Application of Lactiplantibacillus plantarum LP95 as a Functional Starter Culture in Fermented Tofu Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
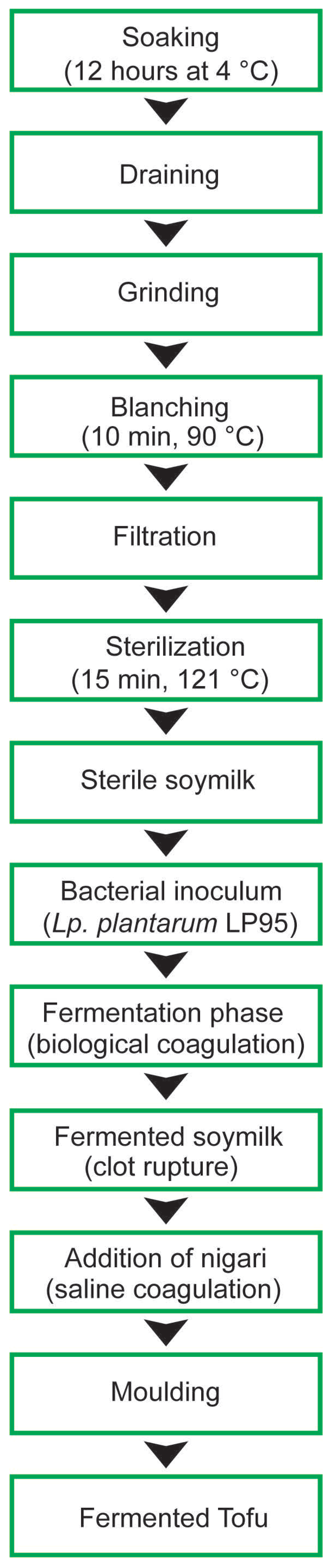
2.2. Production of Fermented Soymilk and Tofu
2.3. Physical Parameters
2.4. Chemical Analysis
2.4.1. Sugar Metabolism
2.4.2. Determination of Isoflavones
2.4.3. Amino Acids Profile
2.4.4. ABTS Antioxidant Activity
2.4.5. DPPH Antioxidant Activity
2.4.6. Total Phenolics
2.5. Statistical Analysis
3. Results
3.1. Fermentation Kinetics
3.2. Physical Parameters
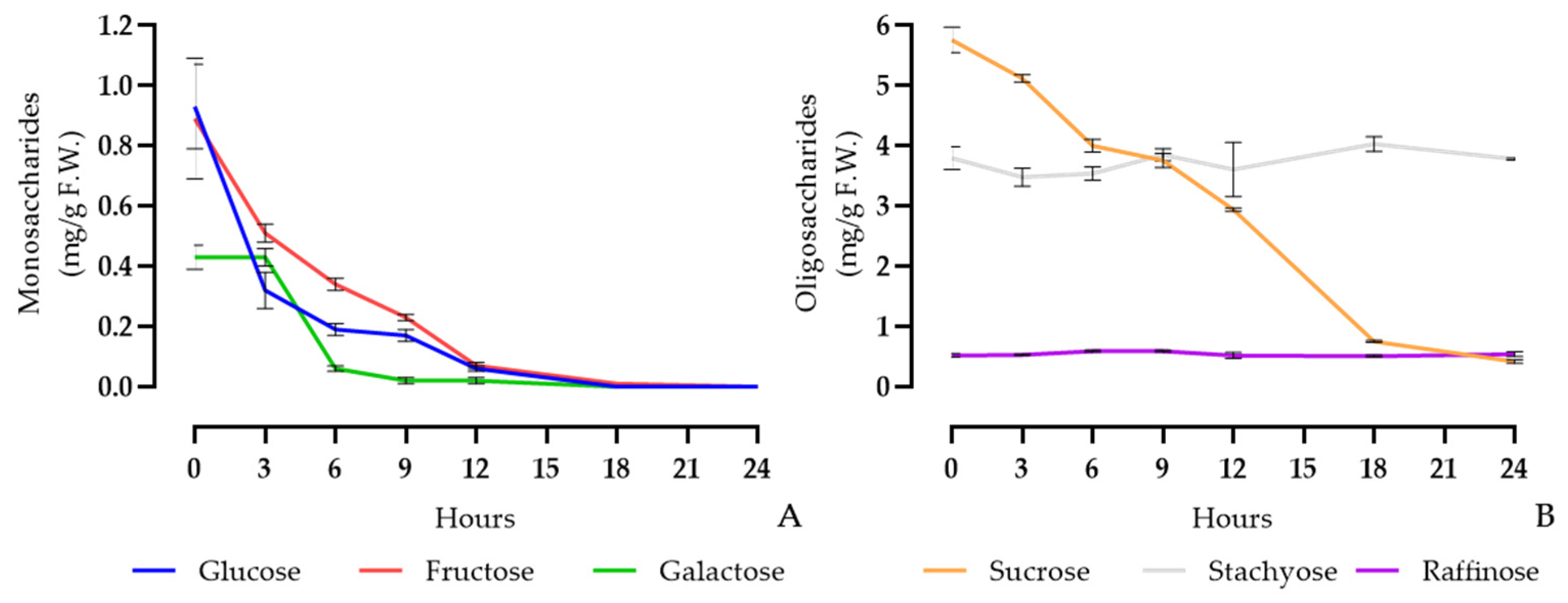
3.3. Quantitative Changes in Sugars during the Fermentation of Soymilk
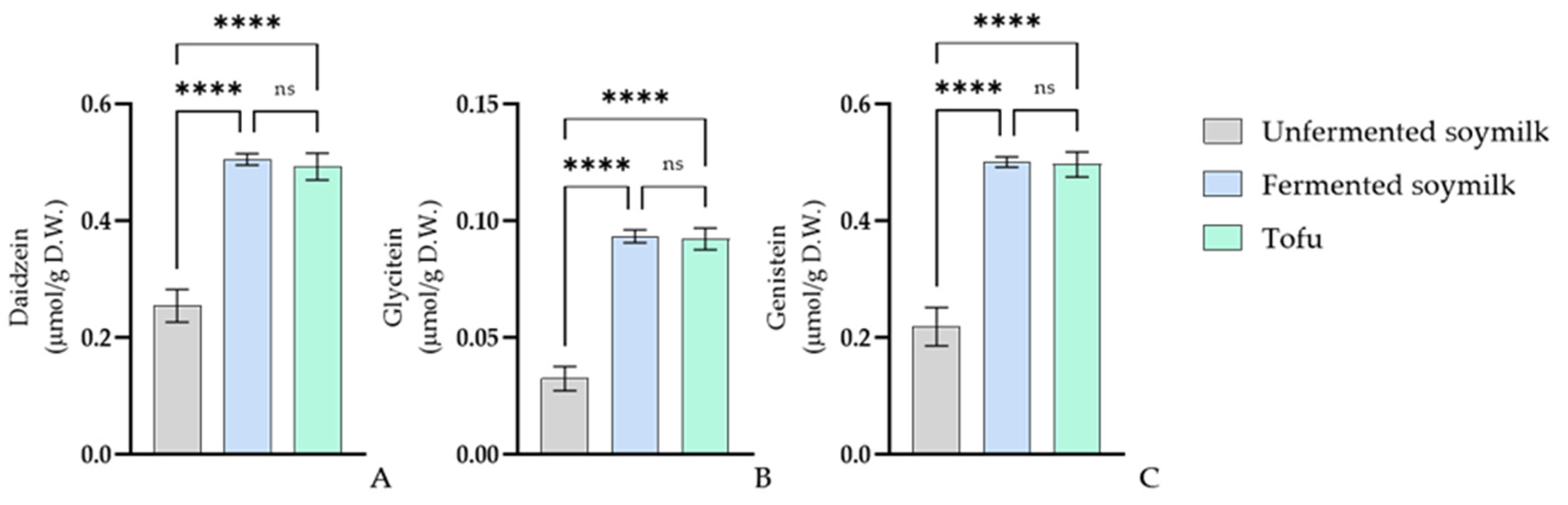
3.4. Bioconversion of Isoflavones
3.5. Free Amino Acid Profiles
3.6. Antioxidant Activity
3.7. Total Phenolic Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, L.; Regenstein, J.M.; Teng, F.; Li, Y. Tofu Products: A Review of Their Raw Materials, Processing Conditions, and Packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3683–3714. [Google Scholar] [CrossRef] [PubMed]
- Rekha, C.; Vijayalakshmi, G. Influence of Processing Parameters on the Quality of Soycurd (Tofu). J. Food Sci. Technol. 2013, 50, 176–180. [Google Scholar] [CrossRef]
- Chang, K.; Hou, H. Science and Technology of Tofu Making. In Handbook of Vegetable Preservation and Processing; CRC Press: Boca Raton, FL, USA, 2003; pp. 530–572. [Google Scholar]
- Zeppa, G.; Tedesco, M.; Bertolino, M.; Çilek Tatar, B. Grape Pomace as a New Coagulant for Tofu Production: Physicochemical and Sensory Effects. Foods 2021, 10, 1857. [Google Scholar] [CrossRef] [PubMed]
- Leroy, F.; De Vuyst, L. Lactic Acid Bacteria as Functional Starter Cultures for the Food Fermentation Industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Serrazanetti, D.I.; Ndagijimana, M.; Miserocchi, C.; Perillo, L.; Guerzoni, M.E. Fermented Tofu: Enhancement of Keeping Quality and Sensorial Properties. Food Control 2013, 34, 336–346. [Google Scholar] [CrossRef]
- Rossi, S.; Turchetti, B.; Sileoni, V.; Marconi, O.; Perretti, G. Evaluation of Saccharomyces cerevisiae Strains Isolated from Non-brewing Environments in Beer Production. J. Inst. Brew. 2018, 124, 381–388. [Google Scholar] [CrossRef]
