Optimizing the Mixing Ratios of Source-Separated Organic Waste and Thickened Waste Activated Sludge in Anaerobic Co-Digestion: A New Approach
Abstract
:1. Introduction
2. Methodology
2.1. Materials
2.2. Experimental Setup
2.3. Analytical Methods
2.4. Calculations
3. Results and Discussion
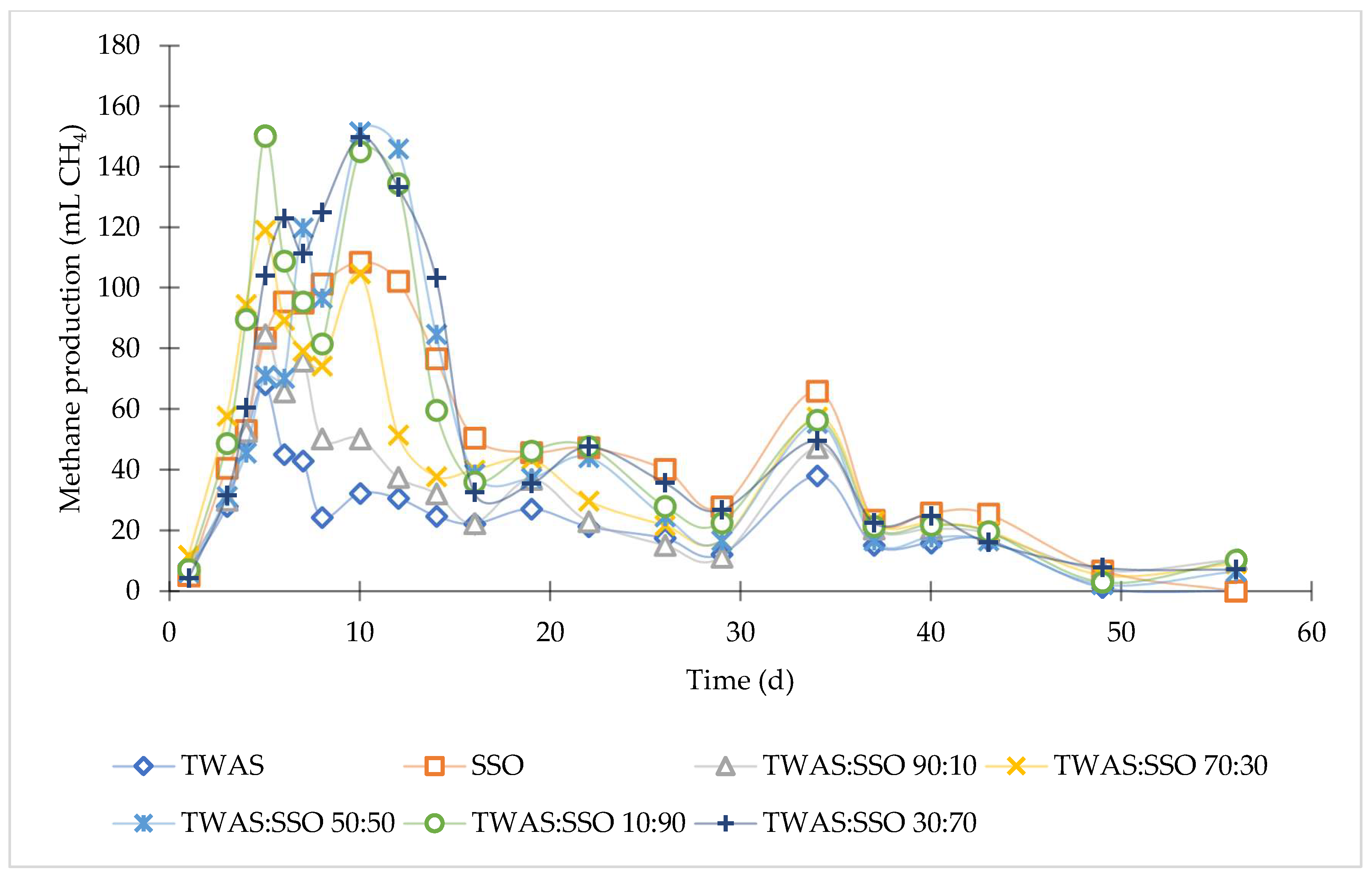
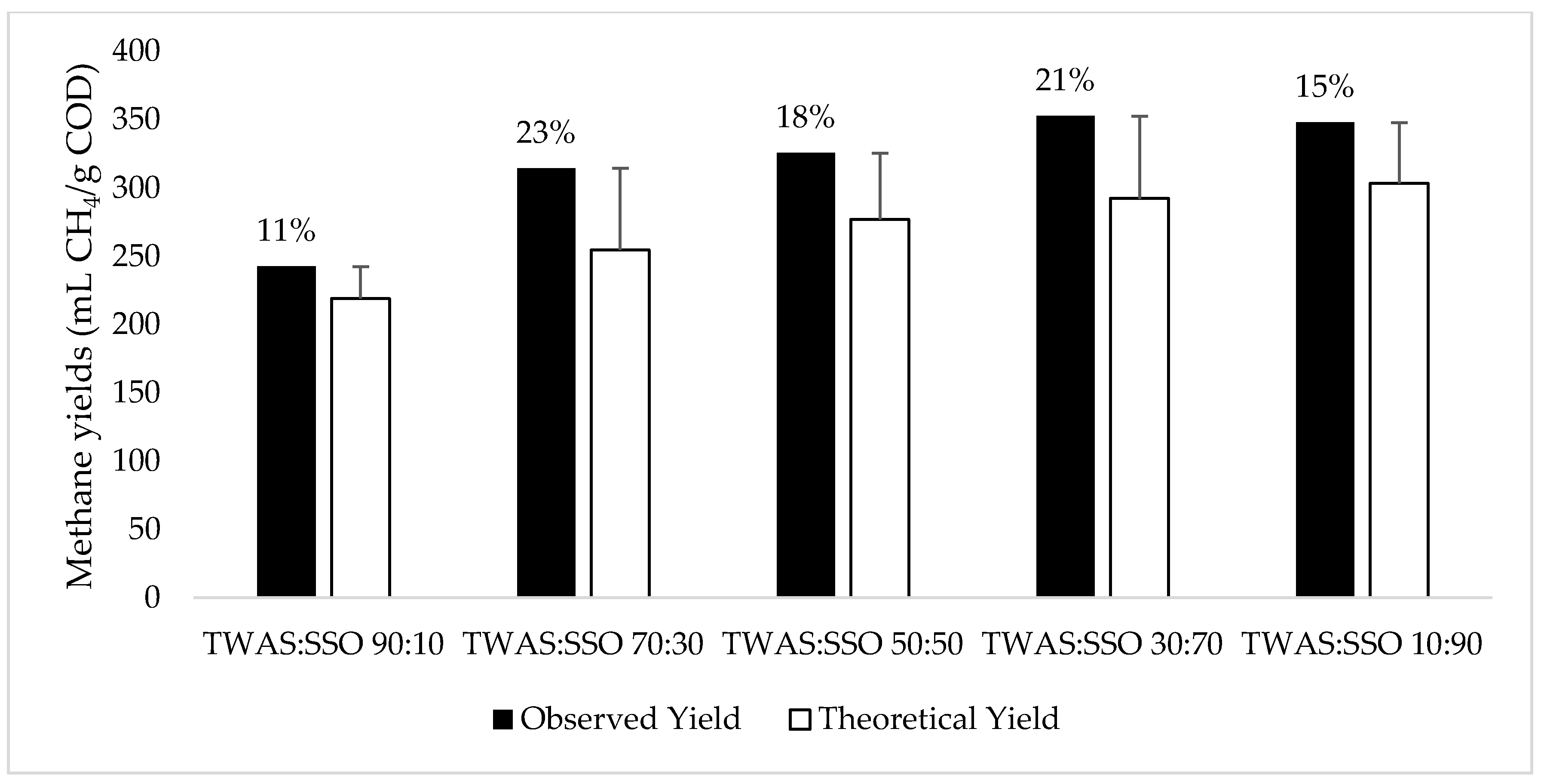
3.1. Anaerobic Degradation and Methane Production
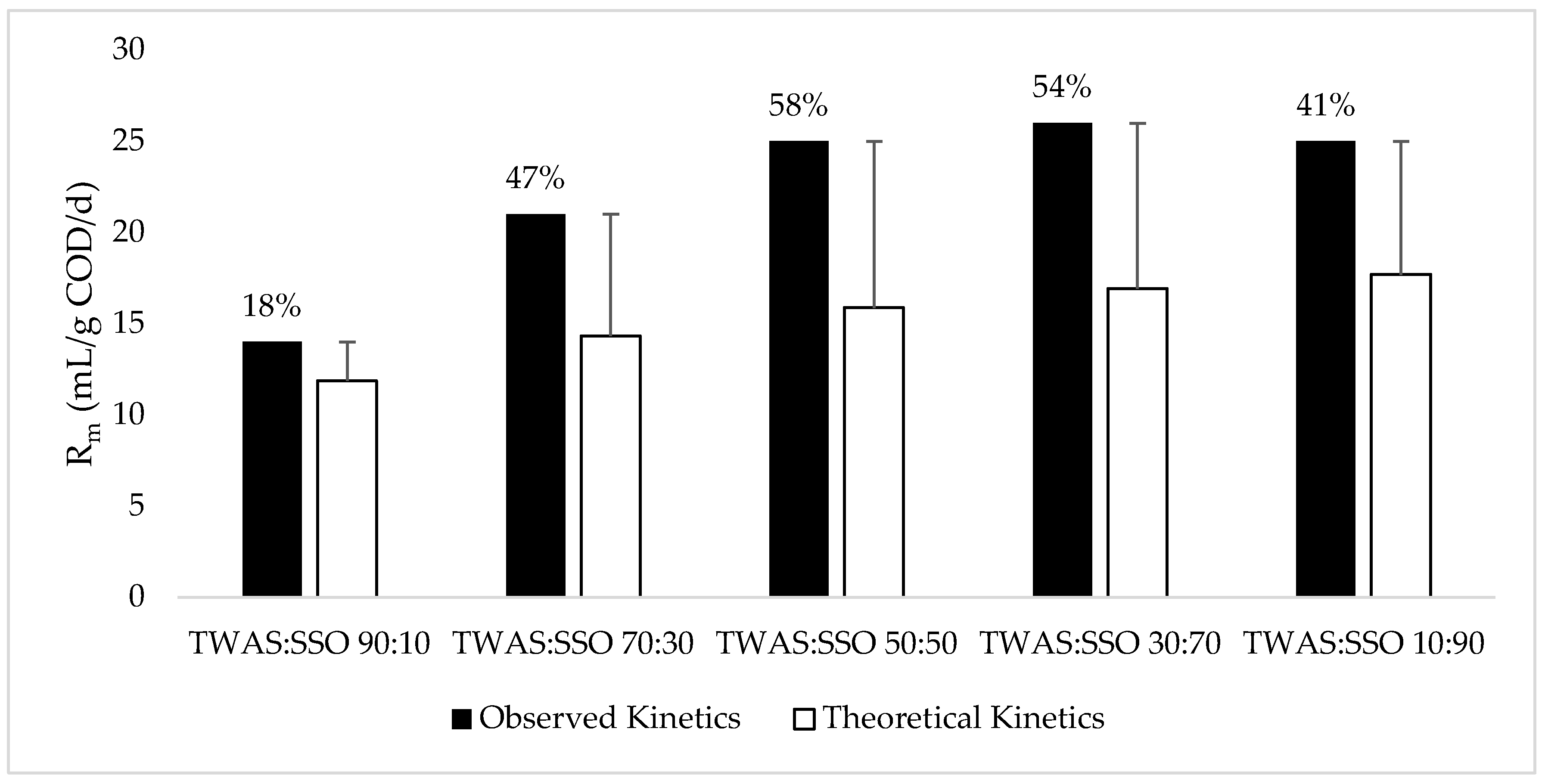
3.2. Kinetic Analysis
3.3. COD/N and L:P:C Ratios
3.4. Synergism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Anaerobic digestion |
| AnCoD | Anaerobic co-digestion |
| C | Carbon |
| FW | Food waste |
| GC | Gas chromatograph |
| HRT | Hydraulic retention time |
| LCFAs | Long-chain fatty acids |
| MSW | Municipal solid waste |
| NHx-N | Ammonia nitrogen |
| N | Nitrogen |
| OFMSW | Organic fraction of municipal solid waste |
