Properties of Carbonic Anhydrase-Containing Active Coatings for CO2 Capture
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of Target Gene
2.2. Expression and Purification of Recombinant Proteins
2.3. Preparation of SazF@SiO2 Particles
2.4. Characterization of SazF@SiO2 Particles
2.5. Preparation of CA-Containing Coatings
2.6. Esterase Activity
2.7. Optimum Temperature
2.8. Thermal Stability
2.9. pH Stability
2.10. CO2 Capture Capacity of SazF
2.11. Stability and Protein Leakage of CA-Containing Coatings
3. Results and Discussion
3.1. Expression and Purification of SazF
3.2. Optimum Temperature of Recombinant Proteins
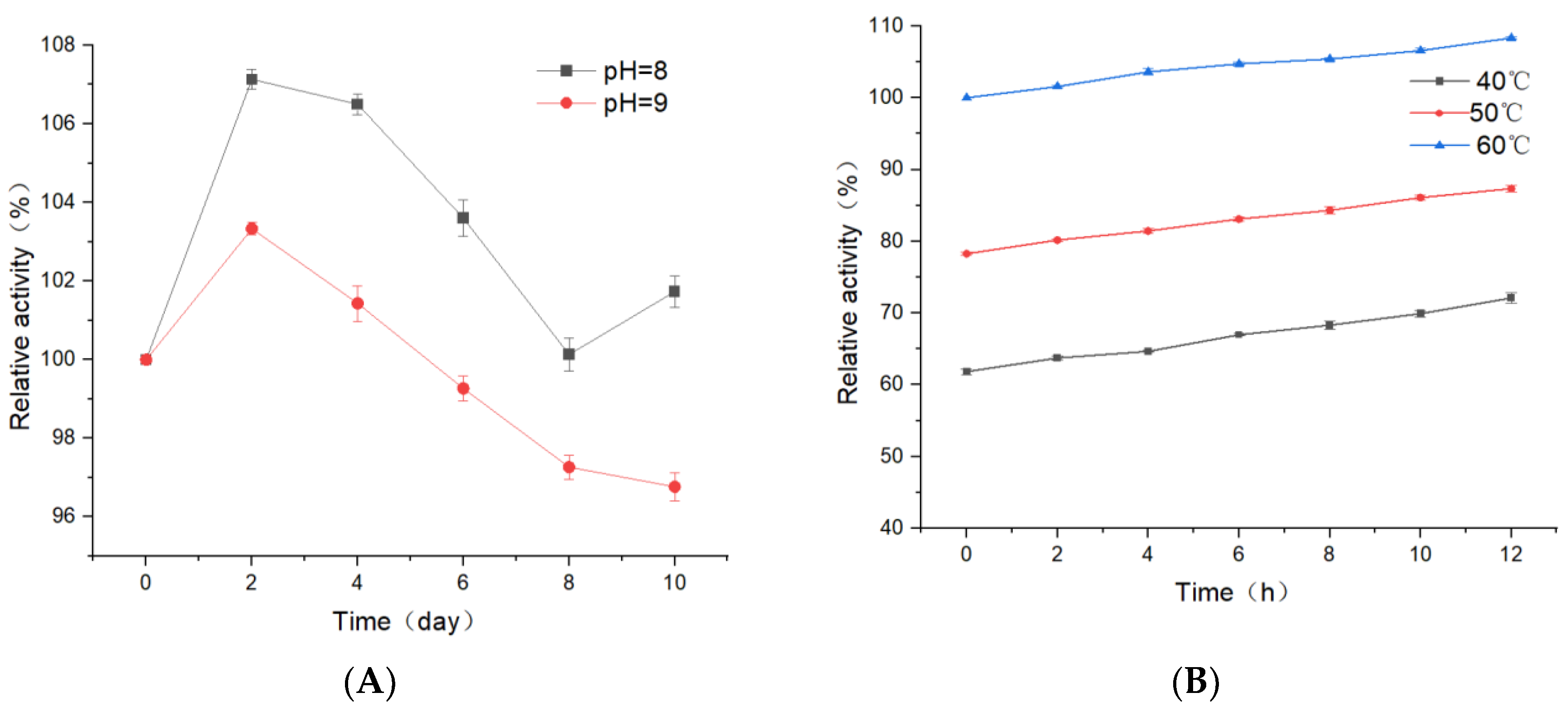
3.3. Temperature and pH Stability of Recombinant Proteins
3.4. CO2 Capture Capacity of SazF
3.5. Characterization of SazF@SiO2 Particles
3.6. Characterization of CA-Containing Coatings
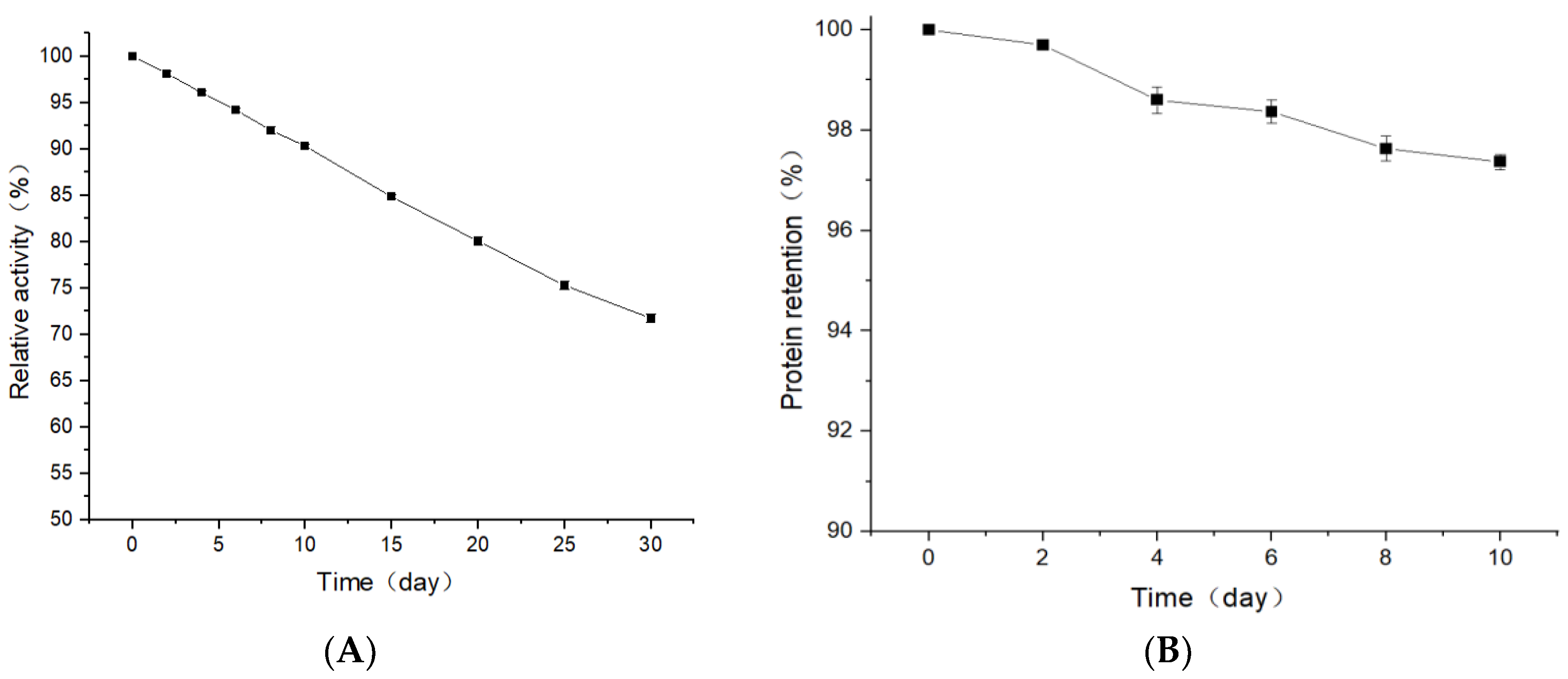
3.7. Thermal Stability and Protein Leakage Rate of CA-Containing Coatings
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CA | carbonic anhydrase |
| CCS | carbon capture and storage |
| CO2 | carbon dioxide |
| 4-NPA | 4-Nitrophthalic acid |
| BDS | biocatalyst delivery system |
| MEA | ethanolamine |
| MDEA | N-Methyldiethanolamine |
| CLEA | cross-linked enzyme aggregates |
| LB | Luria–Bertani |
| TB | Terrific Broth |
| TMOS | tetramethoxysilane |
| PDMS-OH | hydroxyl-capped polydimethylsiloxane |
| SazF | SazCA-Ferritin |
References
- Hua, W.S.; Sha, Y.S.; Zhang, X.L.; Cao, H. Research progress of carbon capture and storage (CCS) technology based on the shipping industry. Ocean Eng. 2023, 281, 114929. [Google Scholar] [CrossRef]
- Liu, S.; Gao, H.X.; He, C.; Liang, Z.W. Experimental evaluation of highly efficient primary and secondary amines with lower energy by a novel method for post-combustion CO2 Capture. Appl. Energy 2019, 233−234, 443–452. [Google Scholar] [CrossRef]
- Smith, K.S.; Ferry, J.G. Prokaryotic carbonic anhydrases. FEMS Microbiol. Rev. 2000, 24, 335–366. [Google Scholar] [CrossRef] [PubMed]
