A Concise Overview of Ultrasound-Assisted Techniques for the Production of 2D Materials
Abstract
:1. Introduction
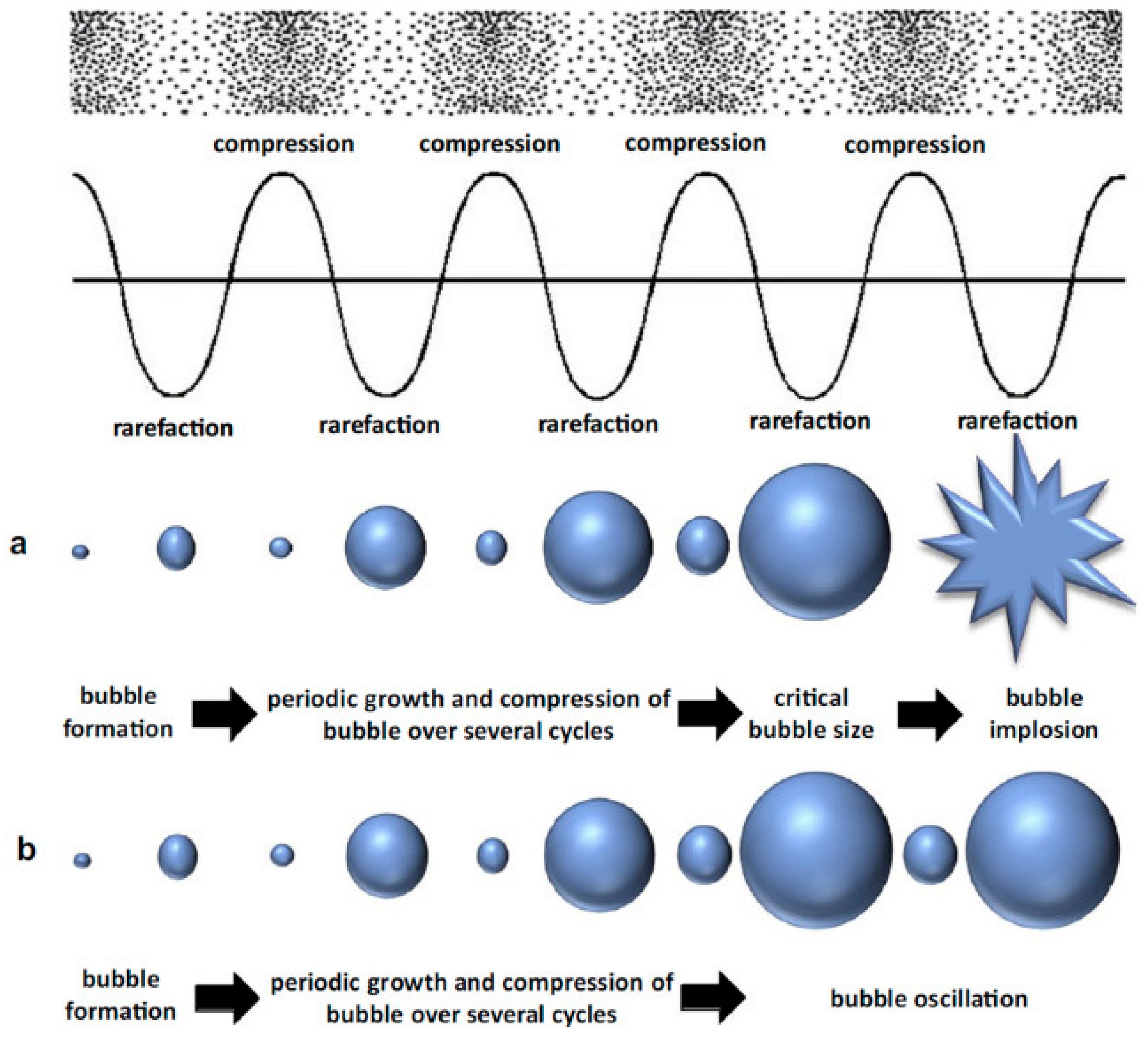
2. The Physiochemical Phenomena of UATs
3. UATs for the Production of 2D Materials: Graphene and Related Materials
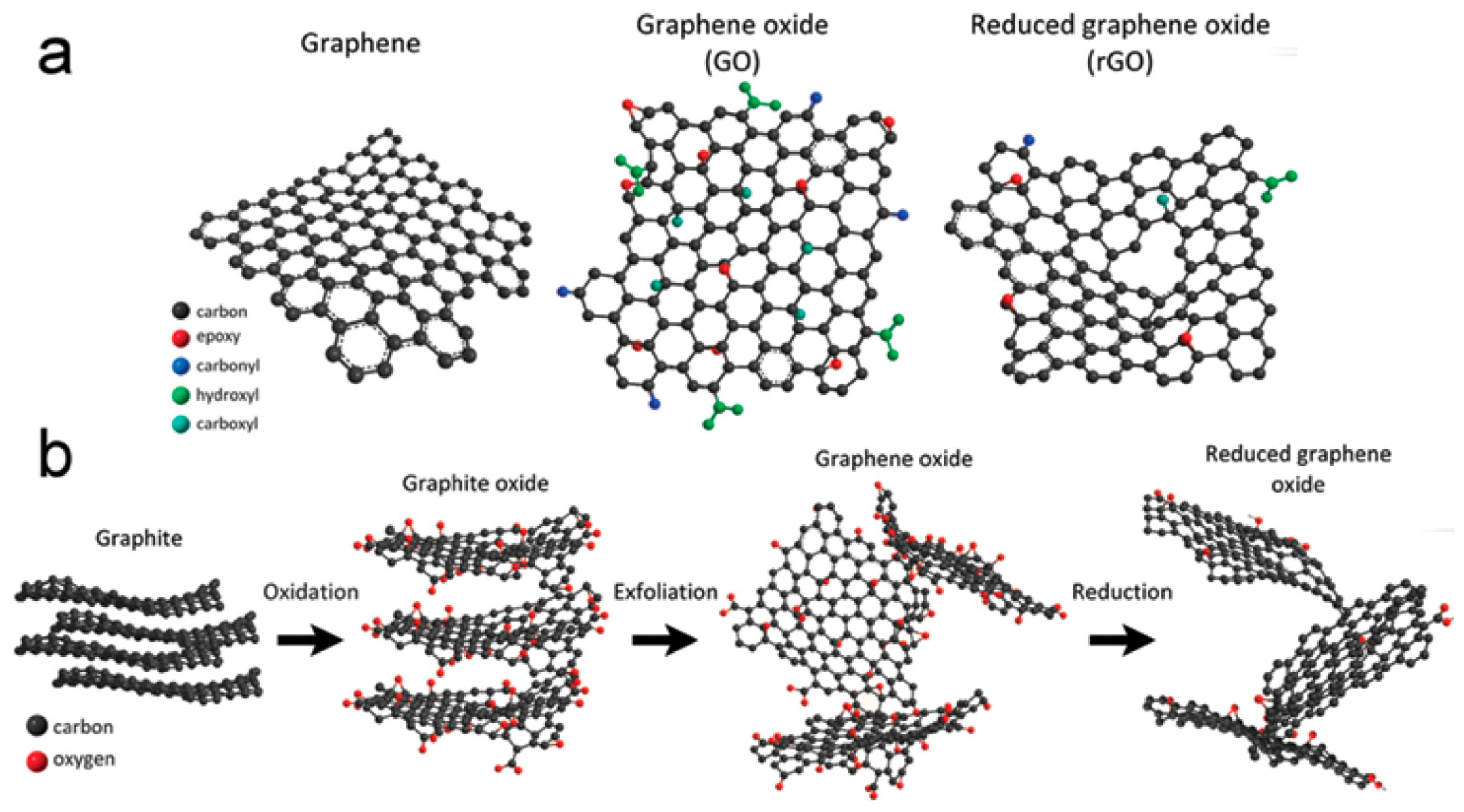
3.1. Exfoliation/Oxidation
3.2. Chemical Tailoring
4. UATs for the Production of 2D Materials: Inorganic Materials
4.1. Metal Nitride Species
4.2. Metal Dichalcogenide Species
4.3. MXenes
5. Advantages and Disadvantages of UATs: A Comparative Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raouf, A. Maintenance quality and environmental performance improvement: An integrated approach. In Handbook of Maintenance Management and Engineering; Springer: Berlin/Heidelberg, Germany, 2009; pp. 649–664. [Google Scholar]
- Smith, P.; Ashmore, M.R.; Black, H.I.; Burgess, P.J.; Evans, C.D.; Quine, T.A.; Thomson, A.M.; Hicks, K.; Orr, H.G. The role of ecosystems and their management in regulating climate, and soil, water and air quality. J. Appl. Ecol. 2013, 50, 812–829. [Google Scholar] [CrossRef]
- Lihua, W.; Tianshu, M.; Yuanchao, B.; Sijia, L.; Zhaoqiang, Y. Improvement of regional environmental quality: Government environmental governance and public participation. Sci. Total Environ. 2020, 717, 137265. [Google Scholar]
- Fallah, B.; Russo, E.; Menz, C.; Hoffmann, P.; Didovets, I.; Hattermann, F.F. Anthropogenic influence on extreme temperature and precipitation in Central Asia. Sci. Rep. 2023, 13, 6854. [Google Scholar] [CrossRef]
- Ghangrekar, M.; Chatterjee, P. Water pollutants classification and its effects on environment. In Carbon Nanotubes for Clean Water; Springer: Berlin/Heidelberg, Germany, 2018; pp. 11–26. [Google Scholar]
- Rasheed, T.; Ahmad, N.; Ali, J.; Hassan, A.A.; Sher, F.; Rizwan, K.; Iqbal, H.M.N.; Bilal, M. Nano and micro architectured cues as smart materials to mitigate recalcitrant pharmaceutical pollutants from wastewater. Chemosphere 2021, 274, 129785. [Google Scholar] [CrossRef] [PubMed]
- Findlay, A. An Introduction to Theoretical and Applied Colloid Chemistry: The World of Neglected Dimensions. By Dr. Wolfgang Ostwald, Privatdozent in the University of Leipsic. Authorised translation from the German by Dr. M. H. Fischer, Eichberg Professor of Physiology in the University of Cincinnati. (New York: John Wiley and Sons, Inc. London: Chapman and Hall, Ltd. 1917.) Price: 11s. 6d. net. J. Soc. Chem. Ind. 1919, 38, 485–486. [Google Scholar] [CrossRef]
- Das, S.; Kim, M.; Lee, J.-W.; Choi, W. Synthesis, properties, and applications of 2-D materials: A comprehensive review. Crit. Rev. Solid State Mater. Sci. 2014, 39, 231–252. [Google Scholar] [CrossRef]
- Shen, P.-C.; Lin, Y.; Wang, H.; Park, J.-H.; Leong, W.S.; Lu, A.-Y.; Palacios, T.; Kong, J. CVD technology for 2-D materials. IEEE Trans. Electron Devices 2018, 65, 4040–4052. [Google Scholar] [CrossRef]
- Baptista, A.; Silva, F.; Porteiro, J.; Míguez, J.; Pinto, G. Sputtering physical vapour deposition (PVD) coatings: A critical review on process improvement and market trend demands. Coatings 2018, 8, 402. [Google Scholar] [CrossRef]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef]
- Huo, C.; Yan, Z.; Song, X.; Zeng, H. 2D materials via liquid exfoliation: A review on fabrication and applications. Sci. Bull. 2015, 60, 1994–2008. [Google Scholar] [CrossRef]
- Khan, K.; Tareen, A.K.; Aslam, M.; Sagar, R.U.R.; Zhang, B.; Huang, W.; Mahmood, A.; Mahmood, N.; Khan, K.; Zhang, H. Recent progress, challenges, and prospects in two-dimensional photo-catalyst materials and environmental remediation. Nano-Micro Lett. 2020, 12, 167. [Google Scholar] [CrossRef] [PubMed]
- Meroni, D.; Djellabi, R.; Ashokkumar, M.; Bianchi, C.L.; Boffito, D.C. Sonoprocessing: From concepts to large-scale reactors. Chem. Rev. 2021, 122, 3219–3258. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B. Ultrasound and Nano-Catalysts: An Ideal and Sustainable Combination to Carry out Diverse Organic Transformations. ChemistrySelect 2019, 4, 2484–2500. [Google Scholar] [CrossRef]
- Amaniampong, P.N.; Jérôme, F. Catalysis under ultrasonic irradiation: A sound synergy. Curr. Opin. Green Sustain. Chem. 2020, 22, 7–12. [Google Scholar] [CrossRef]
