Abstract
Polyethylene terephthalate (PET) microplastics constitute a significant portion of plastic pollution in the environment and pose substantial environmental challenges. In this study, the effectiveness of the Fenton process and post-oxidation coagulation for the removal of non-weathered and UV-weathered PET microplastics (PET MPs) were investigated. A response surface methodology was used to investigate the interplay between PET concentration and ferrous ion (Fe2+) concentration. The models revealed an intricate interplay between these variables, highlighting the need for a balanced system for optimal PET MP removal. For non-weathered PET, the simultaneous increase in the concentrations of both PET microplastics and Fe2+ was found to enhance the removal efficiency. However, this synergistic effect was not observed in UV-weathered PET, which also demonstrated a more pronounced effect from the Fe2+ concentration. The statistical analysis provided a strong basis for the validity of the models. X-ray photoemission spectroscopy (XPS) further elucidated the mechanisms behind these findings, revealing that UV weathering results in surface changes, which facilitate hydroxyl radical oxidation. These findings underline the complexity of the Fenton process in PET microplastic removal and the different behavior of non-weathered and UV-weathered microplastics. This has significant implications for tailoring remediation strategies and underscores the importance of considering environmental weathering in these strategies.
1. Introduction
Microplastics (MPs), tiny plastic fragments less than 5 mm in size, are ubiquitous pollutants that pose a significant threat to aquatic environments globally [1]. MPs can be divided into primary and secondary MPs, i.e., those that are primarily produced at this size and those that are a result of the fragmentation of larger plastic materials. Over time, due to wear and tear, plastic materials break down into smaller particles that can enter the environment. One of the most common plastic materials used worldwide is polyethylene terephthalate (PET), which is a clear, strong, and lightweight plastic widely used in beverage bottles and food packaging. It is also used in synthetic fibers for clothing (i.e., polyester), thermoformed packaging, and in combination with glass fiber for engineered resins. These applications, while beneficial for various industries, contribute substantially to plastic waste and, subsequently, to the increasing presence of PET microplastics in aquatic environments. According to Plastics Europe, Worldwide production of PET was 24.2 million metric tons in 2021 and constituted 6.2% of produced plastics [2]. PET production has been increasing over time, driven by rising global demand. Due to its widespread use and often improper disposal, PET is ubiquitous in the environment and represents a persistent pollutant [3]. PET can end up in the food chain due to ingestion from a range of organisms in the environment, ranging from small zooplankton to larger aquatic and terrestrial animals. Ingestion of MPs, in general, can cause harm to organisms, such as physical harm due to blockage in the digestive tract, accumulation in the gills of fish, and increased cellular oxidative stress [4,5,6,7,8]. In addition, MPs can transport pollutants within the ecosystem. Worryingly, microplastics, and PET in particular, can end up in water and food for human consumption. In a study conducted by Adediran et al. [9], PET MPs have been detected in 45% of drinking water samples and are one of the most prevalent MPs in wastewater effluents. PET MPs have been detected in skim milk powders [10], canned seafood [11], table salt [12], and others [13]. Previous studies have shown that microplastics can absorb harmful pollutants, commonly known as persistent organic pollutants (POPs), and can vector them through the environment. In addition, MPs can also act as vectors for invasive species and pathogens [14,15,16]. The surface properties of microplastics often change when exposed to environmental factors like UV radiation. Such weathering or aging can alter their behavior in the aquatic environment [7,8]. UV weathering of microplastics, a primary environmental factor affecting the properties and behavior of these materials, causes photo-oxidative degradation, which modifies the physico-chemical characteristics of the plastic particles by introducing oxygen-containing moieties (hydroxyl or carboxyl groups). This, in turn, enhances the potential of MPs to adsorb POPs and can affect the release rates [17]. Additionally, these alterations may change the way marine organisms interact with microplastics, influencing their ingestion rates [15].
Since PET has a higher density than freshwater and seawater, unlike many other MPs, it should theoretically be less buoyant and, hence, less mobile in the aquatic environment. Presumably, this is one of the reasons why PET microplastics are somewhat less studied than, e.g., polyethylene (PE). A search on Scopus on 25 March 2024 with the keywords “polyethylene AND microplastic*” returned 5856 results, whereas “*terephthalate AND microplastic*” returned 2393 results. On the other hand, PET is likely more prone to accumulate in the sediment and thus affect benthic organisms to a greater extent.
The investigation of wastewater treatment technologies to prevent the release of MPs, including PET, is of great importance. Wastewater treatment plants (WWTPs) are considered a major source of microplastic pollution in the environment. Microplastics, including PET, enter the wastewater influent through various means, such as during mechanical abrasion of fibers during washing of synthetic clothes, the breakdown of larger plastic items, and from personal care products that contain microbeads. While many WWTPs are designed to remove larger debris and particles, they may not effectively remove smaller microplastic particles [18].
Hence, the study of effective removal methods for PET from wastewater is of great importance. Currently, a small body of research on the applicability of AOPs for the degradation of microplastics exists [19,20]. A potential solution is the Fenton process, a well-established advanced oxidation process (AOP) used for the degradation of organic pollutants in water and wastewater. It involves the generation of hydroxyl radicals (•OH) through the reaction of hydrogen peroxide (H2O2) with ferrous iron (Fe2+) as a catalyst at favorable acidic pH. These hydroxyl radicals are extremely potent and unselective oxidants that can degrade a wide variety of organic compounds [21,22]. Oxidative damage to the polymer chains can lead to the breakdown of the microplastic particles into smaller fragments or even potentially complete mineralization into CO2 and H2O for certain types of plastics [19]. Fenton is a highly attractive AOP for this purpose, as, upon expending H2O2, residual ferric (Fe3+) species need to be removed. Commonly, this is performed by neutralization of the reaction solution, whereby conditions conducive to coagulation occur. In a typical Fenton process for the degradation of other, less persistent pollutants—the ferric sludge is considered to be a problematic aspect. However, the growing particles of hydrated iron oxides can act as a coagulation agent that can entrap and remove particulate matter, i.e., microplastics, from water.
