Preparation, Characterization, and Scattering Characteristics of Mixed Aerosol of Fly Ash and Ammonium Sulfate
Abstract
:1. Introduction
2. Materials and Experimental Methods
2.1. Materials
2.2. Sample Preparation
2.3. Material Characterization
2.4. Laser Levitation and Scattering Measurement System
2.5. Levitation Stability Data Processing Method
2.6. Scattering Signal Processing Methods
3. Results and Discussion
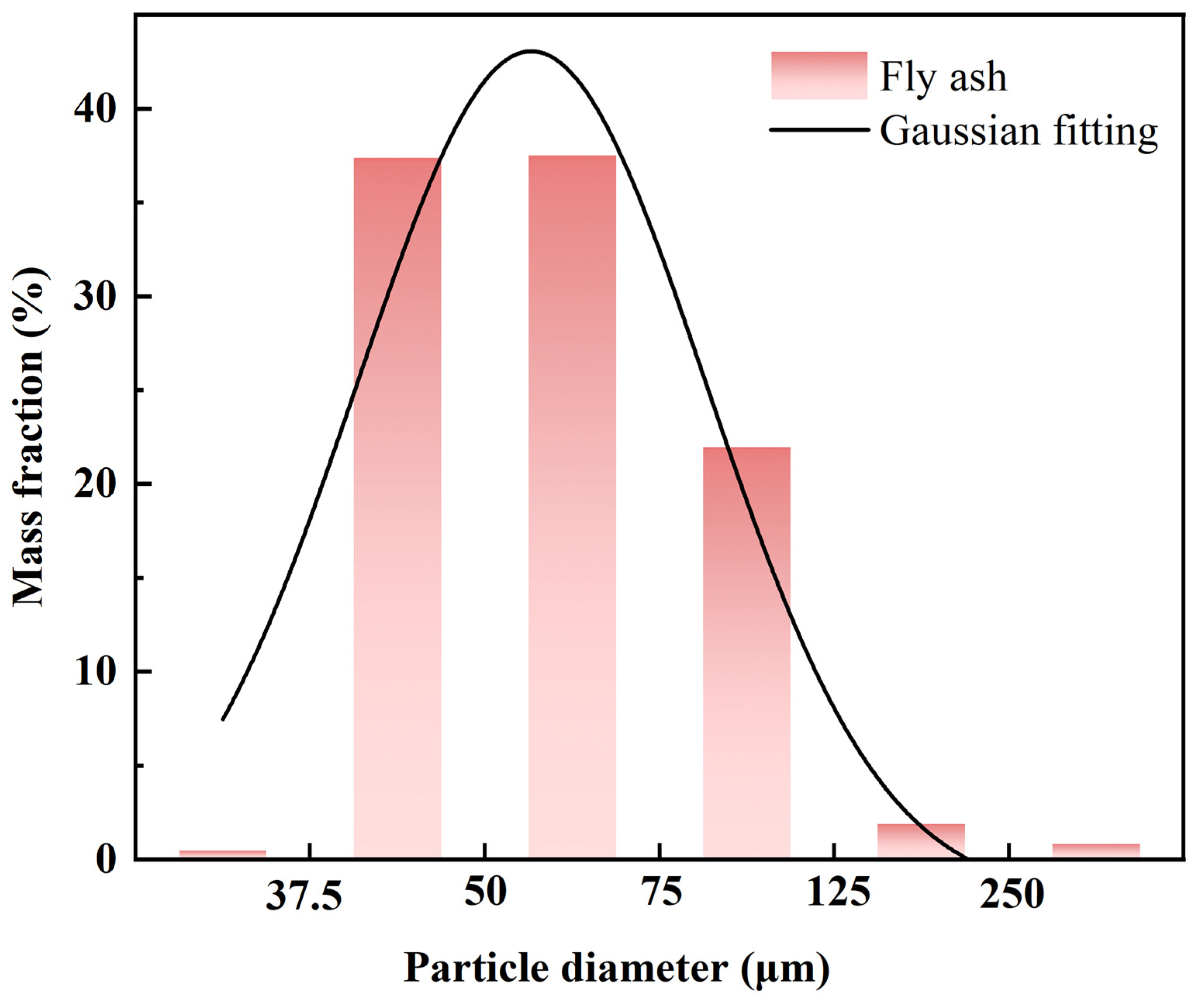
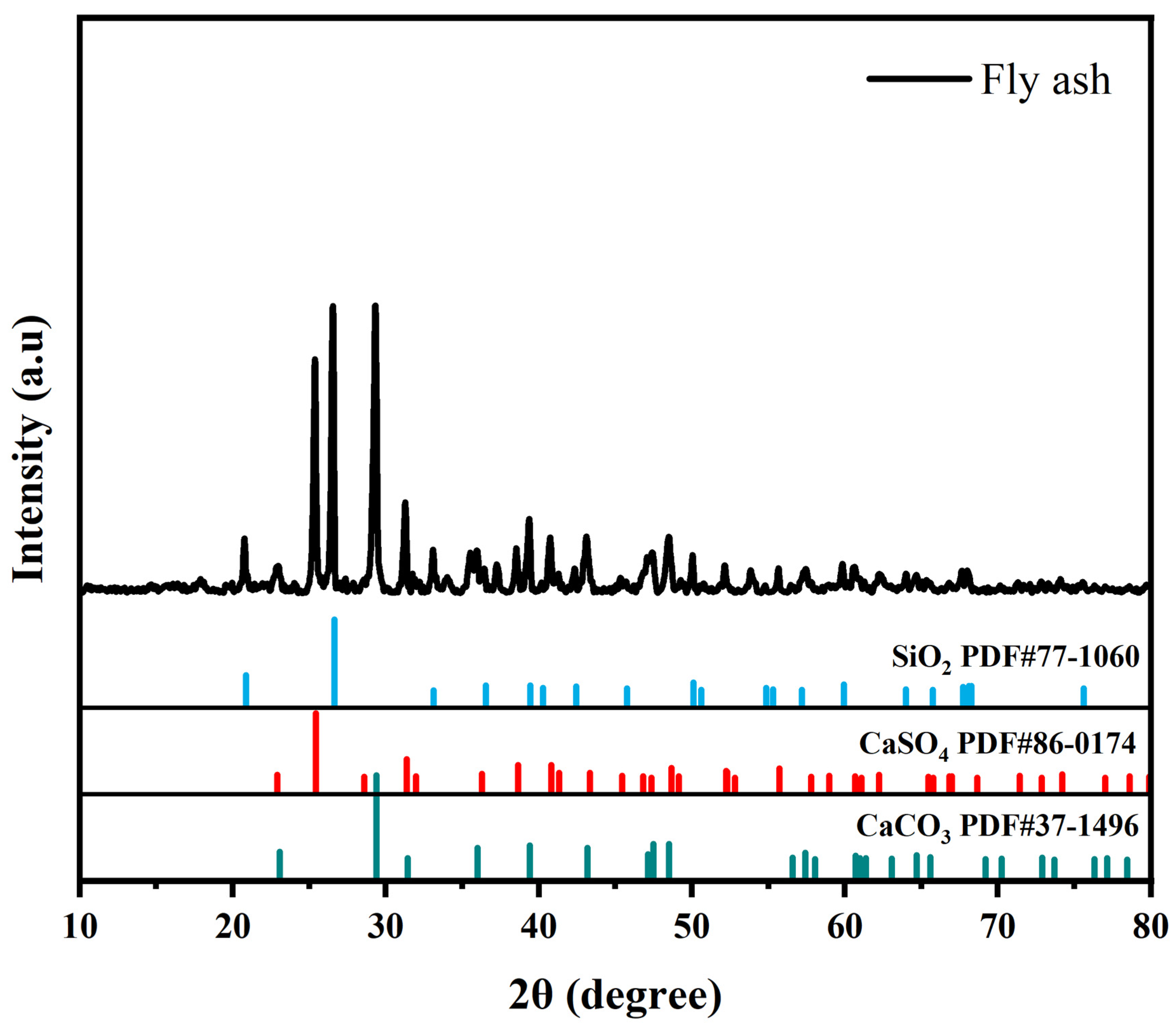
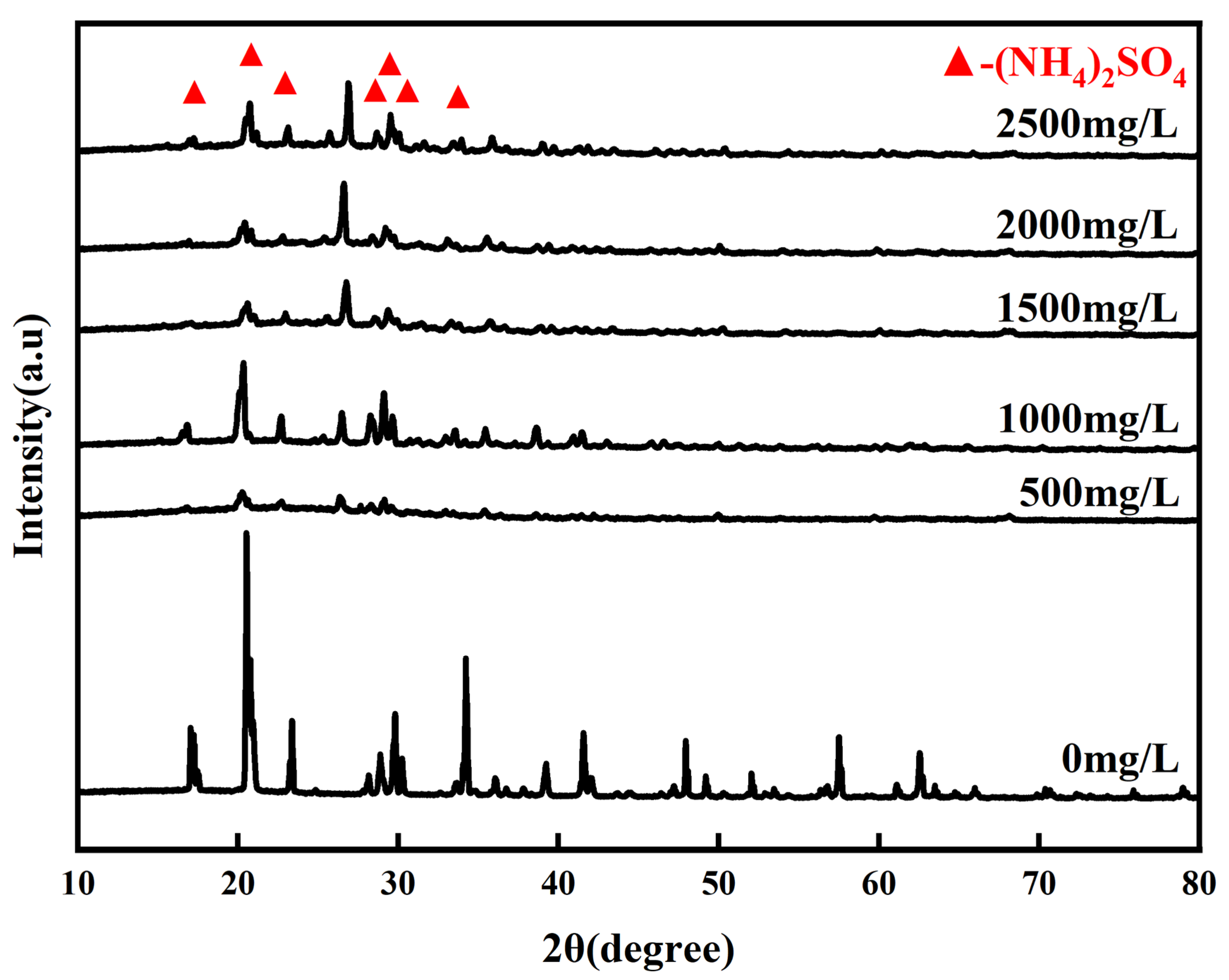
3.1. Sample Characterization
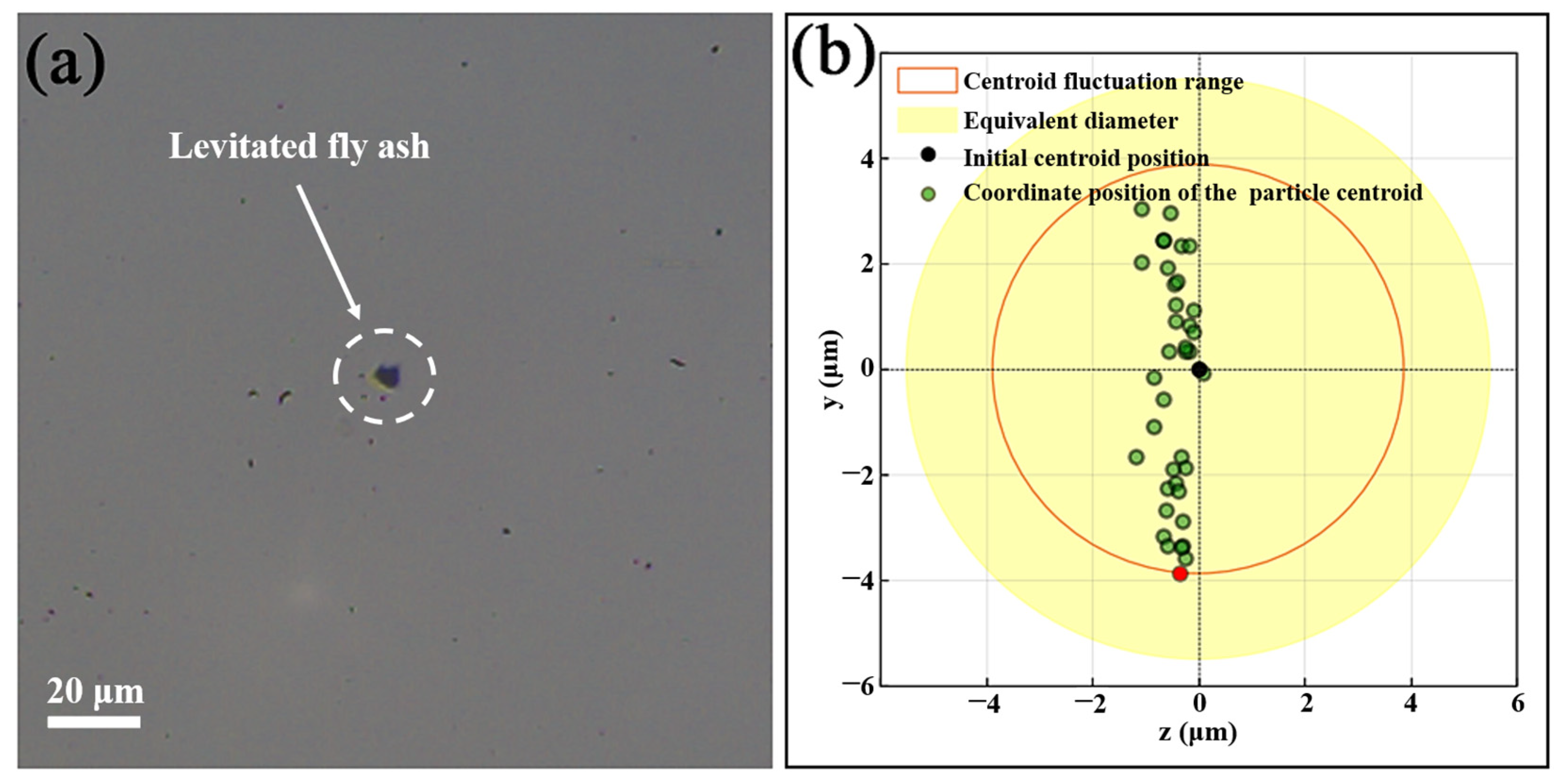
3.2. Stability Analysis of Levitated Particles