- Mintz, K.J.; Bartoli, M.; Rovere, M.; Zhou, Y.; Hettiarachchi, S.D.; Paudyal, S.; Chen, J.; Domena, J.B.; Liyanage, P.Y.; Sampson, R.; et al. A deep investigation into the structure of carbon dots. Carbon 2021, 173, 433–447. [Google Scholar] [CrossRef]
- Arrigo, R.; Bartoli, M.; Torsello, D.; Ghigo, G.; Malucelli, G. Thermal, dynamic-mechanical and electrical properties of UV-LED curable coatings containing porcupine-like carbon structures. Mater. Today Commun. 2021, 28, 102630. [Google Scholar] [CrossRef]
- Bartoli, M.; Giorcelli, M.; Rosso, C.; Rovere, M.; Jagdale, P.; Tagliaferro, A. Influence of Commercial Biochar Fillers on Brittleness/Ductility of Epoxy Resin Composites. Appl. Sci. 2019, 9, 3109. [Google Scholar] [CrossRef]
- Bartoli, M.; Torsello, D.; Piatti, E.; Giorcelli, M.; Sparavigna, A.C.; Rovere, M.; Ghigo, G.; Tagliaferro, A. Pressure-Responsive Conductive Poly(vinyl alcohol) Composites Containing Waste Cotton Fibers Biochar. Micromachines 2022, 13, 125. [Google Scholar] [CrossRef]
- Bartoli, M.; Troiano, M.; Giudicianni, P.; Amato, D.; Giorcelli, M.; Solimene, R.; Tagliaferro, A. Effect of heating rate and feedstock nature on electrical conductivity of biochar and biochar-based composites. Appl. Energy Combust. Sci. 2022, 12, 100089. [Google Scholar] [CrossRef]
- Giorcelli, M.; Bartoli, M. Development of Coffee Biochar Filler for the Production of Electrical Conductive Reinforced Plastic. Polymers 2019, 11, 17. [Google Scholar] [CrossRef]
- Alphandéry, E. Ultrasound and nanomaterial: An efficient pair to fight cancer. J. Nanobiotechnol. 2022, 20, 139. [Google Scholar] [CrossRef] [PubMed]
- Pollet, B.G.; Ashokkumar, M. Introduction to Ultrasound, Sonochemistry and Sonoelectrochemistry; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Pollet, B. Power Ultrasound in Electrochemistry: From Versatile Laboratory Tool to Engineering Solution; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Lorimer, J.P.; Mason, T.J. Sonochemistry. Part 1—The physical aspects. Chem. Soc. Rev. 1987, 16, 239–274. [Google Scholar] [CrossRef]
- Corporation, U.P. Ultrasonic Flow Through Reactors. Available online: https://www.upcorp.com/flow-through-reactors/ (accessed on 7 April 2024).
- Skrabalak, S.E. Ultrasound-assisted synthesis of carbon materials. Phys. Chem. Chem. Phys. 2009, 11, 4930–4942. [Google Scholar] [CrossRef] [PubMed]
- Bhangu, S.K.; Ashokkumar, M. Theory of Sonochemistry. Top. Curr. Chem. 2016, 374, 56. [Google Scholar] [CrossRef]
- Leighton, T. The Acoustic Bubble; Academic Press: London, UK, 1994; pp. 234–243. [Google Scholar]
- Petrier, C.; Luche, J.; Luche, J. Synthetic Organic Sonochemistry; Plenum Press New York: New York, NY, USA, 1998; pp. 53–56. [Google Scholar]
- Apfel, R.E. 7. Acoustic Cavitation. In Methods in Experimental Physics; Edmonds, P.D., Ed.; Academic Press: Cambridge, MA, USA, 1981; Volume 19, pp. 355–411. [Google Scholar]
- Crum, L.A. Sonoluminescence. Phys. Today 1994, 47, 22–29. [Google Scholar] [CrossRef]
- Prosperetti, A. Physics of acoustic cavitation in liquids: H. G. Flynn’s review 35 years later. J. Acoust. Soc. Am. 1998, 103, 2970. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J. Can sonochemistry take place in the absence of cavitation?–A complementary view of how ultrasound can interact with materials. Ultrason. Sonochem. 2019, 52, 2–5. [Google Scholar] [CrossRef]
- Fitzgerald, M.E.; Griffing, V.; Sullivan, J. Chemical effects of ultrasonics—“Hot spot”chemistry. J. Chem. Phys. 1956, 25, 926–933. [Google Scholar] [CrossRef]
- Margulis, M. Sonoluminescence and sonochemical reactions in cavitation fields. A review. Ultrasonics 1985, 23, 157–169. [Google Scholar] [CrossRef]
- Lepoint, T.; Mullie, F. What exactly is cavitation chemistry? Ultrason. Sonochem. 1994, 1, S13–S22. [Google Scholar] [CrossRef]
- Nikitenko, S.I. Plasma Formation during Acoustic Cavitation: Toward a New Paradigm for Sonochemistry. Adv. Phys. Chem. 2014, 2014, 173878. [Google Scholar] [CrossRef]
- Vyas, N.; Manmi, K.; Wang, Q.; Jadhav, A.J.; Barigou, M.; Sammons, R.L.; Kuehne, S.A.; Walmsley, A.D. Which Parameters Affect Biofilm Removal with Acoustic Cavitation? A Review. Ultrasound Med. Biol. 2019, 45, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef] [PubMed]