| OLR | Organic loading rate |
| pH | Potential of hydrogen |
| PS | Primary sludge |
| sCOD | Soluble chemical oxygen demand |
| sN | Soluble nitrogen |
| SNG | Synthetic natural gas |
| SS | Sewage sludge |
| SSO | Source-separated organics |
| TCarbs | Total carbohydrates |
| TCOD | Total chemical oxygen demand |
| TLipids | Total lipids |
| TN | Total nitrogen |
| TProteins | Total proteins |
| TWAS | Thickened waste activated sludge |
| TSs | Total solids |
| TSSs | Total suspended solids |
| VFAs | Volatile fatty acids |
| VSs | Volatile solids |
| VSSs | Volatile suspended solids |
| WAS | Waste activated sludge |
| WWTP | Wastewater treatment plant |
References
- Mehariya, S.; Patel, A.K.; PObulisamy, K.; Punniyakotti, E.; Wong, J.W.C. Co-digestion of food waste and sewage sludge for methane production: Current status and perspective. Bioresour. Technol. 2018, 265, 519–531. [Google Scholar] [CrossRef]
- Thyberg, K.L.; Tonjes, D.J. Drivers of food waste and their implications for sustainable policy development. Resour. Conserv. Recycl. 2016, 106, 110–123. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Dosta, J.; Romero-Güiza, M.S.; Fonoll, X.; Peces, M.; Astals, S. A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- Boguniewicz-Zablocka, J.; Klosok-Bazan, I.; Capodaglio, A.G. Sustainable management of biological solids in small treatment plants: Overview of strategies and reuse options for a solar drying facility in Poland. Environ. Sci. Pollut. Res. 2021, 28, 24680–24693. [Google Scholar] [CrossRef] [PubMed]
- Tiehm, A.; Nickel, K.; Zellhorn, M.; Neis, U. Ultrasonic waste activated sludge disintegration for improving anaerobic stabilization. Water Res. 2001, 35, 2003–2009. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Buhain, J. Bioenergy and biofuels: History, status, and perspective. Renew. Sustain. Energy Rev. 2015, 42, 712–725. [Google Scholar] [CrossRef]
- Razavi, A.S.; Koupaie, E.H.; Azizi, A.; Hafez, H.; Elbeshbishy, E. Bioenergy production data from anaerobic digestion of thermally hydrolyzed organic fraction of municipal solid waste. Data Brief. 2019, 22, 1018–1026. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Dhar, B.R.; Nakhla, G.; Lee, H.S. A critical review on inhibition of dark biohydrogen fermentation. Renew. Sustain. Energy Rev. 2017, 79, 656–668. [Google Scholar] [CrossRef]
- Karlis, P.; Presicce, F.; Giner-Santonja, G.; Brinkmann, T.; Roudier, S. Best Available Techniques (BAT) Reference Document for the Slaughterhouses, Animal By-products and/or Edible Co-products Industries. 2024. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC135916 (accessed on 22 February 2024).