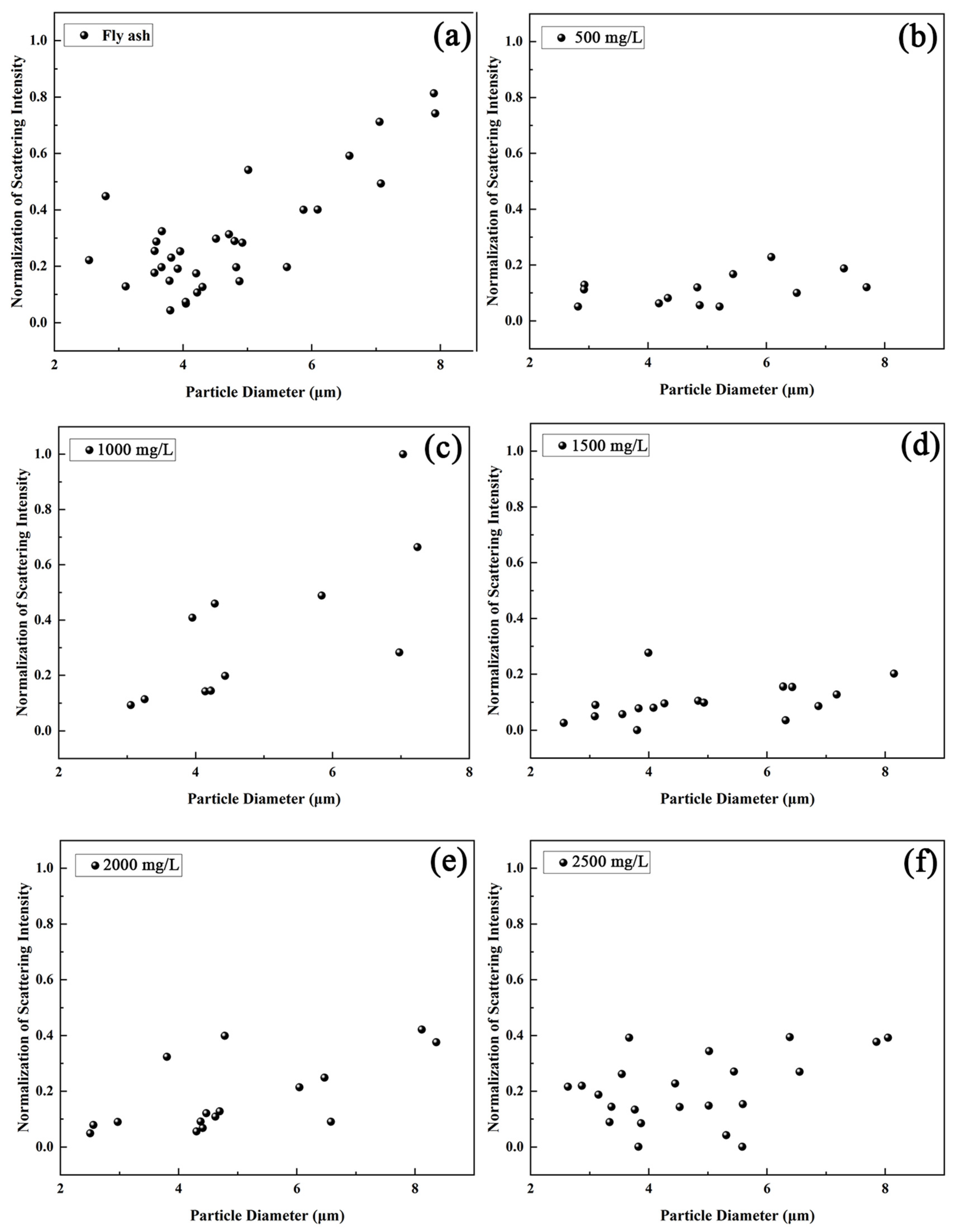
3.3. Measurement of Scattering Characteristics of Mixed Aerosols
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Carlson, B.E.; Yung, Y.L.; Lv, D.; Hansen, J.; Penner, J.E.; Dong, Y. Scattering and absorbing aerosols in the climate system. Nat. Rev. Earth Environ. 2022, 3, 363–379. [Google Scholar] [CrossRef]
- Gueymard, C.A.; Yang, D. Worldwide validation of CAMS and MERRA-2 reanalysis aerosol optical depth products using 15 years of AERONET observations. Atmos. Environ. 2020, 225, 117216. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Zhang, J.; Wu, S.T.; Gao, J.L.; Hao, Z.Q.; Li, C.L. Study on laser scattering depolarization characteristics of typical aerosol particles. Opt. Commun. 