- Crum, L.A. Acoustic cavitation series: Part five rectified diffusion. Ultrasonics 1984, 22, 215–223. [Google Scholar] [CrossRef]
- Grieser, F.; Ashokkumar, M. Sonochemical synthesis of inorganic and organic colloids. In Colloids and Colloid Assemblies: Synthesis, Modification, Organization and Utilization of Colloid Particles; Wiley: Hoboken, NJ, USA, 2006; p. 1842. [Google Scholar]
- Mintmire, J.W.; Dunlap, B.I.; White, C.T. Are fullerene tubules metallic? Phys. Rev. Lett. 1992, 68, 631. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-A.; Ruan, W.; Chou, M. Electron-phonon interactions for optical-phonon modes in few-layer graphene: First-principles calculations. Phys. Rev. B 2009, 79, 115443. [Google Scholar] [CrossRef]
- Dresselhaus, M.; Jorio, A.; Saito, R. Characterizing graphene, graphite, and carbon nanotubes by Raman spectroscopy. Annu. Rev. Condens. Matter Phys. 2010, 1, 89–108. [Google Scholar] [CrossRef]
- Rhee, K.Y. Electronic and Thermal Properties of Graphene. Nanomaterials 2020, 10, 926. [Google Scholar] [CrossRef] [PubMed]
- Lavagna, L.; Meligrana, G.; Gerbaldi, C.; Tagliaferro, A.; Bartoli, M. Graphene and Lithium-Based Battery Electrodes: A Review of Recent Literature. Energies 2020, 13, 4867. [Google Scholar] [CrossRef]
- Catania, F.; Marras, E.; Giorcelli, M.; Jagdale, P.; Lavagna, L.; Tagliaferro, A.; Bartoli, M. A Review on Recent Advancements of Graphene and Graphene-Related Materials in Biological Applications. Appl. Sci. 2021, 11, 614. [Google Scholar] [CrossRef]
- Bartoli, M.; Piatti, E.; Tagliaferro, A. A Short Review on Nanostructured Carbon Containing Biopolymer Derived Composites for Tissue Engineering Applications. Polymers 2023, 15, 1567. [Google Scholar] [CrossRef]
- Lee, H.C.; Liu, W.-W.; Chai, S.-P.; Mohamed, A.R.; Lai, C.W.; Khe, C.-S.; Voon, C.; Hashim, U.; Hidayah, N. Synthesis of single-layer graphene: A review of recent development. Procedia Chem. 2016, 19, 916–921. [Google Scholar] [CrossRef]
- Abu-Nada, A.; McKay, G.; Abdala, A. Recent Advances in Applications of Hybrid Graphene Materials for Metals Removal from Wastewater. Nanomaterials 2020, 10, 595. [Google Scholar] [CrossRef]
- Brisebois, P.; Siaj, M. Harvesting graphene oxide—Years 1859 to 2019: A review of its structure, synthesis, properties and exfoliation. J. Mater. Chem. C 2020, 8, 1517–1547. [Google Scholar] [CrossRef]
- Lee, X.J.; Hiew, B.Y.Z.; Lai, K.C.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Rigby, S. Review on graphene and its derivatives: Synthesis methods and potential industrial implementation. J. Taiwan Inst. Chem. Eng. 2019, 98, 163–180. [Google Scholar] [CrossRef]
- Whitby, R.L. Chemical control of graphene architecture: Tailoring shape and properties. ACS Nano 2014, 8, 9733–9754. [Google Scholar] [CrossRef]
- Lin, L.; Peng, H.; Liu, Z. Synthesis challenges for graphene industry. Nat. Mater. 2019, 18, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Shim, S.J.; Kim, T.H. Scalable Preparation of Low-Defect Graphene by Urea-Assisted Liquid-Phase Shear Exfoliation of Graphite and Its Application in Doxorubicin Analysis. Nanomaterials 2020, 10, 267. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Pan, Y.-H.; Yang, R.; Bao, L.-H.; Meng, L.; Luo, H.-L.; Cai, Y.-Q.; Liu, G.-D.; Zhao, W.-J.; Zhou, Z.; et al. Universal mechanical exfoliation of large-area 2D crystals. Nat. Commun. 2020, 11, 2453. [Google Scholar] [CrossRef]
- Lei, W.; Mochalin, V.N.; Liu, D.; Qin, S.; Gogotsi, Y.; Chen, Y. Boron nitride colloidal solutions, ultralight aerogels and freestanding membranes through one-step exfoliation and functionalization. Nat. Commun. 2015, 6, 8849. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef]
- Price, R.J.; Ladislaus, P.I.; Smith, G.C.; Davies, T.J. A novel ‘bottom-up’synthesis of few-and multi-layer graphene platelets with partial oxidation via cavitation. Ultrason. Sonochem. 2019, 56, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Soltani, T.; Kyu Lee, B. A benign ultrasonic route to reduced graphene oxide from pristine graphite. J. Colloid Interface Sci. 2017, 486, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Kim, G.-S.; Kim, S.J. Graphene nanosheets: Ultrasound assisted synthesis and characterization. Ultrason. Sonochem. 2013, 20, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Entezari, M.H.; Goharshadi, E. Graphene oxide nanosheets synthesized by ultrasound: Experiment versus MD simulation. Appl. Surf. Sci. 2018, 451, 112–120. [Google Scholar] [CrossRef]