In this study, the effectiveness of the Fenton process and post-oxidation coagulation with the residual ferric ions on the removal of PET MPs from water was studied.
2. Materials and Methods
2.1. Chemicals
Iron(II) sulfate heptahydrate (FeSO4 × 7H2O, >99.0%, Sigma-Aldrich, Saint Louis, MO, USA) and hydrogen peroxide (H2O2, w = 30%, T.T.T., Sveta Nedjelja, Croatia) were used as Fenton reagents. Polyethylene terephthalate granules were obtained from Sigma Aldrich (Saint Louis, MO, USA); 0.1 M sulfuric acid (H2SO4, p.a., Lach-Ner, Neratovice, Czech Republic) and 0.1 M sodium hydroxide (NaOH, p.a., Lach-Ner, Neratovice, Czech Republic) were used for pH adjustments. Orthophosphoric acid (H3PO4, w = 85%, Honeywell Fluka, Charlotte, NC, USA) was used for solid sample total organic content (TOC) analysis. The aqueous solutions were prepared with ultra-pure water (>18.0 MΩ × cm) obtained using a Direct-Q3 UV (Merck Millipore, Darmstadt, Germany) ultra-pure water system.
2.2. Preparation of PET MP Samples
The PET granules were first formed into 0.12 mm thick sheets via hot press model 44–226 (Dake Corporation, Grand Haven, MI, USA). One sheet was exposed to UV irradiation in a Suntest CPS weathering chamber (Heraeus, Hanau, Germany) equipped with a xenon arc lamp for 28 days continuously. The weathered material is referred to as PET28, whereas the pristine materials are denoted as PET0. The obtained sheets were then cut up into smaller pieces and ground into microplastics by cryogenic ball milling. For this purpose, a CryoMill (Retsch, Haan, Germany) was programmed to grind the samples using the following conditions: (i) precooling (f = 5 s−1; t = 1 min), (ii) grinding (f = 25 s−1; t = 1 min), followed by intercooling between every grinding cycle (f = 5 s−1; t = 30 s) [23]. Liquid nitrogen (Uljanik tehnički plinovi, Pula, Croatia) was used during the milling process. After milling, the obtained samples were sieved into 100–500 µm fractions using an AS 200 mechanical sieve shaker (Retsch, Haan, Germany).
2.3. MP Removal by Fenton Process
The effectiveness of the Fenton process for the removal of PET MPs from water was investigated using the statistical experimental design approach. The mass concentration of the PET MPs, denoted as coded variable X1, and the concentration of Fe2+, denoted as X2, were varied. pH and the concentration of H2O2 were fixed at pH = 4 and [H2O2] = 80 mM, respectively. A three-level-factorial experimental plan (32) was chosen, where the individual levels of the variable X1 were 125 ppm, 250 ppm, and 375 ppm, whereas the values for X2 were 10 mM, 25 mM, and 40 mM, as shown in Table 1. Design-Expert 10.1 (StatEase, Minneapolis, MN, USA) and Statistica 13.5 (Tibco, München, Germany) software were used to analyze the obtained data and perform response surface modeling and analysis of variance (ANOVA). The experiments were conducted by first weighing the required amount of PET MPs, as per experimental run, and transferring the MPs quantitatively to tall 150 mL beakers, to which a 20 mm × 6 × mm stir bar was previously added. The ratio of the length of the stir bar and the diameter of the beakers was approximately 1:2.7. Then, 100 mL of a freshly made solution containing ferrous ions at the designated concentration according to the experimental plan was added, and the pH was adjusted to pH 4. To this solution, the required amount of H2O2 was added, which was considered to be the beginning of the experiment. For the first 30 min, the suspension was stirred at 450 rpm, after which the pH was increased to 8.5 in order to quench the reaction and initiate precipitation of the hydrated iron oxide species. The reaction mixture was stirred then at 100 rpm for a further 30 min. Then, the stirring was stopped, and the particles were allowed to settle out for a duration of 24 h. All experiments were performed in triplicate in order to ensure higher reproductivity of results.

Table 1.
Experimental design used in the study, with the corresponding levels of the variables.
2.4. Quantification of the PET MP Removal
After the sedimentation process, the supernatant was decanted, and the wet ferric sludge was carefully filtered through a quartz fiber filter paper (Whatman QM-H, r = 37 mm). The obtained filter cake was dried for 24 h at 80 °C. The dried filter cake, containing the entrained PET MPs, was homogenized using an agate pestle and mortar. Then, 20.0 ± 0.5 mg of the dried sample was weighed out in a ceramic sample boat for solid-state TOC analysis. The ceramic sample boats were calcined beforehand at 900 °C for 30 min in an electric muffle furnace (LP-07, Instrumentaria, Zagreb, Croatia) to remove any residual organic or inorganic carbon. The samples were acidified with a few drops of 0.8 M H3PO4 in order to convert inorganic carbon in the form of carbonates to carbon dioxide. The acidified samples were then placed in a DZF-6020 vacuum oven (Lenphan, Zhengzhou, China) heated to 60 °C and evacuated to 0.8 atm for 30 min to outgas evolved CO2. This was performed so as not to include inorganic carbon content during the quantification of the total organic carbon content in the samples, which originates from the PET MPs. After the inorganic carbon was removed, the treated samples were analyzed using TOC-V CPN analyzer (Shimadzu, Kyoto, Japan) coupled with a solid sample combustion unit SSM-5000A (Shimadzu, Kyoto, Japan). The combustion furnace of the solid sample unit was heated to 900 °C. Pristine PET MPs were used as a standard to obtain the calibration of the instrument’s response. The measured total organic carbon of the samples was corrected for the measured carbon content in 0.8 M H3PO4, i.e., the blank sample. The removal of the PET microplastic was determined based on the hypothesized total removal of the PET MPs (TOCtheoretical), i.e., by measuring the total organic carbon content of the PET sample weigh-outs, Equation (1):
All measurements were performed in triplicates; thus, for each experimental point, we had 9 results (3 replicates × 3 measurements); averages with high reproducibility (97.4%) are reported.
