Green Extraction of Phytochemicals from Fresh Vegetable Waste and Their Potential Application as Cosmeceuticals for Skin Health
Abstract
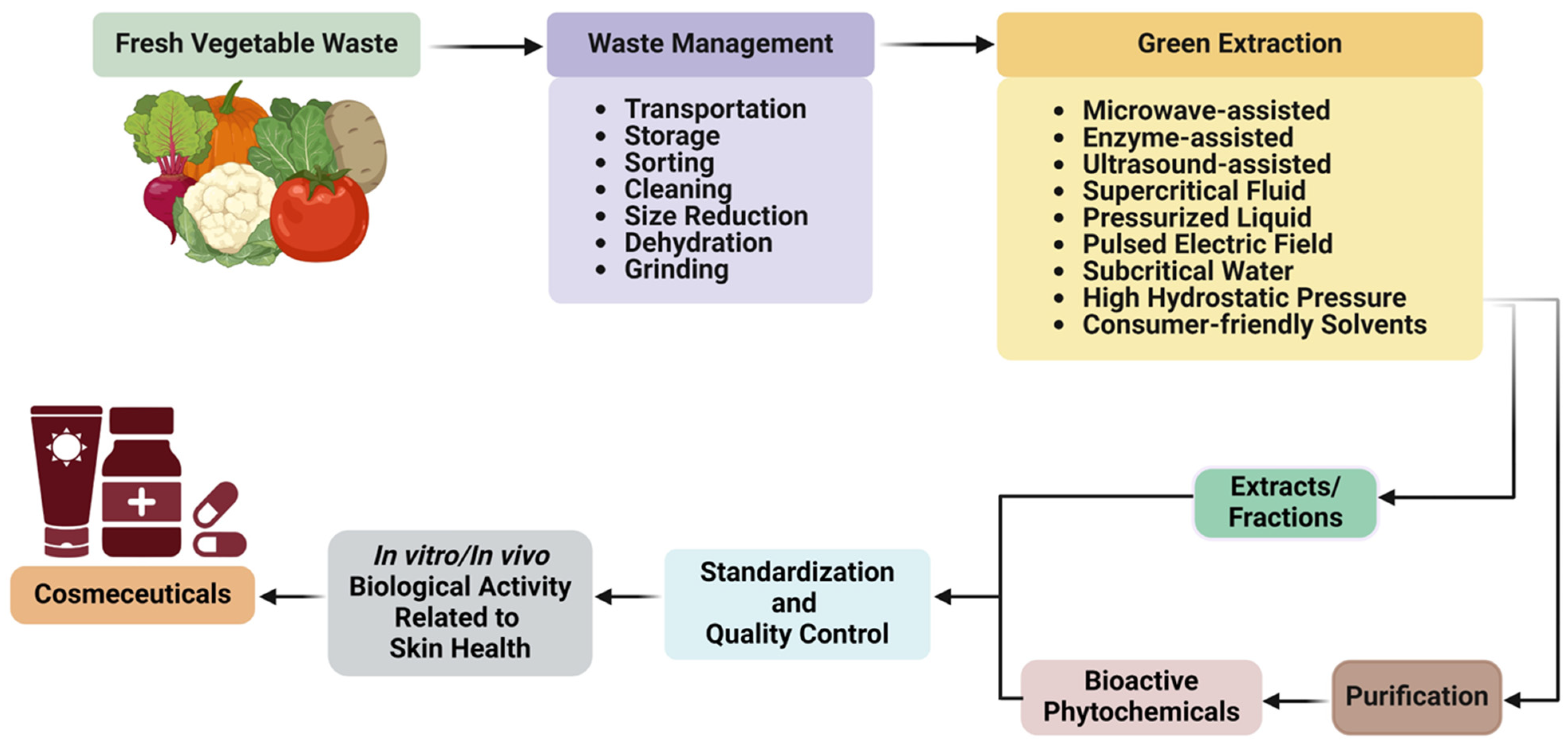
:1. Introduction
2. Methodology
3. Bioactive Compounds Abundant in Vegetables and Root Crops
4. Extraction Methods
4.1. Conventional Extraction Methods
4.2. Advanced Green Extraction Methods
4.2.1. Types of Green Extraction Methods
Microwave-Assisted Extraction (MAE)
Enzyme-Assisted Extraction (EAE)
Ultrasound-Assisted Extraction (UAE)
Supercritical Fluid Extraction (SFE)
Pulsed Electric Field Extraction (PEFE)
Pressurized Liquid Extraction (PLE)
Subcritical Water Extraction (SWE)
High Hydrostatic Pressure Extraction (HHPE)
4.3. Types of Consumer-Friendly Solvents
4.3.1. Food-Grade Ethanol
4.3.2. Ionic Liquids
4.3.3. Deep Eutectic Solvents
4.3.4. Natural Deep Eutectic Solvents
4.3.5. Edible Oil-Based Extractions
5. Recovery of Phytochemicals from Vegetables and Root Crops Using Green Extraction Methods and Its Role in Skin Photoprotection