- Lavagna, L.; Santagati, A.; Bartoli, M.; Suarez-Riera, D.; Pavese, M. Cement-Based Composites Containing Oxidized Graphene Nanoplatelets: Effects on the Mechanical and Electrical Properties. Nanomaterials 2023, 13, 901. [Google Scholar] [CrossRef] [PubMed]
- Ručigaj, A.; Connell, J.G.; Dular, M.; Genorio, B. Influence of the ultrasound cavitation intensity on reduced graphene oxide functionalization. Ultrason. Sonochem. 2022, 90, 106212. [Google Scholar] [CrossRef] [PubMed]
- Sontakke, A.D.; Purkait, M.K. Fabrication of ultrasound-mediated tunable graphene oxide nanoscrolls. Ultrason. Sonochem. 2020, 63, 104976. [Google Scholar] [CrossRef]
- Luche, J. A few questions on the sonochemistry of solutions. Ultrason. Sonochem. 1997, 4, 211–215. [Google Scholar] [CrossRef]
- Domini, C.E.; Álvarez, M.B.; Silbestri, G.F.; Cravotto, G.; Cintas, P. Merging metallic catalysts and sonication: A periodic table overview. Catalysts 2017, 7, 121. [Google Scholar] [CrossRef]
- Shen, J.; Shi, M.; Ma, H.; Yan, B.; Li, N.; Hu, Y.; Ye, M. Synthesis of hydrophilic and organophilic chemically modified graphene oxide sheets. J. Colloid Interface Sci. 2010, 352, 366–370. [Google Scholar] [CrossRef]
- Du, X.; Skachko, I.; Barker, A.; Andrei, E.Y. Approaching ballistic transport in suspended graphene. Nat. Nanotechnol. 2008, 3, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Muthoosamy, K.; Manickam, S. State of the art and recent advances in the ultrasound-assisted synthesis, exfoliation and functionalization of graphene derivatives. Ultrason. Sonochem. 2017, 39, 478–493. [Google Scholar] [CrossRef]
- Jiang, X.; Kuklin, A.V.; Baev, A.; Ge, Y.; Ågren, H.; Zhang, H.; Prasad, P.N. Two-dimensional MXenes: From morphological to optical, electric, and magnetic properties and applications. Phys. Rep 2020, 848, 1–58. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-E.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Guan, J.; Zhang, C.; Shao, H.; Jiang, H.; Zhang, Y.; Xia, H.; Zhang, L.; Hu, J. Features of sonochemistry and its application in electrocatalyst synthesis. J. Alloys Compd. 2023, 957, 170369. [Google Scholar] [CrossRef]
- Matsuura, K. Industrial Applications of Separation through Ultrasonic Atomization. Earozoru Kenkyu 2011, 26, 30–35. [Google Scholar]
- Sato, M.; Matsuura, K.; Fujii, T. Ethanol separation from ethanol-water solution by ultrasonic atomization and its proposed mechanism based on parametric decay instability of capillary wave. J. Chem. Phys. 2001, 114, 2382–2386. [Google Scholar] [CrossRef]
- Yudin, A.; Shatrova, N.; Khaydarov, B.; Kuznetsov, D.; Dzidziguri, E.; Issi, J.-P. Synthesis of hollow nanostructured nickel oxide microspheres by ultrasonic spray atomization. J. Aerosol Sci. 2016, 98, 30–40. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, H.; Xue, J.; Liu, X.; Liu, S.; Li, X.; He, D. Ultrasonic-assisted hydrothermal synthesis of cobalt oxide/nitrogen-doped graphene oxide hybrid as oxygen reduction reaction catalyst for Al-air battery. Ultrason. Sonochem. 2021, 72, 105457. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Lu, M.; Wang, Z.; Jiao, Z.; Hu, P.; Gao, Q.; Jiang, Y.; Cheng, L. Self-assembly of ultrathin MnO2/graphene with three-dimension hierarchical structure by ultrasonic-assisted co-precipitation method. J. Alloys Compd. 2016, 663, 180–186. [Google Scholar] [CrossRef]
- Hudiyanti, D.; Hamidi, N.I.; Anugrah, D.S.B.; Salimah, S.N.M.; Siahaan, P. Encapsulation of vitamin C in sesame liposomes: Computational and experimental studies. Open Chem. 2019, 17, 537–543. [Google Scholar] [CrossRef]
- Zhan, K.; Wang, W.; Li, F.; Cao, J.; Liu, J.; Yang, Z.; Wang, Z.; Zhao, B. Microstructure and properties of graphene oxide reinforced copper-matrix composite foils fabricated by ultrasonic assisted electrodeposition. Mater. Sci. Eng. A 2023, 872, 144995. [Google Scholar] [CrossRef]
