Pyrene-Modified Cyclic Peptides Detect Cu2+ Ions by Fluorescence in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Peptide Synthesis
2.3. Fluorescence Spectrometry
2.4. ESI Mass Spectrometry
2.5. Cyclic Voltammetry
3. Results and Discussion
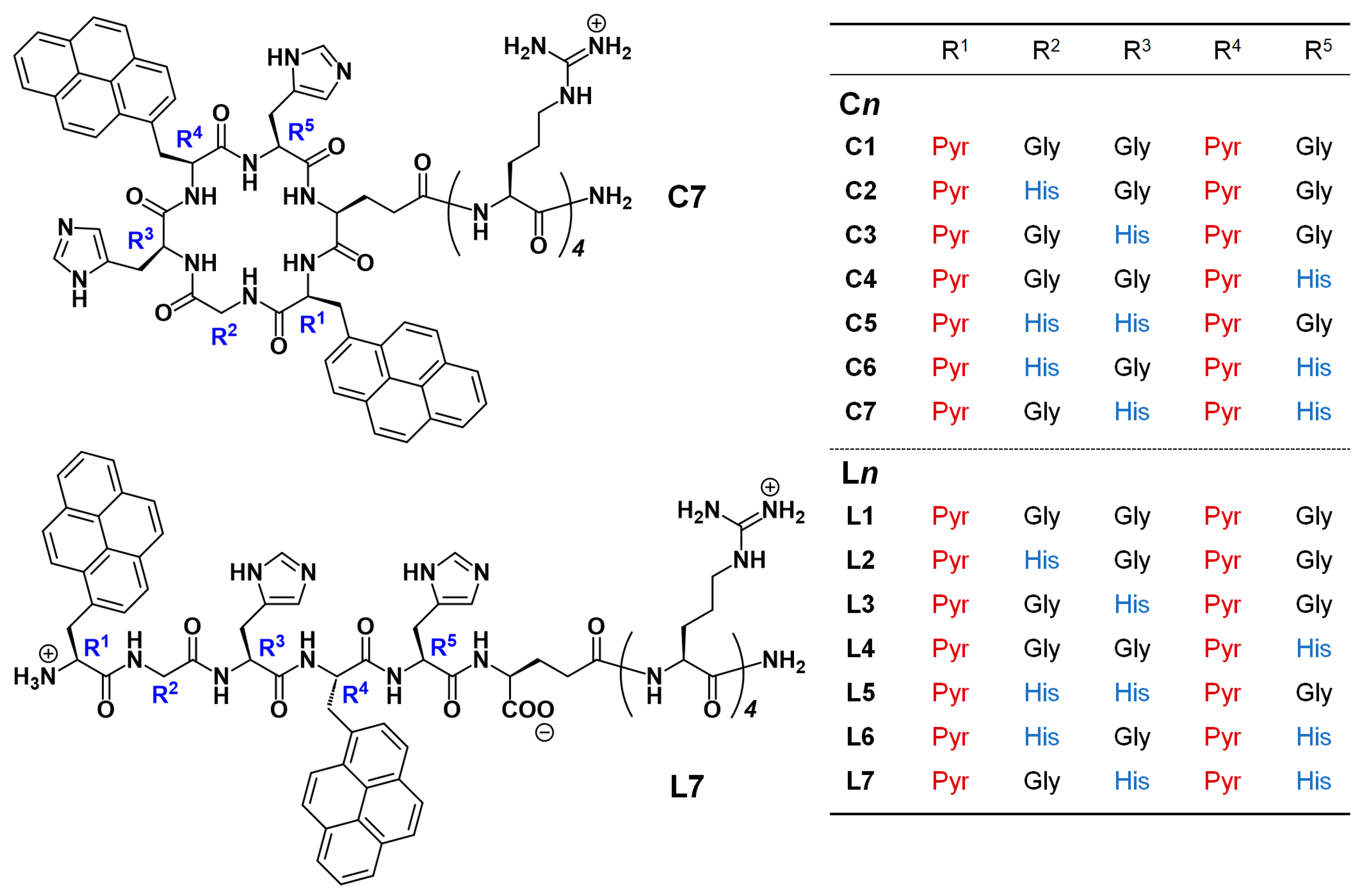
3.1. Design of Pyr-Labeled Peptides
3.2. Fluorescence Detection of Metal Ions for C1 and L1
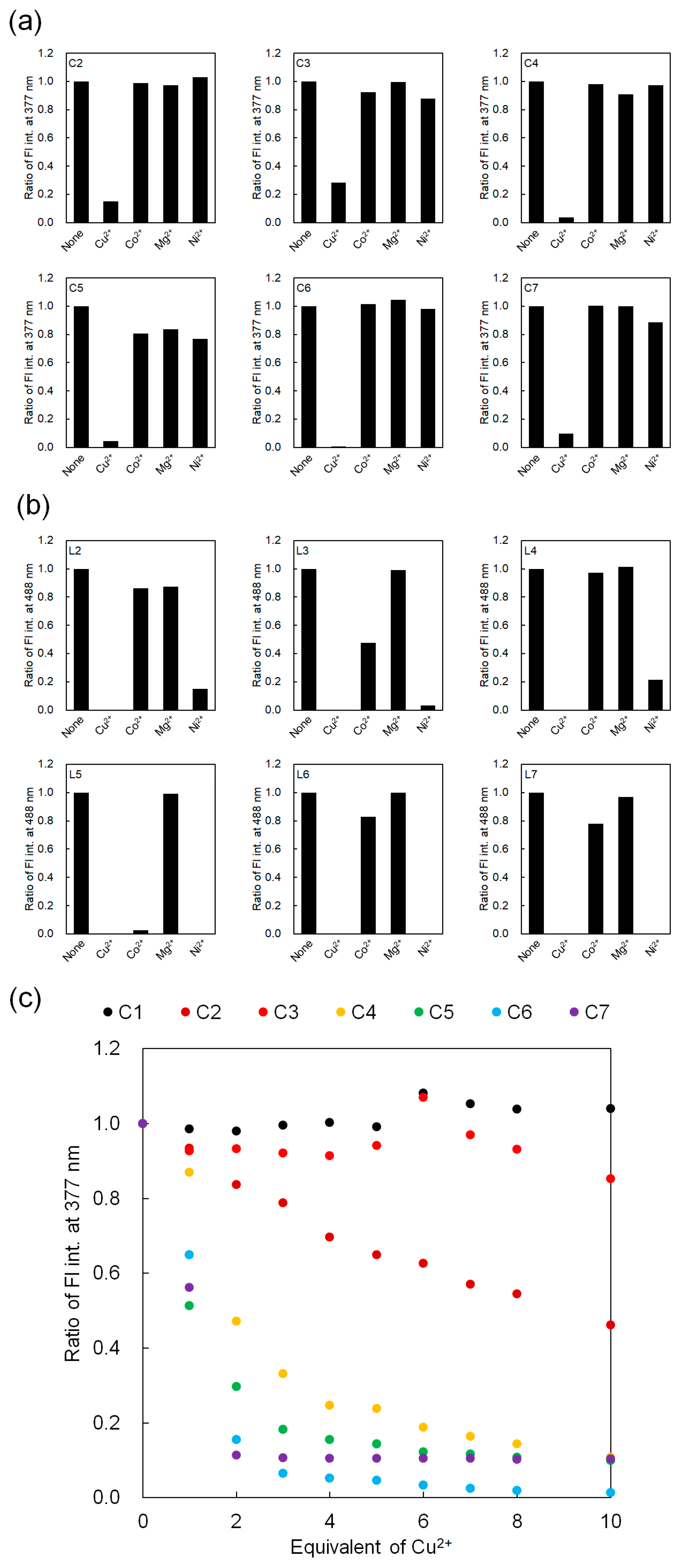
3.3. Fluorescence Detection of Metal Ions for C2–C7 and L2–L7 Containing Histidine Units
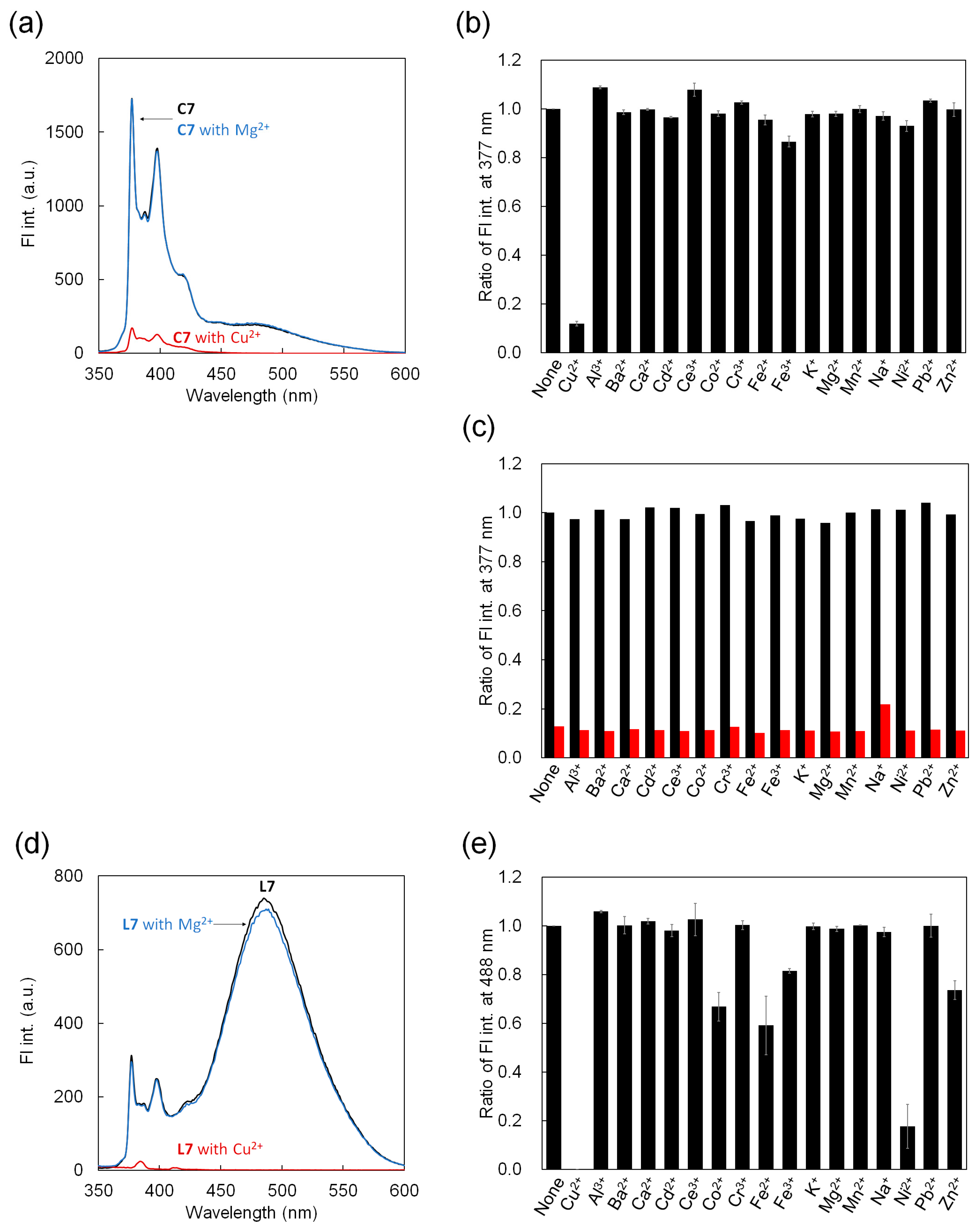
3.4. Fluorescence Detection of Metal Ions for C7 and L7
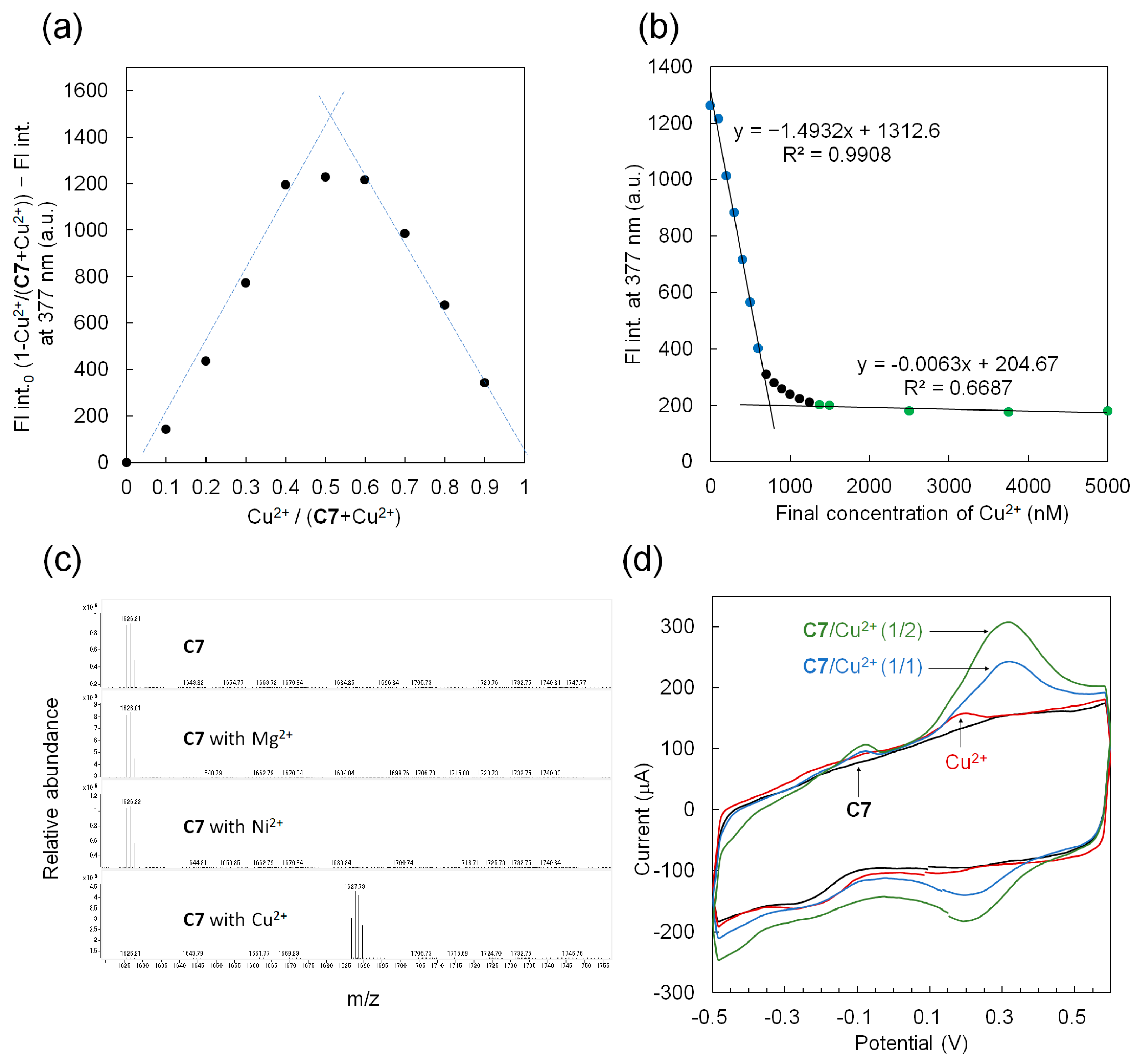
3.5. A 1:1 interaction between C7 and Cu2+
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Izatt, R.M.; Pawlak, K.; Bradshaw, J.S.; Bruening, R.L. Thermodynamic and Kinetic Data for Macrocycle Interactions with Cations and Anions. Chem. Rev. 1991, 91, 1721–2085. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent Chemosensors: The Past, Present and Future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef]
- Wu, S.-P.; Liu, S.-R. A New Water-Soluble Fluorescent Cu(II) Chemosensor Based on Tetrapeptide histidyl-glycyl-glycyl-glycine (HGGG). Sens. Actuators B Chem. 2009, 141, 187–191. [Google Scholar] [CrossRef]