5.1. Kale (Brassica oleracea L. var. acephala)
5.1.1. Green Extraction Methods Are Reviewed for the Identification of Phytochemicals in Kale
5.1.2. Antioxidant Properties of Kale as Bioactive in Skin Health
5.2. Cauliflower (Brassica oleracea var. botrytis)
5.2.1. Different Green Extraction Methods for Phytochemical Recovery from Cauliflower
5.2.2. Plant Bioactive Compounds from the Cauliflower as Powerful Antioxidant in Skin Health
5.3. Beets (Beta vulgaris subsp. vulgaris)
5.3.1. Green Extraction Methods for Extracting Valuable Compounds from Beetroot
5.3.2. Natural Bioactive Compounds from Beets and Their Function in Skin Health
5.4. Sweet Potato (Ipomoea batatas)
5.4.1. Sweet Potato Extraction Methods for Their Bioactive Phytochemicals
5.4.2. Phytochemical Components of Sweet Potato and Its Antioxidant Properties in Skin Health
5.5. Tomato (Lycopersicon esculentum)
5.5.1. Different Green Extraction Techniques of Tomato Extracts
5.5.2. Bioactive Compounds and Biological Activities of Tomato Extracts in Skin Health
5.6. Pumpkin (Cucurbita sp.)
5.6.1. Bioactives of Pumpkin Extracts Using Different Extraction Methods
5.6.2. Potential Phytochemicals in Pumpkin to Fight against Skin Health
6. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Auh, J.-H.; Madhavan, J. Protective effect of a mixture of marigold and rosemary extracts on UV-induced photoaging in mice. Biomed. Pharmacother. 2021, 135, 111178. [Google Scholar] [CrossRef] [PubMed]
- Daré, R.G.; Oliveira, M.M.; Truiti, M.C.; Nakamura, C.V.; Ximenes, V.F.; Lautenschlager, S.O. Abilities of protocatechuic acid and its alkyl esters, ethyl and heptyl protocatechuates, to counteract UVB-induced oxidative injuries and photoaging in fibroblasts L929 cell line. J. Photochem. Photobiol. B Biol. 2020, 203, 111771. [Google Scholar] [CrossRef] [PubMed]
- de Oca, M.K.M.; Pearlman, R.L.; McClees, S.F.; Strickland, R.; Afaq, F. Phytochemicals for the Prevention of Photocarcinogenesis. Photochem. Photobiol. 2017, 93, 956–974. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Cha, J.W.; Shin, J.H.; Yoo, S.J.; Hyun, J.W. Photo-protective effect of americanin B against ultraviolet B-induced damage in cultured human keratinocytes. Environ. Toxicol. Pharmacol. 2014, 38, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural antioxidants: Multiple mechanisms to protect skin from solar radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef] [PubMed]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L.S. Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef]
- Pop, C.; Suharoschi, R.; Pop, O.L. Dietary Fiber and Prebiotic Compounds in Fruits and Vegetables Food Waste. Sustainability 2021, 13, 7219. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2020, 1635, 461770. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Zhang, Z.; Sun, D.-W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. In Vitro Anti-Inflammatory Properties of Selected Green Leafy Vegetables. Biomedicines 2018, 6, 107. [Google Scholar] [CrossRef]
- Pinto, D.; de la Luz Cádiz-Gurrea, M.; Silva, A.M.; Delerue-Matos, C.; Rodrigues, F. Cosmetics—Food waste recovery. In Food Waste Recovery; Elsevier: Amsterdam, The Netherlands, 2021; pp. 503–528. [Google Scholar] [CrossRef]
- Coelho, M.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Extraction of tomato by-products’ bioactive compounds using ohmic technology. Food Bioprod. Process. 2019, 117, 329–339. [Google Scholar] [CrossRef]
- Meinke, M.C.; Nowbary, C.K.; Schanzer, S.; Vollert, H.; Lademann, J.; Darvin, M.E. Influences of Orally Taken Carotenoid-Rich Curly Kale Extract on Collagen I/Elastin Index of the Skin. Nutrients 2017, 9, 775. [Google Scholar] [CrossRef]
- Satheesh, N.; Fanta, S.W. Kale: Review on nutritional composition, bio-active compounds, anti-nutritional factors, health beneficial properties and value-added products. Cogent Food Agric. 2020, 6, 1811048. [Google Scholar] [CrossRef]
- Tian, Q.; Rosselot, R.A.; Schwartz, S.J. Quantitative determination of intact glucosinolates in broccoli, broccoli sprouts, Brussels sprouts, and cauliflower by high-performance liquid chromatography–electrospray ionization–tandem mass spectrometry. Anal. Biochem. 2005, 343, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Trigo, J.P.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M.E. High value-added compounds from fruit and vegetable by-products—Characterization, bioactivities, and application in the development of novel food products. Crit. Rev. Food Sci. Nutr. 2019, 60, 1388–1416. [Google Scholar] [CrossRef]
- Carrillo, C.; Nieto, G.; Martínez-Zamora, L.; Ros, G.; Kamiloglu, S.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Fernández-López, J.; Viuda-Martos, M.; et al. Novel Approaches for the Recovery of Natural Pigments with Potential Health Effects. J. Agric. Food Chem. 2022, 70, 6864–6883. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Bengardino, M.; Fernandez, M.; Nutter, J.; Jagus, R.; Agüero, M. Recovery of bioactive compounds from beet leaves through simultaneous extraction: Modelling and process optimization. Food Bioprod. Process. 2019, 118, 227–236. [Google Scholar] [CrossRef]
- Liu, J.; Mu, T.; Sun, H.; Fauconnier, M.L. Optimization of ultrasonic–microwave synergistic extraction of flavonoids from sweet potato leaves by response surface methodology. J. Food Process. Preserv. 2019, 43, e13928. [Google Scholar] [CrossRef]
- Batool, M.; Ranjha, M.M.A.N.; Roobab, U.; Manzoor, M.F.; Farooq, U.; Nadeem, H.R.; Nadeem, M.; Kanwal, R.; AbdElgawad, H.; Al Jaouni, S.K.; et al. Nutritional Value, Phytochemical Potential, and Therapeutic Benefits of Pumpkin (Cucurbita sp.). Plants 2022, 11, 1394. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef] [PubMed]
- Lemes, A.C.; Egea, M.B.; Filho, J.G.d.O.; Gautério, G.V.; Ribeiro, B.D.; Coelho, M.A.Z. Biological Approaches for Extraction of Bioactive Compounds from Agro-industrial By-products: A Review. Front. Bioeng. Biotechnol. 2022, 9, 802543. [Google Scholar] [CrossRef] [PubMed]
- Soquetta, M.B.; Terra, L.d.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA-J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Saini, A.; Panesar, P.S.; Bera, M.B. Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions-based delivery system. Bioresour. Bioprocess. 2019, 6, 26. [Google Scholar] [CrossRef]
- García, S.L.R.; Raghavan, V. Green extraction techniques from fruit and vegetable waste to obtain bioactive compounds—A review. Crit. Rev. Food Sci. Nutr. 2021, 62, 6446–6466. [Google Scholar] [CrossRef]