- Leimbrink, M.; Nikoleit, K.G.; Spitzer, R.; Salmon, S.; Bucholz, T.; Górak, A.; Skiborowski, M. Enzymatic reactive absorption of CO2 in MDEA by means of an innovative biocatalyst delivery system. Chem. Eng. J. 2018, 334, 1195–1205. [Google Scholar] [CrossRef]
- Gladis, A.; Lomholdt, N.F.; Fosbøl, P.L.; Woodley, J.M.; Solms, N.V. Pilot scale absorption experiments with carbonic anhydrase-enhanced MDEA- Benchmarking with 30 wt% MEA. Int. J. Greenh. Gas Control 2019, 82, 69–85. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, J.; Jose, M.G.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Sánchez-deAlcázar, D.; Liutkus, M.; Cortajarena, A.L. Immobilization of enzymes in protein films. Methods Mol. Biol. 2020, 2100, 211–226. [Google Scholar] [PubMed]
- Cao, L.; Langen, L.V.; Sheldon, R.A. Immobilised enzymes: Carrier-bound or carrier-free? Curr. Opin. Biotechnol. 2003, 14, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Robatjazi, S.M.; Sadri, M.; Mosaabadi, J.M. Covalent immobilization of organophosphorus hydrolase enzyme on chemically modified cellulose microfibers: Statistical optimization and characterization. Polymers 2018, 124, 162–170. [Google Scholar] [CrossRef]
- Hsieh, C.J.; Cheng, J.C.; Hu, C.J.; Yu, C.Y. Entrapment of the fastest known carbonic anhydrase with biomimetic silica and its application for CO2 sequestration. Polymers 2021, 13, 2452. [Google Scholar] [CrossRef]
- Zhang, L.; Lei, L.C.; Zhang, G.Y.; Li, X.L. Oligomerization triggered by foldon to enhance the catalytie efficieney of feruloyl esterase. Chin. J. Biotechnolo. 2019, 35, 816–826. [Google Scholar]
- Wang, X.Z.; Ge, H.H.; Zhang, D.D.; Wu, S.Y.; Zhang, G.Y. Oligomerization triggeredby foldon: A simple method to enhance the catalytic efficiencyof lichenase and xylanase. BMC Biotechnol. 2017, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Chen, X.H.; Hong, J.J.; Tang, A.; Liu, Y.; Xie, N.; Nie, G.; Yan, X.; Liang, M. Biochemistry of mammalian ferritins in the regulation of cellular iron homeostasis andoxidative responses. Sci. China Life Sci. 2021, 64, 352–362. [Google Scholar] [CrossRef]