- Brownson, D.A.; Kampouris, D.K.; Banks, C.E. An overview of graphene in energy production and storage applications. J. Power Sources 2011, 196, 4873–4885. [Google Scholar] [CrossRef]
- Raj, B.G.S.; Ramprasad, R.N.R.; Asiri, A.M.; Wu, J.J.; Anandan, S. Ultrasound assisted synthesis of Mn3O4 nanoparticles anchored graphene nanosheets for supercapacitor applications. Electrochim. Acta 2015, 156, 127–137. [Google Scholar] [CrossRef]
- Choudhury, B.J.; Roy, K.; Moholkar, V.S. Improvement of supercapacitor performance through enhanced interfacial interactions induced by sonication. Ind. Eng. Chem. Res. 2021, 60, 7611–7623. [Google Scholar] [CrossRef]
- Yang, H.; Li, F.; Shan, C.; Han, D.; Zhang, Q.; Niu, L.; Ivaska, A. Covalent functionalization of chemically converted graphene sheets via silane and its reinforcement. J. Mater. Chem. 2009, 19, 4632–4638. [Google Scholar] [CrossRef]
- Qin, X.; Guo, Z.; Liu, Z.; Zhang, W.; Wan, M.; Yang, B. Folic acid-conjugated graphene oxide for cancer targeted chemo-photothermal therapy. J. Photochem. Photobiol. B Biol. 2013, 120, 156–162. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Wang, X. Two-dimensional covalent carbon nitride nanosheets: Synthesis, functionalization, and applications. Energy Environ. Sci. 2015, 8, 3092–3108. [Google Scholar] [CrossRef]
- Rono, N.; Kibet, J.K.; Martincigh, B.S.; Nyamori, V.O. A review of the current status of graphitic carbon nitride. Crit. Rev. Solid State Mater. Sci. 2021, 46, 189–217. [Google Scholar] [CrossRef]
- Cui, J.; Qi, D.; Wang, X. Research on the techniques of ultrasound-assisted liquid-phase peeling, thermal oxidation peeling and acid-base chemical peeling for ultra-thin graphite carbon nitride nanosheets. Ultrason. Sonochem. 2018, 48, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Y.; Bi, Y.; Jin, J.; Ehsan, M.F.; Fu, M.; He, T. Preparation of 2D hydroxyl-rich carbon nitride nanosheets for photocatalytic reduction of CO2. RSC Adv. 2015, 5, 33254–33261. [Google Scholar] [CrossRef]
- Claridge, J.B.; York, A.P.; Brungs, A.J.; Green, M.L. Study of the temperature-programmed reaction synthesis of early transition metal carbide and nitride catalyst materials from oxide precursors. Chem. Mater. 2000, 12, 132–142. [Google Scholar] [CrossRef]
- Tareen, A.K.; Priyanga, G.S.; Behara, S.; Thomas, T.; Yang, M. Mixed ternary transition metal nitrides: A comprehensive review of synthesis, electronic structure, and properties of engineering relevance. Prog. Solid State Chem. 2019, 53, 1–26. [Google Scholar] [CrossRef]
- Chen, Q.; Li, X.; Xie, R.; Xu, L.; Liu, L. Novel rapid synthesis of nanoscale tungsten nitride using non-toxic nitrogen source. Ceram. Int. 2020, 46, 2580–2584. [Google Scholar] [CrossRef]
- Giordano, C.; Antonietti, M. Synthesis of crystalline metal nitride and metal carbide nanostructures by sol–gel chemistry. Nano Today 2011, 6, 366–380. [Google Scholar] [CrossRef]
- Wittmer, M. Properties and microelectronic applications of thin films of refractory metal nitrides. J. Vac. Sci. Technol. A Vac. Surf. Film. 1985, 3, 1797–1803. [Google Scholar] [CrossRef]
- Zhang, R.; Wan, W.; Qiu, L.; Wang, Y.; Zhou, Y. Preparation of hydrophobic polyvinyl alcohol aerogel via the surface modification of boron nitride for environmental remediation. Appl. Surf. Sci. 2017, 419, 342–347. [Google Scholar] [CrossRef]
- Li, J.; Xiao, X.; Xu, X.; Lin, J.; Huang, Y.; Xue, Y.; Jin, P.; Zou, J.; Tang, C. Activated boron nitride as an effective adsorbent for metal ions and organic pollutants. Sci. Rep. 2013, 3, 3208. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, A.; Karim, Z.A.; Ismail, A.F.; Jamil, A.; Said, K.A.M.; Ali, A. Tuneable molecular selective boron nitride nanosheet ultrafiltration lamellar membrane for dye exclusion to remediate the environment. Chemosphere 2022, 303, 135066. [Google Scholar] [CrossRef]
- Hod, O. Graphite and hexagonal boron-nitride have the same interlayer distance. Why? J. Chem. Theory Comput. 2012, 8, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Han, W.-Q.; Wu, L.; Zhu, Y.; Watanabe, K.; Taniguchi, T. Structure of chemically derived mono- and few-atomic-layer boron nitride sheets. Appl. Phys. Lett. 2008, 93, 223103. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Yin, J. Boron nitride nanosheets: Large-scale exfoliation in methanesulfonic acid and their composites with polybenzimidazole. J. Mater. Chem. 2011, 21, 11371–11377. [Google Scholar] [CrossRef]