- Kultys, E.; Kurek, M.A. Green Extraction of Carotenoids from Fruit and Vegetable Byproducts: A Review. Molecules 2022, 27, 518. [Google Scholar] [CrossRef]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Vinatoru, M.; Mason, T.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Brnčić, S.R. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Llompart, M.; Celeiro, M.; Dagnac, T. Microwave-assisted extraction of pharmaceuticals, personal care products and industrial contaminants in the environment. Trends Anal. Chem. 2019, 116, 136–150. [Google Scholar] [CrossRef]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Amran, M.A.; Palaniveloo, K.; Fauzi, R.; Satar, N.M.; Mohidin, T.B.M.; Mohan, G.; Razak, S.A.; Arunasalam, M.; Nagappan, T.; Seelan, J.S.S. Value-Added Metabolites from Agricultural Waste and Application of Green Extraction Techniques. Sustainability 2021, 13, 11432. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C. Enzyme-assisted extractions of polyphenols—A comprehensive review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Kamaruddin, M.S.H.; Chong, G.H.; Daud, N.M.; Putra, N.R.; Salleh, L.M.; Suleiman, N. Bioactivities and green advanced extraction technologies of ginger oleoresin extracts: A review. Food Res. Int. 2023, 164, 112283. [Google Scholar] [CrossRef]
- Rifna, E.J.; Misra, N.N.; Dwivedi, M. Recent advances in extraction technologies for recovery of bioactive compounds derived from fruit and vegetable waste peels: A review. Crit. Rev. Food Sci. Nutr. 2021, 63, 719–752. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Montero-Calderon, A.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M.J. Green solvents and Ultrasound-Assisted Extraction of bioactive orange (Citrus sinensis) peel compounds. Sci. Rep. 2019, 9, 16120. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.P.; Mereddy, R.; Maqsood, S. Recent developments in emerging technologies for beetroot pigment extraction and its food applications. Food Chem. 2021, 356, 129611. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Kittl, T.; Minceva, M. Supercritical CO2 extraction of spices: A systematic study with focus on terpenes and piperamides from black pepper (Piper nigrum L.). Food Chem. 2023, 406, 135090. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.-S.F. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, B.; Shiri, F.; Švec, F.; Nováková, L. Green solvents and approaches recently applied for extraction of natural bioactive compounds. Trends Anal. Chem. 2022, 157, 116732. [Google Scholar] [CrossRef]
- Rovetto, L.J.; Aieta, N.V. Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L. J. Supercrit. Fluids 2017, 129, 16–27. [Google Scholar] [CrossRef]
- Arumugham, T.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Rinklebe, J.; Banat, F. Supercritical carbon dioxide extraction of plant phytochemicals for biological and environmental applications—A review. Chemosphere 2021, 271, 129525. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.d.A.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Fluid Extraction of Carotenoids from Vegetable Waste Matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef] [PubMed]
- Arruda, H.S.; Silva, E.K.; Araujo, N.M.P.; Pereira, G.A.; Pastore, G.M.; Junior, M.R.M. Anthocyanins Recovered from Agri-Food By-Products Using Innovative Processes: Trends, Challenges, and Perspectives for Their Application in Food Systems. Molecules 2021, 26, 2632. [Google Scholar] [CrossRef]
- Tena, N.; Asuero, A.G. Up-To-Date Analysis of the Extraction Methods for Anthocyanins: Principles of the Techniques, Optimization, Technical Progress, and Industrial Application. Antioxidants 2022, 11, 286. [Google Scholar] [CrossRef]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Brunton, N.P.; Rai, D.K. Recent Advances on Application of Ultrasound and Pulsed Electric Field Technologies in the Extraction of Bioactives from Agro-Industrial By-products. Food Bioprocess Technol. 2018, 11, 223–241. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Artés-Hernández, F. By-Products Revalorization with Non-Thermal Treatments to Enhance Phytochemical Compounds of Fruit and Vegetables Derived Products: A Review. Foods 2021, 11, 59. [Google Scholar] [CrossRef]
- Andreu, V.; Picó, Y. Pressurized liquid extraction of organic contaminants in environmental and food samples. Trends Anal. Chem. 2019, 118, 709–721. [Google Scholar] [CrossRef]
- Gallina, L.; Cravotto, C.; Capaldi, G.; Grillo, G.; Cravotto, G. Plant Extraction in Water: Towards Highly Efficient Industrial Applications. Processes 2022, 10, 2233. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef] [PubMed]
- Cvetanović, A. Extractions Without Organic Solvents: Advantages and Disadvantages. Chem. Afr. 2019, 2, 343–349. [Google Scholar] [CrossRef]
- Moreira, S.A.; Pintado, M.; Saraiva, J.A. High hydrostatic pressure-assisted extraction: A review on its effects on bioac-tive profile and biological activities of extracts. In Present and Future of High Pressure Processing; Elsevier: Amsterdam, The Netherlands, 2020; pp. 317–328. [Google Scholar] [CrossRef]
- Navarro-Baez, J.E.; Martínez, L.M.; Welti-Chanes, J.; Buitimea-Cantúa, G.V.; Escobedo-Avellaneda, Z. High Hydrostatic Pressure to Increase the Biosynthesis and Extraction of Phenolic Compounds in Food: A Review. Molecules 2022, 27, 1502. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Daud, N.M.; Putra, N.R.; Jamaludin, R.; Norodin, N.S.M.; Sarkawi, N.S.; Hamzah, M.H.S.; Nasir, H.M.; Zaidel, D.N.A.; Yunus, M.A.C.; Salleh, L.M. Valorisation of plant seed as natural bioactive compounds by various extraction methods: A review. Trends Food Sci. Technol. 2022, 119, 201–214. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which is the best food emerging solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Palaiogiannis, D.; Grigorakis, S.; Bozinou, E.; Lalas, S.I.; Makris, D.P. Food-grade deep eutectic solvent extraction of antioxidant polyphenols from peppermint (Mentha × piperita L.): Screening, optimization and metabolite profile. J. Appl. Res. Med. Aromat. Plants 2023, 33, 100456. [Google Scholar] [CrossRef]
- Renard, C.M. Extraction of bioactives from fruit and vegetables: State of the art and perspectives. LWT 2018, 93, 390–395. [Google Scholar] [CrossRef]