- Yao, H.; Soldano, A.; Fontenot, L.; Donnarumma, F.; Lovell, S.; Chandler, J.R.; Rivera, M. Pseudomonas aeruginosa bacterioferritin is assembled from FtnA and BfrB subunits withthe relative proportions dependent on the environmental oxygenavailability. Biomolecules 2022, 12, 366. [Google Scholar] [CrossRef]
- Honarmand Ebrahimi, K.; Hagedoorn, P.L.; Hagen, W.R. Unity in the biochemistry of the iron-storage proteins ferritin and bacterioferritin. Chem. Rev. 2015, 115, 295–326. [Google Scholar] [CrossRef]
- Chen, H.; Tan, X.; Han, X.; Ma, L.; Dai, H.; Fu, Y.; Zhang, Y. Ferritin nanocage based delivery vehicles: From single-,co- to compartmentalized-encapsulation of bioactive or nutraceutical compounds. Biotechnol. Adv. 2022, 61, 108037. [Google Scholar] [CrossRef]
- Li, H.; Tan, X.; Xia, X.; Zang, J.; El-Seedi, H.; Wang, Z.; Du, M. Improvement of thermal stability of oyster (Crassostrea gigas) ferritin by point mutation. Food Chem. 2021, 346, 128879. [Google Scholar] [CrossRef]
- Mao, L.; Liu, G.Z.; Yuan, H.; Zhang, G.Y. Efficient preparation of carbon anhydrase nanoparticles capable of capturing CO2 and their characteristics. Chem. Eng. J. 2023, 74, 2589–2598. [Google Scholar]
- De Simone, G.; Monti, S.M.; Alterio, V.; Buonanno, M.; De Luca, V.; Rossi, M.; Carginale, V.; Supuran, C.T.; Capasso, C.; Di Fiore, A. Crystal structure of the most catalytically effective carbonic anhydrase enzyme known, SazCA from the thermophilic bacterium Sulfurihydrogenibium azorense. Bioorg. Med. Chem. Lett. 2015, 25, 2002–2006. [Google Scholar] [CrossRef]
- Hempstead, P.D.; Yewdall, S.J.; Fernie, A.R.; Lawson, D.M.; Artymiuk, P.J.; Rice, D.W.; Ford, G.C.; Harrison, P.M. Comparison of the three-dimensional structures of recombinant human H and horse L ferritins at high resolution. J. Mol. Biol. 1997, 268, 424–448. [Google Scholar] [CrossRef]
