Nitrogen Fixation via Plasma-Assisted Processes: Mechanisms, Applications, and Comparative Analysis—A Comprehensive Review
Abstract
:1. Introduction
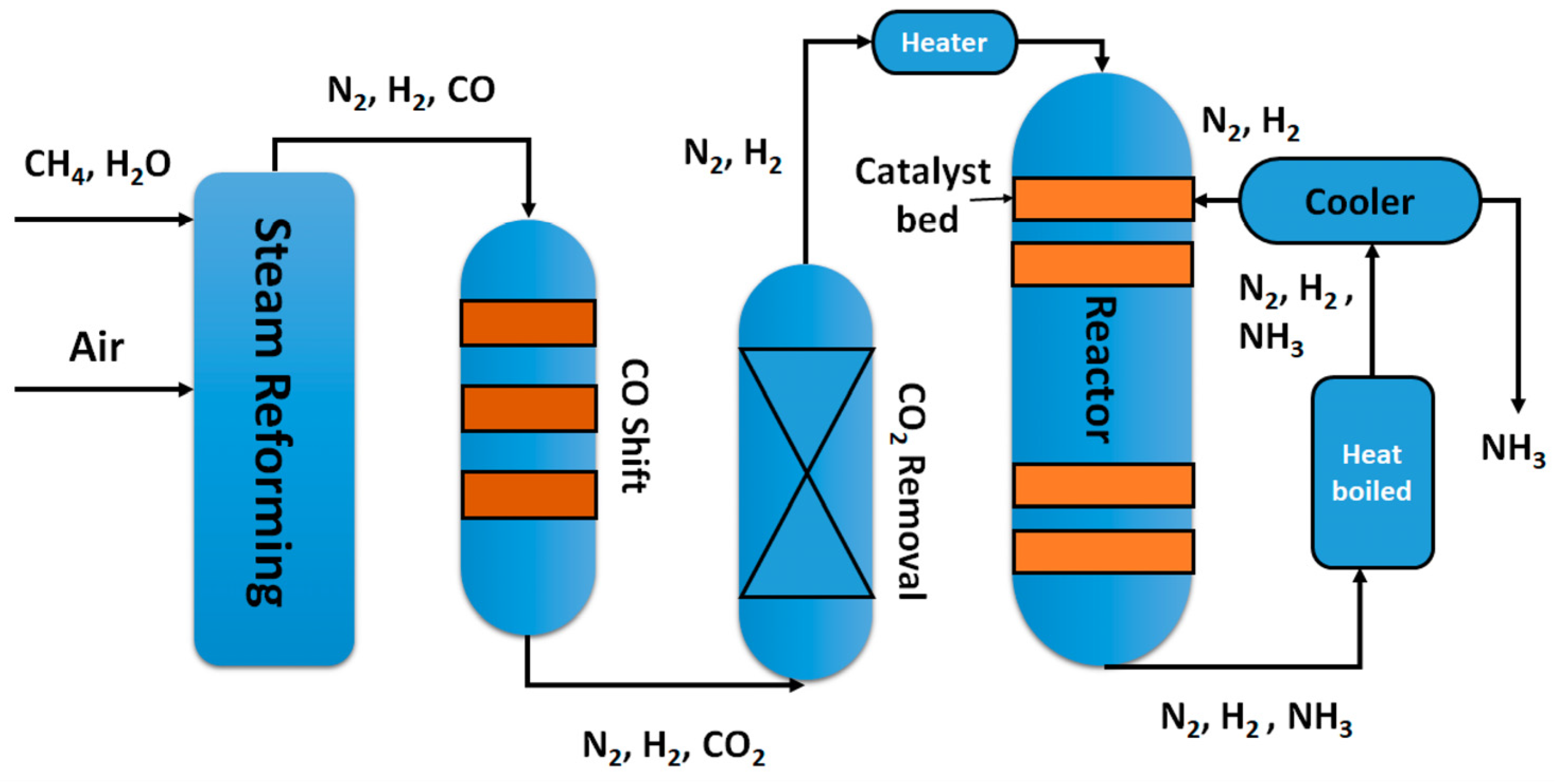
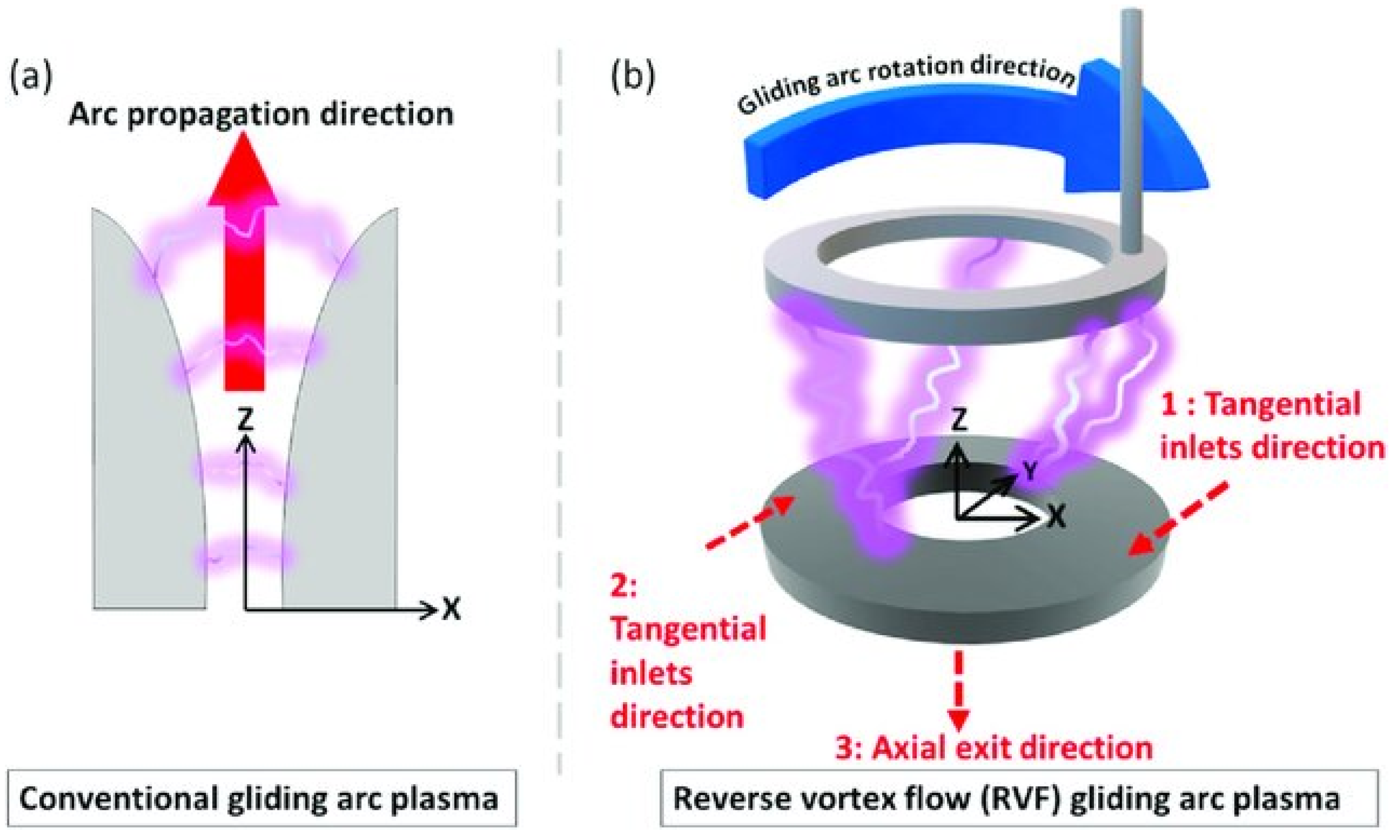
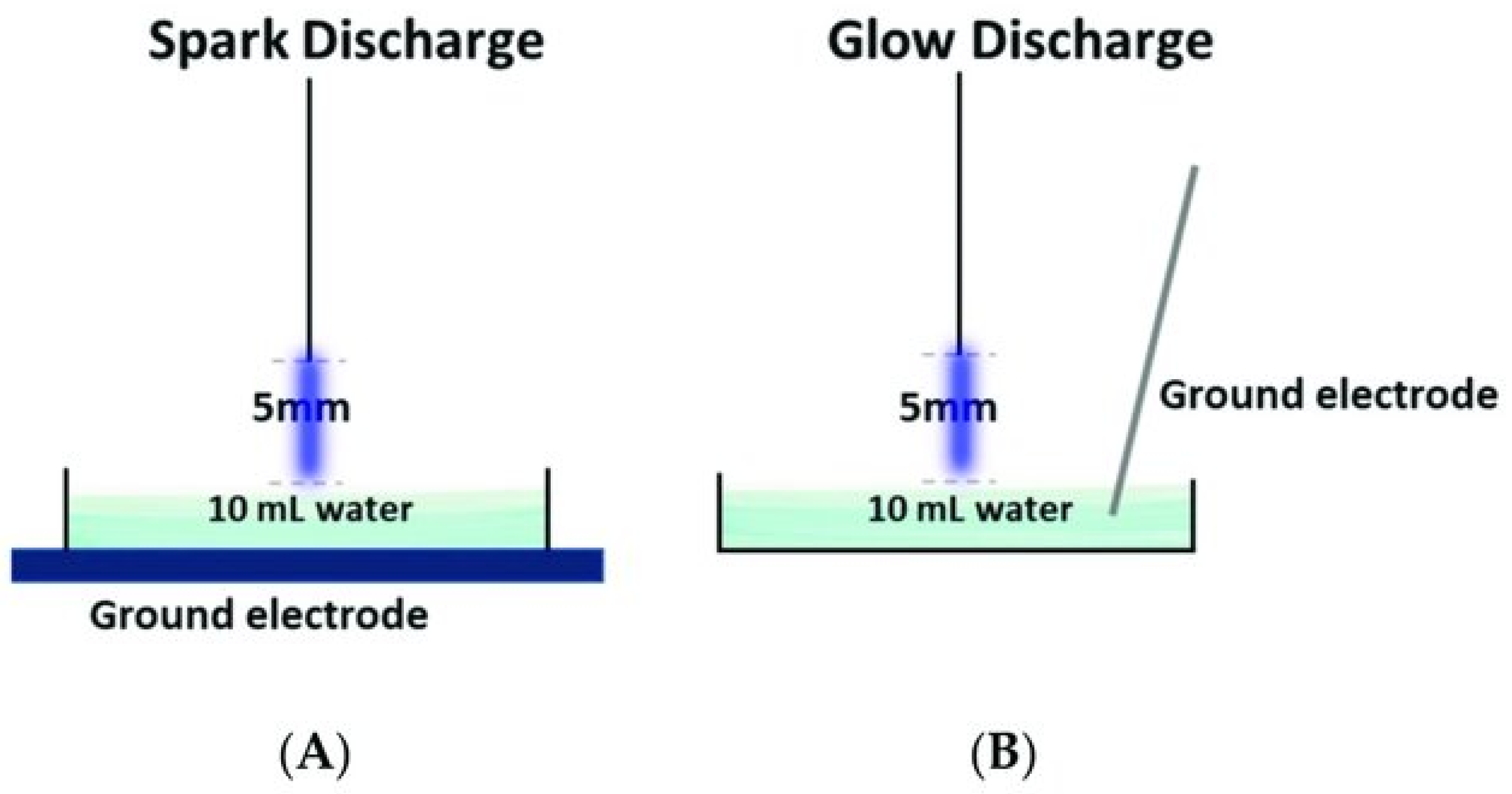
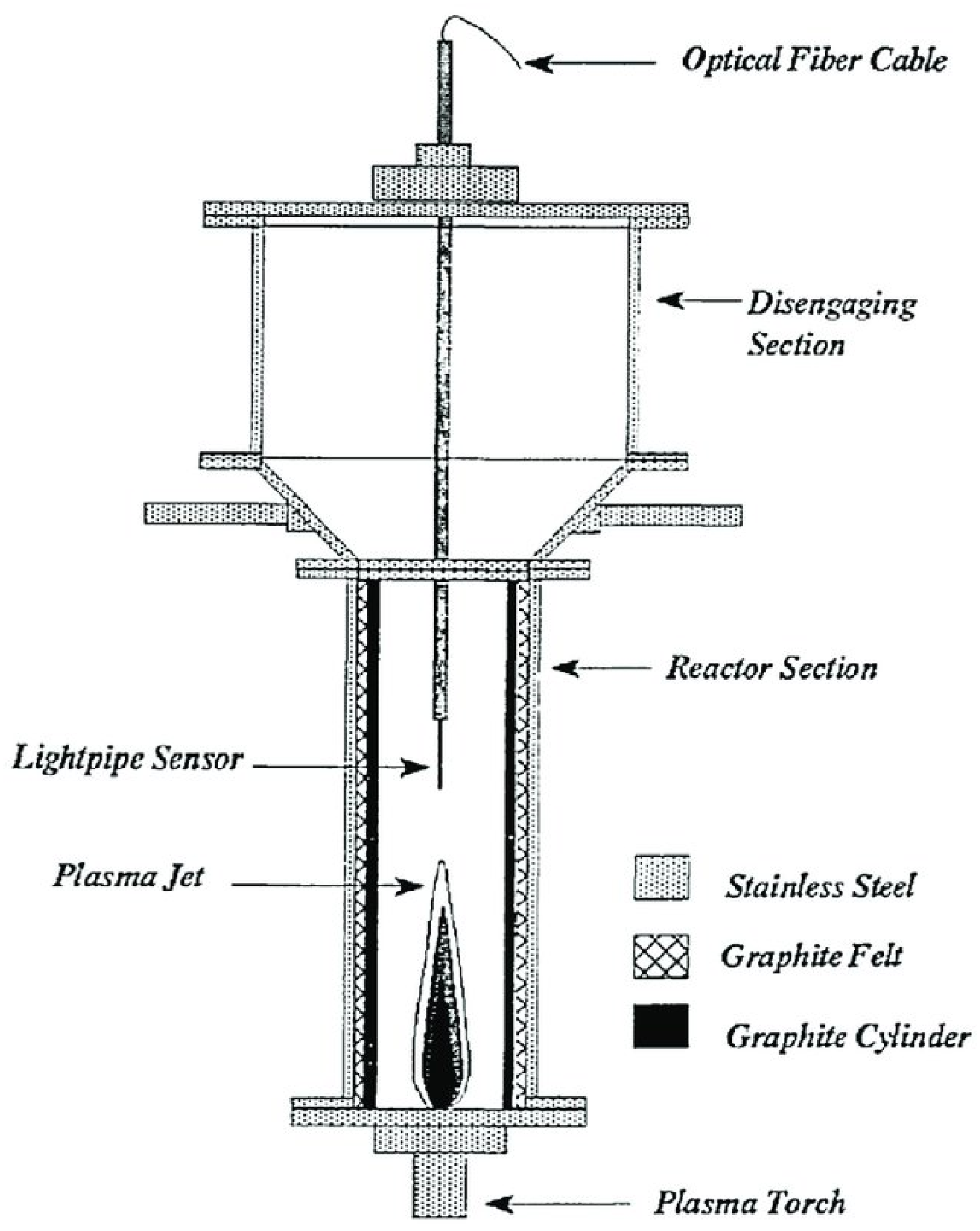
2. Plasma N2 Fixation
3. Prospects of Nitrogen Fixation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagner, S.C. Biological Nitrogen Fixation. Nature Education Knowledge, 3, 15.–References–Scientific Research Publishing. 2011. Available online: https://www.scirp.org/reference/referencespapers?referenceid=1323846 (accessed on 8 May 2023).
- Erisman, J.W.; Galloway, J.N.; Dise, N.B.; Sutton, M.A.; Bleeker, A.; Grizzetti, B.; Leach, A.M.; de Vries, W. Nitrogen: Too Much of a Vital Resource: Science Brief; WWF Netherlands: Zeist, The Netherlands, 2015. [Google Scholar]
- Galloway, J.N.; Cowling, E.B. Reactive Nitrogen and the World: 200 Years of Change. AMBIO A J. Hum. Environ. 2002, 31, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, A. The Nitrogen Cycle: Processes, Players, and Human Impact. Available online: https://www.nature.com/scitable/knowledge/library/the-nitrogen-cycle-processes-players-and-human-15644632/ (accessed on 8 May 2023).