- Onuki, S.; Koziel, J.A.; Jenks, W.S.; Cai, L.; Grewell, D.; Leeuwen, J.H. Taking ethanol quality beyond fuel grade: A review. J. Inst. Brew. 2016, 122, 588–598. [Google Scholar] [CrossRef]
- Azabou, S.; Louati, I.; Ben Taheur, F.; Nasri, M.; Mechichi, T. Towards sustainable management of tomato pomace through the recovery of valuable compounds and sequential production of low-cost biosorbent. Environ. Sci. Pollut. Res. 2020, 27, 39402–39412. [Google Scholar] [CrossRef]
- Lim, J.R.; Chua, L.S.; Mustaffa, A.A. Ionic liquids as green solvent and their applications in bioactive compounds extraction from plants. Process. Biochem. 2022, 122, 292–306. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; Pino, V. Green solvents in analytical chemistry. Curr. Opin. Green Sustain. Chem. 2019, 18, 42–50. [Google Scholar] [CrossRef]
- Tzani, A.; Karadendrou, M.-A.; Kalafateli, S.; Kakokefalou, V.; Detsi, A. Current Trends in Green Solvents: Biocompatible Ionic Liquids. Crystals 2022, 12, 1776. [Google Scholar] [CrossRef]
- Torres-Valenzuela, L.S.; Ballesteros-Gómez, A.; Rubio, S. Green Solvents for the Extraction of High Added-Value Compounds from Agri-food Waste. Food Eng. Rev. 2020, 12, 83–100. [Google Scholar] [CrossRef]
- Krishnan, A.; Gopinath, K.P.; Vo, D.-V.N.; Malolan, R.; Nagarajan, V.M.; Arun, J. Ionic liquids, deep eutectic solvents and liquid polymers as green solvents in carbon capture technologies: A review. Environ. Chem. Lett. 2020, 18, 2031–2054. [Google Scholar] [CrossRef]
- Kaoui, S.; Chebli, B.; Zaidouni, S.; Basaid, K.; Mir, Y. Deep eutectic solvents as sustainable extraction media for plants and food samples: A review. Sustain. Chem. Pharm. 2023, 31, 100937. [Google Scholar] [CrossRef]
- Hang, N.T.; Uyen, T.T.T.; Van Phuong, N. Green extraction of apigenin and luteolin from celery seed using deep eutectic solvent. J. Pharm. Biomed. Anal. 2022, 207, 114406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, W.; Liu, L.; Wen, W.; Jing, X.; Wang, X. Switchable deep eutectic solvents for sustainable extraction of β-carotene from millet. Microchem. J. 2023, 187, 108369. [Google Scholar] [CrossRef]
- Silva, Y.P.A.; Ferreira, T.A.P.C.; Jiao, G.; Brooks, M.S. Sustainable approach for lycopene extraction from tomato processing by-product using hydrophobic eutectic solvents. J. Food Sci. Technol. 2019, 56, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Redha, A.A.; Salauddin, M.; Harahap, I.A.; Rupasinghe, H.P.V. Factors Affecting the Extraction of (Poly)Phenols from Natural Resources Using Deep Eutectic Solvents Combined with Ultrasound-Assisted Extraction. Crit. Rev. Anal. Chem. 2023, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (NADES): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef] [PubMed]
- Percevault, L.; Limanton, E.; Gauffre, F.; Lagrost, C.; Paquin, L. Extraction of plant and algal polyphenols using eutectic solvents. In Deep Eutectic Solvents for Medicine, Gas Solubilization and Extraction of Natural Substances; Fourmentin, S., Gomes, M.C., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 241–306. [Google Scholar] [CrossRef]
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. [Google Scholar] [CrossRef]
- Stupar, A.; Šeregelj, V.; Ribeiro, B.D.; Pezo, L.; Cvetanović, A.; Mišan, A.; Marrucho, I. Recovery of β-carotene from pumpkin using switchable natural deep eutectic solvents. Ultrason. Sonochem. 2021, 76, 105638. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Li, Y.; Balcells, M.; Canela-Garayoa, R.; Fabiano-Tixier, A.-S.; Chemat, F. Vegetable Oils as Alternative Solvents for Green Oleo-Extraction, Purification and Formulation of Food and Natural Products. Molecules 2017, 22, 1474. [Google Scholar] [CrossRef]
- Elik, A.; Yanık, D.K.; Göğüş, F. Microwave-assisted extraction of carotenoids from carrot juice processing waste using flaxseed oil as a solvent. LWT 2020, 123, 109100. [Google Scholar] [CrossRef]
- Tiwari, S.; Upadhyay, N.; Singh, A.K.; Meena, G.S.; Arora, S. Organic solvent-free extraction of carotenoids from carrot bio-waste and its physico-chemical properties. J. Food Sci. Technol. 2019, 56, 4678–4687. [Google Scholar] [CrossRef] [PubMed]
- Portillo-López, R.; Morales-Contreras, B.E.; Lozano-Guzmán, E.; Basilio-Heredia, J.; Muy-Rangel, M.D.; Ochoa-Martínez, L.A.; Rosas-Flores, W.; Morales-Castro, J. Vegetable oils as green solvents for carotenoid extraction from pumpkin (Cucurbita argyrosperma Huber) byproducts: Optimization of extraction parameters. J. Food Sci. 2021, 86, 3122–3136. [Google Scholar] [CrossRef] [PubMed]
- Favela-González, K.M.; Hernández-Almanza, A.Y.; De la Fuente-Salcido, N.M. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef] [PubMed]
- Olsen, H.; Grimmer, S.; Aaby, K.; Saha, S.; Borge, G.I.A. Antiproliferative Effects of Fresh and Thermal Processed Green and Red Cultivars of Curly Kale (Brassica oleracea L. convar. acephala var. sabellica). J. Agric. Food Chem. 2012, 60, 7375–7383. [Google Scholar] [CrossRef] [PubMed]
- Major, N.; Prekalj, B.; Perković, J.; Ban, D.; Užila, Z.; Ban, S.G. The Effect of Different Extraction Protocols on Brassica oleracea var. acephala Antioxidant Activity, Bioactive Compounds, and Sugar Profile. Plants 2020, 9, 1792. [Google Scholar] [CrossRef]
- Šamec, D.; Urlić, B.; Salopek-Sondi, B. Kale (Brassica oleracea var. acephala) as a superfood: Review of the scientific evidence behind the statement. Crit. Rev. Food Sci. Nutr. 2019, 59, 2411–2422. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Olech, M. Optimization of ultrasound-assisted extraction and LC-ESI–MS/MS analysis of phenolic acids from Brassica oleracea L. var. sabellica. Ind. Crop. Prod. 2016, 83, 359–363. [Google Scholar] [CrossRef]
- Olsen, H.; Aaby, K.; Borge, G.I.A. Characterization and Quantification of Flavonoids and Hydroxycinnamic Acids in Curly Kale (Brassica oleracea L. Convar. acephala Var. sabellica) by HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2009, 57, 2816–2825. [Google Scholar] [CrossRef]
- Ligor, M.; Trziszka, T.; Buszewski, B. Study of Antioxidant Activity of Biologically Active Compounds Isolated from Green Vegetables by Coupled Analytical Techniques. Food Anal. Methods 2013, 6, 630–636. [Google Scholar] [CrossRef]
- Doria, E.; Buonocore, D.; Marra, A.; Bontà, V.; Gazzola, A.; Dossena, M.; Verri, M.; Calvio, C. Bacterial-Assisted Extraction of Bioactive Compounds from Cauliflower. Plants 2022, 11, 816. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.B.; Smagghe, G.; Raes, K.; Van Camp, J. Combined Alkaline Hydrolysis and Ultrasound-Assisted Extraction for the Release of Nonextractable Phenolics from Cauliflower (Brassica oleracea var. botrytis) Waste. J. Agric. Food Chem. 2014, 62, 3371–3376. [Google Scholar] [CrossRef] [PubMed]