- Štengl, V.; Henych, J.; Slušná, M.; Ecorchard, P. Ultrasound exfoliation of inorganic analogues of graphene. Nanoscale Res. Lett. 2014, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Zhi, C.; Bando, Y.; Tang, C.; Kuwahara, H.; Golberg, D. Large-Scale Fabrication of Boron Nitride Nanosheets and Their Utilization in Polymeric Composites with Improved Thermal and Mechanical Properties. Adv. Mater. 2009, 21, 2889–2893. [Google Scholar] [CrossRef]
- Marsh, K.; Souliman, M.; Kaner, R.B. Co-solvent exfoliation and suspension of hexagonal boron nitride. Chem. Commun. 2015, 51, 187–190. [Google Scholar] [CrossRef]
- Lin, Y.; Williams, T.V.; Xu, T.-B.; Cao, W.; Elsayed-Ali, H.E.; Connell, J.W. Aqueous Dispersions of Few-Layered and Monolayered Hexagonal Boron Nitride Nanosheets from Sonication-Assisted Hydrolysis: Critical Role of Water. J. Phys. Chem. C 2011, 115, 2679–2685. [Google Scholar] [CrossRef]
- Deshmukh, A.R.; Jeong, J.W.; Lee, S.J.; Park, G.U.; Kim, B.S. Ultrasound-Assisted Facile Green Synthesis of Hexagonal Boron Nitride Nanosheets and Their Applications. ACS Sustain. Chem. Eng. 2019, 7, 17114–17125. [Google Scholar] [CrossRef]
- Vasylyev, M.A.; Chenakin, S.P.; Yatsenko, L.F. Nitridation of Ti6Al4V alloy under ultrasonic impact treatment in liquid nitrogen. Acta Mater. 2012, 60, 6223–6233. [Google Scholar] [CrossRef]
- Ruan, G.; Shen, Y.; Yao, J.; Huang, Y.; Yang, S.; Hu, S.; Wang, H.; Fang, Y.; Cai, X. Vanadium nitride nanocrystals decorated ultrathin, N-rich and hierarchically porous carbon nanosheets as superior polysulfides mediator for stable lithium-sulfur batteries. J. Power Sources 2023, 566, 232922. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Tang, X.; Kou, L. 2D Janus transition metal dichalcogenides: Properties and applications. Phys. Status Solidi (b) 2022, 259, 2100562. [Google Scholar] [CrossRef]
- Fan, J.; Sun, M. Transition Metal Dichalcogenides (TMDCs) Heterostructures: Synthesis, Excitons and Photoelectric Properties. Chem. Rec. 2022, 22, e202100313. [Google Scholar] [CrossRef]
- Peng, W.; Li, Y.; Zhang, F.; Zhang, G.; Fan, X. Roles of two-dimensional transition metal dichalcogenides as cocatalysts in photocatalytic hydrogen evolution and environmental remediation. Ind. Eng. Chem. Res. 2017, 56, 4611–4626. [Google Scholar] [CrossRef]
- Rehman, F.; Hussain Memon, F.; Ullah, S.; Jafar Mazumder, M.A.; Al-Ahmed, A.; Khan, F.; Hussain Thebo, K. Recent Development in Laminar Transition Metal Dichalcogenides-Based Membranes Towards Water Desalination: A Review. Chem. Rec. 2022, 22, e202200107. [Google Scholar] [CrossRef]
- Li, W.; Yang, Z.; Sun, M.; Dong, J. Interlayer interactions in transition metal dichalcogenides heterostructures. Rev. Phys. 2022, 9, 100077. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Gadore, V. MoS2 based nanocomposites: An excellent material for energy and environmental applications. J. Environ. Chem. Eng. 2021, 9, 105836. [Google Scholar] [CrossRef]
- Vignesh; Kaushik, S.; Tiwari, U.K.; Kant Choubey, R.; Singh, K.; Sinha, R.K. Study of Sonication Assisted Synthesis of Molybdenum Disulfide (MoS2) Nanosheets. Mater. Today Proc. 2020, 21, 1969–1975. [Google Scholar] [CrossRef]
- Qiao, W.; Yan, S.; He, X.; Song, X.; Li, Z.; Zhang, X.; Zhong, W.; Du, Y. Effects of ultrasonic cavitation intensity on the efficient liquid-exfoliation of MoS2 nanosheets. RSC Adv. 2014, 4, 50981–50987. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, H.; Dong, S.; Liu, Y.; Tai Nai, C.; Suk Shin, H.; Young Jeong, H.; Liu, B.; Ping Loh, K. High yield exfoliation of two-dimensional chalcogenides using sodium naphthalenide. Nat. Commun. 2014, 5, 2995. [Google Scholar] [CrossRef]
- Das, S.; Tama, A.M.; Dutta, S.; Ali, M.S.; Basith, M. Facile high-yield synthesis of MoS2 nanosheets with enhanced photocatalytic performance using ultrasound driven exfoliation technique. Mater. Res. Express 2019, 6, 125079. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, K. Few-layers MoS2 nanosheets modified thin film composite nanofiltration membranes with improved separation performance. J. Membr. Sci. 2020, 595, 117526. [Google Scholar] [CrossRef]