- Klopsch, I.; Yuzik-Klimova, E.Y.; Schneider, S. Functionalization of N2 by Mid to Late Transition Metals via N–N Bond Cleavage. Nitrogen Fixat. 2017, 71–112. [Google Scholar] [CrossRef]
- Jiang, J.; He, X.; Li, L.; Li, J.; Shao, H.; Xu, Q.; Ye, R.; Dong, Y. Effect of Cold Plasma Treatment on Seed Germination and Growth of Wheat. Plasma Sci. Technol. 2014, 16, 54–58. [Google Scholar] [CrossRef]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a Century of Ammonia Synthesis Changed the World. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Bezdek, M.J.; Chirik, P.J. Expanding Boundaries: N2 Cleavage and Functionalization beyond Early Transition Metals. Angew. Chem. Int. Ed. 2016, 55, 7892–7896. [Google Scholar] [CrossRef] [PubMed]
- Smil, V. Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production; MIT Press: Cambridge, UK, 2004. [Google Scholar]
- Smil, V. Nitrogen and Food Production: Proteins for Human Diets. AMBIO A J. Hum. Environ. 2002, 31, 126–131. [Google Scholar] [CrossRef] [PubMed]
- World Population Prospects: The 2017 Revision. UN DESA Publications. Available online: https://desapublications.un.org/publications/world-population-prospects-2017-revision (accessed on 8 May 2023).
- Travis, A.S.; Springerlink (Online Service). Nitrogen Capture: The Growth of an International Industry (1900–1940); Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Tamaru, K. The History of the Development of Ammonia Synthesis; Springer: Boston, MA, USA, 1991; pp. 1–18. [Google Scholar] [CrossRef]
- Travis, A.S. Electric Arcs, Cyanamide, Carl Bosch and Fritz Haber. In SpringerBriefs in Molecular Science; Springer: Cham, Switzerland, 2015; pp. 17–72. [Google Scholar] [CrossRef]
- Leigh, G.J. Nitrogen Fixation at the Millennium; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Ernst, F.A. Fixation of Atmospheric Nitrogen; D. Van Nostrand Company: New York, NY, USA, 1928. [Google Scholar]
- Patil, B.S.; Wang, Q.; Hessel, V.; Lang, J. Plasma N2-Fixation: 1900–2014. Catal. Today 2015, 256, 49–66. [Google Scholar] [CrossRef]
- The Haber-Bosch Heritage: The Ammonia Production Technology. Fertilizer. Available online: https://www.fertilizer.org/resource/the-haber-bosch-heritage-the-ammonia-production-technology (accessed on 8 May 2023).