- Gelegenis, J.; Georgakakis, D.; Angelidaki, I.; Christopoulou, N.; Goumenaki, M. Optimization of biogas production from olive-oil mill wastewater, by codigesting with diluted poultry-manure. Appl. Energy 2007, 84, 646–663. [Google Scholar] [CrossRef]
- Igoni, A.H.; Ayotamuno, M.J.; Eze, C.L.; Ogaji, S.O.T.; Probert, S.D. Designs of anaerobic digesters for producing biogas from municipal solid-waste. Appl. Energy 2008, 85, 430–438. [Google Scholar] [CrossRef]
- Karagiannidis, A.; Perkoulidis, G. A multi-criteria ranking of different technologies for the anaerobic digestion for energy recovery of the organic fraction of municipal solid wastes. Bioresour. Technol. 2009, 100, 2355–2360. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Bayr, S.; Ojanperä, M.; Kaparaju, P.; Rintala, J. Long-term thermophilic mono-digestion of rendering wastes and co-digestion with potato pulp. Waste Manag. 2014, 34, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Mata-Alvarez, J.; Dosta, J.; Macé, S.; Astals, S. Codigestion of solid wastes: A review of its uses and perspectives including modeling. Crit. Rev. Biotechnol. 2011, 31, 99–111. [Google Scholar] [CrossRef]
- Silvestre, G.; Illa, J.; Fernández, B.; Bonmatí, A. Thermophilic anaerobic co-digestion of sewage sludge with grease waste: Effect of long chain fatty acids in the methane yield and its dewatering properties. Appl. Energy 2014, 117, 87–94. [Google Scholar] [CrossRef]
- Shah, F.A.; Mahmood, Q.; Rashid, N.; Pervez, A.; Raja, I.A.; Shah, M.M. Co-digestion, pretreatment and digester design for enhanced methanogenesis. Renew. Sustain. Energy Rev. 2015, 42, 627–642. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Macé, S.; Llabrés, P. Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Bioresour. Technol. 2000, 76, 3–16. [Google Scholar] [CrossRef]
- Divya, D.; Gopinath, L.R.; Merlin, P. Christy A review on current aspects and diverse prospects for enhancing biogas production in sustainable means. Renew. Sustain. Energy Rev. 2015, 42, 690–699. [Google Scholar] [CrossRef]
- Secondi, L.; Principato, L.; Laureti, T. Household food waste behaviour in EU-27 countries: A multilevel analysis. Food Policy 2015, 56, 25–40. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Shi, Y.; Zheng, L.; Gao, X.; Qiao, W.; Zhou, Y. Pilot-scale anaerobic co-digestion of municipal biomass waste and waste activated sludge in China: Effect of organic loading rate. Waste Manag. 2012, 32, 2056–2060. [Google Scholar] [CrossRef]
- Kim, M.; Chowdhury, M.M.I.; Nakhla, G.; Keleman, M. Synergism of co-digestion of food wastes with municipal wastewater treatment biosolids. Waste Manag. 2017, 61, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Al bkoor Alrawashdeh, K.; Pugliese, A.; Slopiecka, K.; Pistolesi, V.; Massoli, S.; Bartocci, P.; Bidini, G.; Fantozzi, F. Codigestion of untreated and treated sewage sludge with the organic fraction of municipal solid wastes. Fermentation 2017, 3, 35. [Google Scholar] [CrossRef]