- Smith, R.J.; King, P.J.; Lotya, M.; Wirtz, C.; Khan, U.; De, S.; O’Neill, A.; Duesberg, G.S.; Grunlan, J.C.; Moriarty, G.; et al. Large-Scale Exfoliation of Inorganic Layered Compounds in Aqueous Surfactant Solutions. Adv. Mater. 2011, 23, 3944–3948. [Google Scholar] [CrossRef]
- Deng, R.; Yi, H.; Fan, F.; Fu, L.; Zeng, Y.; Wang, Y.; Li, Y.; Liu, Y.; Ji, S.; Su, Y. Facile exfoliation of MoS2 nanosheets by protein as a photothermal-triggered drug delivery system for synergistic tumor therapy. RSC Adv. 2016, 6, 77083–77092. [Google Scholar] [CrossRef]
- Gopalakrishnan, D.; Damien, D.; Shaijumon, M.M. MoS2 Quantum Dot-Interspersed Exfoliated MoS2 Nanosheets. ACS Nano 2014, 8, 5297–5303. [Google Scholar] [CrossRef]
- Štengl, V.; Tolasz, J.; Popelková, D. Ultrasonic preparation of tungsten disulfide single-layers and quantum dots. RSC Adv. 2015, 5, 89612–89620. [Google Scholar] [CrossRef]
- Jha, R.K.; Guha, P.K. Liquid exfoliated pristine WS2 nanosheets for ultrasensitive and highly stable chemiresistive humidity sensors. Nanotechnology 2016, 27, 475503. [Google Scholar] [CrossRef]
- Qin, J.-K.; Ren, D.-D.; Shao, W.-Z.; Li, Y.; Miao, P.; Sun, Z.-Y.; Hu, P.; Zhen, L.; Xu, C.-Y. Photoresponse Enhancement in Monolayer ReS2 Phototransistor Decorated with CdSe–CdS–ZnS Quantum Dots. ACS Appl. Mater. Interfaces 2017, 9, 39456–39463. [Google Scholar] [CrossRef]
- Hu, Z.; Hu, X.; He, P.; Chen, J.; Huang, J.; Xie, Z.; Zhao, Y.; Tao, L.; Hao, M.; He, J. NbS2-nanosheet-based saturable absorber for 1.5 µm and 2 µm ultrafast fiber lasers. Photonics Nanostruct. Fundam. Appl. 2023, 54, 101117. [Google Scholar] [CrossRef]
- Miremadi, B.K.; Morrison, S.R. The intercalation and exfoliation of tungsten disulfide. J. Appl. Phys. 1988, 63, 4970–4974. [Google Scholar] [CrossRef]
- Pan, L.; Liu, Y.T.; Xie, X.M.; Ye, X.Y. Facile and green production of impurity-free aqueous solutions of WS2 nanosheets by direct exfoliation in water. Small 2016, 12, 6703–6713. [Google Scholar] [CrossRef]
- Li, Z.; Dong, J.; Zhang, H.; Zhang, Y.; Wang, H.; Cui, X.; Wang, Z. Sonochemical catalysis as a unique strategy for the fabrication of nano-/micro-structured inorganics. Nanoscale Adv. 2021, 3, 41–72. [Google Scholar] [CrossRef]
- Bayat, A.; Saievar-Iranizad, E. Synthesis of blue photoluminescent WS2 quantum dots via ultrasonic cavitation. J. Lumin. 2017, 185, 236–240. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, R.; Wang, B.; Liu, X.; Wang, H. Dual Regulation of Metal Doping and Adjusting Cut-Off Voltage for MoSe2 to Achieve Reversible Sodium Storage. Small 2022, 18, 2200437. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Zhu, X.-D.; Xie, X.-M. Direct Exfoliation of High-Quality, Atomically Thin MoSe2 Layers in Water. Adv. Sustain. Syst. 2018, 2, 1700107. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Liu, F.; Zou, Y.; Tang, X.; Mao, L.; Du, D.; Wang, H.; Zhang, M.; Wang, Z.; Yao, N.; Zhao, W.; et al. Phase Engineering and Alkali Cation Stabilization for 1T Molybdenum Dichalcogenides Monolayers. Adv. Funct. Mater. 2022, 32, 2204601. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y. Potential environmental applications of MXenes: A critical review. Chemosphere 2021, 271, 129578. [Google Scholar] [CrossRef]
- Yu, S.; Tang, H.; Zhang, D.; Wang, S.; Qiu, M.; Song, G.; Fu, D.; Hu, B.; Wang, X. MXenes as emerging nanomaterials in water purification and environmental remediation. Sci. Total Environ. 2022, 811, 152280. [Google Scholar] [CrossRef]
- Feng, W.; Luo, H.; Wang, Y.; Zeng, S.; Tan, Y.; Zhang, H.; Peng, S. Ultrasonic assisted etching and delaminating of Ti3C2 Mxene. Ceram. Int. 2018, 44, 7084–7087. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Rajavel, K.; Ke, T.; Yang, K.; Lin, D. Condition optimization for exfoliation of two dimensional titanium carbide (Ti3C2Tx). Nanotechnology 2018, 29, 095605. [Google Scholar] [CrossRef]
- Mashtalir, O.; Lukatskaya, M.R.; Zhao, M.Q.; Barsoum, M.W.; Gogotsi, Y. Amine-assisted delamination of Nb2C MXene for Li-ion energy storage devices. Adv. Mater. 2015, 27, 3501–3506. [Google Scholar] [CrossRef]