- Cherkasov, N.; Ibhadon, A.O.; Fitzpatrick, P. A Review of the Existing and Alternative Methods for Greener Nitrogen Fixation. Chem. Eng. Process. Process Intensif. 2015, 90, 24–33. [Google Scholar] [CrossRef]
- Vu, M.-H.; Sakar, M.; Do, T.-O. Insights into the Recent Progress and Advanced Materials for Photocatalytic Nitrogen Fixation for Ammonia (NH3) Production. Catalysts 2018, 8, 621. [Google Scholar] [CrossRef]
- Schrock, R.R. Reduction of Dinitrogen. Proc. Natl. Acad. Sci. USA 2006, 103, 17087. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Nishibayashi, Y. Developing More Sustainable Processes for Ammonia Synthesis. Coord. Chem. Rev. 2013, 257, 2551–2564. [Google Scholar] [CrossRef]
- Pfromm, P.H. Towards Sustainable Agriculture: Fossil-Free Ammonia. J. Renew. Sustain. Energy 2017, 9, 034702. [Google Scholar] [CrossRef]
- Barbir, F. PEM Electrolysis for Production of Hydrogen from Renewable Energy Sources. Sol. Energy 2005, 78, 661–669. [Google Scholar] [CrossRef]
- Egeland, A.; Burke, W.J.; Springerlink (Online Service). Kristian Birkeland: The First Space Scientist; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2005. [Google Scholar]
- Fridman, A.A. Plasma Chemistry; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing; John Wiley & Sons: New Jersey, NJ, USA, 2005. [Google Scholar]
- Fauchais, P.; Rakowitz, J. Physics on Plasma Chemistry. J. Phys. Colloq. 1979, 40, C7-289–C7-312. [Google Scholar] [CrossRef]
- Hippler, R.; Pfau, S.; Schmidt, M.; Schoenbach, K.H. Low Temperature Plasma Physics; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Birkeland, K. On the Oxidation of Atmospheric Nitrogen in Electric Arcs. Trans. Faraday Soc. 1906, 2, 98. [Google Scholar] [CrossRef]
- Eyde, S. Oxidation of Atmospheric Nitrogen and Development of Resulting Industries in Norway. J. Ind. Eng. Chem. 1912, 4, 771–774. [Google Scholar] [CrossRef]
- Eremin, E.N.; Maltsev, A.N. Thermodynamic equilibrium concentrations of nitrogen oxide. Russ. J. Phys. Chem. 1956, 30, 1179–1181. [Google Scholar]
- International Energy Agency. World Energy Outlook 2020–Analysis. IEA. Available online: https://www.iea.org/reports/world-energy-outlook-2020 (accessed on 29 December 2023).
- Rusanov, V.D.; Fridman, A.A.; Sholin, G.V. The Physics of a Chemically Active Plasma with Nonequilibrium Vibrational Excitation of Molecules. Sov. Phys. Uspekhi 1981, 24, 447–474. [Google Scholar] [CrossRef]
- Rusanov, V.D.; Fridman, A.A.; Sholin, G.V. Nitrogen Oxide Synthesis in nonequilibrium Plasma Chemical Systems, Ed. Smirnov Smirnov BM “At.” Mosc. 1978, 5, 222–240. [Google Scholar]
- Li, S.; Medrano Jimenez, J.; Hessel, V.; Gallucci, F. Recent Progress of Plasma-Assisted Nitrogen Fixation Research: A Review. Processes 2018, 6, 248. [Google Scholar] [CrossRef]
- Lamichhane, P.; Paneru, R.; Nguyen, L.N.; Lim, J.S.; Bhartiya, P.; Adhikari, B.C.; Mumtaz, S.; Choi, E.H. Plasma-Assisted Nitrogen Fixation in Water with Various Metals. React. Chem. Eng. 2020, 5, 2053–2057. [Google Scholar] [CrossRef]
- Winter, L.R.; Chen, J.G. N2 Fixation by Plasma-Activated Processes. Joule 2021, 5, 300–315. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, D.; Wu, A.; Lin, X.; Li, X. Review of Low-Temperature Plasma Nitrogen Fixation Technology. Waste Dispos. Sustain. Energy 2021, 3, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xiao, A.; Liu, D.; Lu, X.; Ostrikov, K. Plasma-Water-Based Nitrogen Fixation: Status, Mechanisms, and Opportunities. Plasma Process. Polym. 2022, 19, 2100198. [Google Scholar] [CrossRef]
- Frost, D.C.; McDowell, C.A. The Dissociation Energy of the Nitrogen Molecule. Proceedings of the Royal Society of London. Series A. Math. Phys. Sci. 1956, 236, 278–284. [Google Scholar] [CrossRef]
- Rapakoulias, D.; Amouroux, J. Processus Catalytiques Dans Un Réacteur à Plasma Hors d’Équilibre II. Fixation de l’Azote Dans Le Système N2-O2. Rev. Phys. Appl. 1980, 15, 1261–1265. [Google Scholar] [CrossRef]
- Rapakoulias, D.; Amouroux, J. Réacteur de Synthèse et de Trempe Dans Un Plasma Hors d’Équilibre: Application à La Synthèse de C2H2 et HCN. Rev. Phys. Appl. 1979, 14, 961. [Google Scholar] [CrossRef]
- Cavadias, S.; Amouroux, J. Synthesis of nitrogen oxides in plasma reactors. Bull. Soc. Chim. Fr. 1986, 2, 147–158. [Google Scholar]
- Pollo, I.; Hoffmann-Fedenczuk, K.; Fedenczuk, L. The influence of gas-inlet and quenching systems on the nitrogen oxides production in air plasmas. Int. Symp. Plasma Chem. 1981, 2, 756–760. [Google Scholar]
- Pollo, I.; Banasik, S. Experiments on the synthesis of nitric oxide in an argon plasma. Chem. Plazmy 1978, 259–270. [Google Scholar]
- Krop, J.; Pollo, I. Chemical reactors for synthesis of nitrogen oxide in a stream of low-temperature plasma. III. Reactor to freeze reaction products by injection of water. Chemia 1981, 678, 51–59. [Google Scholar]
- Krop, J.; Pollo, I. Chemical reactors for synthesis of nitrogen oxide in low temperature of air plasma jet. II, Efficiency of nitrogen oxide synthesis in reactors with rotating disk. Chemia 1980, 633, 25–33. [Google Scholar]
- Krop, J.; Krop, E.; Pollo, I. Calculated amounts of nitric oxide in a nitrogen–oxygen plasma jet. Chem. Plazmy 1979, 242–249. [Google Scholar]
- Amouroux, J.; Cavvadias, S.; Rapakoulias, D. Réacteur de Synthèse et de Trempe Dans Un Plasma Hors d’Équilibre: Application à La Synthèse Des Oxydes D’azote. Rev. Phys. Appl. 1979, 14, 969–976. [Google Scholar] [CrossRef]
- Production of Nitric Monoxide in Dry Air Using Pulsed Discharge. IEEE Conference Publication. IEEE Xplore. Available online: https://ieeexplore.ieee.org/document/823768 (accessed on 21 June 2023).
- Production of Nitric Oxide Using a Pulsed Arc Discharge. IEEE Journals & Magazine. IEEE Xplore. Available online: https://ieeexplore.ieee.org/document/1178015 (accessed on 21 June 2023).