2.5. X-ray Photoemission Spectroscopy Characterization
In order to assess the effects of the Fenton treatment on the functional groups on the surface of microplastics, X-ray photoemission spectroscopy (XPS) analysis of the samples was performed. The samples were placed onto adhesive graphite tapes and were analyzed without a pre-treatment to coat the samples with a conductive layer by vapor phase deposition. A PHI VersaProbe III (Version AD) (PHI, Chanhassen, MN, USA) equipped with a hemispherical analyzer and an Al Kα monochromatic X-ray source were used. Survey spectra were recorded using 224 eV pass energy at a step of 0.8 eV, whereas Fe 2p core level spectra were analyzed at a pass energy of 27 eV at a step of 0.1 eV. Data acquisition was performed with ESCApe 1.4 software. The fitting of the C 1s and Fe 2p level spectra was performed using CasaXPS 2.3.15. software.
3. Results and Discussion
3.1. Removal of PET MPs by the Fenton Process
The aim of this study was to model the removal efficiency of pristine and UV-weathered polyethylene terephthalate (PET) microplastics using the Fenton process and post-Fenton coagulation facilitated by the precipitation of ferric species. The model parameters under consideration were the concentrations of the pristine (PET0) and weathered (PET28) PET microplastics (X1) and ferrous ions (Fe2+) (X2) as the catalyst for the Fenton reaction, while the concentration of H2O2 was kept constant. Response surface methodology (RSM) was used to generate a second-order polynomial model for the removal efficiency (Y1) by applying the multiple regression analysis on the full factorial matrix and corresponding experimental results, i.e., removal extents, presented in Table S1 (Supplementary Information). In such a way, a complex relationship between the removal of PET microplastics and the designated variables was revealed and presented as 3D-surface plots (Figure 1). In order to successfully apply RSM for the intended action, the first postulate is to have accurate, significant, and predictive models. Usually, RSM models are evaluated on the basis of analysis of variance (ANOVA), an essential test providing a list of statistical parameters such as: Fisher F-test value (F), its probability value (p), regression coefficients (pure; R2, adjusted; Radj2, predicted; Rpre2), t-test value, etc. Most commonly, initial screening of model significance and accuracy may be provided through pertaining p, R2, and Radj2 values [24,25,26]. The corresponding response surface model equation (Y1) for the removal of pristine PET MPs is shown in Equation (2):
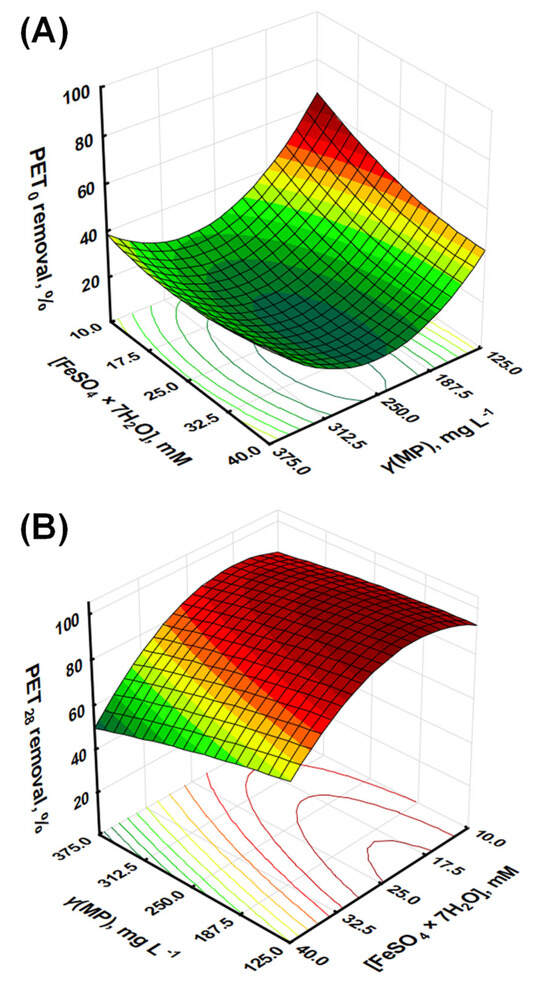
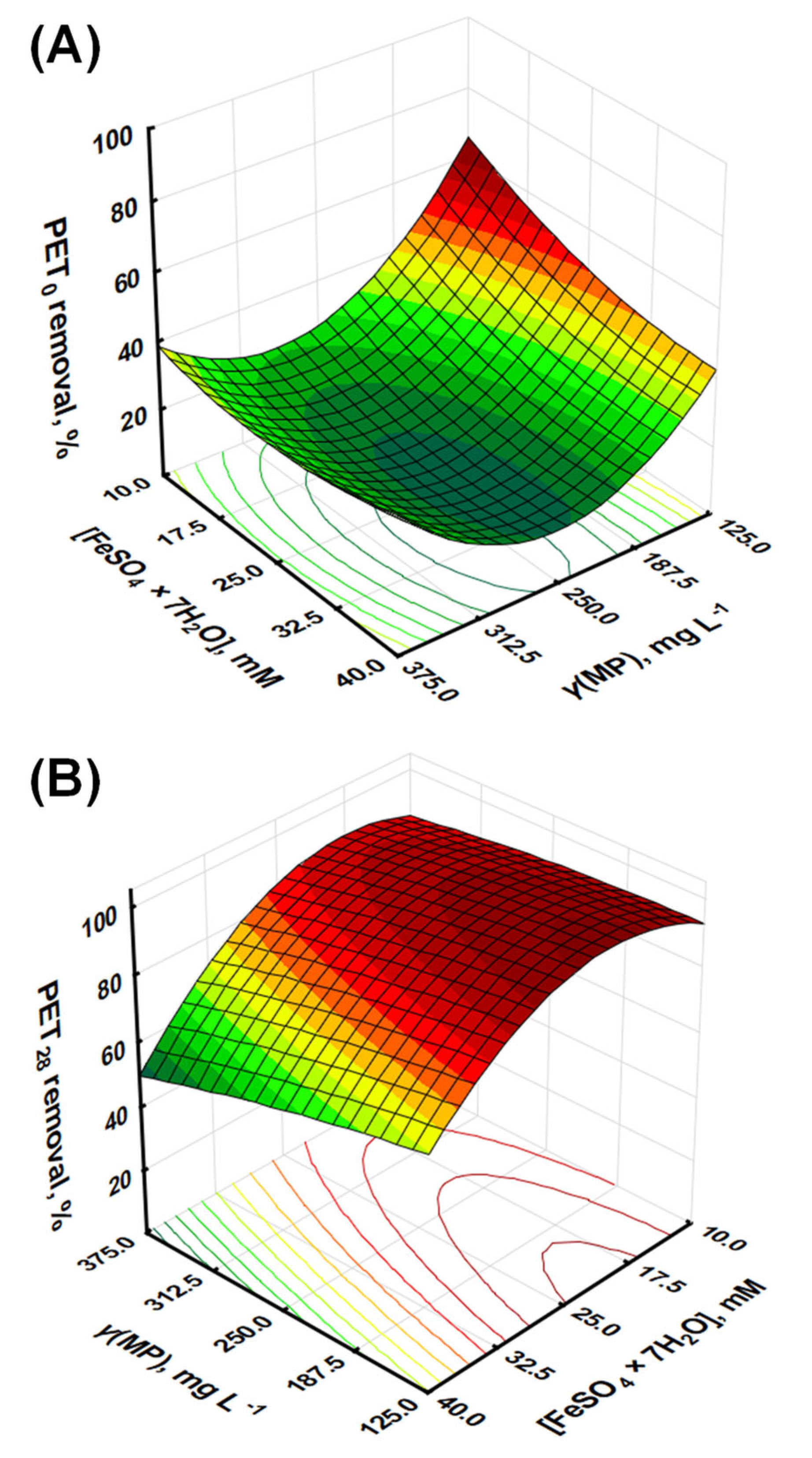
Figure 1.
Response surface models for the removal effectiveness of (A) PET0 and (B) PET28 by response surface modeling.
Statistically, the model showed excellent goodness-of-fit, with an R2 of 0.9895, an adjusted R2 of 0.9721, and a predicted R2 of 0.8791. These values indicate that the model explains a significant portion of the variability in the data and can predict new observations with reasonable accuracy. The F-value of the model was 56.80, with a p-value of 0.0036, suggesting a statistically significant relationship between the variables and the response (Table 2).

Table 2.
Analysis of variance (ANOVA) of RSM models R1 and R2 predicting removal effectiveness of PET0 and PET28, respectively, by Fe2+/H2O2 process.
