Modification of Copper Benzene-1,3,5-tricarboxy Late (Cu-BTC) Composites with Multiwalled Carbon Nanotubes and Amino Groups for Enhanced CO2/CH4 Selective Adsorption Performance and Water Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cu-BTC
2.3. Preparation of NH2-Cu-BTC
2.4. Preparation of CNT-NH2-Cu-BTC
2.5. Wet Treatment
2.6. Desorption of CNT-NH2-BTC Composites in Cyclic Experiments
2.7. Characterization
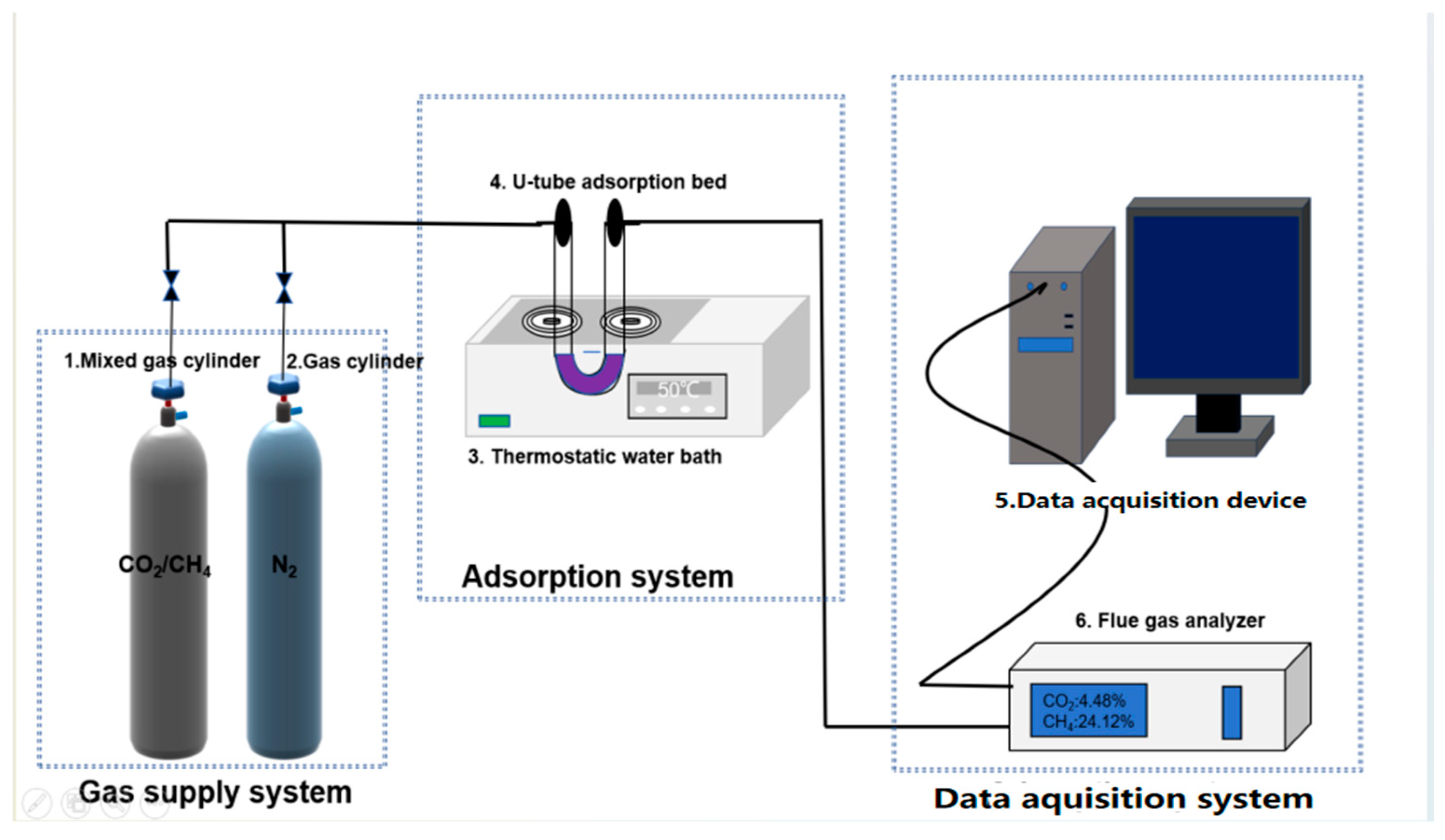
2.8. CO2 and CH4 Adsorption Experiments
3. Results and Discussion
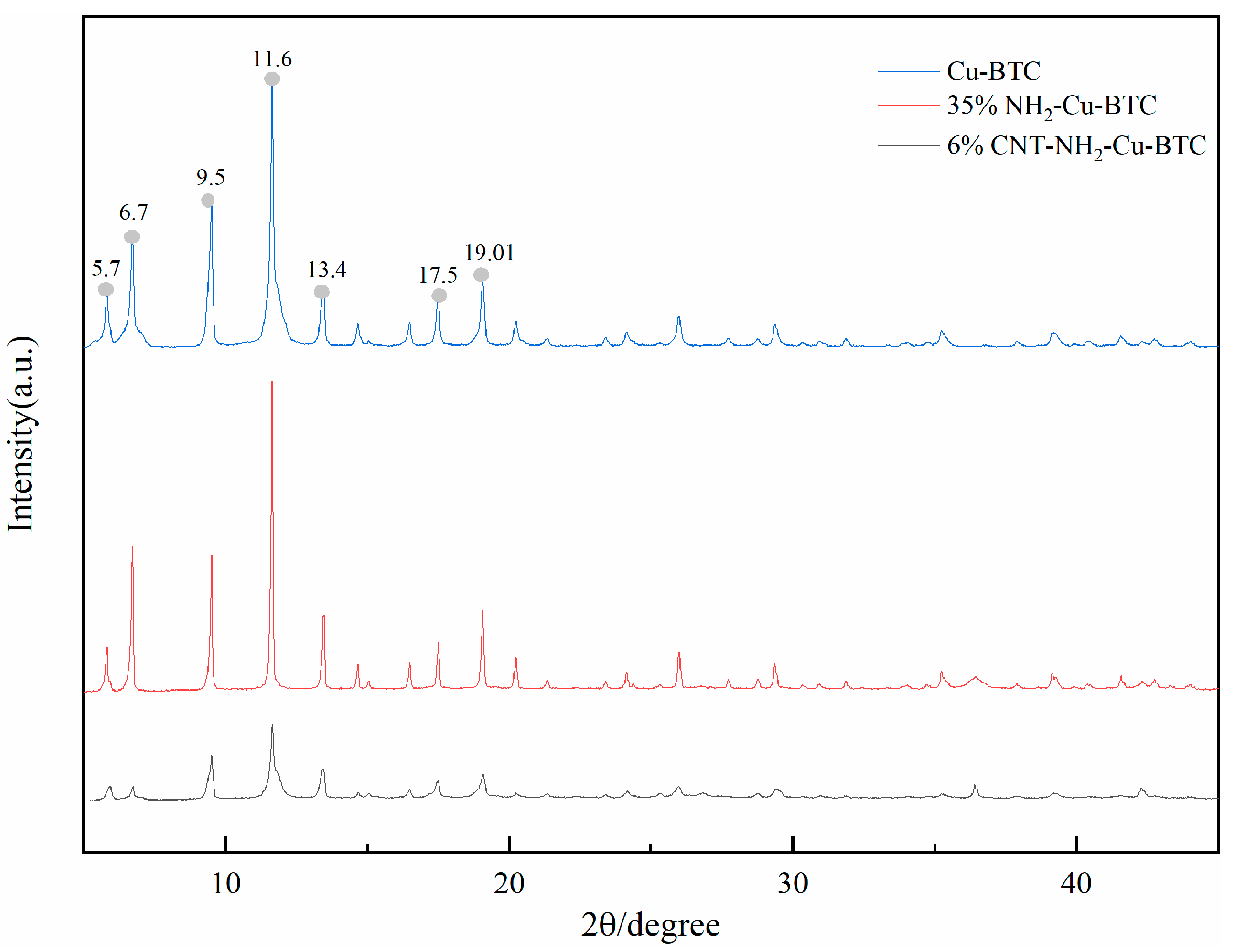
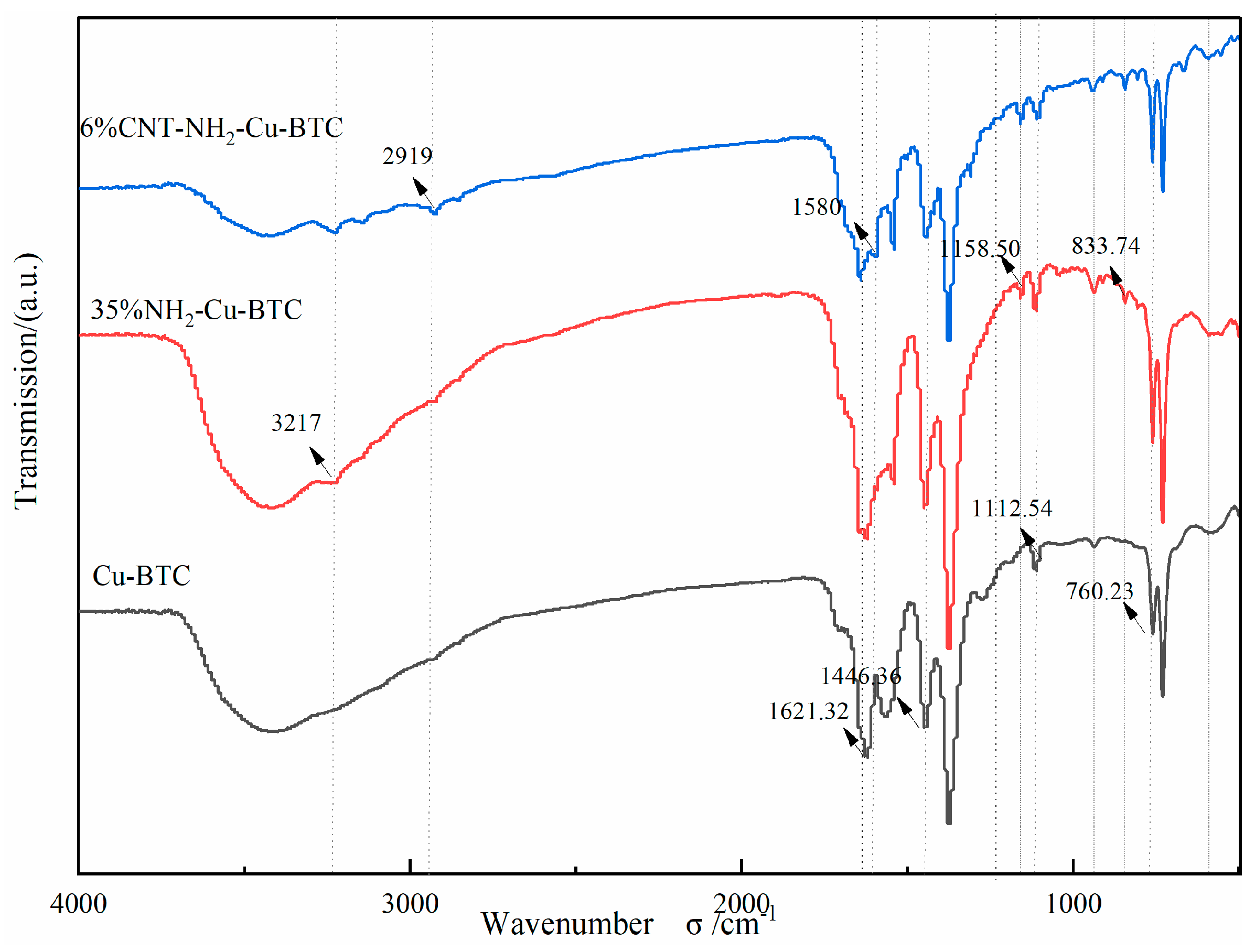
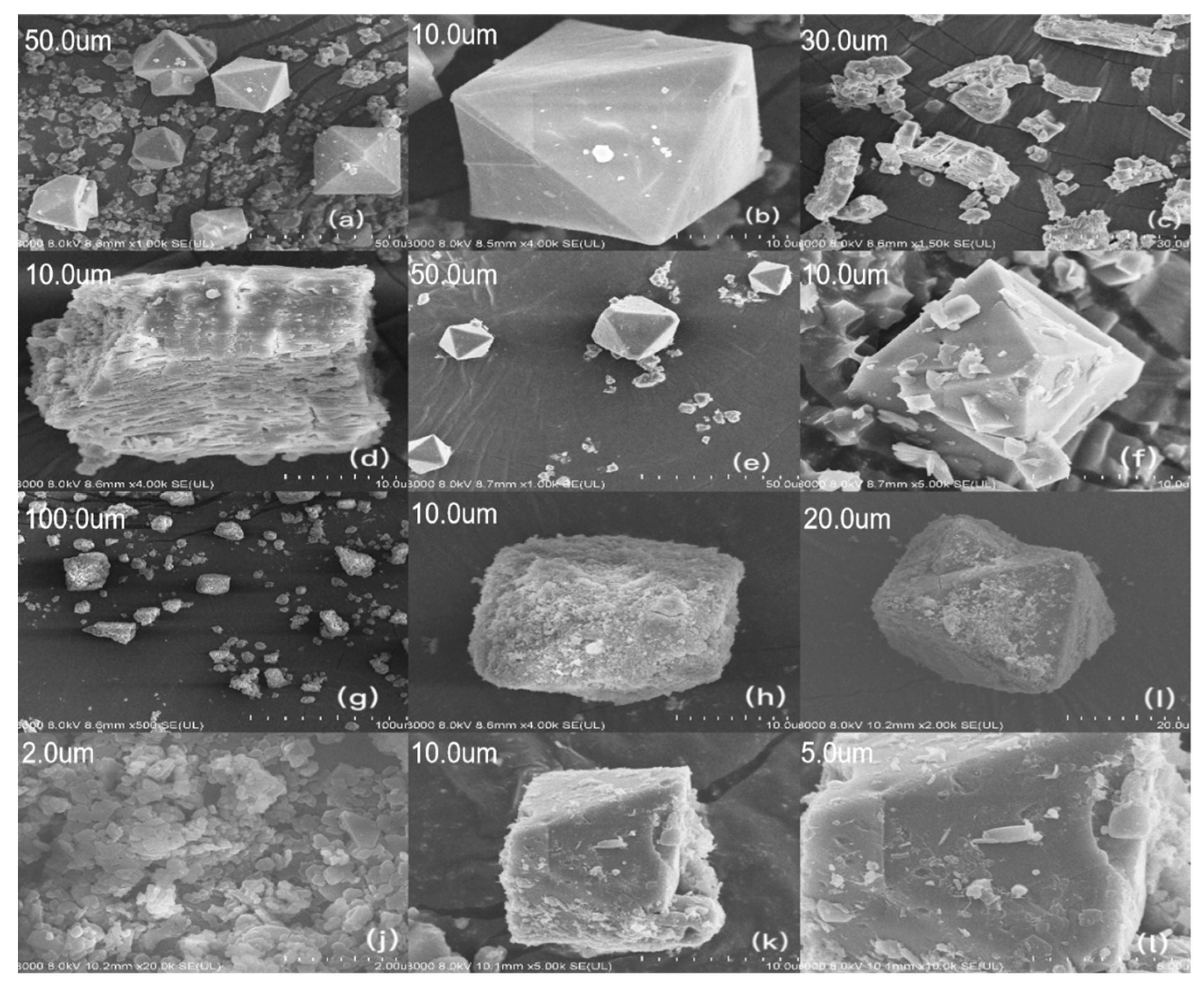
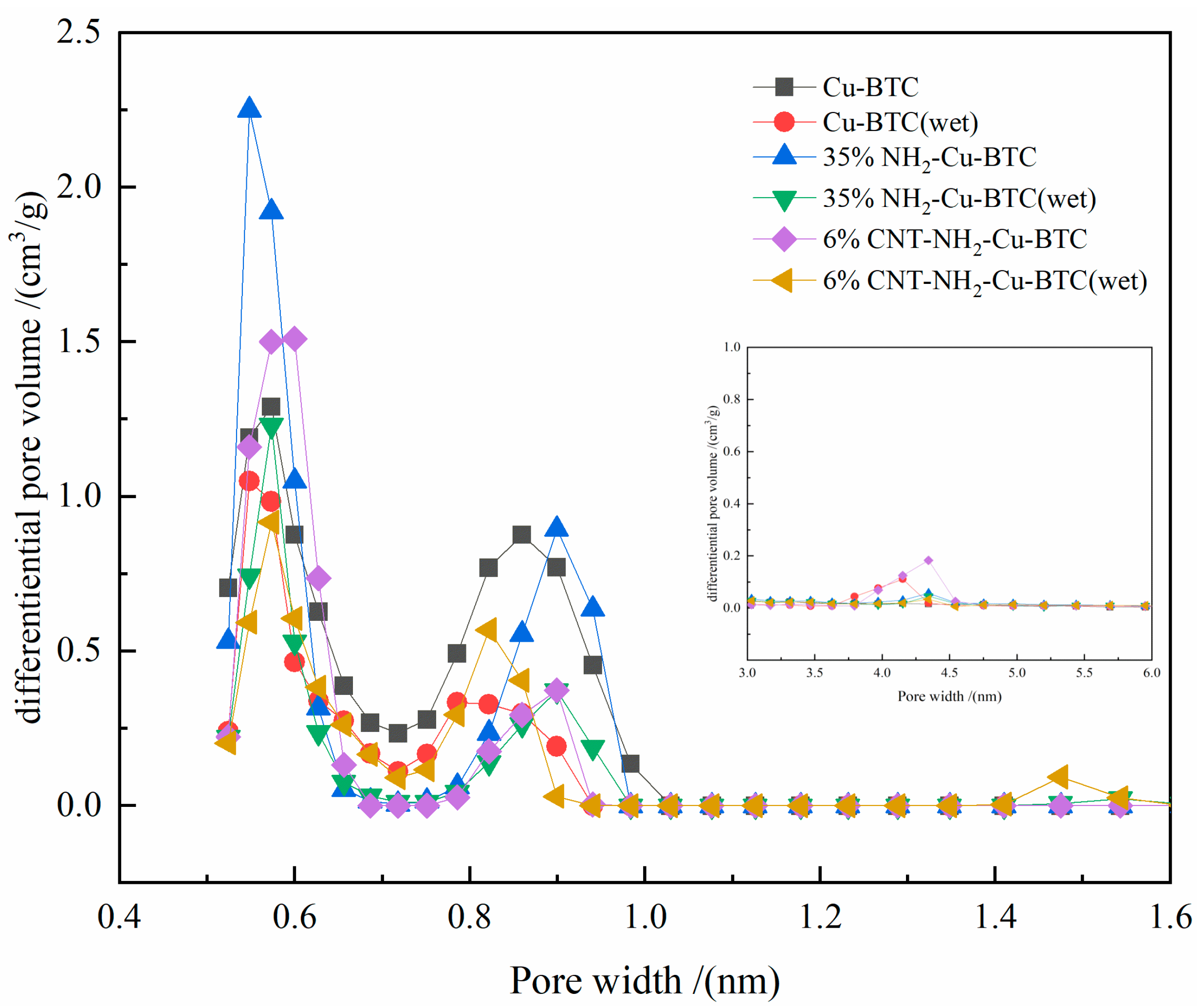
3.1. Adsorbent Characterization
3.2. Adsorption of CO2/CH4 Mixtures and Selectivity
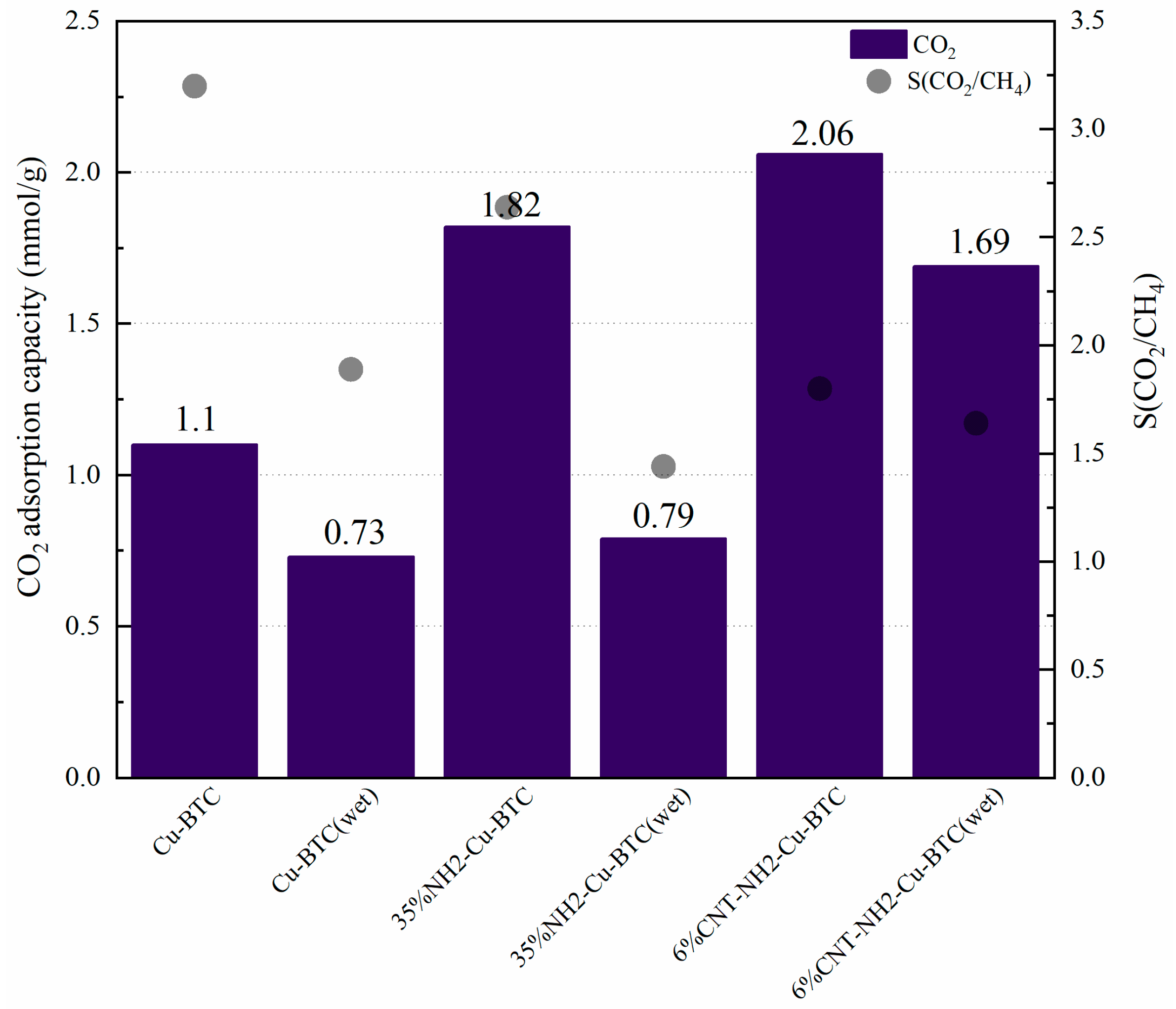
3.2.1. CO2 Adsorption Capacity and CO2/CH4 Selective Adsorption of NH2-Cu-BTC and CNT-NH2-Cu-BTC
- (1)
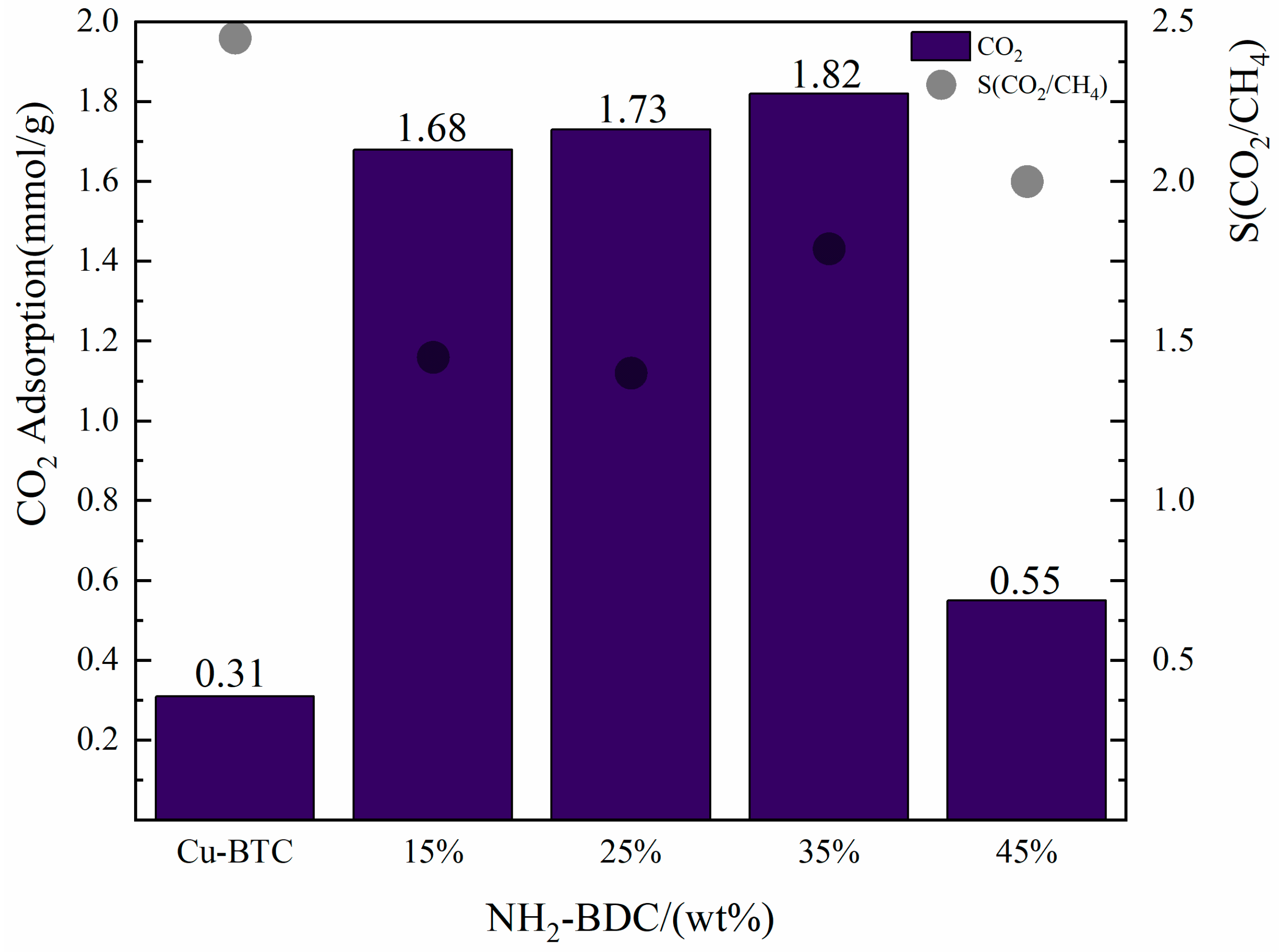
- Effect of NH2-BDC addition on the CO2/CH4 selective adsorption of NH2-Cu-BTC
- (2)