- Riciputi, Y.; Serrazanetti, D.I.; Verardo, V.; Vannini, L.; Caboni, M.F.; Lanciotti, R. Effect of Fermentation on the Content of Bioactive Compounds in Tofu-Type Products. J. Funct. Foods 2016, 27, 131–139. [Google Scholar] [CrossRef]
- Benucci, G.M.N.; Wang, X.; Zhang, L.; Bonito, G.; Yu, F. Yeast and Lactic Acid Bacteria Dominate the Core Microbiome of Fermented ‘Hairy’Tofu (Mao Tofu). Diversity 2022, 14, 207. [Google Scholar] [CrossRef]
- Li, C.; Rui, X.; Zhang, Y.; Cai, F.; Chen, X.; Jiang, M. Production of Tofu by Lactic Acid Bacteria Isolated from Naturally Fermented Soy Whey and Evaluation of Its Quality. LWT-Food Sci. Technol. 2017, 82, 227–234. [Google Scholar] [CrossRef]
- Liu, S.; Han, Y.; Zhou, Z. Lactic Acid Bacteria in Traditional Fermented Chinese Foods. Food Res. Int. 2011, 44, 643–651. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shima, T.; Fukuda, M. Metabolite Profile of Lactic Acid-Fermented Soymilk. Food Nutr. 2018, 9, 1327–1340. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Li, L. A New Style of Fermented Tofu by Lactobacillus casei Combined with Salt Coagulant. 3 Biotech 2020, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. BioMed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus plantarum and Its Probiotic and Food Potentialities. Probiotics Antimicrob. Proteins 2017, 9, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Paventi, G.; Di Martino, C. Biosynthesis of Gamma-Aminobutyric Acid (GABA) by Lactiplantibacillus plantarum in Fermented Food Production. Curr. Issues Mol. Biol. 2023, 46, 200–220. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Di Martino, C.; Letizia, F.; Crawford, T.W., Jr.; Paventi, G. Production of Conjugated Linoleic Acid (CLA) by Lactiplantibacillus plantarum: A Review with Emphasis on Fermented Foods. Foods 2024, 13, 975. [Google Scholar] [CrossRef]
- Letizia, F.; Albanese, G.; Testa, B.; Vergalito, F.; Bagnoli, D.; Di Martino, C.; Carillo, P.; Verrillo, L.; Succi, M.; Sorrentino, E. In Vitro Assessment of Bio-Functional Properties from Lactiplantibacillus plantarum Strains. Curr. Issues Mol. Biol. 2022, 44, 2321–2334. [Google Scholar] [CrossRef]
- Letizia, F.; Fratianni, A.; Cofelice, M.; Testa, B.; Albanese, G.; Di Martino, C.; Panfili, G.; Lopez, F.; Iorizzo, M. Antioxidative Properties of Fermented Soymilk Using Lactiplantibacillus plantarum LP95. Antioxidants 2023, 12, 1442. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Yu, R.-C.; Chou, C.-C. Antioxidative Activities of Soymilk Fermented with Lactic Acid Bacteria and Bifidobacteria. Food Microbiol. 2006, 23, 128–135. [Google Scholar] [CrossRef]