The further judgment of RSM models accuracy, i.e., adequacy of model fitting to empirical values and corresponding lack of fit, includes the so-called “residual diagnostic” (RD), which is based on normal probability test, Levene’s test, and constant variance test. The RD for the Y1 model revealed that: (i) there were no violations in the assumptions that the errors were normally distributed and independent of each other, (ii) the error variances were homogeneous, and (iii) residuals were independent, while the demonstration of performed graphical analysis of RD is given in Figure S1 (Supplementary Material). Taking into account the above-given information on Y1, it can be concluded that the derived model can be used hereinafter as a tool to enlighten the influence of studied parameters on Fenton treatment effectiveness and provide more information on occurring mechanisms and process chemistry. The evaluation of the influence of individual model terms representing process parameters was performed. As seen from Table 2, the individual linear model terms have p-values below 0.05, i.e., X1 (p = 0.0068) and X2 (p = 0.0162), indicating that process variables represented by both terms are significantly related to the removal of PET0 MPs. Quadratic model term X12, representing PET0 mass concentration, was also found to be highly significant, with a p-value of 0.0009, as was the model term representing the interaction between PET0 mass concentration and iron catalyst concentration (p = 0.0180) (Table 2). The latter clearly indicates the necessity for applying the RSM approach to investigate the influence of the studied parameters on the effectiveness of MP removal by the Fenton process instead of the commonly applied “one-parameter-at-the-time” approach. Such an approach does not consider mutual interactions between studied parameters and may provide misleading information [27]. As can be seen from Equation (1), the negative coefficients associated with the linear terms, i.e., X1 and X2, suggest that an increase in either the concentration of PET MPs or Fe2+ individually decreases the removal efficiency. The negative impact of the increase of PET MP concentrations is unsurprising, as the removal capacity of the combined Fenton and coagulation process diminishes as the concentration of MPs increases. The negative impact of X2 is somewhat surprising, as one would expect that higher concentrations of Fe2+ would produce more •OH. However, an excess amount of Fe2+ may lead to the production of less reactive species, such as hydroperoxyl radicals (HO2•) or peroxide ions (O22⁻) through the Fenton–Weiss or the Haber–Weiss reactions. In addition, recombination reactions between the •OH can become more significant [28,29]. Interestingly, the interaction term between X1 and X2 (X1 × X2) had a positive coefficient, implying that a simultaneous increase in the concentrations of both PET microplastics and Fe2+ enhances the removal efficiency. This synergy could potentially stem from the need for a balanced ratio between PET concentration and Fe2+ for optimal removal effectiveness during oxidation and the post-oxidation coagulation step, as a higher concentration of Fe2+ would lead to better removal effectiveness. Likewise, at higher concentrations, it is more likely that growing hydrated iron oxide species will entrap PET MPs. The quadratic terms of X1 and X2, i.e., X12 and X22 (although the latter is not highly significant for the derived Y1 model), further highlight the complexity of the system. Both terms showed positive coefficients, suggesting a parabolic relationship between the variables and the response. This indicates the existence of an optimum concentration for both parameters, where any deviation would result in a decrease in removal efficiency. These insights underline the intricate interdependencies of the variables in the Fenton process for microplastic removal, emphasizing the need for a well-balanced reaction system. An increase in Fe2+ concentration or PET concentration individually leads to reduced removal, while a joint increase improves it. Furthermore, the quadratic terms suggest an optimal concentration for each parameter, stressing the importance of fine-tuning the reaction conditions to achieve maximum microplastic removal.