- Banki, M.R.; Wood, D.W. Inteins and affinity resin substitutes for protein purification and scale up. Microb. Cell Fact. 2005, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Ki, M.R.; Min, K.; Kanth, B.K.; Lee, J.; Pack, S.P. Expression, reconstruction and characterization of codon-optimized carbonic anhydrase from Hahella chejuensis for CO2 sequestration application. Bioprocess Biosyst. Eng. 2013, 36, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.Y.; Kim, S.H.; Kanth, B.K.; Lee, J.; Pack, S.P. Expression and characterization of a codon-optimized alkaline-stable carbonic anhydrase from Aliivibrio salmonicida for CO2 sequestration applications. Bioprocess Biosyst. Eng. 2017, 40, 413–421. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, C.; Guo, H.; Wang, Z. Performance Analysis of Ship Exhaust Gas Temperature Differential Power Generation. Energies 2022, 15, 3900. [Google Scholar] [CrossRef]
- Melnikov, S.M.; Stein, M. The effect of CO2 loading on alkanolamine absorbents in aqueous solutions. Phys. Chem. Chem. Phys. 2019, 21, 18386–18392. [Google Scholar] [CrossRef]
- Luca, V.D.; Vullo, D.; Scozzafava, A.; Carginale, V.; Rossi, M.; Supuran, C.T.; Capasso, C. An α-carbonic anhydrase from the thermophilic bacterium Sulphurihydrogenibium azorense is the fastest enzyme known for the CO2 hydration reaction. Bioorg. Med. Chem. 2013, 21, 1465–1469. [Google Scholar] [CrossRef]
- Jo, B.H.; Seo, J.H.; Cha, H.J. Bacterial extremo-α-carbonic anhydrases from deep-sea hydrothermal vents as potential biocatalysts for CO2 sequestration. J. Mol. Catal. B Enzym. 2014, 109, 31–39. [Google Scholar] [CrossRef]
- Parra-Cruz, R.; Jäger, C.M.; Lau, P.L.; Gomes, R.L.; Pordea, A. Engineering of Thermovibrio ammonificans carbonic anhydrase mutants with increased thermostability. J. CO2 Util. 2020, 37, 1–8. [Google Scholar] [CrossRef]
- Heuer, J.; Kraus, Y.; Vučak, M.; Zeng, A.P. Enhanced sequestration of carbon dioxide into calcium carbonate using pressure and a carbonic anhydrase from alkaliphilic Coleofasciculus chthonoplastes. Eng. Life Sci. 2021, 22, 178–191. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Ai, M.; Jia, X. Surface display of carbonic anhydrase on Escherichia coli for CO2 capture and mineralization. Synth. Syst. Biotechnol. 2022, 7, 460–473. [Google Scholar] [CrossRef]