- Amatayakul, T.; Halmos, A.; Sherkat, F.; Shah, N. Physical Characteristics of Yoghurts Made Using Exopolysaccharide-Producing Starter Cultures and Varying Casein to Whey Protein Ratios. Int. Dairy J. 2006, 16, 40–51. [Google Scholar] [CrossRef]
- Cui, L.; Chang, S.K.; Nannapaneni, R. Comparative Studies on the Effect of Probiotic Additions on the Physicochemical and Microbiological Properties of Yoghurt Made from Soymilk and Cow’s Milk during Refrigeration Storage (R2). Food Control 2021, 119, 107474. [Google Scholar] [CrossRef]
- Jung, S.; Murphy, P.A.; Sala, I. Isoflavone Profiles of Soymilk as Affected by High-Pressure Treatments of Soymilk and Soybeans. Food Chem. 2008, 111, 592–598. [Google Scholar] [CrossRef]
- Singh, P.; Bilyeu, L.; Krishnaswamy, K. Spray Drying Process Optimization: Drought Resistant Variety (W82) Soymilk Powder Using Response Surface Methodology (RSM). LWT 2022, 166, 113760. [Google Scholar] [CrossRef]
- Fahmi, R.; Khodaiyan, F.; Pourahmad, R.; Emam-Djomeh, Z. Effect of Ultrasound Assisted Extraction upon the Genistin and Daidzin Contents of Resultant Soymilk. J. Food Sci. Technol. 2014, 51, 2857–2861. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Dell’Aversana, E.; D’Amelia, L.; Colla, G.; Caruso, G.; De Pascale, S.; Rouphael, Y. Sensory and Functional Quality Characterization of Protected Designation of Origin ‘Piennolo Del Vesuvio’Cherry Tomato Landraces from Campania-Italy. Food Chem. 2019, 292, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Kyratzis, A.; Kyriacou, M.C.; Dell’Aversana, E.; Fusco, G.M.; Corrado, G.; Rouphael, Y. Biostimulatory Action of Arbuscular Mycorrhizal Fungi Enhances Productivity, Functional and Sensory Quality in ‘Piennolo Del Vesuvio’Cherry Tomato Landraces. Agronomy 2020, 10, 911. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Khurshid Alam, A. In Vitro Antioxidant and Free Radical Scavenging Activity of Different Parts of Tabebuia pallida Growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Santos, F.; Segundo, M.A.; Reis, S.; Lima, J.L. Rapid Microplate High-Throughput Methodology for Assessment of Folin-Ciocalteu Reducing Capacity. Talanta 2010, 83, 441–447. [Google Scholar] [CrossRef]