- Mutel, B.; Dessaux, O.; Goudmand, P. Energy Cost Improvement of the Nitrogen Oxides Synthesis in a Low Pressure Plasma. Rev. Phys. Appl. 1984, 19, 461–464. [Google Scholar] [CrossRef]
- Pollo, I.; Banasik, S. Some problems of energy utilization in a plasma reactor with a direct current argon plasma generator. Chemia 1979, 631, 377–378. [Google Scholar]
- Jardali, F.; Van Alphen, S.; Creel, J.; Ahmadi Eshtehardi, H.; Axelsson, M.; Ingels, R.; Snyders, R.; Bogaerts, A. NOx Production in a Rotating Gliding Arc Plasma: Potential Avenue for Sustainable Nitrogen Fixation. Green Chem. 2021, 23, 1748–1757. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Jardali, F.; Bogaerts, A.; Lefferts, L. From the Birkeland–Eyde Process towards Energy-Efficient Plasma-Based NOX Synthesis: A Techno-Economic Analysis. Energy Environ. Sci. 2021, 14, 2520–2534. [Google Scholar] [CrossRef]
- Rehbein, N.; Cooray, V. NOx Production in Spark and Corona Discharges. J. Electrost. 2001, 51, 333–339. [Google Scholar] [CrossRef]
- Pei, X.; Gidon, D.; Yang, Y.-J.; Xiong, Z.; Graves, D.B. Reducing Energy Cost of NO Production in Air Plasmas. Chem. Eng. J. 2019, 362, 217–228. [Google Scholar] [CrossRef]
- Janda, M.; Martišovitš, V.; Hensel, K.; Machala, Z. Generation of Antimicrobial NOx by Atmospheric Air Transient Spark Discharge. Plasma Chem. Plasma Process. 2016, 36, 767–781. [Google Scholar] [CrossRef]
- Pavlovich, M.J.; Ono, T.; Galleher, C.; Curtis, B.; Clark, D.S.; Machala, Z.; Graves, D.B. Air Spark-like Plasma Source for Antimicrobial NOxGeneration. J. Phys. D Appl. Phys. 2014, 47, 505202. [Google Scholar] [CrossRef]
- Abdelaziz, A.A.; Teramoto, Y.; Nozaki, T.; Kim, H.-H. Toward Reducing the Energy Cost of NOx Formation in a Spark Discharge Reactor through Pinpointing Its Mechanism. ACS Sustain. Chem. Eng. 2023, 11, 4106–4118. [Google Scholar] [CrossRef]
- Zhang, S.; Zong, L.; Zeng, X.; Zhou, R.; Liu, Y.; Zhang, C.; Pan, J.; Cullen, P.J.; Ostrikov, K.; Shao, T. Sustainable Nitrogen Fixation with Nanosecond Pulsed Spark Discharges: Insights into Free-Radical-Chain Reactions. Green Chem. 2022, 24, 1534–1544. [Google Scholar] [CrossRef]
- Pei, X.; Gidon, D.; Graves, D.B. Specific energy cost for nitrogen fixation as NOx using DC glow discharge in air. J. Phys. D Appl. Phys. 2019, 53, 044002. [Google Scholar] [CrossRef]
- Nitrogen Fixation into Water by Pulsed High-Voltage Discharge. IEEE Journals & Magazine. IEEE Xplore. Available online: https://ieeexplore.ieee.org/document/4735609 (accessed on 14 October 2023).
- Rahman, M.; Cooray, V. NOx Generation in Laser-Produced Plasma in Air as a Function of Dissipated Energy. Opt. Laser Technol. 2003, 35, 543–546. [Google Scholar] [CrossRef]
- Partridge, W.S.; Parlin, R.B.; Zwolinski, B.J. Fixation of Nitrogen in a Crossed Discharge. Ind. Eng. Chem. 1954, 46, 1468–1471. [Google Scholar] [CrossRef]
- Patil, B.S.; Cherkasov, N.; Lang, J.; Ibhadon, A.O.; Hessel, V.; Wang, Q. Low Temperature Plasma-Catalytic NO X Synthesis in a Packed DBD Reactor: Effect of Support Materials and Supported Active Metal Oxides. Appl. Catal. B Environ. 2016, 194, 123–133. [Google Scholar] [CrossRef]
- Roy, N.C.; Maira, N.; Pattyn, C.; Remy, A.; Delplancke, M.-P.; Reniers, F. Mechanisms of Reducing Energy Costs for Nitrogen Fixation Using Air-Based Atmospheric DBD Plasmas over Water in Contact with the Electrode. Chem. Eng. J. 2023, 461, 141844. [Google Scholar] [CrossRef]
- Li, Y.; Qin, L.; Wang, H.-L.; Li, S.-S.; Yuan, H.; Yang, D.-Z. High Efficiency NOx Synthesis and Regulation Using Dielectric Barrier Discharge in the Needle Array Packed Bed Reactor. Chem. Eng. J. 2023, 461, 141922. [Google Scholar] [CrossRef]
- Vervloessem, E.; Aghaei, M.; Jardali, F.; Hafezkhiabani, N.; Bogaerts, A. Plasma-Based N2 Fixation into NOx: Insights from Modeling toward Optimum Yields and Energy Costs in a Gliding Arc Plasmatron. ACS Sustain. Chem. Eng. 2020, 8, 9711–9720. [Google Scholar] [CrossRef]
- Coudert, J.; Bourdin, E.; Baronnet, J.-M.; Rakowitz, J.; Fauchais, P. Chemical kinetics study of nitrogen oxide synthesis in a DC plasma jet: A proposed model. J. Physique. Colloq. 1979, 40, C7-355–C7-356. [Google Scholar] [CrossRef]
- Patil, B.S.; Peeters, F.J.J.; van Rooij, G.J.; Medrano, J.A.; Gallucci, F.; Lang, J.; Wang, Q.; Hessel, V. Plasma Assisted Nitrogen Oxide Production from Air: Using Pulsed Powered Gliding Arc Reactor for a Containerized Plant. AIChE J. 2017, 64, 526–537. [Google Scholar] [CrossRef]
- Wang, W.; Patil, B.; Heijkers, S.; Hessel, V.; Bogaerts, A. Nitrogen Fixation by Gliding Arc Plasma: Better Insight by Chemical Kinetics Modelling. ChemSusChem 2017, 10, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Tsonev, I.; O’Modhrain, C.; Bogaerts, A.; Gorbanev, Y. Nitrogen Fixation by an Arc Plasma at Elevated Pressure to Increase the Energy Efficiency and Production Rate of NOx. ACS Sustain. Chem. Eng. 2023, 11, 1888–1897. [Google Scholar] [CrossRef]