- Wang, P.; Wu, J.; Di, C.; Zhou, R.; Zhang, H.; Su, P.; Xu, C.; Zhou, P.; Ge, Y.; Liu, W.; et al. A Novel Peptide-based Fluorescence Chemosensor for Selective Imaging of Hydrogen Sulfide Both in Living Cells and Zebrafish. Biosens. Bioelectron. 2017, 92, 602–609. [Google Scholar] [CrossRef]
- Ghosh, S.; Singh, P.; Verma, S. Morphological Consequences of Metal Ion–Peptide Vesicle Interaction. Tetrahedron 2008, 64, 1250–1256. [Google Scholar] [CrossRef]
- Xu, J.; Liu, N.; Hao, C.; Han, Q.; Duan, Y.; Wu, J. A Novel Fluorescence “On-Off-On” Peptide-based Chemosensor for Simultaneous Detection of Cu2+, Ag+ and S2−. Sens. Actuators B Chem. 2019, 280, 129–137. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, Y.; He, Y.; Lin, Q.; Ren, J.; Cao, D.; Zhang, L. A Label-free Fluorescent Peptide Probe for Sensitive and Selective Determination of Copper and Sulfide Ions in Aqueous Systems. RSC Adv. 2021, 11, 7426–7435. [Google Scholar] [CrossRef]
- Gui, S.; Huang, Y.; Zhu, Y.; Jin, Y.; Zhao, R. Biomimetic Sensing System for Tracing Pb2+ Distribution in Living Cells Based on the Metal−Peptide Supramolecular Assembly. ACS Appl. Mater. Interfaces 2019, 11, 5804–5811. [Google Scholar] [CrossRef]
- Ramezanpour, S.; Barzinmehr, H.; Shiri, P.; Meier, C.; Ayatollahi, S.A.; Mehrazar, M. Highly Selective Fluorescent Peptide–based Chemosensors for Aluminium Ions in Aqueous Solution. Anal. Bioanal. Chem. 2021, 413, 3881–3891. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, Z.; Gao, L.; Zhang, B.; Wang, L.; Kong, J.; Li, L. A Highly Selective and Sensitive Peptide-Based Fluorescent Ratio Sensor for Ag+. J. Fluoresc. 2021, 31, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.P.; Park, J.; Lee, W.I.; Lee, K.-H. Ratiometric and Turn-on Monitoring for Heavy and Transition Metal Ions in Aqueous Solution with a Fluorescent Peptide Sensor. Talanta 2009, 78, 903–909. [Google Scholar] [CrossRef]
- Nischan, N.; Herce, H.D.; Natale, F.; Bohlke, N.; Budisa, N.; Cardoso, M.C.; Hackenberger, C.P.R. Covalent Attachment of Cyclic TAT Peptides to GFP Results in Protein Delivery into Live Cells with Immediate Bioavailability. Angew. Chem. Int. Ed. 2015, 54, 1950–1953. [Google Scholar] [CrossRef]
- Qian, Z.; Martyna, A.; Hard, R.L.; Wang, J.; Appiah-Kubi, G.; Coss, C.; Phelps, M.A.; Rossman, J.S.; Pei, D. Discovery and Mechanism of Highly Efficient Cyclic Cell-Penetrating Peptides. Biochemistry 2016, 55, 2601–2612. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Uyeda, A.; Kidera, A.; Kikuchi, M. Functional Analysis and Modeling of a Conformationally Constrained Arg-Gly-Asp Sequence Inserted into Human Lysozyme. Biochemistry 1994, 33, 11678–11683. [Google Scholar] [CrossRef]