- Bhimanapati, G.R.; Lin, Z.; Meunier, V.; Jung, Y.; Cha, J.; Das, S.; Xiao, D.; Son, Y.; Strano, M.S.; Cooper, V.R. Recent advances in two-dimensional materials beyond graphene. ACS Nano 2015, 9, 11509–11539. [Google Scholar] [CrossRef]
- Soleymaniha, M.; Shahbazi, M.A.; Rafieerad, A.R.; Maleki, A.; Amiri, A. Promoting role of MXene nanosheets in biomedical sciences: Therapeutic and biosensing innovations. Adv. Healthc. Mater. 2019, 8, 1801137. [Google Scholar] [CrossRef]
- Naguib, M.; Unocic, R.R.; Armstrong, B.L.; Nanda, J. Large-scale delamination of multi-layers transition metal carbides and carbonitrides “MXenes”. Dalton Trans. 2015, 44, 9353–9358. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Wu, Y.; Huang, H.; Li, G.; Zhang, X.; Wang, Z. Surface modified MXene Ti3C2 multilayers by aryl diazonium salts leading to large-scale delamination. Appl. Surf. Sci. 2016, 384, 287–293. [Google Scholar] [CrossRef]
- Li, C.; Kota, S.; Hu, C.; Barsoum, M. On the synthesis of low-cost, titanium-based MXenes. J. Ceram. Sci. Technol 2016, 7, 301–306. [Google Scholar]
- Dong, Z.; Delacour, C.; Mc Carogher, K.; Udepurkar, A.P.; Kuhn, S. Continuous ultrasonic reactors: Design, mechanism and application. Materials 2020, 13, 344. [Google Scholar] [CrossRef]
- Tyurnina, A.V.; Tzanakis, I.; Morton, J.; Mi, J.; Porfyrakis, K.; Maciejewska, B.M.; Grobert, N.; Eskin, D.G. Ultrasonic exfoliation of graphene in water: A key parameter study. Carbon 2020, 168, 737–747. [Google Scholar] [CrossRef]
- Asakura, Y.; Nishida, T.; Matsuoka, T.; Koda, S. Effects of ultrasonic frequency and liquid height on sonochemical efficiency of large-scale sonochemical reactors. Ultrason. Sonochem. 2008, 15, 244–250. [Google Scholar] [CrossRef]
- Nickel, K.; Neis, U. Ultrasonic disintegration of biosolids for improved biodegradation. Ultrason. Sonochem. 2007, 14, 450–455. [Google Scholar] [CrossRef]
- Aymonier, C.; Bottreau, M.; Berdeu, B.; Cansell, F. Ultrasound for hydrothermal treatments of aqueous wastes: Solution for overcoming salt precipitation and corrosion. Ind. Eng. Chem. Res. 2000, 39, 4734–4740. [Google Scholar] [CrossRef]
- Zou, X.; Schmitt, T.; Perloff, D.; Wu, N.; Yu, T.-Y.; Wang, X. Nondestructive corrosion detection using fiber optic photoacoustic ultrasound generator. Measurement 2015, 62, 74–80. [Google Scholar] [CrossRef]
- Martina, K.; Tagliapietra, S.; Barge, A.; Cravotto, G. Combined microwaves/ultrasound, a hybrid technology. In Sonochemistry: From Basic Principles to Innovative Applications; Springer: Berlin/Heidelberg, Germany, 2017; pp. 175–201. [Google Scholar]
- Bartoli, M.; Frediani, F.; Briens, C.; Berruti, F.; Rosi, L. An Overview of Temperature Issues in Microwave-Assisted Pyrolysis. Processes 2019, 7, 658. [Google Scholar] [CrossRef]
- Narula, U.; Tan, C.M. Engineering a PVD-based graphene synthesis method. IEEE Trans. Nanotechnol. 2017, 16, 784–789. [Google Scholar] [CrossRef]
- Wang, X.; You, H.; Liu, F.; Li, M.; Wan, L.; Li, S.; Li, Q.; Xu, Y.; Tian, R.; Yu, Z. Large-scale synthesis of few-layered graphene using CVD. Chem. Vap. Depos. 2009, 15, 53–56. [Google Scholar] [CrossRef]

| Technique | Advantages | Disadvantages |
|---|---|---|
| UATs |
|
|
| MATs |
|
|
| Hydrothermal methods |
|
|
| CVD |
|
|
| PVD |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzotta, S.; Lettieri, S.; Ferraro, G.; Bartoli, M.; Etzi, M.; Pirri, C.F.; Bocchini, S. A Concise Overview of Ultrasound-Assisted Techniques for the Production of 2D Materials. Processes 2024, 12, 759. https://doi.org/10.3390/pr12040759
Mazzotta S, Lettieri S, Ferraro G, Bartoli M, Etzi M, Pirri CF, Bocchini S. A Concise Overview of Ultrasound-Assisted Techniques for the Production of 2D Materials. Processes. 2024; 12(4):759. https://doi.org/10.3390/pr12040759
Chicago/Turabian StyleMazzotta, Silvia, Stefania Lettieri, Giuseppe Ferraro, Mattia Bartoli, Marco Etzi, Candido Fabrizio Pirri, and Sergio Bocchini. 2024. "A Concise Overview of Ultrasound-Assisted Techniques for the Production of 2D Materials" Processes 12, no. 4: 759. https://doi.org/10.3390/pr12040759
APA StyleMazzotta, S., Lettieri, S., Ferraro, G., Bartoli, M., Etzi, M., Pirri, C. F., & Bocchini, S. (2024). A Concise Overview of Ultrasound-Assisted Techniques for the Production of 2D Materials. Processes, 12(4), 759. https://doi.org/10.3390/pr12040759