- Šeremet, D.; Durgo, K.; Jokić, S.; Huđek, A.; Vojvodić Cebin, A.; Mandura, A.; Jurasović, J.; Komes, D. Valorization of banana and red beetroot peels: Determination of basic macrocomponent composition, application of novel extraction methodology and assessment of biological activity in vitro. Sustainability 2020, 12, 4539. [Google Scholar] [CrossRef]
- Fernando, G.S.N.; Wood, K.; Papaioannou, E.H.; Marshall, L.J.; Sergeeva, N.N.; Boesch, C. Application of an Ultrasound-Assisted Extraction Method to Recover Betalains and Polyphenols from Red Beetroot Waste. ACS Sustain. Chem. Eng. 2021, 9, 8736–8747. [Google Scholar] [CrossRef]
- Nutter, J.; Fernandez, M.V.; Jagus, R.J.; Agüero, M.V. Development of an aqueous ultrasound-assisted extraction process of bioactive compounds from beet leaves: A proposal for reducing losses and increasing biomass utilization. J. Sci. Food Agric. 2021, 101, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Goyeneche, R.; Di Scala, K.; Ramirez, C.L.; Fanovich, M.A. Recovery of bioactive compounds from beetroot leaves by supercritical CO2 extraction as a promising bioresource. J. Supercrit. Fluids 2020, 155, 104658. [Google Scholar] [CrossRef]
- Lasta, H.F.B.; Lentz, L.; Rodrigues, L.G.G.; Mezzomo, N.; Vitali, L.; Ferreira, S.R.S. Pressurized liquid extraction applied for the recovery of phenolic compounds from beetroot waste. Biocatal. Agric. Biotechnol. 2019, 21, 101353. [Google Scholar] [CrossRef]
- Zhu, Z.; Guan, Q.; Koubaa, M.; Barba, F.J.; Roohinejad, S.; Cravotto, G.; Yang, X.; Li, S.; He, J. HPLC-DAD-ESI-MS2 analytical profile of extracts obtained from purple sweet potato after green ultrasound-assisted extraction. Food Chem. 2017, 215, 391–400. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Q.; Belwal, T.; Li, L.; Aalim, H.; Wu, Q.; Duan, Z.; Zhang, X.; Luo, Z. Ultrasonic impact on viscosity and extraction efficiency of polyethylene glycol: A greener approach for anthocyanins recovery from purple sweet potato. Food Chem. 2019, 283, 59–67. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef]
- Pellicanò, T.M.; Sicari, V.; Loizzo, M.R.; Leporini, M.; Falco, T.; Poiana, M. Optimizing the supercritical fluid extraction process of bioactive compounds from processed tomato skin by-products. Food Sci. Technol. 2020, 40, 692–697. [Google Scholar] [CrossRef]
- Pataro, G.; Carullo, D.; Ferrari, G. Effect of PEF pre-treatment and extraction temperature on the recovery of carotenoids from tomato wastes. Chem. Eng. Trans. 2019, 75, 139–144. [Google Scholar] [CrossRef]
- Sharma, M.; Bhat, R. Extraction of Carotenoids from Pumpkin Peel and Pulp: Comparison between Innovative Green Extraction Technologies (Ultrasonic and Microwave-Assisted Extractions Using Corn Oil). Foods 2021, 10, 787. [Google Scholar] [CrossRef]
- Ferreira, D.F.; Barin, J.S.; Binello, A.; Veselov, V.V.; Cravotto, G. Highly efficient pumpkin-seed extraction with the simultaneous recovery of lipophilic and hydrophilic compounds. Food Bioprod. Process. 2019, 117, 224–230. [Google Scholar] [CrossRef]
- Salami, A.; Asefi, N.; Kenari, R.E.; Gharekhani, M. Addition of pumpkin peel extract obtained by supercritical fluid and subcritical water as an effective strategy to retard canola oil oxidation. J. Food Meas. Charact. 2020, 14, 2433–2442. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Zha, X.; Mei, Y.; Xia, J.; Jiao, Z. Supercritical carbon dioxide extraction of β-carotene and α-tocopherol from pumpkin: A Box–Behnken design for extraction variables. Anal. Methods 2017, 9, 294–303. [Google Scholar] [CrossRef]
- Wang, L.; Kim, H.S.; Oh, J.Y.; Je, J.G.; Jeon, Y.-J.; Ryu, B. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against UVB-induced damage in vitro in human dermal fibroblasts and in vivo in zebrafish. Food Chem. Toxicol. 2020, 136, 110963. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.-G.; Byun, S. Quercetin Directly Targets JAK2 and PKCδ and Prevents UV-Induced Photoaging in Human Skin. Int. J. Mol. Sci. 2019, 20, 5262. [Google Scholar] [CrossRef]
- Wölfle, U.; Esser, P.R.; Simon-Haarhaus, B.; Martin, S.F.; Lademann, J.; Schempp, C.M. UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free Radic. Biol. Med. 2011, 50, 1081–1093. [Google Scholar] [CrossRef]
- González, S.; Astner, S.; An, W.; Pathak, M.A.; Goukassian, D. Dietary Lutein/Zeaxanthin Decreases Ultraviolet B-Induced Epidermal Hyperproliferation and Acute Inflammation in Hairless Mice. J. Investig. Dermatol. 2003, 121, 399–405. [Google Scholar] [CrossRef]
- Wu, P.-Y.; Lyu, J.-L.; Liu, Y.-J.; Chien, T.-Y.; Hsu, H.-C.; Wen, K.-C.; Chiang, H.-M. Fisetin Regulates Nrf2 Expression and the Inflammation-Related Signaling Pathway to Prevent UVB-Induced Skin Damage in Hairless Mice. Int. J. Mol. Sci. 2017, 18, 2118. [Google Scholar] [CrossRef] [PubMed]
- Chawalitpong, S.; Ichikawa, S.; Uchibori, Y.; Nakamura, S.; Katayama, S. Long-Term Intake of Glucoraphanin-Enriched Kale Suppresses Skin Aging via Activating Nrf2 and the TβRII/Smad Pathway in SAMP1 Mice. J. Agric. Food Chem. 2019, 67, 9782–9788. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Ma, H.; Chen, H.; Zhang, X.; Ye, M. Luteolin Prevents UVB-Induced Skin Photoaging Damage by Modulating SIRT3/ROS/MAPK Signaling: An in vitro and in vivo Studies. Front. Pharmacol. 2021, 12, 728261. [Google Scholar] [CrossRef]
- Balupillai, A.; Nagarajan, R.P.; Ramasamy, K.; Govindasamy, K.; Muthusamy, G. Caffeic acid prevents UVB radiation induced photocarcinogenesis through regulation of PTEN signaling in human dermal fibroblasts and mouse skin. Toxicol. Appl. Pharmacol. 2018, 352, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Al-Barnawi, H.M. Kinetics of Sulforaphane Supplementation in an Immortalizedhuman Keratinocyte (HaCaT) Cell Line Exposed to UVB Irradiation. J. Med.–Clin. Res. Rev. 2018, 2, 1–6. [Google Scholar] [CrossRef]