- Strauss, J.; Daub, J. Optically Active Cyclic Hexapeptides with Covalently Attached Pyrene Probes: Selective Alkaline Earth Metal Ion Recognition Using Excimer Emission. Org. Lett. 2002, 4, 683–686. [Google Scholar] [CrossRef]
- Gahan, L.R.; Cusack, R.M. Metal Complexes of Synthetic Cyclic Peptides. Polyhedron 2018, 153, 1–23. [Google Scholar] [CrossRef]
- Kotynia, A.; Pap, J.; Brasun, J. The Binding Abilities of Homodetic Cyclic His-Peptides toward Copper Ions. Inorganica Chim. Acta 2018, 472, 3–11. [Google Scholar] [CrossRef]
- Qin, C.; Xu, C.; Zhang, R.; Niu, W.; Shang, X. On-resin Cyclization and Antimicrobial Activity of Laterocidin and Its Analogues. Tetrahedron Lett. 2010, 51, 1257–1261. [Google Scholar] [CrossRef]
- Berthelot, T.; Gonçalves, M.; Laïn, G.; Estieu-Gionnet, K.; Déléris, G. New Strategy towards the Efficient Solid Phase Synthesis of Cyclopeptides. Tetrahedron 2006, 62, 1124–1130. [Google Scholar] [CrossRef]
- Johnson, T.; Liley, M.; Cheeseright, T.J.; Begum, F. Problems in the Synthesis of Cyclic Peptides through Use of the Dmab Protecting Group. J. Chem. Soc. Perkin Trans. 2000, 1, 2811–2820. [Google Scholar] [CrossRef]
- Sgolastra, F.; Backlund, C.M.; Ozay, I.E.; deRonde, B.M.; Minter, L.M.; Tew, G.N. Sequence Segregation Improves Non-Covalent Protein Delivery. J. Control. Release 2017, 254, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Heitz, F.C.; Morris, M.C.; Fesquet, D.; Cavadore, J.; Dorée, M.; Divita, G. Interactions of Cyclins with Cyclin-Dependent Kinases: A Common Interactive Mechanism. Biochemistry 1997, 36, 4995–5003. [Google Scholar] [CrossRef] [PubMed]
- Sugime, H.; Sato, T.; Nakagawa, R.; Hayashi, T.; Inoue, Y.; Noda, S. Ultra-Long Carbon Nanotube Forest via in Situ Supplements of Iron and Aluminum Vapor Sources. Carbon 2021, 172, 772–780. [Google Scholar] [CrossRef]
- Ghosh, P.; Rosenberg, I.; Maayan, G. Sequence-function Relationship within Water-soluble Peptoid Chelators for Cu2+. J. Inorg. Biochem. 2021, 217, 111388–111394. [Google Scholar] [CrossRef]
- Bar-Or, D.; Curtis, G.; Rao, N.; Bampos, N.; Lau, E. Characterization of the Co2+ and Ni2+ Binding Amino-acid Residues of the N-Terminus of Human Albumin. Eur. J. Biochem. 2001, 268, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Mirzapour-Kouhdasht, A.; Yun, H.; Park, J.H.; Min, H.J.; Lee, C.W. Development of Cobalt-Binding Peptide Chelate from Human Serum Albumin: Cobalt-Binding Properties and Stability. Int. J. Mol. Sci. 2022, 23, 719. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Kong, X.; Wang, Y.; Chao, J.; Li, C.; Dong, C.; Wang, Y.; Shuang, S. An Anthraquinone-based Highly Selective Colorimetric and Fluorometric Sensor for Sequential Detection of Cu2+ and S2− with Intracellular Application. J. Mater. Chem. B 2017, 5, 8957–8966. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.A.; Schilsky, M.L. AASLD Practice Guidelines: Diagnosis and Treatment of Wilson Disease: An Update. Hepatology 2008, 47, 2089–2111. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.F. Wilson’s Disease. Handb. Clin. Neurol. 2011, 100, 681–709. [Google Scholar]
- Brewer, G.J.; Dick, R.D.; Johnson, V.D.; Fink, J.K.; Kluin, K.J.; Daniels, S. Treatment of Wilson’s Disease with Zinc XVI. Treatment during the Pediatric Years. J. Lab. Clin. Med. 2001, 137, 191–198. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maekawa, Y.; Sakura, S.; Furutani, Y.; Fujihara, R.; Sugime, H.; Ohtsuki, T.; Kitamatsu, M. Pyrene-Modified Cyclic Peptides Detect Cu2+ Ions by Fluorescence in Water. Processes 2024, 12, 746. https://doi.org/10.3390/pr12040746
Maekawa Y, Sakura S, Furutani Y, Fujihara R, Sugime H, Ohtsuki T, Kitamatsu M. Pyrene-Modified Cyclic Peptides Detect Cu2+ Ions by Fluorescence in Water. Processes. 2024; 12(4):746. https://doi.org/10.3390/pr12040746
Chicago/Turabian StyleMaekawa, Yuhi, Sora Sakura, Yuji Furutani, Rento Fujihara, Hisashi Sugime, Takashi Ohtsuki, and Mizuki Kitamatsu. 2024. "Pyrene-Modified Cyclic Peptides Detect Cu2+ Ions by Fluorescence in Water" Processes 12, no. 4: 746. https://doi.org/10.3390/pr12040746







