MALDI-TOF Mass Spectrometry-Based Identification of Aerobic Mesophilic Bacteria in Raw Unpreserved and Preserved Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Milk Sampling
2.2. Classical Method of Determining the Aerobic Mesophilic Bacteria Count in Raw Milk
2.3. MALDI-TOF Mass Spectrometry
2.4. Data Analysis
3. Results and Discussion
3.1. Classical Method of Determining the Number of Aerobic Mesophilic Bacteria in Raw and Preserved Milk
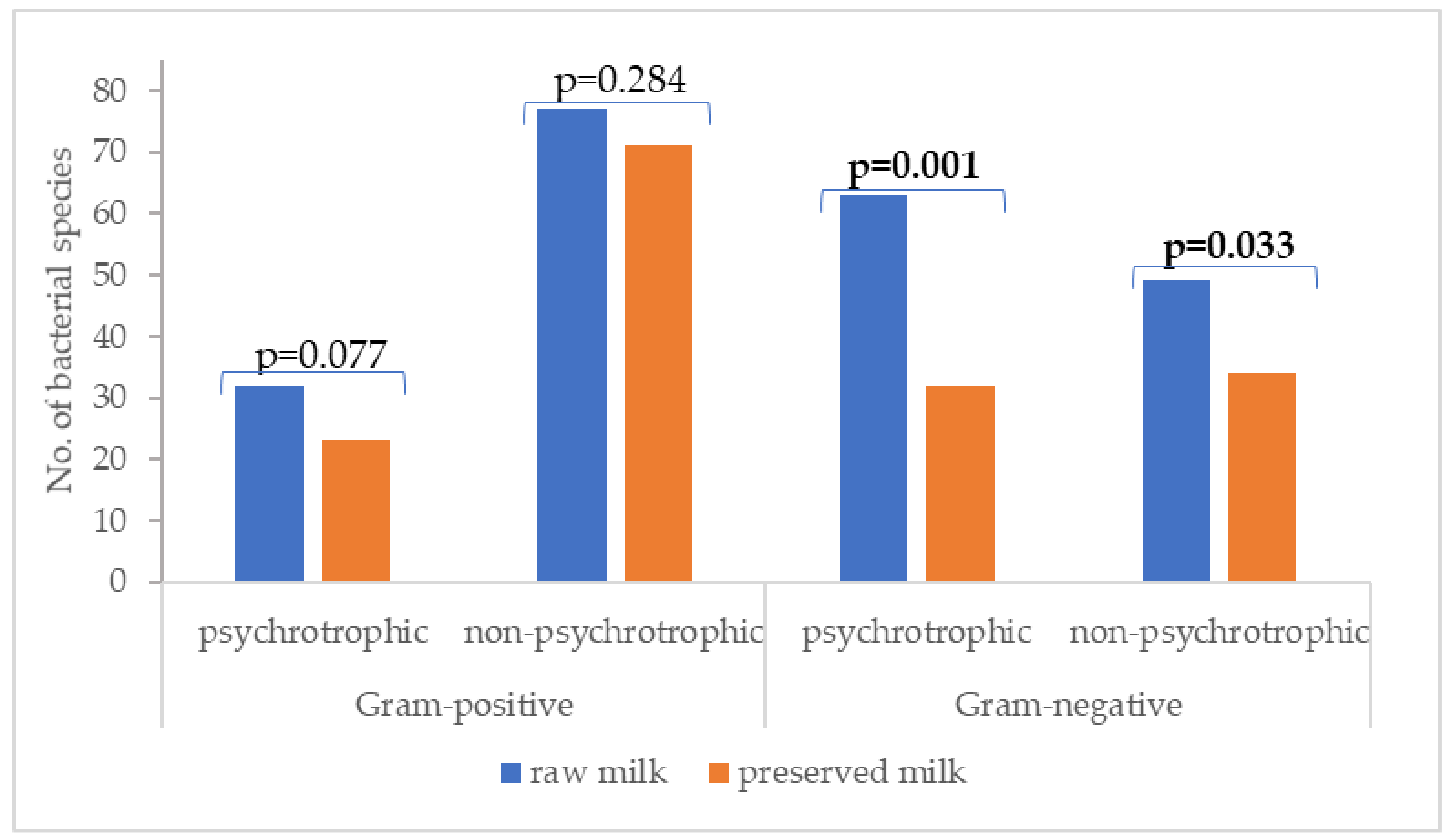
3.2. MALDI-TOF Mass Spectrometry Identification of Aerobic Mesophilic Bacteria
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Antunac, N.; Havranek, J. Milk. Chemistry, Physics and Microbiology; Internal Script; University of Zagreb, Faculty of Agriculture: Zagreb, Croatia, 2013. [Google Scholar]
- Herrera, A.G. Mesophilic Aerobic Microorganisms. In Food Microbiology Protocols, Methods in Biotechnology; Spencer, J.F.T., de Ragout Spencer, A.L., Eds.; Humana Press: Totowa, NJ, USA, 2001; Volume 14. [Google Scholar] [CrossRef]
- Samaržija, D. Dairy Microbiology—General Properties of Bacteria, Yeasts, Molds and Viruses with Basics of Taxonomy; Internal Script; University of Zagreb, Faculty of Agriculture: Zagreb, Croatia, 2018. [Google Scholar]
- Weisglass, H. Bakterije i Bolesti Čovjeka: Specijalna Bakteriologija; Školska Knjiga: Zagreb, Croatia, 1983. [Google Scholar]
- Perin, L.M.; Pereira, J.G.; Bersot, L.S.; Nero, L.A. Chapter 3—The Microbiology of Raw Milk. In Raw Milk: Balance between Hazards and Benefits; Nero, L.A., De Carvalho, A.F., Eds.; Academic Press: Oxford, UK, 2019; pp. 45–64. [Google Scholar] [CrossRef]
- Ministarstvo Poljoprivrede. Pravilnik o Utvrđivanju Sastava Sirovog Mlijeka. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2020_12_136_2605.html (accessed on 22 February 2024).
- Upadhyay, N.; Goyal, A.; Kumar, A.; Ghai, D.L.; Singh, R. Preservation of milk and milk products for analytical purposes. Food Rev. Int. 2014, 30, 203–224. [Google Scholar] [CrossRef]
- Antunac, N.; Mikulec, N.; Zamberlin, Š.; Sović, J.Š.; Blažek, A.; Horvat, I. Influence of various methods of milk sample preservation on the number of somatic cells. Milchwissenschaft 2010, 65, 313–316. [Google Scholar]
- Samaržija, D.; Antunac, N.; Pogačić, T.; Sikora, S. Determination of the total number of bacteria in raw milk by flow cytometry. Dairy 2004, 54, 39–51. Available online: https://hrcak.srce.hr/clanak/4022 (accessed on 22 February 2024).