- Aziz, T.; Xingyu, H.; Sarwar, A.; Khan, A.A.; Shahzad, M. Assessing the Probiotic Potential, Antioxidant, and Antibacterial Activities of Oat and Soy Milk Fermented with Lactiplantibacillus plantarum Strains Isolated from Tibetan Kefir. Front. Microbiol. 2023, 14, 1265188. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Xiao, L.; Wang, S.; Wang, X.; Ma, K.; Ji, F.; Azarpazhooh, E.; Ajami, M.; Rui, X. Effects of Lactiplantibacillus plantarum with Different Phenotypic Features on the Nutrition, Flavor, Gel Properties, and Digestion of Fermented Soymilk. Food Biosci. 2023, 55, 103026. [Google Scholar] [CrossRef]
- Guan, Z.; Zhang, J.; Zhang, S.; He, Y.; Li, Y.; Regenstein, J.M.; Xie, Y.; Zhou, P. Effect of Coagulant and Treatment Conditions on the Gelation and Textural Properties of Acidic Whey Tofu. Foods 2023, 12, 918. [Google Scholar] [CrossRef]
- Yilmaz, B.; Bangar, S.P.; Echegaray, N.; Suri, S.; Tomasevic, I.; Manuel Lorenzo, J.; Melekoglu, E.; Rocha, J.M.; Ozogul, F. The Impacts of Lactiplantibacillus plantarum on the Functional Properties of Fermented Foods: A Review of Current Knowledge. Microorganisms 2022, 10, 826. [Google Scholar] [CrossRef]
- Fiocco, D.; Capozzi, V.; Collins, M.; Gallone, A.; Hols, P.; Guzzo, J.; Weidmann, S.; Rieu, A.; Msadek, T.; Spano, G. Characterization of the CtsR Stress Response Regulon in Lactobacillus plantarum. J. Bacteriol. 2010, 192, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, L.; Wang, J.; Zhang, Q.; Zhou, Q.; Sun, T.; Chen, W.; Zhang, H. Fermentation Characteristics of Six Probiotic Strains in Soymilk. Ann. Microbiol. 2012, 62, 1473–1483. [Google Scholar] [CrossRef]
- Conte, F.; van Buuringen, N.; Voermans, N.C.; Lefeber, D.J. Galactose in Human Metabolism, Glycosylation and Congenital Metabolic Diseases: Time for a Closer Look. Biochim. Biophys. Acta BBA-Gen. Subj. 2021, 1865, 129898. [Google Scholar] [CrossRef]
- Li, C.; Fan, Y.; Li, S.; Zhou, X.; Park, K.-Y.; Zhao, X.; Liu, H. Antioxidant Effect of Soymilk Fermented by Lactobacillus plantarum HFY01 on D-Galactose-Induced Premature Aging Mouse Model. Front. Nutr. 2021, 8, 667643. [Google Scholar] [CrossRef]
- Fatemi, I.; Khaluoi, A.; Kaeidi, A.; Shamsizadeh, A.; Heydari, S. Protective Effect of Metformin on D-Galactose-Induced Aging Model in Mice. Iran. J. Basic Med. Sci. 2018, 21, 19. [Google Scholar]