- Muzammil, I.; Lee, D.H.; Dinh, D.K.; Kang, H.; Roh, S.A.; Kim, Y.N.; Choi, S.; Jung, C.; Song, Y.-H. A Novel Energy Efficient Path for Nitrogen Fixation Using a Non-Thermal Arc. RSC Adv. 2021, 11, 12729–12738. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, A.; Mathieu, S.; Gao, P.; Li, X.; Xu, B.Z.; Yan, J.; Tu, X. Highly Efficient Nitrogen Fixation Enabled by an Atmospheric Pressure Rotating Gliding Arc. Plasma Process. Polym. 2021, 18, 2000200. [Google Scholar] [CrossRef]
- Van Alphen, S.; Eshtehardi, H.A.; O’Modhrain, C.; Bogaerts, J.; Van Poyer, H.; Creel, J.; Delplancke, M.P.; Snyders, R.; Bogaerts, A. Effusion Nozzle for Energy-Efficient NOx Production in a Rotating Gliding Arc Plasma Reactor. Chem. Eng. J. 2022, 443, 136529. [Google Scholar] [CrossRef]
- Kim, T.; Song, S.; Kim, J.; Iwasaki, R. Formation of NOx from Air and N2/O2 Mixtures Using a Nonthermal Microwave Plasma System. Jpn. J. Appl. Phys. 2010, 49, 126201. [Google Scholar] [CrossRef]
- Asisov, R.I.; Givotov, V.K.; Rusanov, V.D.; Fridman, A. High energy chemistry (Khimia Vysokikh energij). Sov. Phys. 1980, 14, 366. [Google Scholar]
- Peng, P.; Chen, P.; Addy, M.; Cheng, Y.; Zhang, Y.; Anderson, E.; Zhou, N.; Schiappacasse, C.; Hatzenbeller, R.; Fan, L.; et al. In Situ Plasma-Assisted Atmospheric Nitrogen Fixation Using Water and Spray-Type Jet Plasma. Chem. Commun. 2018, 54, 2886–2889. [Google Scholar] [CrossRef] [PubMed]
- Gorbanev, Y.; Vervloessem, E.; Nikiforov, A.; Bogaerts, A. Nitrogen Fixation with Water Vapor by Nonequilibrium Plasma: Toward Sustainable Ammonia Production. ACS Sustain. Chem. Eng. 2020, 8, 2996–3004. [Google Scholar] [CrossRef]
- Toth, J.R.; Abuyazid, N.H.; Lacks, D.J.; Renner, J.N.; Mohan Sankaran, R. A Plasma-Water Droplet Reactor for Process-Intensified, Continuous Nitrogen Fixation at Atmospheric Pressure. ACS Sustain. Chem. Eng. 2020, 8, 14845. [Google Scholar] [CrossRef]
- Peng, P.; Schiappacasse, C.; Zhou, N.; Addy, M.; Cheng, Y.; Zhang, Y.; Anderson, E.; Chen, D.; Wang, Y.; Liu, Y.; et al. Plasma in Situ Gas–Liquid Nitrogen Fixation Using Concentrated High-Intensity Electric Field. J. Phys. D Appl. Phys. 2019, 52, 494001. [Google Scholar] [CrossRef]
- Kubota, Y.; Kogo, K.; Ohno, M.; Hara, T. Synthesis of Ammonia through Direct Chemical Reactions between an Atmospheric Nitrogen Plasma Jet and a Liquid. Plasma Fusion Res. 2010, 5, 042. [Google Scholar] [CrossRef]
- Kumari, S.; Pishgar, S.; Schwarting, M.E.; Paxton, W.F.; Spurgeon, J.M. Synergistic Plasma-Assisted Electrochemical Reduction of Nitrogen to Ammonia. Chem. Commun. 2018, 54, 13347–13350. [Google Scholar] [CrossRef] [PubMed]
- Hawtof, R.; Ghosh, S.; Guarr, E.; Xu, C.; Mohan Sankaran, R.; Renner, J.N. Catalyst-Free, Highly Selective Synthesis of Ammonia from Nitrogen and Water by a Plasma Electrolytic System. Sci. Adv. 2019, 5, eaat5778. [Google Scholar] [CrossRef] [PubMed]
- Haruyama, T.; Namise, T.; Shimoshimizu, N.; Uemura, S.; Takatsuji, Y.; Hino, M.; Yamasaki, R.; Kamachi, T.; Kohno, M. Non-Catalyzed One-Step Synthesis of Ammonia from Atmospheric Air and Water. Green Chem. 2016, 18, 4536–4541. [Google Scholar] [CrossRef]
- Sakakura, T.; Uemura, S.; Hino, M.; Kiyomatsu, S.; Takatsuji, Y.; Yamasaki, R.; Morimoto, M.; Haruyama, T. Excitation of H2O at the plasma/water interface by UV irradiation for the elevation of ammonia production. Green Chem. 2017, 20, 627–633. [Google Scholar] [CrossRef]
- Sakakura, T.; Murakami, N.; Takatsuji, Y.; Morimoto, M.; Haruyama, T. Contribution of Discharge Excited Atomic N, N2*, and N2+ to a Plasma/Liquid Interfacial Reaction as Suggested by Quantitative Analysis. Chemphyschem 2019, 20, 1467–1474. [Google Scholar] [CrossRef]
- Sakakura, T.; Murakami, N.; Takatsuji, Y.; Haruyama, T. Nitrogen Fixation in a Plasma/Liquid Interfacial Reaction and Its Switching between Reduction and Oxidation. J. Phys. Chem. C 2020, 124, 9401–9408. [Google Scholar] [CrossRef]
- Tsonev, I.; Ahmadi Eshtehardi, H.; Delplancke, M.-P.; Bogaerts, A. Scaling Up Energy-Efficient Plasma-Based Nitrogen Fixation. Soc. Sci. Res. Netw. 2023. [Google Scholar] [CrossRef]
- Bogaerts, A.; Neyts, E.C. Plasma Technology: An Emerging Technology for Energy Storage. ACS Energy Lett. 2018, 3, 1013–1027. [Google Scholar] [CrossRef]
- Czernichowski, A. Gliding arc: Applications to engineering and environment control. Pure Appl. Chem. 1994, 66, 1301–1310. [Google Scholar] [CrossRef]
- Ramakers, M.; Trenchev, G.; Heijkers, S.; Wang, W.; Bogaerts, A. Gliding Arc Plasmatron: Providing an Alternative Method for Carbon Dioxide Conversion. ChemSusChem 2017, 10, 2642–2652. [Google Scholar] [CrossRef] [PubMed]