- Cabrol, L.; Quemeneur, M.; Misson, B. Inhibitory effects of sodium azide on microbial growth in experimental resuspension of marine sediment. J. Microbiol. Methods 2017, 133, 62–65. [Google Scholar] [CrossRef]
- Cieśla, Z.; Mardarowicz, K.; Klopotowski, T. Inhibition of DNA synthesis and cell division in Salmonella typhimurium by azide. Mol. Gen. Genet. 1974, 135, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D.B.; Cabelli, R.J.; Dolan, K.M.; Jarosik, G.P. Azide-resistant mutants of Escherichia coli alter the SecA protein, an azide-sensitive component of the protein export machinery. Proc. Natl. Acad. Sci. USA 1990, 87, 8227–8231. [Google Scholar] [CrossRef] [PubMed]
- Chandler, R.; Jamshad, M.; Yule, J.; Robinson, A.; Alam, F.; Dunne, K.A.; Nabi, N.; Henderson, I.; Huber, D. Genetic screen suggests an alternative mechanism for azide-mediated inhibition of SecA. bioRxiv 2018, 173039. [Google Scholar] [CrossRef]
- Suresh, A.; Pan, C.; Ng, W.J. Sodium azide inhibition of microbial activities and impact on sludge floc destabilization. Chemosphere 2020, 244, 125452. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, S.; Girault, H.H. Revealing the effects of three different antimicrobial agents on E. coli biofilms by using Soft-Probe Scanning Electrochemical Microscopy. Appl. Nano 2023, 4, 260–279. [Google Scholar] [CrossRef]
- Sobrun, Y.; Bhaw-Luximon, A.; Jhurry, D.; Puchooa, D. Isolation of lactic acid bacteria from sugar cane juice and production of lactic acid from selected improved strains. Adv. Biosci. Biotechnol. 2012, 3, 398–407. [Google Scholar] [CrossRef]
- Sešķēna, R.J.; Jankevica, L. Influence of chemical preservatives on the quality and composition indices of raw milk samples. Acta Univ. Latv. 2007, 723, 171–180. [Google Scholar]
- Sinaga, H.; Deeth, H.; Bhandari, B. Effect of sodium azide addition and aging storage on casein micelle size. IOP Conf. Ser. Earth Environ. Sci. 2018, 122, 012083. [Google Scholar] [CrossRef]
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- Gonzalo, C.; Martínez, J.R.; Carriedo, J.A.; San Primitivo, F. Fossomatic Cell-Counting on Ewe Milk: Comparison with Direct Microscopy and Study of Variation Factors. J. Dairy Sci. 2003, 86, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Croatian Agency for Agriculture and Food (HAPIH). Annual Report for 2022; Editorial Center for Quality Control of Livestock Products: Osijek, Croatia, 2023. [Google Scholar]