Taking into account the analogy used in Y1 construction, the response equation (Y2) fitted from the data in Table S2 (Supplementary Information) for the removal of PET28 is shown in Equation (3):
The ANOVA results for the R2 model indicate a good fit for the model (R2 = 0.9868, adjusted R2 = 0.9647, predicted R2 = 0.8542), with the F-value (44.71) and p-value (0.0051) providing evidence of a statistically significant relationship between the variables and the response (Table 2). The RD for the Y2 model is presented graphically in Figure S1 (Supplementary Materials). Based on the results, the conclusions are similar to that above for R1; thus, Y2 can be used as a tool to enlighten the influence of the studied parameters on Fenton treatment effectiveness for treating PET28 samples. In the following step, the significance of the individual model terms is characterized. The p-values for X1 (0.0114) and X2 (0.0016) are below 0.05, indicating significant main effects, while the p-value for X1 × X2 (0.0932) suggests a possible interaction effect at the 10% level. The quadratic terms show a significant effect for X22 (p = 0.0042), while X12 (p = 0.5362) does not seem to be significant (Table 2). From the given second-order polynomial model, i.e., Y2, simulating the removal of UV-weathered PET microplastics over 28 consecutive days, we saw a somewhat different pattern of variable effects compared to the non-weathered PET microplastics (Y1). Similar to the model for Y1, the negative coefficients for the linear terms of X1 and X2 (−7.21 for X1 and −14.33 for X2) suggest that an increase in either the concentration of PET microplastics or Fe2+ individually results in a decrease in removal efficiency. This effect appears to be more pronounced for UV-weathered PET, especially with regards to the Fe2+ concentration (X2), as indicated by the larger negative coefficient (−14.33 for Y2 compared to −5.34 for Y1). The interaction term (X1 × X2) was also negative (−3.85), implying that a simultaneous increase in the concentrations of PET microplastics and Fe2+ leads to a further decrease in removal efficiency, in contrast to the non-weathered case, where this interaction was positive. This suggests that the synergistic effect observed for the non-weathered PET microplastics does not occur for the UV-weathered PET microplastics or might even be reversed. The quadratic terms (X12 and X22) were also negative (−1.56 for X12 and −17.79 for X22), which suggests a downward-opening parabola and indicates that removal efficiency decreases at both low and high concentrations of the variables, with a maximum at some intermediate concentration. This is similar to the pattern observed for non-weathered PET microplastics, although the effect appears to be more pronounced for UV-weathered PET, especially for X2 ([Fe2+]).
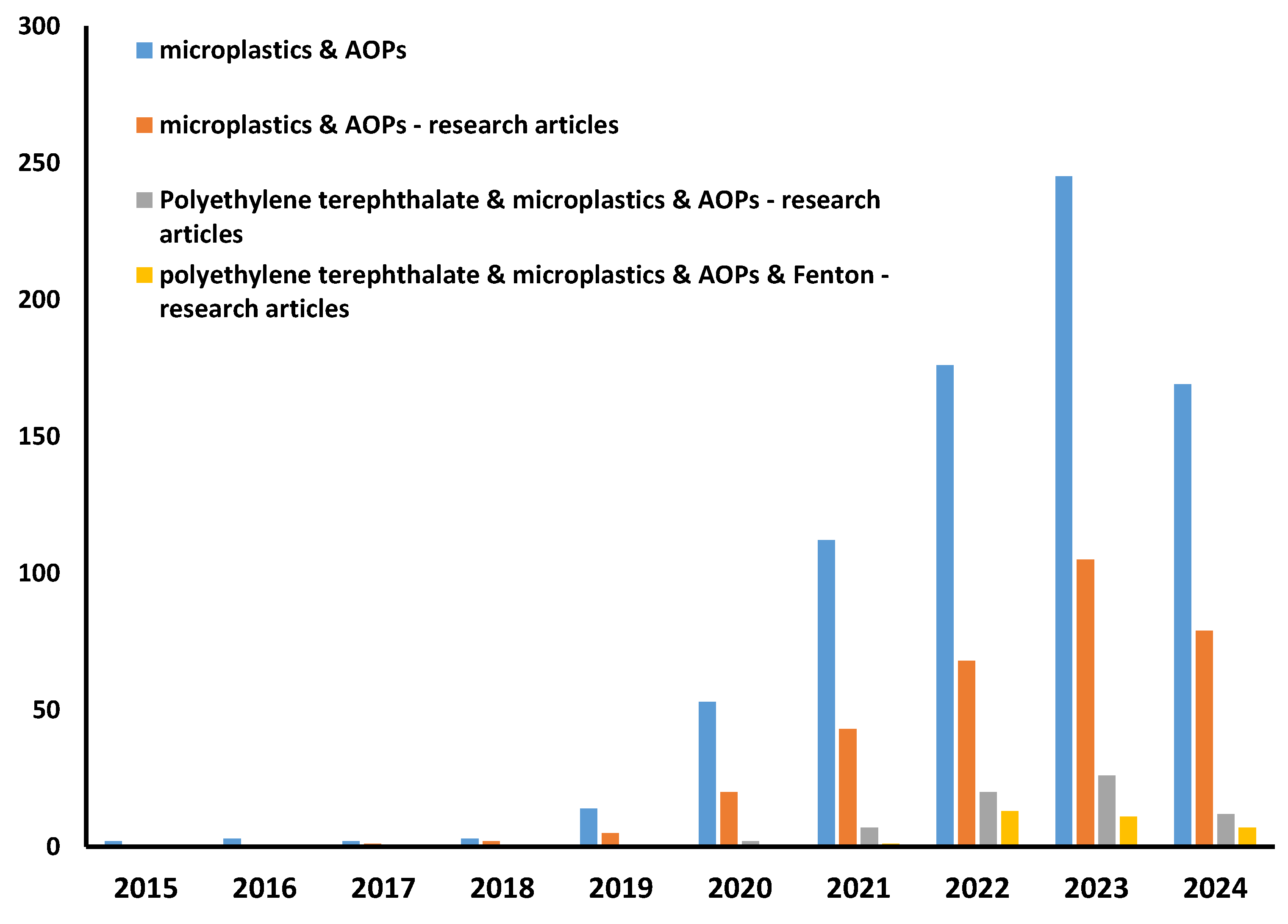
Table 3 provides a comparative literature review of MP removal by various advanced oxidation processes. It should be noted that research on MPs, including their removal by various methods, is a “hot topic”; thus, many studies are available (Figure 2). However, there are no standards for the systematic approach; thus, different MPs, in both type and size, are studied, while conditions varied greatly, even between the same/similar processes, as well as experimental set-up configurations and targeted environmental standards for monitoring MP removal. Additionally, many studies are not research articles but reviews tackling MP occurrence, fate, behavior, and remediation methods. In addition, studies on PET removal by advanced oxidation processes are rather scarce (Figure 2).