2022, 518, 128183. [Google Scholar] [CrossRef]
- Liu, D.T.; Whitehead, J.; Alfarra, M.R.; Reyes-Villegas, E.; Spracklen, D.V.; Reddington, C.L.; Allan, J.D. Black-carbon absorption enhancement in the atmosphere determined by particle mixing state. Nat. Geosci. 2017, 10, 184–188. [Google Scholar] [CrossRef]
- Martins, J.V.; Artaxo, P.; Liousse, C.; Reid, J.S.; Hobbs, P.V.; Kaufman, Y.J. Effects of black carbon content, particle size, and mixing on light absorption by aerosol particles from biomass burning in Brazil. J. Geophys. Res. Atmos. 1998, 103, 32041–32050. [Google Scholar] [CrossRef]
- Pósfai, M.; Anderson, J.R.; Buseck, P.R.; Sievering, H. Soot and sulfate aerosol particles in the remote marine troposphere. J. Geophys. Res. Atmos. 1999, 104, 21685–21693. [Google Scholar] [CrossRef]
- Mishchenko, M.I.; Liu, L.; Mackowski, D.W. T-matrix modeling of linear depolarization by morphologically complex soot and soot-containing aerosols. J. Quant. Spectrosc. Radiat. Transf. 2013, 123, 135–144. [Google Scholar] [CrossRef]
- Zhang, X.L.; Huang, Y.B.; Rao, R.Z. Equivalence of light scattering by one internal-mixed model for aerosol particles. Acta Optica Sinica 2012, 32, 0629001. [Google Scholar] [CrossRef]