- Cleiren, E.; Heijkers, S.; Ramakers, M.; Bogaerts, A. Dry Reforming of Methane in a Gliding Arc Plasmatron: Towards a Better Understanding of the Plasma Chemistry. ChemSusChem 2017, 10, 4025–4036. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Piavis, W.; Turn, S. Reforming of biogas using a non-thermal, gliding-arc, plasma in reverse vortex flow and fate of hydrogen sulfide contaminants. Fuel Process. Technol. 2019, 193, 378–391. [Google Scholar] [CrossRef]
- Janda, M.; Martišovitš, V.; Hensel, K.; Dvonč, L.; Machala, Z. Measurement of the Electron Density in Transient Spark Discharge. Plasma Sources Sci. Technol. 2014, 23, 065016. [Google Scholar] [CrossRef]
- Janda, M.; Hoder, T.; Sarani, A.; Brandenburg, R.; Machala, Z. Cross-Correlation Spectroscopy Study of the Transient Spark Discharge in Atmospheric Pressure Air. Plasma Sources Sci. Technol. 2017, 26, 055010. [Google Scholar] [CrossRef]
- Tsoukou, E.; Delit, M.; Treint, L.; Bourke, P.; Boehm, D. Distinct Chemistries Define the Diverse Biological Effects of Plasma Activated Water Generated with Spark and Glow Plasma Discharges. Appl. Sci. 2021, 11, 1178. [Google Scholar] [CrossRef]
- Mansouri, F.; Khavanin, A.; Jafari, A.J.; Asilian, H.; Ghomi, H.R.; Mousavi, S.M. Energy efficiency improvement in nitric oxide reduction by packed DBD plasma: Optimization and modeling using response surface methodology(RSM). Environ. Sci. Pollut. Res. 2020, 27, 16100–16109. [Google Scholar] [CrossRef]
- Bruggeman, P.; Iza, F.; Brandenburg, R. Foundations of Atmospheric Pressure Non-Equilibrium Plasmas. Plasma Sources Sci. Technol. 2017, 26, 123002. [Google Scholar] [CrossRef]
- van Alphen, S.; Vermeiren, V.; Butterworth, T.D.; van den Bekerom, D.C.; van Rooij, G.J.; Bogaerts, A. Power Pulsing to Maximize Vibrational Excitation Efficiency in N2 Microwave Plasma: A Combined Experimental and Computational Study. J. Phys. Chem. C 2019, 124, 1765–1779. [Google Scholar] [CrossRef]
- Mehdizadeh, M. Plasma Applicators at RF and Microwave Frequencies; William Andrew Publishers: Norwich, NY, USA, 2015; pp. 335–363. [Google Scholar] [CrossRef]
- Vervloessem, E.; Gorbanev, Y.; Nikiforov, A.; Geyter, N.D.; Bogaerts, A. Sustainable NOx Production from Air in Pulsed Plasma: Elucidating the Chemistry behind the Low Energy Consumption. Green Chem. 2022, 24, 916–929. [Google Scholar] [CrossRef]
- Lee, J.; Sun, H.; Im, S.-K.; Bak, M.S. Formation of nitrogen oxides from atmospheric electrodeless microwave plasmas in nitrogen–oxygen mixtures. J. Appl. Phys. 2017, 122, 083303. [Google Scholar] [CrossRef]
- Na, Y.H.; Kumar, N.; Kang, M.-H.; Cho, G.S.; Choi, E.H.; Park, G.; Uhm, H.S. Production of nitric oxide using a microwave plasma torch and its application to fungal cell differentiation. J. Phys. D Appl. Phys. 2015, 48, 195401. [Google Scholar] [CrossRef]
- Abdelaziz, A.A.; Teramoto, Y.; Nozaki, T.; Kim, H.-H. Performance of High-Frequency Spark Discharge for Efficient NO Production with Tunable Selectivity. Chem. Eng. J. 2023, 470, 144182. [Google Scholar] [CrossRef]
- Li, Z.; Wu, E.; Nie, L.; Liu, D.; Lu, X. Magnetic Field Stabilized Atmospheric Pressure Plasma Nitrogen Fixation: Effect of Electric Field and Gas Temperature. Phys. Plasmas 2023, 30, 083502. [Google Scholar] [CrossRef]
- Malik, M.A.; Jiang, C.; Heller, R.; Lane, J.; Hughes, D.; Schoenbach, K.H. Ozone-Free Nitric Oxide Production Using an Atmospheric Pressure Surface Discharge–a Way to Minimize Nitrogen Dioxide Co-Production. Chem. Eng. J. 2016, 283, 631–638. [Google Scholar] [CrossRef]
- Li, K.; Javed, H.; Zhang, G. Calculation of Ozone and NOx Production under AC Corona Discharge in Dry Air Used for Faults Diagnostic; Atlantis Press: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Amouroux, J.; Cavadias, S. Process and Installation for Heating a Fluidized Bed by Plasma Injection. 1984. Available online: www.osti.gov (accessed on 14 October 2023).
- Rodygin, K.S.; Vikenteva, Y.A.; Ananikov, V.P. Calcium-Based Sustainable Chemical Technologies for Total Carbon Recycling. ChemSusChem 2019, 12, 1483–1516. [Google Scholar] [CrossRef] [PubMed]
- Gicquel, A.; Cavadias, S.; Amouroux, J. Heterogeneous Catalysis in Low-Pressure Plasmas. J. Phys. D Appl. Phys. 1986, 19, 2013–2042. [Google Scholar] [CrossRef]
- Sun, Q.; Zhu, A.; Yang, X.; Niu, J.; Xu, Y. Formation of NOx from N2 and O2 in Catalyst-Pellet Filled Dielectric Barrier Discharges at Atmospheric Pressure. Chem. Commun. 2003, 12, 1418–1419. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Li, Y.; Luo, Y.; Man, C.; Zhang, Y.; Lu, X.; Graves, D.B. Nitrogen Fixation as NOx Using Air Plasma Coupled with Heterogeneous Catalysis at Atmospheric Pressure. Plasma Process. Polym. 2023, 21, 2300135. [Google Scholar] [CrossRef]
- O’Hare, L.R. Nitrogen Fixation by Plasma and Catalyst. 1984. Available online: www.osti.gov (accessed on 21 June 2023).
- O’Hare, L.R. Nitrogen Fixation by Electric Arc and Catalyst. Available online: https://patents.google.com/patent/US4877589A/en (accessed on 21 June 2023).