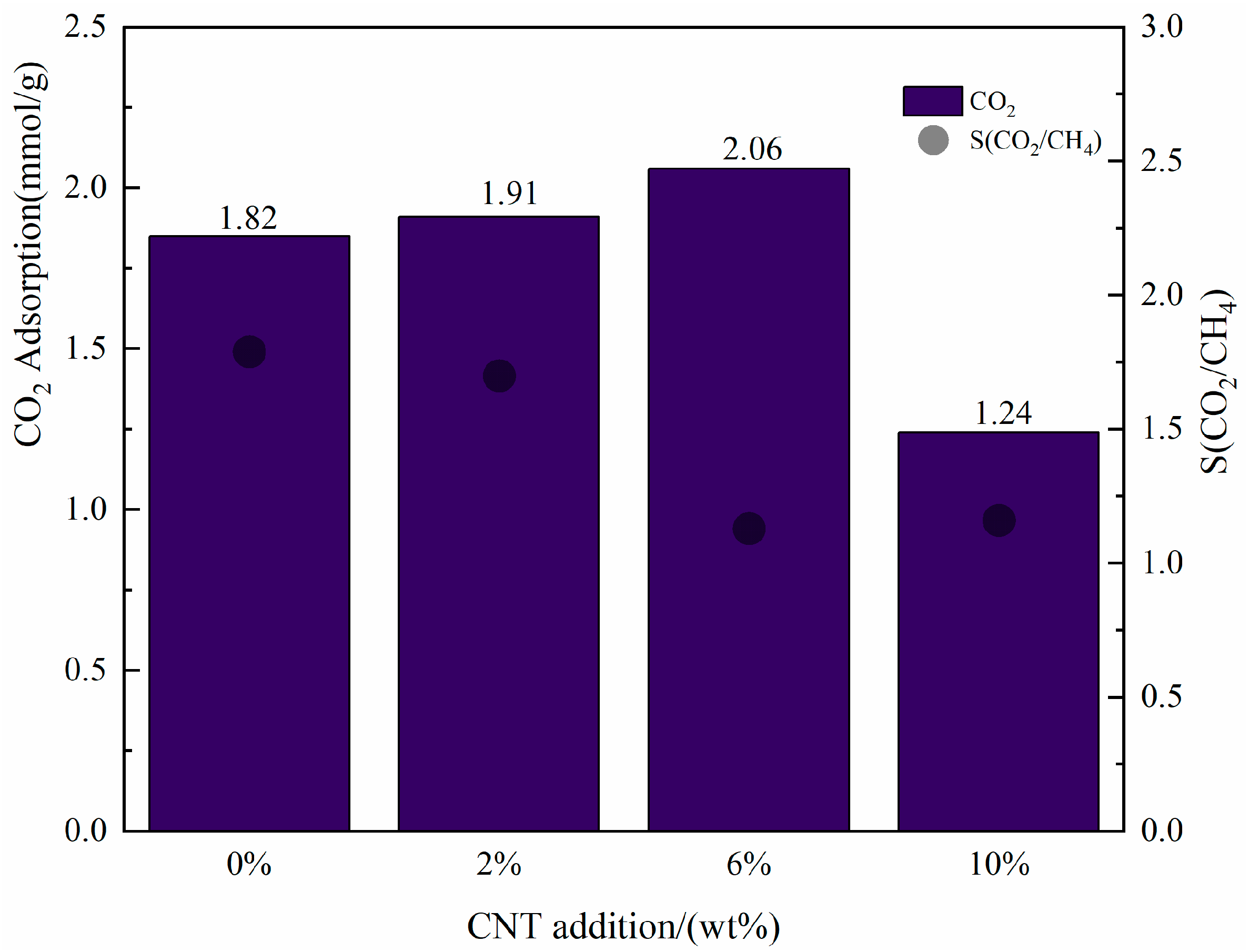
- Effect of MWCNT loading on the CO2/CH4 selective adsorption of CNT-NH2-Cu-BTC
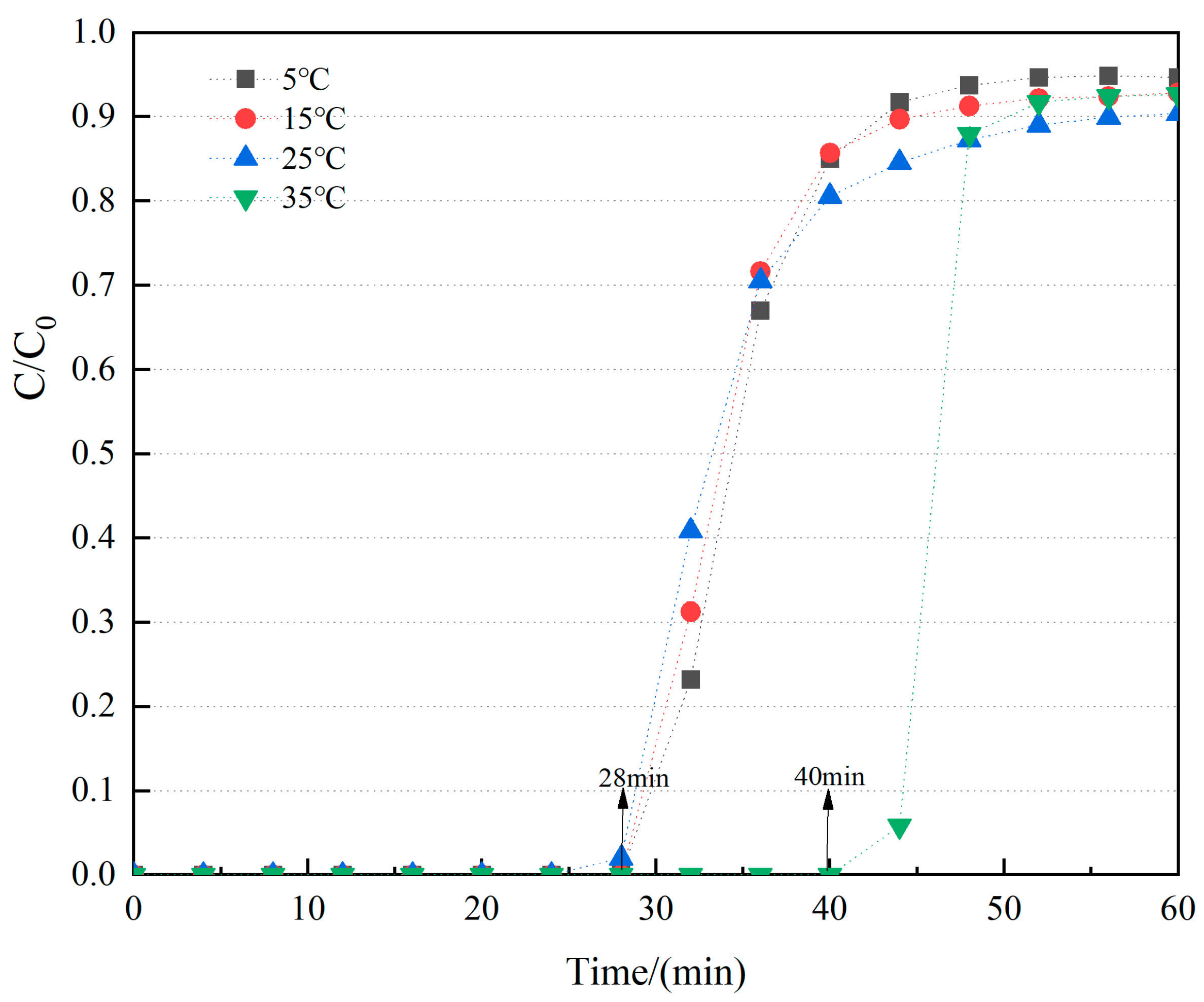
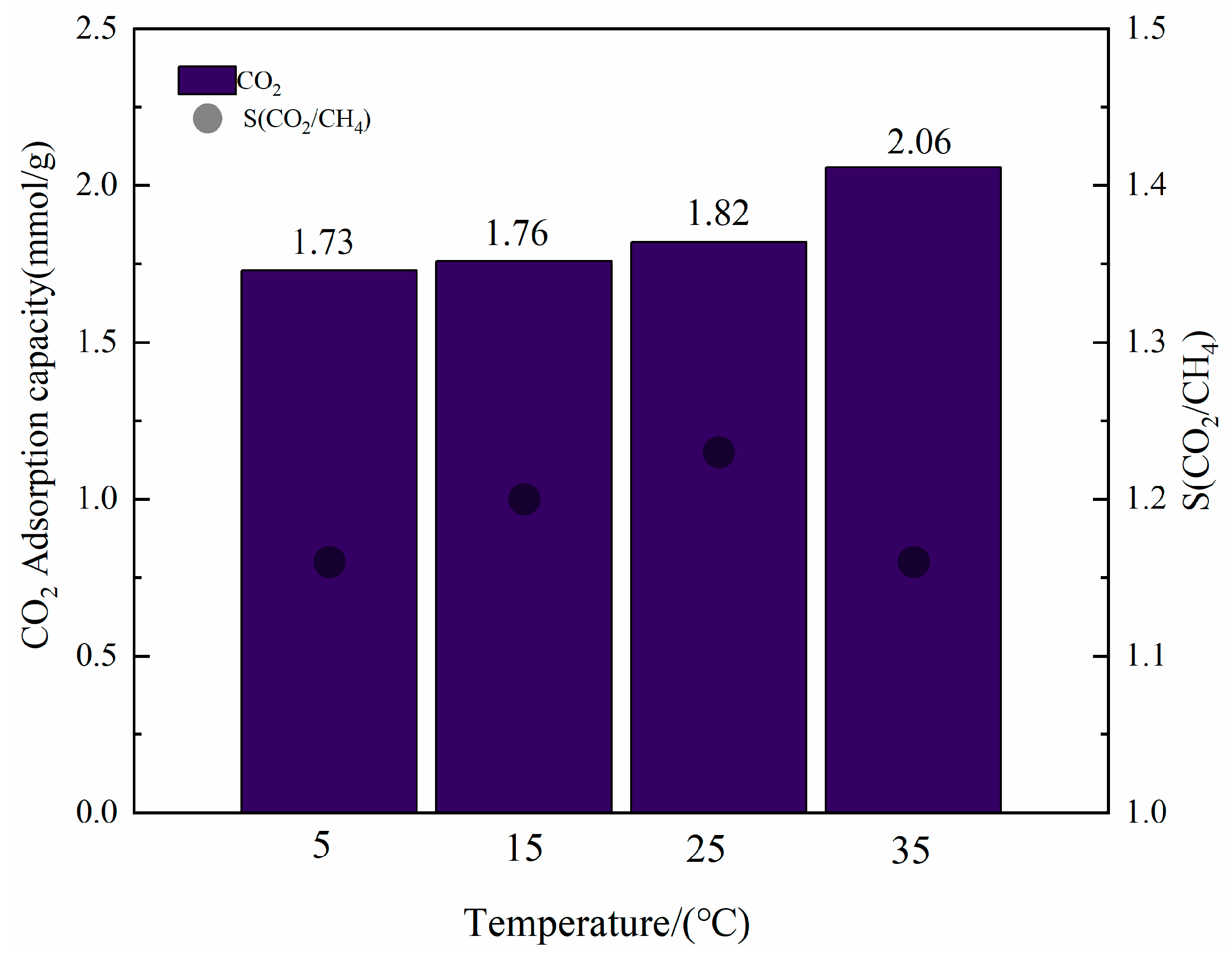
3.2.2. Effect of Temperature on the CO2/CH4 Selective Adsorption of CNT-NH2-Cu-BTC
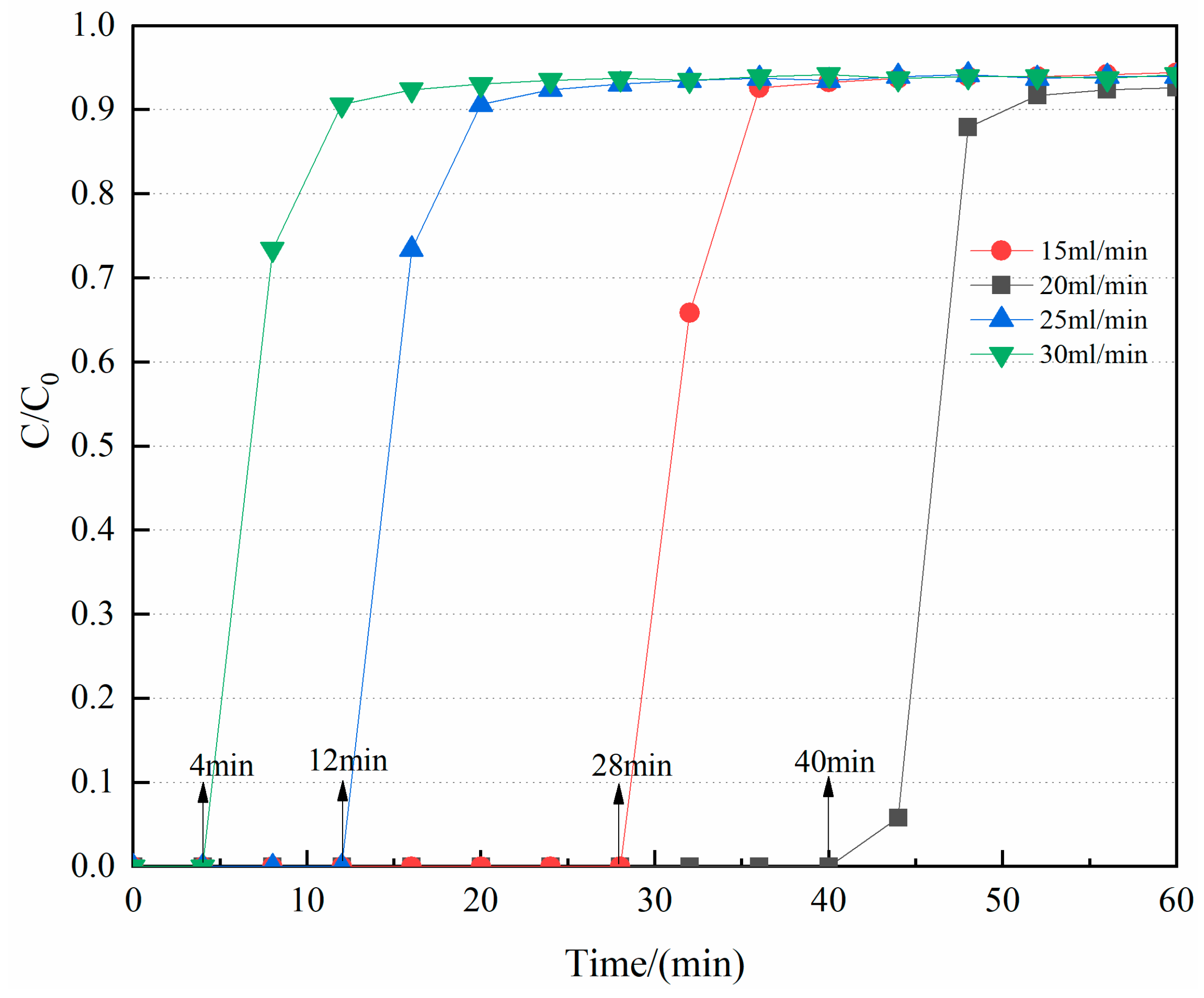
3.2.3. Effect of the Gas Flow Rate on the CO2/CH4 Selective Adsorption of CNT-NH2-Cu-BTC
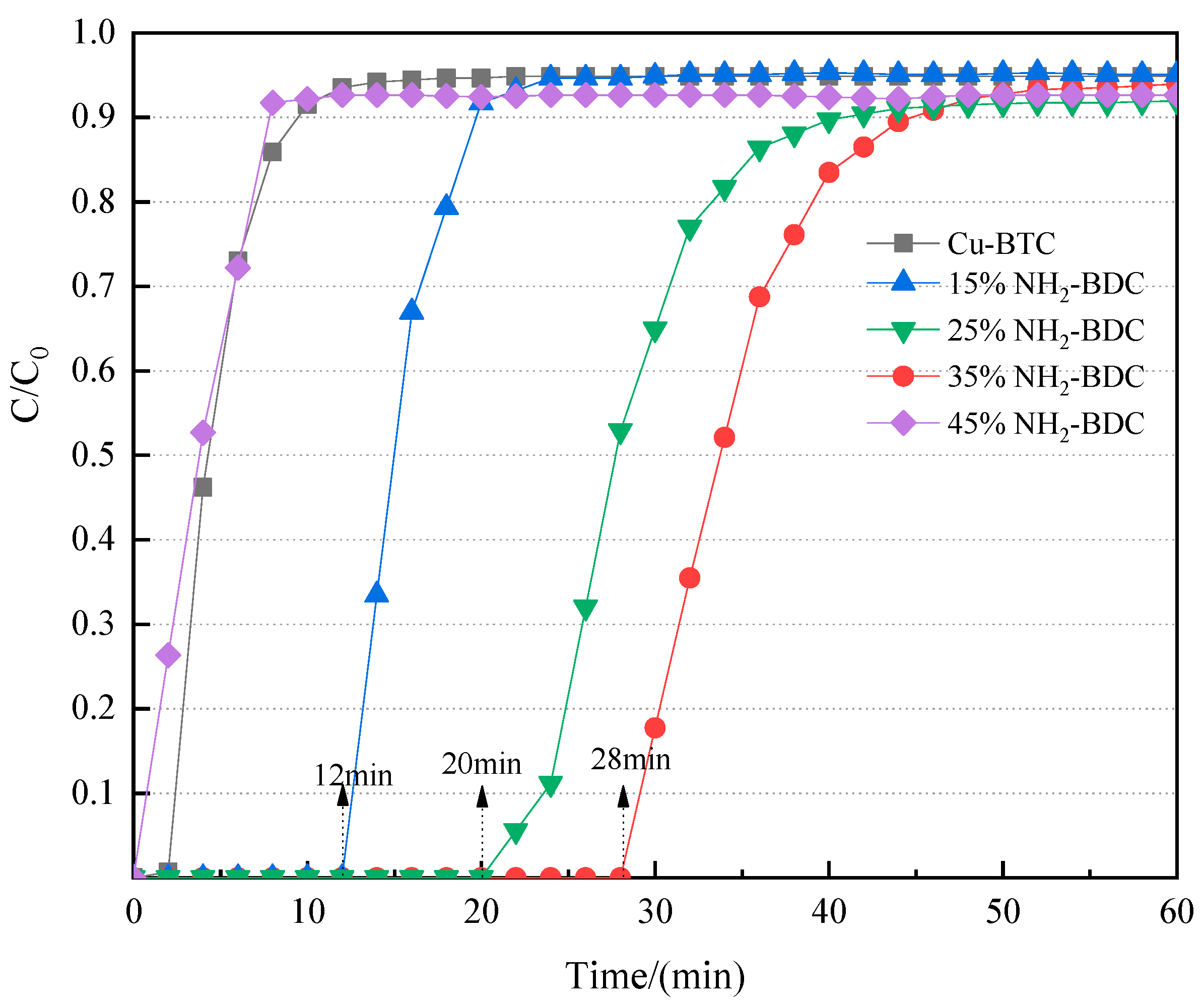
3.2.4. Water Stability of CNT-NH2-Cu-BTC
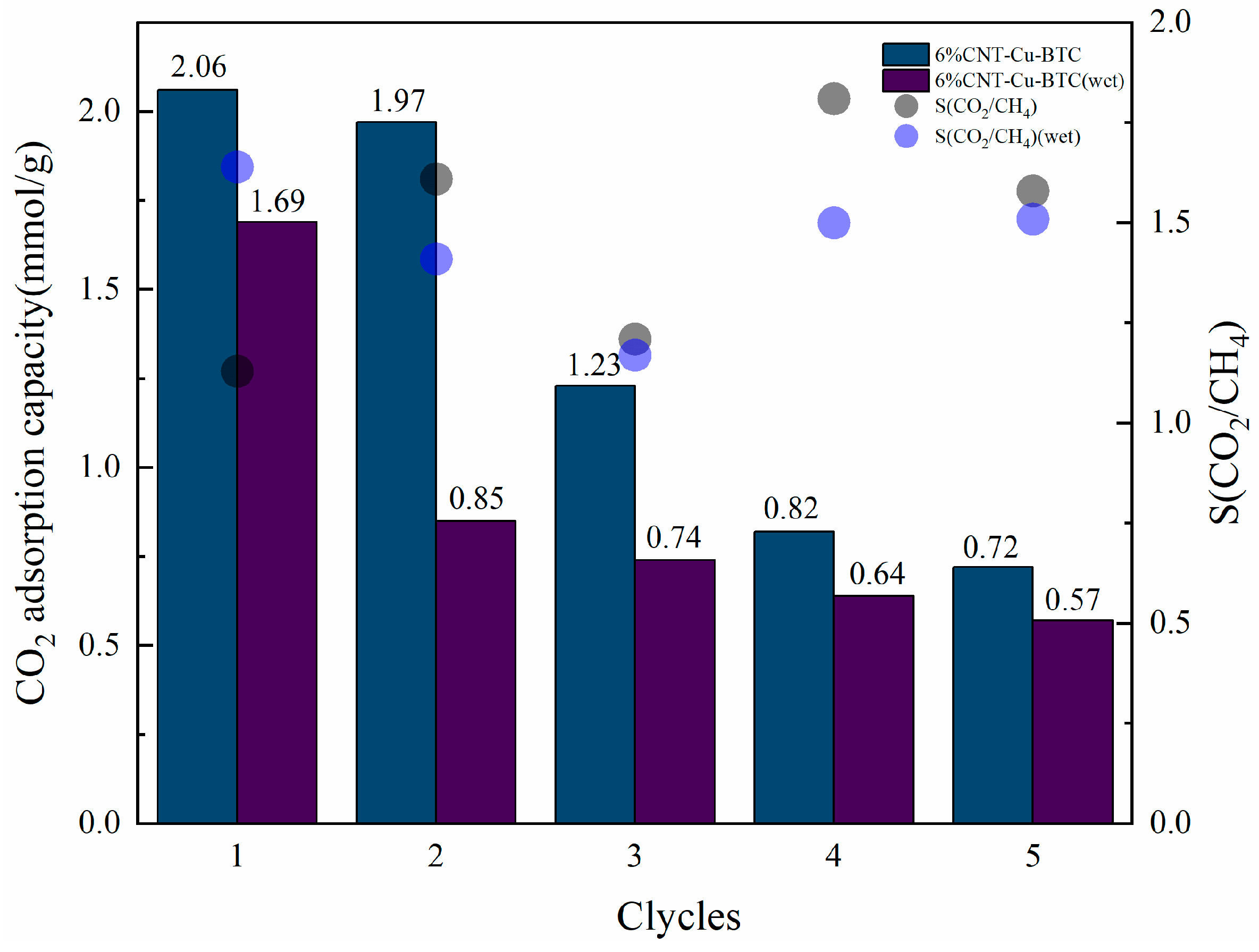
3.2.5. Adsorption Cycles of CNT-NH2-Cu-BTC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, B.; Wang, C.; Yu, G.; Dou, K. Reflections on the development of biogas industry in Europe. China’s Energy 2019, 41, 5. [Google Scholar]
- Zheng, Y.; Zhang, Y.; Sun, B.; Zhang, B.; Zhang, S.; Jin, S.; Xiao, Z.; Chu, S.; Jing, Y.; Zhang, Z. Corrosion Behavior and Mechanical Performance of Drill Pipe Steel in a CO2/H2S-Drilling-Fluid Environment. Processes 2024, 12, 502. [Google Scholar] [CrossRef]