- Hao, L.; Yang, W.; Wu, T.W.; Shi, G.Y.; Zhao, J.Q.; Tan, Y. Study on radiative property of soot-sulfate mixed aerosol. Plateau Meteor. 2010, 29, 1238–1245. [Google Scholar]
- Freedman, M.A.; Hasenkopf, C.A.; Beaver, M.R.; Margaret, A. Optical Properties of Internally Mixed Aerosol Particles Composed of Dicarboxylic Acids and Ammonium Sulfate. J. Phys. Chem. A 2009, 113, 13584–13592. [Google Scholar] [CrossRef]
- Malm, W.; Day, D.; Kreidenweis, S. Light scattering characteristics of aerosols as a function of relative humidity: Part I—A comparison of measured scattering and aerosol concentrations using the theoretical models. J. Air Waste Manag. Assoc. 2000, 50, 686–700. [Google Scholar] [CrossRef]
- Carruthers, A.E.; Reid, J.P.; Orr-Ewing, A.J. Longitudinal optical trapping and sizing of aerosol droplets. Opt. Express 2010, 18, 14238–14244. [Google Scholar] [CrossRef]
- Jones, S.H.; King, M.D.; Ward, A.D. Determining the unique refractive index properties of solid polystyrene aerosol using broadband Mie scattering from optically trapped beads. Phys. Chem. Chem. Phys. 2013, 15, 20735–20741. [Google Scholar] [CrossRef] [PubMed]
- GB/T 212-2008; Proximate Analysis of Coal. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China; Standardization Administration of the People’s Republic of China; Standards Press of China: Beijing, China, 2008.