- Elizondo, J.; Aldunate, A.; Ezcurra, P.; Gallego, I.; Saigos, E.; Ulayar, E.; Izco, J.M. Efficiency of the proportion of azidiol on preservation in ewe’s milk samples for analysis. Food Control 2007, 18, 185–190. [Google Scholar] [CrossRef]
- Zajác, P.; Zubrická, S.; Čapla, J.; Zeleňáková, L.; Židek, R.; Čurle, J. Effect of preservatives on milk composition determination. Int. Dairy J. 2016, 61, 239–244. [Google Scholar] [CrossRef]
- Lichstein, H.C.; Soule, M.H. Studies of the effect of sodium azide on microbic growth and respiration: I. The action of sodium azide on microbic growth. J. Bacteriol. 1944, 47, 221–230. [Google Scholar] [CrossRef]
- Dobranić, V.; Kazazić, S.; Filipović, I.; Mikulec, N.; Zdolec, N. Composition of raw cow’s milk microbiota and identification of enterococci by MALDI-TOF MS—Short communication. Vet. Arh. 2016, 86, 581–590. Available online: https://hrcak.srce.hr/166060 (accessed on 5 March 2024).
- Nacef, M.; Chevalier, M.; Chollet, S.; Drider, D.; Flahaut, C. MALDI-TOF mass spectrometry for the identification of lactic acid bacteria isolated from a French cheese: The Maroilles. Int. J. Food Microbiol. 2017, 247, 2–8. [Google Scholar] [CrossRef]
- Pukančíková, L.; Lipničanová, S.; Kačániová, M.; Chmelová, D.; Ondrejovič, M. Natural microflora of raw cow milk and their enzymatic spoilage potential. Nova Biotechnol. Chim. 2016, 15, 142–155. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Godočíková, L.; Puchalski, C.; Kunová, S.; Kluz, M.; Kordiaka, R.; Haščík, P. Identification of lactic acid bacteria in milk and milk products with MALDI-TOF mass spectrometry. Sci. Pap. Anim. Sci. Biotechnol. 2017, 50, 115–120. [Google Scholar]
- Regalado, N.G.; Martin, G.; Antony, S.J. Acinetobacter lwoffii: Bacteremia associated with acute gastroenteritis. Travel Med. Infect. Dis. 2009, 7, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Júnior, J.C.; Tamanini, R.; de Oliveira, A.L.M.; Alfieri, A.A.; Beloti, V. Genetic diversity of thermoduric spoilage microorganisms of milk from Brazilian dairy farms. J. Dairy Sci. 2018, 101, 6927–6936. [Google Scholar] [CrossRef]
- Hahne, J.; Kloster, T.; Rathmann, S.; Weber, M.; Lipski, A. Isolation and characterization of Corynebacterium spp. from bulk tank raw cow’s milk of different dairy farms in Germany. PLoS ONE 2018, 13, e0194365. [Google Scholar] [CrossRef] [PubMed]
