimization Conditions for the Recovery of Bioactive Compounds from Levisticum officinale WDJ Koch Roots Using Green and Sustainable Ultrasound-Assisted Extraction. Processes. 2024; 12(2):275. https://doi.org/10.3390/pr12020275
Chicago/Turabian StylePlawgo, Michał, Sławomir Kocira, and Andrea Bohata. 2024. "Multi-Criteria Optimization Conditions for the Recovery of Bioactive Compounds from Levisticum officinale WDJ Koch Roots Using Green and Sustainable Ultrasound-Assisted Extraction" Processes 12, no. 2: 275. https://doi.org/10.3390/pr12020275
APA StylePlawgo, M., Kocira, S., & Bohata, A. (2024). Multi-Criteria Optimization Conditions for the Recovery of Bioactive Compounds from Levisticum officinale WDJ Koch Roots Using Green and Sustainable Ultrasound-Assisted Extraction. Processes, 12(2), 275. https://doi.org/10.3390/pr12020275