- Gu, J.; Liu, R.; Cheng, Y.; Stanisavljevic, N.; Li, L.; Djatkov, D.; Peng, X.; Wang, X. Anaerobic co-digestion of food waste and sewage sludge under mesophilic and thermophilic conditions: Focusing on synergistic effects on methane production. Bioresour. Technol. 2020, 301, 122765. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Moody, L.; Burns, R.; Wu-Haan, W.; Spajić, R. Use of biochemical methane potential (BMP) assays for predicting and enhancing anaerobic digester performance. In Proceedings of the 4th International and 44th Croatian Symposium of Agriculture, Opatija, Croatia, 16–20 February 2009. [Google Scholar]
- Owen, W.F.; Stuckey, D.C.; Healy, J.B.; Young, L.Y.; McCarty, P.L. Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1979, 13, 485–492. [Google Scholar] [CrossRef]
- Metcalf & Eddy. Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill Professional: New York, NY, USA, 2014. [Google Scholar]
- Baird, R.B. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Water Environment Federation, American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Smith Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bougrier, C.; Delgenès, J.P.; Carrère, H. Impacts of thermal pre-treatments on the semi-continuous anaerobic digestion of waste activated sludge. Biochem. Eng. J. 2007, 34, 20–27. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, T.; Ma, Y. New insights into the co-locating concept on synergistic co-digestion of sewage sludge and food waste towards energy self-sufficient in future WWTPs. Bioresour. Technol. Rep. 2020, 10, 100351. [Google Scholar] [CrossRef]
- Ismail, A.; Kakar, F.L.; Elbeshbishy, E.; Nakhla, G. Combined thermal hydrolysis pretreatment and anaerobic co-digestion of waste activated sludge and food waste. Renew. Energy 2022, 195, 528–539. [Google Scholar] [CrossRef]
- Weemaes, M.P.J.; Verstraete, W.H. Evaluation of current wet sludge disintegration techniques. J. Chem. Technol. Biotechnol. 1998, 73, 83–92. [Google Scholar] [CrossRef]
- Rittmann, B.E.; McCarty, P.L. Environmental Biotechnology: Principles and Applications, 1st ed.; McGraw-Hill Education: New York, NY, USA, 2001; Available online: https://www.accessengineeringlibrary.com/content/book/9781260440591 (accessed on 22 February 2024).
- Koch, K.; Helmreich, B.; Drewes, J.E. Co-digestion of food waste in municipal wastewater treatment plants: Effect of different mixtures on methane yield and hydrolysis rate constant. Appl. Energy 2015, 137, 250–255. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Dutta, S.; Sherpa, K.C.; Jayaswal, K.; Saravanabhupathy, S.; Mohanty, K.T.; Banerjee, R.; Kumar, J.; Rajak, R.C. Chapter 7—Co-digestion processes of waste: Status and perspective. In Bio-Based Materials and Waste for Energy Generation and Resource Management; Hussain, C.M., Kushwaha, A., Bharagava, R.N., Goswami, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 207–241. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Li, F.; Yang, G. Effects of Temperature and Carbon-Nitrogen (C/N) Ratio on the Performance of Anaerobic Co-Digestion of Dairy Manure, Chicken Manure and Rice Straw: Focusing on Ammonia Inhibition. PLoS ONE 2014, 9, e97265. [Google Scholar] [CrossRef] [PubMed]