- Lei, X.; Cheng, H.; Nie, L.; Xian, Y.; Lu, X.P. Plasma-Catalytic NOx Production in a Three-Level Coupled Rotating Electrodes Air Plasma Combined with Nano-Sized TiO2. J. Phys. D Appl. Phys. 2021, 55, 115201. [Google Scholar] [CrossRef]
- Belova, V.M.; Eremin, E.N.; Maltsev, A.N. Heterogeneous catalytic oxidation of nitrogen in a glow discharge II 1: 1 nitrogen-oxygen mixture. Russ. J. Phys. Chem. 1978, 52, 968–970. [Google Scholar]
- Alamaro, M. Production of Nitric Oxides. Available online: https://patents.google.com/patent/US4287040 (accessed on 21 June 2023).
- Bayer, B.N.; Bruggeman, P.J.; Bhan, A. NO Formation by N2/O2 Plasma Catalysis: The Impact of Surface Reactions, Gas-Phase Reactions, and Mass Transport. Chem. Eng. J. 2024, 482, 149041. [Google Scholar] [CrossRef]
- Yu, S.; Cornelis, S.; von Keudell, A. Controlled Synthesis of NO in an Atmospheric Pressure Plasma by Suppressing NO Destruction Channels by Plasma Catalysis. J. Phys. D Appl. Phys. 2024, 57, 245203. [Google Scholar] [CrossRef]
- Kim, H.-H.; Teramoto, Y.; Ogata, A.; Takagi, H.; Nanba, T. Atmospheric-Pressure Nonthermal Plasma Synthesis of Ammonia over Ruthenium Catalysts. Plasma Process. Polym. 2016, 14, 1600157. [Google Scholar] [CrossRef]
- Carman, R.J.; Kane, D.M.; Ward, B.K. Enhanced Performance of an EUV Light Source (λ = 84 Nm) Using Short-Pulse Excitation of a Windowless Dielectric Barrier Discharge in Neon. J. Phys. D Appl. Phys. 2009, 43, 025205. [Google Scholar] [CrossRef]
- Popov, N.A. Dissociation of Nitrogen in a Pulse-Periodic Dielectric Barrier Discharge at Atmospheric Pressure. Plasma Phys. Rep. 2013, 39, 420–424. [Google Scholar] [CrossRef]
- A High Voltage Nanosecond Pulser with Variable Pulse Width and Pulse Repetition Frequency Control for Nonequilibrium Plasma Applications. IEEE Conference Publication. IEEE Xplore. Available online: https://ieeexplore.ieee.org/document/7012774 (accessed on 14 October 2023).
- Anastasopoulou, A.; Wang, Q.; Hessel, V.; Lang, J. Energy Considerations for Plasma-Assisted N-Fixation Reactions. Processes 2014, 2, 694–710. [Google Scholar] [CrossRef]
- Jianli, Z.; Juncheng, Z.; Ji, S.; Hongchen, G.; Xiangsheng, W.; Weimin, G. Scale-up Synthesis of Hydrogen Peroxide from H2/O2 with Multiple Parallel DBD Tubes. Plasma Sci. Technol. 2009, 11, 181–186. [Google Scholar] [CrossRef]
- Yao, S.; Fushimi, C.; Kodama, S.; Yamamoto, S.; Mine, C.; Fujioka, Y.; Madokoro, K.; Naito, K.; Kim, Y.-H. On the Scale-up of Uneven DBD Reactor on Removal of Diesel Particulate Matter. Int. J. Chem. React. Eng. 2009, 7. [Google Scholar] [CrossRef]
- Anastasopoulou, A.; Butala, S.; Patil, B.; Suberu, J.; Fregene, M.; Lang, J.; Wang, Q.; Hessel, V. Techno-Economic Feasibility Study of Renewable Power Systems for a Small-Scale Plasma-Assisted Nitric Acid Plant in Africa. Processes 2016, 4, 54. [Google Scholar] [CrossRef]
- Anastasopoulou, A.; Butala, S.; Lang, J.; Hessel, V.; Wang, Q. Life Cycle Assessment of the Nitrogen Fixation Process Assisted by Plasma Technology and Incorporating Renewable Energy. Ind. Eng. Chem. Res. 2016, 55, 8141–8153. [Google Scholar] [CrossRef]




| Plasma Type | Energy Consumption (MJ/mol N) | Reference |
|---|---|---|
| Electric arc (Birkeland–Eyde) | 2.4–3.1 | [31,33,57] |
| Spark discharge | 20.27, 40, 1.9–4.4 | [58,61,62] |
| Plate-to-plate ns-pulsed spark discharge | 22.18–25.72 | [63] |
| Pin-to-plane ns-pulsed spark discharge | 5.0–7.7 | [59] |
| Transient spark discharge | 8.6 | [60] |
| (Positive/negative) DC corona discharge | 1057/1673 | [58] |
| Pulsed corona discharge | 186 | [65] |
| Radio-frequency crossed discharge | 24–108 | [67] |
| Laser-produced discharge | 8.9 | [66] |
| Pin-to-plane DC glow discharge | 7, 2.8–4.8 | [59,92] |
| Pin-to-pin DC glow discharge | 2.8 | [64] |
| Dielectric barrier discharge | 56–140, 20.7 | [59,69] |
| Packed dielectric barrier discharge | 18, 17–33 | [68,70] |
| DC plasma arc jet | 3.6 | [72] |
| Propeller arc | 4.2 | [59] |
| Pulsed milli-scale gliding arc | 2.8–4.8 | [73,74] |
| Gliding arc plasmatron | 3.6 | [71] |
| Rotating gliding arc | 2.5, 1.8, 0.67, 4.2, 2.1 | [68,75,76,77,78] |
| Microwave plasma | 3.76 | [79] |
| Microwave plasma with catalyst | 0.84 | [54] |
| Electron cyclotron resonance | 0.28 | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimek, A.; Piercey, D.G. Nitrogen Fixation via Plasma-Assisted Processes: Mechanisms, Applications, and Comparative Analysis—A Comprehensive Review. Processes 2024, 12, 786. https://doi.org/10.3390/pr12040786
Klimek A, Piercey DG. Nitrogen Fixation via Plasma-Assisted Processes: Mechanisms, Applications, and Comparative Analysis—A Comprehensive Review. Processes. 2024; 12(4):786. https://doi.org/10.3390/pr12040786
Chicago/Turabian StyleKlimek, Angelique, and Davin G. Piercey. 2024. "Nitrogen Fixation via Plasma-Assisted Processes: Mechanisms, Applications, and Comparative Analysis—A Comprehensive Review" Processes 12, no. 4: 786. https://doi.org/10.3390/pr12040786
APA StyleKlimek, A., & Piercey, D. G. (2024). Nitrogen Fixation via Plasma-Assisted Processes: Mechanisms, Applications, and Comparative Analysis—A Comprehensive Review. Processes, 12(4), 786. https://doi.org/10.3390/pr12040786