- Benedict, A.L.; Knatko, E.V.; Kostov, R.V.; Zhang, Y.; Higgins, M.; Kalra, S.; Fahey, J.W.; Ibbotson, S.H.; Proby, C.M.; Talalay, P.; et al. The multifaceted role of sulforaphane in protection against UV radiation-mediated skin damage in broccoli. In Cultivation, Nutritional Properties and Effects on Health; Nova Science Publishers, Inc.: Hapog, NY, USA, 2016; pp. 185–208. [Google Scholar]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Benedict, A.L.; Jenkins, S.N.; Ye, L.; Wehage, S.L.; Talalay, P. Dietary glucoraphanin-rich broccoli sprout extracts protect against UV radiation-induced skin carcinogenesis in SKH-1 hairless mice. Photochem. Photobiol. Sci. 2010, 9, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, G.J.; Azuine, M.A.; Sridhar, R.; Okuda, Y.; Tsuruta, A.; Ichiishi, E.; Mukainake, T.; Takasaki, M.; Konoshima, T.; Nishino, H.; et al. Chemoprevention of DMBA-induced UV-B promoted, NOR-1-induced TPA promoted skin carcinogenesis, and DEN-induced phenobarbital promoted liver tumors in mice by extract of beetroot. Pharmacol. Res. 2003, 47, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Her, Y.; Lee, T.-K.; Kim, J.D.; Kim, B.; Sim, H.; Lee, J.-C.; Ahn, J.H.; Park, J.H.; Lee, J.-W.; Hong, J.; et al. Topical Application of Aronia melanocarpa Extract Rich in Chlorogenic Acid and Rutin Reduces UVB-Induced Skin Damage via Attenuating Collagen Disruption in Mice. Molecules 2020, 25, 4577. [Google Scholar] [CrossRef]
- Jung, S.K.; Lee, K.W.; Kim, H.Y.; Oh, M.H.; Byun, S.; Lim, S.H.; Heo, Y.-S.; Kang, N.J.; Bode, A.M.; Dong, Z.; et al. Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochem. Pharmacol. 2010, 79, 1455–1461. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, Y.; Wu, S.; Chen, T.; He, Y.; Sun, J.; Jiao, R.; Jiang, X.; Huang, Y.; Deng, L.; et al. Protective Effect of Cyanidin-3-O-Glucoside against Ultraviolet B Radiation-Induced Cell Damage in Human HaCaT Keratinocytes. Front. Pharmacol. 2016, 7, 301. [Google Scholar] [CrossRef]
- Wertz, K.; Seifert, N.; Hunziker, P.B.; Riss, G.; Wyss, A.; Lankin, C.; Goralczyk, R. β-carotene inhibits UVA-induced matrix metalloprotease 1 and 10 expression in keratinocytes by a singlet oxygen-dependent mechanism. Free. Radic. Biol. Med. 2004, 37, 654–670. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Karadeniz, F.; Lee, J.I.; Seo, Y.; Kong, C. Protective effect of 3,5-dicaffeoyl-epi-quinic acid against UVB-induced photoaging in human HaCaT keratinocytes. Mol. Med. Rep. 2019, 20, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, W.; Son, Y.-O.; Sun, L.; Lu, J.; Kim, D.; Wang, X.; Yao, H.; Wang, L.; Pratheeshkumar, P.; et al. Quercitrin protects skin from UVB-induced oxidative damage. Toxicol. Appl. Pharmacol. 2013, 269, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, U.; Wiebusch, M.; Tronnier, H.; Gärtner, C.; Eichler, O.; Sies, H.; Stahl, W. Supplementation with β-Carotene or a Similar Amount of Mixed Carotenoids Protects Humans from UV-Induced Erythema. J. Nutr. 2003, 133, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Stahl, W.; Krutmann, J. Molecular evidence that oral supplementation with lycopene or lutein protects human skin against ultraviolet radiation: Results from a double-blinded, placebo-controlled, crossover study. Br. J. Dermatol. 2017, 176, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Syed, D.N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Kweon, M.-H.; Khan, N.; Abu Zaid, M.; Mukhtar, H. Delphinidin, an Anthocyanidin in Pigmented Fruits and Vegetables, Protects Human HaCaT Keratinocytes and Mouse Skin Against UVB-Mediated Oxidative Stress and Apoptosis. J. Investig. Dermatol. 2007, 127, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.-M.; Chan, S.-Y.; Chu, Y.; Wen, K.-C. Fisetin Ameliorated Photodamage by Suppressing the Mitogen-Activated Protein Kinase/Matrix Metalloproteinase Pathway and Nuclear Factor-κB Pathways. J. Agric. Food Chem. 2015, 63, 4551–4560. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Wang, J.; Xu, X.; Zhai, L.; Li, Z.; Liu, J.; Zhao, D.; Jiang, R.; Sun, L. Panax ginseng Meyer cv. Silvatica phenolic acids protect DNA from oxidative damage by activating Nrf2 to protect HFF-1 cells from UVA-induced photoaging. J. Ethnopharmacol. 2023, 302, 115883. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.J.; Lee, J.; Park, J.; Lee, N.H.; Kim, Y.E.; Song, K.-M.; Chang, P.-S.; Jeong, C.-H.; Jung, S.K. Syringic acid prevents skin carcinogenesis via regulation of NoX and EGFR signaling. Biochem. Pharmacol. 2018, 154, 435–445. [Google Scholar] [CrossRef]
- Kappler, K.; Grothe, T.; Srivastava, S.; Jagtap, M. Evaluation of the Efficacy and Safety of Blue Fenugreek Kale Extract on Skin Health and Aging: In-vitro and Clinical Evidences. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2051–2064. [Google Scholar] [CrossRef]
- Tanjung, N.L.O.S.; Ruma, I.M.W.; Dewi, N.N.A. The extract of Red Beetroot (Beta vulgaris L.) Prevent Tumor Necrosis Factor-Alpha (TNF-?) Levels and Prevent Increased Sunburn in Epidermal Cell in Rats Exposed to UVB. Int. J. Res. Publ. 2022, 105, 11. [Google Scholar] [CrossRef]
- Zhi, Q.; Lei, L.; Li, F.; Zhao, J.; Yin, R.; Ming, J. The anthocyanin extracts from purple-fleshed sweet potato exhibited anti-photoaging effects on ultraviolent B-irradiated BALB/c-nu mouse skin. J. Funct. Foods 2020, 64, 103640. [Google Scholar] [CrossRef]
- Muntafiah, L.; Shabrina, B.A.; Sulistyowati, D.; Asshagab, M.R.N.; Jenie, R.I. Anti-Aging Activity of Cucurbita moschata Ethanolic Extract Towards NIH3T3 Fibroblast Cells Induced By Doxorubicin. Indones. J. Cancer Chemoprevent. 2017, 7, 49–53. [Google Scholar] [CrossRef]
- Ahmed, F.A.; Ali, R.F.M. Bioactive Compounds and Antioxidant Activity of Fresh and Processed White Cauliflower. BioMed Res. Int. 2013, 2013, 367819. [Google Scholar] [CrossRef] [PubMed]
- Drabińska, N.; Jeż, M.; Nogueira, M. Variation in the Accumulation of Phytochemicals and Their Bioactive Properties among the Aerial Parts of Cauliflower. Antioxidants 2021, 10, 1597. [Google Scholar] [CrossRef]
- Abbaoui, B.; Lucas, C.R.; Riedl, K.M.; Clinton, S.K.; Mortazavi, A. Cruciferous Vegetables, Isothiocyanates, and Bladder Cancer Prevention. Mol. Nutr. Food Res. 2018, 62, e1800079. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, A.; Razis, A.F.A. Apoptosis as a Mechanism of the Cancer Chemopreventive Activity of Glucosinolates: A Review. Asian Pac. J. Cancer Prev. 2018, 19, 1439–1448. [Google Scholar] [CrossRef]
- Avato, P.; Argentieri, M.P. Brassicaceae: A rich source of health improving phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Nartea, A.; Fanesi, B.; Giardinieri, A.; Campmajó, G.; Lucci, P.; Saurina, J.; Pacetti, D.; Fiorini, D.; Frega, N.G.; Núñez, O. Glucosinolates and Polyphenols of Colored Cauliflower as Chemical Discriminants Based on Cooking Procedures. Foods 2022, 11, 3041. [Google Scholar] [CrossRef]