- Viotti, P.; Di Genova, P.; Falcioli, F. Numerical analysis of the anaerobic co-digestion of the organic fraction from municipal solid waste and wastewater: Prediction of the possible performances at Olmeto plant in Perugia (Italy). Waste Manag. Res. 2004, 22, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Zamanzadeh, M.; Hagen, L.H.; Svensson, K.; Linjordet, R.; Horn, S.J. Biogas production from food waste via co-digestion and digestion- effects on performance and microbial ecology. Sci. Rep. 2017, 7, 17664. [Google Scholar] [CrossRef]

| Parameter † | SSO * | TWAS | Inoculum |
|---|---|---|---|
| TSs | 62,300 ± 0.02 | 38,800 ± 0.12 | 21,500 ± 0.03 |
| VSs | 43,500 ± 0.02 | 31,200 ± 0.01 | 13,100 ± 0.02 |
| TSSs | 53,800 ± 0.12 | 31,500 ± 0.1 | 17,000 ± 0.02 |
| VSSs | 38,500 ± 0.1 | 25,600 ± 0.1 | 10,900 ± 0.02 |
| TCOD | 110,000 ± 0.1 | 40,000 ± 0.1 | 16,400 ± 0.03 |
| sCOD | 44,400 | 360 ± 0.1 | 362 ± 0.05 |
| TN | 4170 ± 0.18 | 2900 ± 0.14 | 2030 ± 0.1 |
| sN | 1790 ± 0.02 | 420 ± 0.15 | 696 ± 0.16 |
| NHx-N | 1740 | 255 ± 0.12 | 1500 ± 0.03 |
| TCarbs | 40,400 ± 0.1 | 1290 ± 0.1 | 961 ± 0.1 |
| TProteins | 2020 ± 0.1 | 1460 ± 0.2 | 585 ± 0.1 |
| TLipids | 18,600 ± 0.12 | 551 ± 0.1 | 1920 ± 0.1 |
| Alkalinity (CaCO3) | 7700 ± 0.07 | 1950 ± 0.1 | 3940 ± 0.12 |
| pH | 5.6 | 6.3 | 7.2 |
| Condition | TWAS | |||||||
|---|---|---|---|---|---|---|---|---|
| 0% | 10% | 30% | 50% | 70% | 90% | 100% | ||
| SSO | 0% | R8 | ||||||
| 10% | R7 | |||||||
| 30% | R6 | |||||||
| 50% | R5 | |||||||
| 70% | R4 | |||||||
| 90% | R3 | |||||||
| 100% | R1 | |||||||
| Feedstock | P (mL CH4/g COD) | Rm (mL CH4/g COD/d) | λ (d) | R2 |
|---|---|---|---|---|
| TWAS | 181 | 10 | 0.00 | 0.985 |
| SSO | 292 | 18 | 1.95 | 0.993 |
| TWAS: SSO 90:10 | 222 | 14 | 0.80 | 0.985 |
| TWAS: SSO 70:30 | 293 | 21 | 0.97 | 0.989 |
| TWAS: SSO 50:50 | 310 | 25 | 3.11 | 0.996 |
| TWAS: SSO 30:70 | 329 | 26 | 2.73 | 0.994 |
| TWAS: SSO 10:90 | 333 | 25 | 1.85 | 0.993 |
| Feedstock | COD/N | L:P:C | P (mL CH4/g COD) |
|---|---|---|---|
| TWAS | 14 | 1:3:2 | 181 |
| SSO | 28 | 9:1:20 | 292 |
| TWAS: SSO 90:10 | 16 | 2:1:4 | 222 |
| TWAS: SSO 70:30 | 19 | 4:1:8 | 293 |
| TWAS: SSO 50:50 | 21 | 6:1:12 | 310 |
| TWAS: SSO 30:70 | 26 | 7:1:15 | 329 |
| TWAS: SSO 10:90 | 24 | 8:1:18 | 333 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabii, A.; El Sayed, A.; Ismail, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. Optimizing the Mixing Ratios of Source-Separated Organic Waste and Thickened Waste Activated Sludge in Anaerobic Co-Digestion: A New Approach. Processes 2024, 12, 794. https://doi.org/10.3390/pr12040794
Rabii A, El Sayed A, Ismail A, Aldin S, Dahman Y, Elbeshbishy E. Optimizing the Mixing Ratios of Source-Separated Organic Waste and Thickened Waste Activated Sludge in Anaerobic Co-Digestion: A New Approach. Processes. 2024; 12(4):794. https://doi.org/10.3390/pr12040794
Chicago/Turabian StyleRabii, Anahita, Ahmed El Sayed, Amr Ismail, Saad Aldin, Yaser Dahman, and Elsayed Elbeshbishy. 2024. "Optimizing the Mixing Ratios of Source-Separated Organic Waste and Thickened Waste Activated Sludge in Anaerobic Co-Digestion: A New Approach" Processes 12, no. 4: 794. https://doi.org/10.3390/pr12040794