- Woudstra, S.; Lücken, A.; Wente, N.; Zhang, Y.; Leimbach, S.; Gussmann, M.K.; Kirkeby, C.; Krömker, V. Reservoirs of Corynebacterium spp. in the Environment of Dairy Cows. Pathogens 2023, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Dean, C.J.; Slizovskiy, I.B.; Crone, K.K.; Pfennig, A.X.; Heins, B.J.; Caixeta, L.S.; Noyes, N.R. Investigating the cow skin and teat canal microbiomes of the bovine udder using different sampling and sequencing approaches. J. Dairy Sci. 2021, 104, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Gvozdyak, O.R.; Nogina, T.M.; Schumann, P. Taxonomic study of the genus Brachybacterium: Brachybacterium nesterenkovii sp. nov. Int. J. Syst. Microbiol. 1992, 42, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Lee, K.S.; Shin, D.S.; Han, J.H.; Park, K.S.; Lee, C.H.; Park, K.H.; Kim, S.B. Four new species of Chryseobacterium from the rhizosphere of coastal sand dune plants, Chryseobacterium elymi sp. nov., Chryseobacterium hagamense sp. nov., Chryseobacterium lathyri sp. nov. and Chryseobacterium rhizosphaerae sp. nov. Syst. Appl. Microbiol. 2010, 33, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.G.; da Silva, F.L.; Bazzolli, D.M.S.; Heyndrickx, M.; Costa, P.M.d.A.; Vanetti, M.C.D. Pseudomonas spp. and Serratia liquefaciens as predominant spoilers in cold raw milk. J. Food Sci. 2015, 80, M1842–M1849. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, M.K.; Singleton, I.; Goodfellow, M.; Lee, S.-T. Enhanced biodegradation of diesel oil by a newly identified Rhodococcus baikonurensis EN3 in the presence of mycolic acid. J. Appl. Microbiol. 2006, 100, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Miwa, H.; Ahmed, I.; Yokota, A.; Fujiwara, T. Rhodococcus baikonurensis BTM4c, a boron-tolerant actinobacterial strain isolated from soil. Biosci. Biotechnol. Biochem. 2010, 74, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, C.; Milesi, S.; Iacumin, L. Description of new pigmented bacteria in raw milk. Ind. Aliment. 2006, 45, 1133–1136. [Google Scholar]
- Hartmann, G. Ein Beitrag zur Reinzüchtung von Mastitisstreptokokken aus verunreinigtem Material. Milchw. Forsch. 1936, 18, 116–122. [Google Scholar]
- Milanović, V.; Cardinali, F.; Boban, A.; Gajdoš Kljusurić, J.; Osimani, A.; Aquilanti, L.; Garofalo, C.; Budić-Leto, I. White grape variety Maraština as a promising source of non-Saccharomyces yeasts intended as starter cultures. Food Biosci. 2023, 55, 103033. [Google Scholar] [CrossRef]
- Samaržija, D.; Zamberlin, Š.; Pogačić, T. Psychrotrophic bacteria and milk and dairy products quality. Mljekarstvo/Dairy 2012, 62, 77–95. Available online: https://hrcak.srce.hr/83325 (accessed on 15 February 2024).