- Zin, M.M.; Anucha, C.B.; Bánvölgyi, S. Recovery of Phytochemicals via Electromagnetic Irradiation (Microwave-Assisted-Extraction): Betalain and Phenolic Compounds in Perspective. Foods 2020, 9, 918. [Google Scholar] [CrossRef]
- Azeredo, H.M. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Khan, M.I. Plant Betalains: Safety, Antioxidant Activity, Clinical Efficacy, and Bioavailability. Compr. Rev. Food Sci. Food Saf. 2016, 15, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, M.; Chung, I.-M.; Samynathan, R.; Chandar, S.R.H.; Venkidasamy, B.; Sarkar, T.; Rebezov, M.; Gorelik, O.; Shariati, M.A.; Simal-Gandara, J. A comprehensive review of beetroot (Beta vulgaris L.) bioactive components in the food and pharmaceutical industries. Crit. Rev. Food Sci. Nutr. 2024, 64, 708–739. [Google Scholar] [CrossRef]
- Lechner, J.F.; Stoner, G.D. Red Beetroot and Betalains as Cancer Chemopreventative Agents. Molecules 2019, 24, 1602. [Google Scholar] [CrossRef] [PubMed]
- Masih, D.; Singh, N.; Singh, A. Red beetroot: A source of natural colourant and antioxidants: A review. J. Pharmacogn. Phytochem. 2019, 8, 162–166. [Google Scholar]
- Noviendri, D.; Hasrini, R.F.; Taher, M. Fucoxanthin: A marine carotenoid has anticancer activities and apoptosis-inducing effect (a review). IOP Conf. Ser. Earth Environ. Sci. 2021, 674, 012093. [Google Scholar] [CrossRef]
- Egbuna, C.; Tupas, G.D. (Eds.) Functional Foods and Nutraceuticals: Bioactive Components, Formulations and Innovations; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Yildiz, Z.I.; Topuz, F.; Kilic, M.E.; Durgun, E.; Uyar, T. Encapsulation of antioxidant beta-carotene by cyclodextrin complex electrospun nanofibers: Solubilization and stabilization of beta-carotene by cyclodextrins. Food Chem. 2023, 423, 136284. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Islamian, J.P.; Mehrali, H. Lycopene as a carotenoid provides radioprotectant and antioxidant effects by quenching radiation-induced free radical singlet oxygen: An overview. Cell J. 2015, 16, 386. [Google Scholar] [CrossRef]
- Adhami, V.M.; Syed, D.N.; Khan, N.; Afaq, F. Phytochemicals for Prevention of Solar Ultraviolet Radiation-induced Damages†. Photochem. Photobiol. 2008, 84, 489–500. [Google Scholar] [CrossRef]
- Kaur, S.; Panghal, A.; Garg, M.K.; Mann, S.; Khatkar, S.K.; Sharma, P.; Chhikara, N. Functional and nutraceutical properties of pumpkin—A review. Nutr. Food Sci. 2019, 50, 384–401. [Google Scholar] [CrossRef]

| Extraction Method | Advantages | Disadvantages |
|---|---|---|
| MAE | Quick, efficient, low-cost, room-temperature operation | Poor non-polar solvents, not for thermally labile compounds |
| EAE | High efficiency and selectivity, eco-friendly, low temperature | Expensive enzymes, time-consuming, pH and temperature sensitive |
| UAE | Easy to use, efficient, eco-friendly, versatile solvent selection | Multiple extractions needed, generate radicals, difficult to scale up |
| SFE | Non-toxic, cost-effective, rapid, recyclable supercritical fluid | High initial cost, limited for non-polar phytochemicals, parameter optimization needed |
| PEF | Shorter extraction time, high quality, non-thermal, increases cell permeability | High equipment maintenance cost, parameters are dependent on the texture and electric conductivity of raw material |
| PLE | Energy efficient, non-toxic solvents, simple equipment | High equipment cost, potential compound inactivation |
| SWE | Cost-effective, efficient, water as solvent | Higher temperatures may degrade sensitive compounds |
| HHP | Low time, high solubility, high yield | Possible structural changes in fragile materials |
| Extraction Method | Optimized Extraction Conditions | Recovered Phytochemicals | Yield | References |
|---|---|---|---|---|
| Kale (Brassica oleracea L. var. acephala) | ||||
| UAE | 80% aqueous Ethanol, 60 °C, 20 kHz, 1 h | Benzoic acid (0.49 µg/g DW), Vanillic acid (1.46 µg/g DW), Trans-ferulic acid (3.38 µg/g DW), Trans coumaric acid (2.49 µg/g DW) | [91] | |
| UAE | 15 mL methanol, 10 °C, 15 min | Kaempferol (58 ± 4 mg/100 g FW), Quercetin (44 ± 4 mg/100 g FW), Total Flavonoids (646 ± 12 mg/100 g FW) | [92] | |
| SFE | CO2 with 5% methanol, 35 °C, 30 min | Lutein (0.69 ± 0.03 mg/g DW) | [93] | |
| Cauliflower (Brassica oleracea var. botrytis) | ||||
| EAE | Pre-treatment: 85% phosphoric acid, 50 °C, pre-treatment: 16 h, extraction: 5 h | Isothiocyanates (0.49 mg/g FW) | [94] | |
| UAE | 2 M NaOH, 60 °C, 30 min | Non-extractable phenolics (Blade: 6109 µg GAE/g DW; Petiole: 940 µg GAE/g DW) | [95] | |
| UAE | 70% aqueous ethanol, 70 °C, 30 min | Glucosinolates (3.22 µmol/g FW) | [19] | |
| Beets (Beta vulgaris subsp. vulgaris) | ||||
| MAE UAE SWE | Sample/solvent (1:20), 50 °C, 5 min Sample/solvent (1:20), 80 °C, 30 min Sample/solvent (1:20), 150 °C, 5 min | Betacyanins (3.08 mg/g) DW, Betaxanthins (1.74 mg/g DW) Betacyanins (3.87 mg betanin/g DW) DW, Betaxanthins (8.61 mg betaxanthin/g DW) Betacyanins (0.03 mg/g DW, Betaxanthins (0.19 mg/g DW) | [96] | |
| UAE | 30% ethanol, 30 °C, 30 min, 44 kHz | Betalains (6.86 mg/g DW) | [97] | |
| UAE | 1:20 solid–liquid ratio, 30 °C, 16 min | Betaxanthin, Betacyanin (949 µg/g DW, 562 µg/g DW) | [98] | |
| SFE | 20 mL ethanol as co-solvent, 35 °C, 400 bars | Phenolic content (2.80 mg/g DW) | [99] | |
| PLE | Ethanol, 40 °C, pressure—10 MPa, flowrate—3 mL/min | Phenolic content (252 mg GAE/g DW) | [100] | |
| Sweet potato (Ipomoea batatas) | ||||
| UAE | 58% ethanol, 80 °C, 40 min, 178 W | Polyphenols (3.87 mg/g FW) | [101] | |
| UME | 72% ethanol, 57 °C, 76 s | Flavonoids (91.6 ± 3.37% w/w DW) | [24] | |
| UAE | 83% polyethylene glycol, 64 °C, 80 min | Phenolic compounds (83.7 CE/100 g DW) | [102] | |
| Tomato (Lycopersicon esculentum) | ||||
| SFE | 5% ethanol, 55 °C, 120 min | β-carotene, Lycopene 53.9 mg/g DW | [103] | |
| SFE | CO2 solvent, 60 °C, 0.79 bar, 80 min | Lycopene (0.86 mg/ 100 g DW), Oleoresin (1.5 mg/100 g DW) | [104] | |
| SFE | 15.5% ethanol, 59 °C, 30 min, 350 bar | β-carotene (99 ± 2.8%,), lutein (98.5 ± 2.2%,), lycopene (36.2 ± 6.2%) | [50] | |
| PEF | Acetone, solid–liquid ratio 1:40 g/mL, 50 °C, 480 min | Carotenoids (84.0 ± 8.3 mg/100 g FW) | [105] | |
| UAE | NaDES DL-methanol and lactic acid—8:1 mol BBA /mol HBD, 70 °C, 10 min | Lycopene (1446 µg/g DW) | [77] | |
| Pumpkin (Cucurbita sp.) | ||||