- Hai, Q.; Jian, W.; Xie, W.; Tong, X.; Sun, Y. Research progress on adsorption of CO2 by porous solid materials. J. Liaoning P Univ. 2022, 42, 30–77. [Google Scholar]
- Granados-Pichardo, A.; Granados-Correa, F.; Sanchez-Mendieta, V.; Hernded-Mendoza, H. New CaO-based adsorbents prepared by solution combustion and high-energy ball-milling processes for CO2 adsorption: Textural and structural influences. Arab. J. Chem. 2017, 13, 171–183. [Google Scholar] [CrossRef]
- Wang, S.; Shen, H.; Fan, S.; Zhao, Y.; Ma, X.; Gong, J. Enhanced CO2 adsorption capacity and stability using CaO-based adsorbents treated by hydration. AIChE J. 2013, 59, 3586–3593. [Google Scholar] [CrossRef]
- Cláudio, R.; Soria, M.; Luís, M. Doping of hydrotalcite-based sorbents with different interlayer anions for CO2 capture. Sep. Purif. Technol. 2020, 235, 1116140. [Google Scholar]
- Khosravi, T.; Omidkhah, M.; Kaliaguine, S.; Rodrigue, D. Amine-Functionalized CuBTC/poly(ether-b-amide-6) (Pebax MH 1657) Mixed Matrix Membranes for CO2/CH4 Separatio. Can. J. Chem. Eng. 2017, 95, 2024–2033. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarathi, R.; Ramaprabhu, S. Magnesium oxide modified nitrogen-doped porous carbon composite as an efficient candidate for high pressure carbon dioxide capture and methane storage. J. Colloid Interface Sci. 2019, 539, 245–256. [Google Scholar] [CrossRef]
- Guo, A.; Ban, Y.; Yang, K.; Yang, V. Metal-organic framework-based mixed matrix membranes: Synergetic effect of adsorption and diffusion for CO2/CH4 separation. J. Membr. Sci. 2018, 562, 76–84. [Google Scholar] [CrossRef]
- Agbaje, T.; Singh, S.; Reddy, K.S.K.; Polychronopoulou, K.; Vega, L.F.; Khaleel, M.; Wang, K.; Karanikolos, G.N. Salt-free synthesis of Cu-BTC metal-organic framework exhibiting mesoporosity and enhanced carbon dioxide adsorption. Microporous Mesoporous Mater. 2021, 324, 111265. [Google Scholar] [CrossRef]
- Ahmadi, R.; Ardjmand, M.; Rashidi, A.; Rafizadeh, M. High performance novel nano adsorbents derived–Natural cellulose fibers for superior CO2 adsorption and CO2/CH4 separation. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–19. [Google Scholar] [CrossRef]
- Mutyala, S.; Jonnalagadda, M.; Ibrahim, S.M. Effect of modification of UiO-66 for CO2 adsorption and separation of CO2/CH4. J. Mol. Struct. 2021, 1227, 129506. [Google Scholar] [CrossRef]
- Salehi, S.; Anbia, M. High CO2 Adsorption Capacity and CO2/CH4 Selectivity by Nanocomposites of MOF-199. Energy Fuels 2017, 31, 5376–5384. [Google Scholar] [CrossRef]
- Ullah, S.; Shariff, A.M.; Bustam, A.; Elkhalifah, A.E.I.; Gonfa, G.; Kareem, F.A.A. The Role of Multiwall Carbon Nanotubes in Cu-BTC Metal-Organic Frameworks for CO2 Adsorption. J. Chin. Chem. Soc. 2016, 63, 1022–1032. [Google Scholar] [CrossRef]
- Zhang, Y.; Wibowo, H.; Zhong, L.; Horttanainen, M.; Wang, Z.; Yu, C.; Yan, M. Cu-BTC-based composite adsorbents for selective adsorption of CO2 from syngas. Sep. Purif. Technol. 2021, 279, 119644. [Google Scholar] [CrossRef]
- Eshraghi, F.; Anbia, M.; Salehi, S. Dative post synthetic methods on SBUs of MWCNT@MOFs hybrid composite and its effect on CO2 uptake properties. J. Environ. Chem. Eng. 2017, 5, 4516–4523. [Google Scholar] [CrossRef]