Table 3.
A comparison of microplastic (MP) removal by various advanced oxidation treatments.
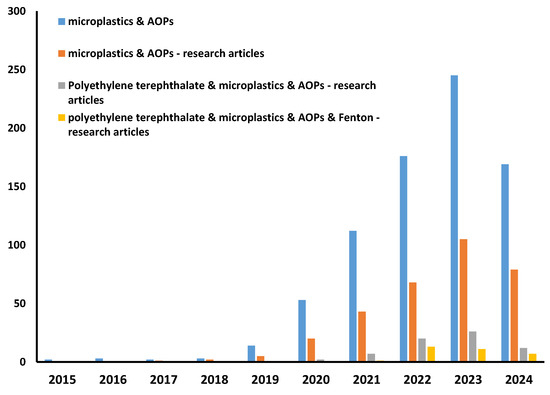
Figure 2.
Results of literature search using terms “microplastics”, “AOPs”, “polyethylene terephthalate”, and “Fenton” (Science Direct base, 16 April 2024).
Accordingly, we have compared available data obtained from studies where Fenton processes (at different conditions) were applied for the treatment of different MP types, including PET, targeting mineralization degrees as the evaluation parameter for process effectiveness due to the easier comparison (Table 3). As can be seen, the Fenton process was shown to be rather powerful in mineralizing MP pollution; between 70 and 99% were obtained depending on the conditions of the Fenton process and type and presence of additional treatment empowerment (e.g., irradiation, heat). Similarly, high removal rates were recorded and thereafter modeled via the RSM approach in our study as well, with 68 and 98% PET removals for pristine and aged samples, respectively (Figure 1). Another process with promising PET removal was the hybrid photocatalysis process, empowered by the addition of strong oxidants, where over 90% of mass losses were recorded for PET MPs (Table 3). However, practical and economic constraints may favor Fenton treatment over photocatalysis; irradiation is energy-consumptive, while the removal of fine powder from nano-scale photocatalysts may also increase the cost of treatment.
3.2. X-ray Photoemission Spectroscopy Analysis of the PET MPs
To better understand the changes induced in PET MPs by UV weathering and the Fenton process, we performed X-ray photoemission spectroscopy analyses. First, pristine PET0 and UV-weathered PET28 samples were analyzed. As can be seen in Table 4, deconvoluted C 1s and, consequently, integrated XPS spectra (spectra provided in Figure S2, Supplementary Information) reveal changes in the composition of the UV-weathered PET28 samples. A decrease in the relative areas, i.e., the amount of the C-O-C/C-OH bonds and O-C=O bonds, implies that UV-weathering has effectively cleaved ester bonds within the polymer and has additionally led to a loss of the terminal –OH and –COOH groups from the ends of the PET macromolecular chains [28]. Conversely, the increasing areas of the peaks corresponding to C=C and conjugated π-π systems after UV-weathering reveals the formation of unsaturated carbon–carbon bonds and enhanced π-π interactions. Decarboxylation and subsequent chain scission result in the formation of smaller oligomers, which have fewer degrees of freedom. In turn, these smaller fragments have fewer ways to rotate around their bonds, which promotes π-π stacking interactions [29]. Moreover, the chain scission process creates oligomer radicals that can cross-link, thus explaining the increase in the amount of C-C bonds. The UV-weathered PET sample is, thus, more likely to undergo reactions with •OH radicals due to a greater abundance of unsaturated C=C bonds and saturated C-C, C-H bonds, whereas less reactive C-OH and O-C=O, which may, in particular, inhibit the Fenton reaction, are less abundant.

Table 4.
Areas corresponding to the deconvoluted C 1s core level peaks in X-ray photoemission spectroscopy (XPS) analysis for untreated samples PET0 and PET28.
XPS analysis of Fenton-treated samples, namely those corresponding to the maximum X1 and X2-coded variable values, i.e., initial PET MP concentration and Fe2+ concentration, were analyzed by XPS; the results are shown in Table 5, and the spectra are provided in Supplementary Information. These two experiments have a marked difference in the achieved removal extent, as evident in Figure 1.

Table 5.
Areas corresponding to the deconvoluted C 1s core level peaks in X-ray photoemission spectroscopy (XPS) analysis for Fenton-treated samples PET0 and PET28.