| MAE UAE | 5 g in 50 mL corn oil (1:10), 45 °C, 30 min 5 g in 50 mL corn oil (1:10), 22–25 °C, 30 min | Carotenoids (Pulp: 31.1 μg/g DW, Peel: 34.9 μg/g DW), phenolic compounds (Pulp: 527 mg GAE/g DW, Peel: 554.5 mg GAE/g DW) Carotenoids (Pulp: 32.7 μg/g DW, Peel: 38.1 μg/g DW), phenolic compounds (Pulp: 555 mg GAE/g DW, Peel: 588 mg GAE/g DW) | [106] | |
| MAE UAE | 6 g in 60% ethanol, 2.45 GHz, 100 °C, 20 min 60% ethanol, 40 °C, 20 min | Phenolic compounds (35.0 mg/g DW) Phenolic compounds (34.2 mg/g DW) | [107] | |
| SFE SWE | CO2 fluid, ethanol: water (80:20), 70 °C, 3 h Water, 120 °C, 3 h at 5 MPa | Carotenoids (11.5 mg/100 g DW), phenolic compounds (353 mg GAE/100 g DW) Carotenoids (15.2 mg/100 g DW), phenolic compounds (213 mg GAE/100 g DW) | [108] | |
| SFE | CO2 with ethanol, 47.7 °C, 60 min | β-carotene (20.5 mg/100 g DW), α-tocopherol (9.5 mg/100 g DW) | [109] | |
| UAE | NaDES caprylic acid: capric acid of C8:C10 (3:1), 50 °C, 52.5 w/cm3 power, 10 min | β-carotene (151.4 µg/mL DW) | [82] | |
| Phytochemicals | Experimental Model | Marker Measured | References |
|---|---|---|---|
| Kale (Brassica oleracea L. var. acephala) | |||
| Kaempferol | UV radiation on HDFs and HaCaT cells | ↑Radical scavenging, ↓IL-6 and TNF-α, ↑Cytokines inhibitory potency | [110] |
| Quercetin | UVA-induced skin aging in skin cells and human skin tissue | ↓PKCδ and JAK2 Kinase, ↓MMP-1, ↓COX-2, ↓collagen degradation, ↓AP-1, ↓NF-κB, ↓ERK/JNK/Akt/STAT3 | [111] |
| Fisetin | UVB-induced photodamage in hairless mice | ↓MMP-1/2, ↓COX-2, ↓IL-6, ↓NF-κB, ↑Nrf2 | [114] |
| Luteolin | UVB-induced DNA damage in HaCaT cells | ↓cyclobutane pyrimidine dimers, ↓COX-2, ↓prostaglandin E2 production | [112] |
| Lutein | UVB-induced acute inflammation in hairless mice | ↓acute inflammatory responses, ↓epidermal hyperproliferation | [113] |
| Cauliflower (Brassica oleracea var. botrytis) | |||
| Luteolin | UVB-induced skin barrier damage in SD rats and HDFs | ↑Collagen expression, ↓SIRT3, ↓ROS, ↓MAPK, ↓MMPs | [116] |
| Caffeic acid | UVB-induced skin carcinogenesis in HDFs and Swiss albino mice skin | ↓CPD formation, ↓DNA damage, ↓ROS, ↑PTEN, ↓PI3K and AKT Kinase | [117] |
| Sulforaphane | UVB-induced skin aging in HaCaT cells UVR-mediated skin damage in SKH-1 hairless mice | ↓AP-1, ↑Nrf2, No significant protection with post-UVB supplementation ↑Nrf2, ↓NF-kB signaling, ↓MIF | [118] [119] |
| Glucoraphanin | UVB-induced skin carcinogenesis in SKH-1 hairless mice | ↓AP-1, ↑Nrf2, ↓TPA-induced ornithine decarboxylase, ↓tumor incidence | [120] |
| Beets (Beta vulgaris subsp. vulgaris) | |||
| Caffeic acid | UVB-induced photo carcinogenesis in mouse skin | ↓Lipid peroxidation, ↓iNOS/VEGF/TGF-β, ↓tumor multiplicity, ↑PPARγ, ↓COX-2, ↓ NF-κB | [117] |
| Betanin | DMBA–UV-B-induced skin carcinogenesis in female hairless mice | ↓Skin tumors, ↓skin papillomas, ↑tumor latency | [121] |
| Rutin | UVB-induced skin damage in mice | ↓ROS; ↓MMP-1; ↑Collagen I/III; ↓ COX-2; ↓iNOS | [122] |
| Sweet potato (Ipomoea batatas) | |||
| Myricetin | UVB-induced wrinkle formation in SKH-1 hairless mice | ↓MMP-9, ↓Raf kinase, ↓MEK/ERK | [123] |
| Cyanidin-3-O-glucoside, | UVB-induced cell damage in human HaCaT cells | ↑Bcl-2, ↓p53, ↓ROS, ↓Caspase-3 | [124] |
| β-carotene | UVA-induced photoaging in HaCaT cells | ↓MMP-1, ↓MMP-3, ↓MMP-10 | [125] |
| Caffeoylquinic acid (CQA) | UVB-induced damage in human HaCaT cells | ↓ROS, ↓Nrf2, ↓TNF-α, ↓COX-2, ↓IL-6, ↓IL-1β | [126] |
| Tomato (Lycopersicon esculentum) | |||
| Quercetin | UVB-induced oxidative damage in JB6 P+ mouse epidermal C141 cells | ↓ROS, ↑Catalase, ↑GSH/GSSG, ↓DNA Damage/Apoptosis, ↓NF-κB | [127] |
| β-carotene | 36 healthy adults | Diminished erythema intensity 24 h post-irradiation | [128] |
| Lycopene | 65 healthy volunteers | ↓HO1, ↓ICAM1, ↓MMP1 | [129] |
| Delphinidin | UVB-mediated oxidative stress in Human HaCaT Keratinocytes and mouse skin | ↓Apoptosis/DNA damage, ↓Nuclear Antigen, ↑Lipid peroxidation, ↑Caspases/Bax/Bid/Bak, ↓Bcl-2/Bcl-xL | [130] |
| Fisetin | UV-induced photodamage in human skin fibroblasts | ↓ERK/JNK/p38 phosphorylation, ↓IκB, p65 (NF-κB subunit), ↓ROS/PGE2/NO | [131] |
| Pumpkin (Cucurbita sp.) | |||
| Syringic acid, Ferulic acid, | UVA-induced photoaging in HFF-1 cells | ↓ROS, ↑GSH, ↑GPx, ↑SOD, ↑CAT, ↓MDA, ↓Keap1 protein, | [132] |
| Caffeic acid | UVB radiation effects in human HaCaT keratinocytes | ↓COX-2, ↓MMP-1, ↓PGE2, ↓MAPK, ↓EGFR | [133] |
| Extracts | |||
| Blue fenugreek kale extract | UVA and H2O2 induced protein carbonylation (PA) in HDFs, followed by a randomized, double-blind, placebo-controlled clinical trial on 59 volunteers for 56 days. | ↓PA, ↓TEWL, ↑skin moisture content level, ↑skin hydration. No significant difference was observed in wrinkle severity, skin sagging, elasticity, and inflammatory markers. | [134] |
| Glucoraphanin-enriched kale (GEK) extract | GEK diet on skin aging in senescence-accelerated mouse prone 1 (SAMP1) mouse. | ↑Nrf2, ↑HO-1, ↑collagen production, ↑thickness of the epidermis | [115] |
| Beetroot extract | UVB-induced sunburn in epidermal cells in rats | ↓TNF-α, ↓sunburn, ↓IL-6 | [135] |
| Purple-fleshed sweet potato anthocyanin extract (PSP-AE) | UVB-induced photoaging in BALB/c- mouse skin | ↓TNF-α, ↓IL-6, ↓Kinase, ↓MMP-1, ↓NF-κB | [136] |
| Pumpkin seed extract | Pumpkin seed extract Doxorubicin-induced normal NIH-3T3 fibroblasts | Dose-dependent reduction in cell senescence percentages | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valisakkagari, H.; Chaturvedi, C.; Rupasinghe, H.P.V. Green Extraction of Phytochemicals from Fresh Vegetable Waste and Their Potential Application as Cosmeceuticals for Skin Health. Processes 2024, 12, 742. https://doi.org/10.3390/pr12040742
Valisakkagari H, Chaturvedi C, Rupasinghe HPV. Green Extraction of Phytochemicals from Fresh Vegetable Waste and Their Potential Application as Cosmeceuticals for Skin Health. Processes. 2024; 12(4):742. https://doi.org/10.3390/pr12040742
Chicago/Turabian StyleValisakkagari, Harichandana, Chandrika Chaturvedi, and H. P. Vasantha Rupasinghe. 2024. "Green Extraction of Phytochemicals from Fresh Vegetable Waste and Their Potential Application as Cosmeceuticals for Skin Health" Processes 12, no. 4: 742. https://doi.org/10.3390/pr12040742
APA StyleValisakkagari, H., Chaturvedi, C., & Rupasinghe, H. P. V. (2024). Green Extraction of Phytochemicals from Fresh Vegetable Waste and Their Potential Application as Cosmeceuticals for Skin Health. Processes, 12(4), 742. https://doi.org/10.3390/pr12040742