- Huang, X.; Chen, C.; Li, J.; Zhang, M.; Li, S. Measurement of scattering intensity distribution of single microparticles/nanoclusters based on laser levitation. Acta Phys. Sin. 2023, 72, 174201. [Google Scholar] [CrossRef]
- China, S.; Mazzoleni, C.; Gorkowski, K.; Aiken, A.C.; Dubey, M.K. Morphology and mixing state of individual freshly emitted wildfire carbonaceous particles. Nat. Commun. 2013, 4, 2122. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Buseck, P.R. Internally mixed soot, sulfates, and organic matter in aerosol particles from Mexico City. Atmos. Chem. Phys. 2008, 8, 6469–6481. [Google Scholar] [CrossRef]
- Rożek, P.; Król, M.; Mozgawa, W. Spectroscopic studies of fly ash-based geopolymers. Spectrochim. Acta A 2018, 198, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yuan, L. Analysis of characteristics of Mie scattering. Opt. Tech. 2010, 36, 936–939. [Google Scholar]
- Hu, S.; Gao, T.C.; Liu, L. Analysis on scattering characteristics and equivalent Mie scattering errors of non-spherical aerosol. J. Meteorol. Res. 2014, 34, 612–619. [Google Scholar]
- Niklasson, G.A.; Granqvist, C.G.; Hunderi, O. Effective medium models for the optical properties of inhomogeneous materials. Appl. Opt. 1981, 20, 26–30. [Google Scholar] [CrossRef] [PubMed]





| Moisture | Ash Content | Volatile Matter | Fixed Carbon | |
|---|---|---|---|---|
| Method | GB/T 212-2008 “Proximate analysis of coal” [14] | |||
| Instrument | Electric blast drying oven | Muffle furnace | Muffle furnace | Muffle furnace |
| Results | 1.73% | 64.14% | 13.29% | 20.84% |
| Sample | Fly Ash Addition (g) | 0.33 wt.% (NH4)2SO4 Volume (L) | Fly Ash Solid Mass Concentration (mg/L) |
|---|---|---|---|
| 1 | 0 | 0.10 | 0 |
| 2 | 0.05 | 0.10 | 500 |
| 3 | 0.10 | 0.10 | 1000 |
| 4 | 0.15 | 0.10 | 1500 |
| 5 | 0.20 | 0.10 | 2000 |
| 6 | 0.25 | 0.10 | 2500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, S.; Zhang, M.; Wang, Z.; Shu, Y.; Liu, J.; Huang, X. Preparation, Characterization, and Scattering Characteristics of Mixed Aerosol of Fly Ash and Ammonium Sulfate. Processes 2024, 12, 769. https://doi.org/10.3390/pr12040769
Li J, Li S, Zhang M, Wang Z, Shu Y, Liu J, Huang X. Preparation, Characterization, and Scattering Characteristics of Mixed Aerosol of Fly Ash and Ammonium Sulfate. Processes. 2024; 12(4):769. https://doi.org/10.3390/pr12040769
Chicago/Turabian StyleLi, Jiaxin, Shengji Li, Minqi Zhang, Zhangtao Wang, Yanbokang Shu, Jie Liu, and Xuefeng Huang. 2024. "Preparation, Characterization, and Scattering Characteristics of Mixed Aerosol of Fly Ash and Ammonium Sulfate" Processes 12, no. 4: 769. https://doi.org/10.3390/pr12040769
APA StyleLi, J., Li, S., Zhang, M., Wang, Z., Shu, Y., Liu, J., & Huang, X. (2024). Preparation, Characterization, and Scattering Characteristics of Mixed Aerosol of Fly Ash and Ammonium Sulfate. Processes, 12(4), 769. https://doi.org/10.3390/pr12040769