- Faridi, S.; Satyanarayana, T. Novel alkalistable α-carbonic anhydrase from the polyextremophilic bacterium Bacillus halodurans: Characteristics and applicability in flue gas CO2 sequestration. Environ. Sci. Pollut. Res. 2016, 23, 15236–15249. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.J.; Chen, H.; Ying, Q.; Zhang, S.H. Structure–Activity relationship of carbonic anhydrase enzyme immobilized on various silica-based mesoporous molecular sieves for CO2 absorption into a potassium carbonate solution. Energy Fuels 2020, 34, 2089–2096. [Google Scholar] [CrossRef]
- Ying, Q.; Chen, H.; Shao, P.; Zhou, X.; He, X.; Ye, J.; Zhang, S.; Chen, J.; Wang, L. Core-shell magnetic ZIF-8@Fe3O4-carbonic anhydrase biocatalyst for promoting CO2 absorption into MDEA solution. J. CO2 Util. 2021, 49, 101565. [Google Scholar] [CrossRef]
- Molina-Fernandez, C.; Peters, A.P.; Debecker, D.P.; Luis, P. Immobilization of carbonic anhydrase in a hydrophobic poly (ionic liquid): A new functional solid for CO2 capture. Biochem. Eng. J. 2022, 187, 108639. [Google Scholar] [CrossRef]






| BET Surface Area | Pore Volume | Pore Size |
|---|---|---|
| 0.2032 m2/g | 0.003246 cm3/g | 63.8903 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhou, R.; Yang, H.; Liang, Z.; Yao, Y.; Yu, Z.; Du, M.; Lou, D.; Li, K. Properties of Carbonic Anhydrase-Containing Active Coatings for CO2 Capture. Processes 2024, 12, 810. https://doi.org/10.3390/pr12040810
Li X, Zhou R, Yang H, Liang Z, Yao Y, Yu Z, Du M, Lou D, Li K. Properties of Carbonic Anhydrase-Containing Active Coatings for CO2 Capture. Processes. 2024; 12(4):810. https://doi.org/10.3390/pr12040810
Chicago/Turabian StyleLi, Xiaobo, Rui Zhou, Haoran Yang, Zimu Liang, Yuxiang Yao, Zhipeng Yu, Mingsai Du, Diming Lou, and Ke Li. 2024. "Properties of Carbonic Anhydrase-Containing Active Coatings for CO2 Capture" Processes 12, no. 4: 810. https://doi.org/10.3390/pr12040810
APA StyleLi, X., Zhou, R., Yang, H., Liang, Z., Yao, Y., Yu, Z., Du, M., Lou, D., & Li, K. (2024). Properties of Carbonic Anhydrase-Containing Active Coatings for CO2 Capture. Processes, 12(4), 810. https://doi.org/10.3390/pr12040810