- Florowska, A.; Krygier, K.; Florowski, T.; Dłużewska, E. Prebiotics as Functional Food Ingredients Preventing Diet-Related Diseases. Food Funct. 2016, 7, 2147–2155. [Google Scholar] [CrossRef]
- Mustafa, S.E.; Mustafa, S.; Ismail, A.; Abas, F.; Abd Manap, M.Y.; Hamdi, O.A.A.; Elzen, S.; Nahar, L.; Sarker, S.D. Impact of Prebiotics on Equol Production from Soymilk Isoflavones by Two Bifidobacterium Species. Heliyon 2020, 6, e05298. [Google Scholar] [CrossRef]
- Kanwal, F.; Ren, D.; Kanwal, W.; Ding, M.; Su, J.; Shang, X. The Potential Role of Nondigestible Raffinose Family Oligosaccharides as Prebiotics. Glycobiology 2023, 33, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Yuan, J.; Li, D. Biological Activity of Galacto-Oligosaccharides: A Review. Front. Microbiol. 2022, 13, 993052. [Google Scholar] [CrossRef]
- Cheng, I.-C.; Shang, H.-F.; Lin, T.-F.; Wang, T.-H.; Lin, H.-S.; Lin, S.-H. Effect of Fermented Soy Milk on the Intestinal Bacterial Ecosystem. World J. Gastroenterol. WJG 2005, 11, 1225. [Google Scholar] [CrossRef] [PubMed]
- Moraes Filho, M.; Busanello, M.; Garcia, S. Optimization of the Fermentation Parameters for the Growth of Lactobacillus in Soymilk with Okara Flour. LWT 2016, 74, 456–464. [Google Scholar] [CrossRef]
- Hsiao, Y.-H.; Ho, C.-T.; Pan, M.-H. Bioavailability and Health Benefits of Major Isoflavone Aglycones and Their Metabolites. J. Funct. Foods 2020, 74, 104164. [Google Scholar] [CrossRef]
- Kudełka, W.; Kowalska, M.; Popis, M. Quality of Soybean Products in Terms of Essential Amino Acids Composition. Molecules 2021, 26, 5071. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.-K.; Rocha, J.M. Recent Developments in Polyphenol Applications on Human Health: A Review with Current Knowledge. Plants 2023, 12, 1217. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhang, Y.; Wu, H.; Wang, Z.; Dai, Y.; Zhou, J.; Liu, X.; Dong, M.; Xia, X. Improvement of the Phenolic Content, Antioxidant Activity, and Nutritional Quality of Tofu Fermented with Actinomucor Elegans. LWT 2020, 133, 110087. [Google Scholar] [CrossRef]
- Bei, Q.; Chen, G.; Lu, F.; Wu, S.; Wu, Z. Enzymatic Action Mechanism of Phenolic Mobilization in Oats (Avena sativa L.) during Solid-State Fermentation with Monascus Anka. Food Chem. 2018, 245, 297–304. [Google Scholar] [CrossRef]