A stark contrast in the composition of the post-Fenton treatment was evident. Immediately, we observed that UV-weathered PET was further oxidized to a much greater extent in the Fenton process, which was clearly demonstrated by greater contributions of the C-O-C and C-OH bonds in the PET28 sample and, in turn, a much lower C=C content. However, pristine PET was also oxidized to a greater extent, i.e., the contribution of the O-C=O bonds was much greater for the Fenton-treated PET0 sample (Table 5). The growing contribution of the O-C=O moiety for the Fenton-treated PET0 sample implies effective fragmentation of the PET MPs. Additionally, •OH facilitates the formation of cross-linked moieties, as the contributions of the C-C and C-H groups more than doubled for PET0 alone. However, the greater carboxyl moiety content of the PET0 samples post-Fenton treatment gives an insight into why the removal effectiveness is lower in relation to PET28. The greater abundance of oligomers with carboxyl groups more than likely leads to the formation of stable and inactive Fe3+ chelate complexes, thus greatly reducing the effectiveness of the Fenton process [38,39]. XPS analysis and deconvolution of the Fe 2p3/2 core-level spectra support this notion.
4. Conclusions
The response surface models indicate a complex relationship between the removal effectiveness of PET microplastics and the concentrations of PET MPs and Fe2+. The effects of these variables appear to be markedly different for non-weathered and UV-weathered PET. For non-weathered PET, there seems to be a synergistic effect between PET and Fe2+ concentration, which is not observed for UV-weathered PET. Also, the effect of Fe2+ concentration appears to be more pronounced for UV-weathered PET. These findings suggest that weathering may change the physicochemical properties of PET microplastics, altering their reactivity in the Fenton process. Indeed, XPS analysis has revealed that UV-weathering predisposes PET MPs to Fenton degradation; specifically, the greater abundance of C=C and C-C bonds facilitates more effective degradation by OH radicals. In addition, the abundance of O-C=O decreases, which in turn does not lead to the formation of inactive Fe3+ chelate complexes. Therefore, it is crucial to take into account the potential effects of environmental weathering when developing and optimizing microplastic removal strategies. Further experimental research is needed to fully understand these effects; however, these results indicate that the Fenton treatment is a viable means for the removal of PET MPs. In addition, these findings, especially that UV weathering has enhanced the effectiveness of the Fenton process, have far-reaching implications for the treatment processes concerned with the removal of polyester-based microplastics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr12040844/s1, Table S1. Full-factorial experimental plan for the removal of pristine PET MPs (PET0) with the Fenton process; Table S2. Full-factorial experimental plan for the removal of PET MPs obtained from UV-weathered PET for 28 days (PET28) with the Fenton process; Figure S1. Deconvoluted XPS C 1s spectra for (A) PET0 MPs, and (B) PET28 MPs. Figure S2. Deconvoluted XPS C 1s spectra for samples (A) PET0 MPs, and (B) PET28 MPs corresponding to experiment #8 in Tables S1 and S2, respectively, after the Fenton treatment. Figure S3. Deconvoluted XPS Fe 2p3/2 core-level spectra for samples (A) PET0 MPs, and (B) PET28 MPs corresponding to experiment #8 in Tables S1 and S2, respectively, after the Fenton treatment.
Author Contributions
Conceptualization, A.L.B. and M.K.; methodology, M.K. and Z.K.; software, M.K.; validation, M.K., H.K., B.G. and A.L.B.; formal analysis, M.K. and Z.K.; investigation, A.T., S.T., J.P.Z., and A.P.; resources, B.G. and A.L.B.; data curation, M.K. and A.T.; writing—original draft preparation, M.K.; writing—review and editing, A.L.B.; visualization, M.K.; supervision, A.L.B.; project administration, A.L.B.; funding acquisition, A.L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Croatian Science Foundation, grant number IP-2020-02-6033.
Data Availability Statement
The data will be available upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Plastics—The Facts. 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 12 March 2024).
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro(nano)plastics: A threat to human health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Bhuyan, M.S. Effects of Microplastics on Fish and in Human Health. Front. Environ. Sci. 2022, 10, 827289. [Google Scholar] [CrossRef]
- Yin, X.; Wu, J.; Liu, Y.; Chen, X.; Xie, C.; Liang, Y.; Li, J.; Jiang, Z. Accumulation of microplastics in fish guts and gills from a large natural lake: Selective or non-selective? Environ. Pollut. 2022, 309, 119785. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chang, X.; Hu, M.; Fang, J.K.-H.; Sokolova, I.M.; Huang, W.; Xu, E.G.; Wang, Y. Is microplastic an oxidative stressor? Evidence from a meta-analysis on bivalves. J. Hazard. Mater. 2022, 423, 127211. [Google Scholar] [CrossRef]
- Adediran, G.A.; Cox, R.; Jürgens, M.D.; Morel, E.; Cross, R.; Carter, H.; Pereira, M.G.; Read, D.S.; Johnson, A.C. Fate and behaviour of Microplastics (>25 µm) within the water distribution network, from water treatment works to service reservoirs and customer taps. Water Res. 2024, 255, 121508. [Google Scholar] [CrossRef] [PubMed]
- Visentin, E.; Manuelian, C.L.; Niero, G.; Benetti, F.; Perini, A.; Zanella, M.; Pozza, M.; De Marchi, M. Characterization of microplastics in skim-milk powders. J. Dairy Sci. 2024. [Google Scholar] [CrossRef]
- Silva, D.M.; Almeida, C.M.R.; Guardiola, F.A.; Pereira, R.; Rodrigues, S.M.; Ramos, S. Uncovering microplastics contamination in canned seafood. Food Chem. 2024, 448, 139049. [Google Scholar] [CrossRef]