- Xiang, Z.; Peng, X.; Cheng, X.; Li, X.; Cao, D. CNT@Cu3(BTC)2 and Metal–Organic Frameworks for Separation of CO2/CH4 Mixture. J. Phys. Chem. C 2011, 115, 19864–19871. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; Zhou, Y.; Tian, N.; Liang, G.; Ma, N.; Dai, W. Highly Improved Water Resistance and Congo Red Uptake Capacity with a Zn/Cu-BTC@MC Composite Adsorbent. J. Chem. Eng. Data 2019, 64, 3323–3330. [Google Scholar] [CrossRef]
- Li, H.; Lin, Z.; Zhou, X.; Wang, X.; Li, Y.; Wang, H.; Li, Z. Ultrafast room temperature synthesis of novel composites Imi@Cu-BTC with improved stability against moisture. Chem. Eng. J. 2017, 307, 537–543. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Q.; Dai, W.; Tian, N.; Ma, N. Efficient thiophene capture with a hydrophobic Cu-BTC-(n)Br adsorbent in the presence of moisture. Microporous Mesoporous Mater. 2018, 266, 7–13. [Google Scholar] [CrossRef]
- Baosheng, G.; Yanyan, X.; Haoru, Z.; Sun, H.; Guo, Y.; Wang, W. High Performance Gas Separation Mixed Matrix Membrane Fabricated by Incorporation of Functionalized Submicrometer-Sized Metal-Organic Framework. Materials 2018, 11, 1421. [Google Scholar] [CrossRef]