- Yuan, H.; Han, S.; Zhang, S.; Xue, Y.; Zhang, Y.; Lu, H.; Wang, S. Microbial Properties of Raw Milk throughout the Year and their Relationships to Quality Parameters. Foods 2022, 11, 3077. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Hudson, J.A.; Korpela, R.; de los Reyes-Gavilán, C.G. Impact on human health of microorganisms present in fermented dairy products: An overview. BioMed Res. Int. 2015, 2015, 412714. [Google Scholar] [CrossRef] [PubMed]
- Wenning, M.; Breitenwieser, F.; Konrad, R.; Huber, I.; Busch, U.; Scherer, S. Identification and differentiation of food-related bacteria: A comparison of FTIR spectroscopy and MALDI-TOF mass spectrometry. J. Microbiol. Methods 2014, 103, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Barberis, C.; Almuzara, M.; Join-Lambert, O.; Ramírez, M.S.; Famiglietti, A.; Vay, C. Comparison of the Bruker MALDI-TOF mass spectrometry system and conventional phenotypic methods for identification of Gram-positive rods. PLoS ONE 2014, 9, e106303. [Google Scholar] [CrossRef] [PubMed]




| Sample | Batch 1 | Batch 2 | Batch 3 | Batch 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Raw Milk | Preserved Milk | Raw Milk | Preserved Milk | Raw Milk | Preserved Milk | Raw Milk | Preserved Milk | |
| 1 | 44,000 | 43,000 | 141,000 | 105,000 | 29,000 | 25,000 | 77,000 | 74,000 |
| 2 | 50,000 | 46,000 | 67,000 | 35,000 | 201,000 | 170,000 | 188,000 | 142,000 |
| 3 | 43,000 | 36,000 | 82,000 | 79,000 | 232,000 | 151,000 | 90,000 | 50,000 |
| 4 | 48,000 | 43,000 | 145,000 | 111,000 | 920,000 | 830,000 | 120,000 | 117,000 |
| 5 | 46,000 | 26,000 | 58,000 | 19,000 | 1,900,000 | 830,000 | 116,000 | 76,000 |
| 6 | 80,000 | 56,000 | 74,000 | 57,000 | 2,720,000 | 1,340,000 | 910,000 | 730,000 |
| 7 | 52,000 | 52,000 | 123,000 | 63,000 | 248,000 | 191,000 | 234,000 | 93,000 |
| 8 | 430,000 | 300,000 | 8000 | 6000 | 460,000 | 127,000 | 229,000 | 156,000 |
| 9 | 1,500,000 | 620,000 | 59,000 | 20,000 | 120,000 | 100,000 | 225,000 | 221,000 |
| 10 | 93,000 | 48,000 | 36,000 | 12,000 | 1,190,000 | 950,000 | 263,000 | 204,000 |
| Average ± Standard deviation | 2.39 × 105 ± 4.59 × 105 A | 1.27 × 105 ± 1.91 × 105 a | 7.93 × 104 ± 4.48 × 104 B | 5.07 × 104 ± 3.84 × 104 a,* | 8.02 × 105 ± 8.96 × 105 A | 4.71 × 105 ± 4.68 × 105 b,* | 2.45 × 105 ± 2.43 × 105 A | 1.86 × 105 ± 1.99 × 105 a,* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikulec, N.; Špoljarić, J.; Plavljanić, D.; Lovrić, N.; Oštarić, F.; Gajdoš Kljusurić, J.; Sarim, K.M.; Zdolec, N.; Kazazić, S. MALDI-TOF Mass Spectrometry-Based Identification of Aerobic Mesophilic Bacteria in Raw Unpreserved and Preserved Milk. Processes 2024, 12, 731. https://doi.org/10.3390/pr12040731
Mikulec N, Špoljarić J, Plavljanić D, Lovrić N, Oštarić F, Gajdoš Kljusurić J, Sarim KM, Zdolec N, Kazazić S. MALDI-TOF Mass Spectrometry-Based Identification of Aerobic Mesophilic Bacteria in Raw Unpreserved and Preserved Milk. Processes. 2024; 12(4):731. https://doi.org/10.3390/pr12040731
Chicago/Turabian StyleMikulec, Nataša, Jasminka Špoljarić, Dijana Plavljanić, Nina Lovrić, Fabijan Oštarić, Jasenka Gajdoš Kljusurić, Khan Mohd. Sarim, Nevijo Zdolec, and Snježana Kazazić. 2024. "MALDI-TOF Mass Spectrometry-Based Identification of Aerobic Mesophilic Bacteria in Raw Unpreserved and Preserved Milk" Processes 12, no. 4: 731. https://doi.org/10.3390/pr12040731
APA StyleMikulec, N., Špoljarić, J., Plavljanić, D., Lovrić, N., Oštarić, F., Gajdoš Kljusurić, J., Sarim, K. M., Zdolec, N., & Kazazić, S. (2024). MALDI-TOF Mass Spectrometry-Based Identification of Aerobic Mesophilic Bacteria in Raw Unpreserved and Preserved Milk. Processes, 12(4), 731. https://doi.org/10.3390/pr12040731