- Syamsu, D.A.; Deswati, D.; Syafrizayanti, S.; Putra, A.; Suteja, Y. Presence of microplastics contamination in table salt and estimated exposure in humans. Glob. J. Environ. Sci. Manag. 2024, 10, 205–224. [Google Scholar]
- Espiritu, E.Q.; Pauco, J.L.R.; Bareo, R.S.; Palaypayon, G.B.; Capistrano, H.A.M.; Jabar, S.R.; Coronel, A.S.O.; Rodolfo, R.S.; Enriquez, E.P. Microplastics contamination in selected staple consumer food products. J. Food Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “Plastisphere”: Microbial Communities on Plastic Marine Debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.K.; Law, J.C.F.; Zhang, T.; Leung, K.S.Y. Effects of Weathering on the Sorption Behavior and Toxicity of Polystyrene Microplastics in Multi-solute Systems. Water Res. 2020, 187, 116419. [Google Scholar] [CrossRef]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, N.d.O.; Busquets, R.; Campos, L.C. Insights into the removal of microplastics and microfibres by Advanced Oxidation Processes. Sci. Total Environ. 2023, 861, 160665. [Google Scholar] [CrossRef] [PubMed]
- Bule Možar, K.; Miloloža, M.; Martinjak, V.; Cvetnić, M.; Kušić, H.; Bolanča, T.; Kučić Grgić, D.; Ukić, Š. Potential of Advanced Oxidation as Pretreatment for Microplastics Biodegradation. Separations 2023, 10, 132. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Kusic, H.; Koprivanac, N.; Srsan, L. Azo dye degradation using Fenton type processes assisted by UV irradiation: A kinetic study. J. Photochem. Photobiol. A Chem. 2006, 181, 195–202. [Google Scholar] [CrossRef]
- Papac Zjačić, J.; Tonković, S.; Pulitika, A.; Katančić, Z.; Kovačić, M.; Kušić, H.; Hrnjak Murgić, Z.; Lončarić Božić, A. Effect of Aging on Physicochemical Properties and Size Distribution of PET Microplastic: Influence on Adsorption of Diclofenac and Toxicity Assessment. Toxics 2023, 11, 615. [Google Scholar] [CrossRef]
- Dopar, M.; Kusic, H.; Koprivanac, N. Treatment of simulated industrial wastewater by photo-Fenton process. Part I: The optimization of process parameters using design of experiments (DOE). Chem. Eng. J. 2011, 173, 267–279. [Google Scholar] [CrossRef]
- Kovacic, M.; Salaeh, S.; Kusic, H.; Suligoj, A.; Kete, M.; Fanetti, M.; Stangar, U.L.; Dionysiou, D.D.; Bozic, A.L. Solar-driven photocatalytic treatment of diclofenac using immobilized TiO2-based zeolite composites. Environ. Sci. Pollut. Res. 2016, 23, 17982–17994. [Google Scholar] [CrossRef]
- Tomic, A.; Cvetnic, M.; Kovacic, M.; Kusic, H.; Karamanis, P.; Bozic, A.L. Structural features promoting adsorption of contaminants of emerging concern onto TiO2 P25: Experimental and computational approaches. Environ. Sci. Pollut. Res. 2022, 29, 87628–87644. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Edge, M.; Wiles, R.; Allen, N.S.; McDonald, W.A.; Mortlock, S.V. Characterisation of the species responsible for yellowing in melt degraded aromatic polyesters—I: Yellowing of poly(ethylene terephthalate). Polym. Degrad. Stab. 1996, 53, 141–151. [Google Scholar] [CrossRef]
- Hermann, J.; Alfè, D.; Tkatchenko, A. Nanoscale π–π stacked molecules are bound by collective charge fluctuations. Nat. Commun. 2017, 8, 14052. [Google Scholar] [CrossRef]
- Hu, K.; Zhou, P.; Yang, Y.; Hall, T.; Nie, G.; Yao, Y.; Duan, X.; Wang, S. Degradation of Microplastics by a Thermal Fenton Reaction. ACS ES&T Eng. 2022, 2, 110–120. [Google Scholar]
- Feng, H.-M.; Zheng, J.-C.; Lei, N.-Y.; Yu, L.; Kong, K.H.-K.; Yu, H.-Q.; Lau, T.-C.; Lam, M.H.W. Photoassisted Fenton Degradation of Polystyrene. Environ. Sci. Technol. 2011, 45, 744–750. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Ding, J.; Song, Z.; Yang, B.; Zhang, C.; Guan, B. Degradation of nano-sized polystyrene plastics by ozonation or chlorination in drinking water disinfection processes. Chem. Eng. J. 2022, 427, 131690. [Google Scholar] [CrossRef]
- Ortiz, D.; Munoz, M.; Nieto-Sandoval, J.; Romera-Castillo, C.; de Pedro, Z.M.; Casas, J.A. Insights into the degradation of microplastics by Fenton oxidation: From surface modification to mineralization. Chemosphere 2022, 309, 136809. [Google Scholar] [CrossRef]
- Allé, P.H.; Garcia-Muñoz, P.; Adouby, K.; Keller, N.; Robert, D. Efficient photocatalytic mineralization of polymethylmethacrylate and polystyrene nanoplastics by TiO2/β-SiC alveolar foams. Environ. Chem. Lett. 2021, 19, 1803–1808. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, L.; Zhang, F.; Wu, J.; Wang, H.; Yang, J. Feasible Degradation of Polyethylene Terephthalate Fiber-Based Microplastics in Alkaline Media with Bi2O3@N-TiO2 Z-Scheme Photocatalytic System. Adv. Sustain. Syst. 2022, 6, 2100516. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, H.; Liu, J.; Liu, X.; Song, X.; Zhou, W.; Zhang, J.; Huo, P. Enhanced degradation of polyethylene terephthalate plastics by CdS/CeO2 heterojunction photocatalyst activated peroxymonosulfate. J. Hazard. Mater. 2023, 452, 131375. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, H.; Liu, J.; Li, J.; Zhou, W.; Zhang, J.; Liu, X.; Song, X.; Wang, H.; Huo, P. Removal of polyethylene terephthalate plastics waste via Co–CeO2 photocatalyst–activated peroxymonosulfate strategy. Chem. Eng. J. 2024, 479, 147781. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Inhibition of Fe2+- and Fe3+- induced hydroxyl radical production by the iron-chelating drug deferiprone. Free Radic. Biol. Med. 2015, 78, 118–122. [Google Scholar] [CrossRef]
- Milovac, N.; Juretic, D.; Kusic, H.; Dermadi, J.; Bozic, A.L. Photooxidative degradation of aromatic carboxylic acids in water: Influence of hydroxyl substituents. Ind. Eng. Chem. Res. 2014, 53, 10590–10598. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).