- Zukal, A.; Opanasenko, M.; Rubeš, M.; Nachtigall, P.; Jagiello, J. Adsorption of pentane isomers on metal-organic frameworks Cu-BTC and Fe-BTC. Catal. Today 2015, 243, 69–75. [Google Scholar] [CrossRef]
- Saleh, T.; Gupta, V. Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide. J. Colloid Interface Sci. 2012, 371, 101–106. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, P.; Hao, L.; Xu, Y. Amine-modified SBA-15(P): A promising adsorbent for CO2 capture. J. CO2 Util. 2018, 24, 22–33. [Google Scholar] [CrossRef]




| Sample | BET Surface Area (m2/g) | Relative Reduction in the BET Surface Area | Pore Volume (cm3/g) | Relative Reduction in Pore Volume |
|---|---|---|---|---|
| Cu-BTC | 990.6 | -- | 0.48 | -- |
| Cu-BTC (wet) | 454.1 | 54% | 0.34 | 29% |
| 35%NH2-Cu-BTC | 726.6 | -- | 0.50 | -- |
| 35%NH2-Cu-BTC (wet) | 379.0 | 48% | 0.32 | 36% |
| 6%CNT-NH2-Cu-BTC | 515.2 | -- | 0.42 | -- |
| 6%CNT-NH2-Cu-BTC (wet) | 444.6 | 14% | 0.38 | 10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, W.; Hai, Q.; Youlidaxi, A.; Liu, T.; Ma, D.; Jia, F. Modification of Copper Benzene-1,3,5-tricarboxy Late (Cu-BTC) Composites with Multiwalled Carbon Nanotubes and Amino Groups for Enhanced CO2/CH4 Selective Adsorption Performance and Water Stability. Processes 2024, 12, 745. https://doi.org/10.3390/pr12040745
Jian W, Hai Q, Youlidaxi A, Liu T, Ma D, Jia F. Modification of Copper Benzene-1,3,5-tricarboxy Late (Cu-BTC) Composites with Multiwalled Carbon Nanotubes and Amino Groups for Enhanced CO2/CH4 Selective Adsorption Performance and Water Stability. Processes. 2024; 12(4):745. https://doi.org/10.3390/pr12040745
Chicago/Turabian StyleJian, Weiwei, Qiuyan Hai, Adili Youlidaxi, Tianqiang Liu, Danzhu Ma, and Fengrui Jia. 2024. "Modification of Copper Benzene-1,3,5-tricarboxy Late (Cu-BTC) Composites with Multiwalled Carbon Nanotubes and Amino Groups for Enhanced CO2/CH4 Selective Adsorption Performance and Water Stability" Processes 12, no. 4: 745. https://doi.org/10.3390/pr12040745