- Zhao, D.; Shah, N.P. Changes in Antioxidant Capacity, Isoflavone Profile, Phenolic and Vitamin Contents in Soymilk during Extended Fermentation. LWT-Food Sci. Technol. 2014, 58, 454–462. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, Y.-F.; Zhou, Z.-Q. Phenolic and Flavonoid Contents of Mandarin (Citrus reticulata Blanco) Fruit Tissues and Their Antioxidant Capacity as Evaluated by DPPH and ABTS Methods. J. Integr. Agric. 2018, 17, 256–263. [Google Scholar] [CrossRef]
| Days of Soymilk Fermentation | Days of Tofu Storage | ||||
|---|---|---|---|---|---|
| 0 | 1 | 7 | 14 | 21 | |
| Viable cell count | 8.07 ± 0.06 b | 9.52 ± 0.11 a | 7.76 ± 0.29 b | 6.00 ± 0.50 c | 5.83 ± 0.23 c |
| L-Amino Acids (µmol/g D.W.) | Unfermented Soymilk | Fermented Soymilk | Tofu |
|---|---|---|---|
| Alanine | 3.03 ± 0.52 a | 0.50 ± 0.06 b | 0.64 ± 0.09 b |
| Arginine | 6.01 ± 1.23 a | 7.11 ± 1.11 a | 4.93± 1.05 a |
| Asparagine | 10.8 ± 1.62 a | 2.91 ± 0.42 b | 2.18 ± 0.39 b |
| Aspartic acid | 1.20 ± 0.20 a | 0.07 ± 0.01 b | 0.08 ± 0.01 b |
| γ-aminobutyric acid | 8.21 ± 1.53 a | 8.87 ± 1.65 a | 6.75 ± 1.16 a |
| Glutamine | 0.15 ± 0.01 a | 0.12 ± 0.03 a | 0.08 ± 0.04 b |
| Glutamic acid | 2.73 ± 0.43 a | 0.00 ± 0.01 b | 0.00 ± 0.01 b |
| Glycine | 0.80 ± 0.11 a | 0.28 ± 0.11 b | 0.22 ± 0.09 b |
| Histidine | 0.95 ± 0.15 a | 1.02 ± 0.23 a | 0.78 ± 0.20 a |
| lsoleucine | 0.85 ± 0.13 a | 0.09 ± 0.01 b | 0.07 ± 0.01 b |
| Leucine | 1.33 ± 0.21 a | 0.15 ± 0.02 b | 0.13 ± 0.02 b |
| Lysine | 0.76 ± 0.14 a | 1.13 ± 0.41 a | 0.98 ± 0.29 a |
| Monoethanolamine | 0.84 ± 0.15 a | 0.96 ± 0.16 a | 0.79 ± 0.12 a |
| Methionine | 0.32 ± 0.04 a | 0.10 ± 0.02 b | 0.07 ± 0.01 b |
| Ornithine | 0.45 ± 0.05 a | 0.59 ± 0.04 a | 0.48 ± 0.13 a |
| Phenylalanine | 1.17 ± 0.20 a | 0.02 ± 0.01 b | 0.02 ± 0.01 b |
| Proline | 5.60 ± 0.49 a | 5.70 ± 0.85 a | 3.85 ± 1.00 b |
| Serine | 1.05 ± 0.09 a | 0.17 ± 0.03 b | 0.15 ± 0.03 b |
| Threonine | 4.67 ± 0.90 a | 5.74 ± 1.10 a | 4.46 ± 0.71 a |
| Tryptophan | 1.65 ± 0.29 a | 0.15 ± 0.04 b | 0.13 ± 0.04 b |
| Tyrosine | 0.93 ± 0.13 a | 0.02 ± 0.01 b | 0.01 ± 0.00 b |
| Valine | 1.36 ± 0.20 a | 0.08 ± 0.01 b | 0.06 ± 0.01 b |
| Unfermented Soymilk | Fermented Soymilk | Tofu | |
|---|---|---|---|
| * ABTS | 0.69 ± 0.02 a | 0.63 ± 0.02 b | 0.48 ± 0.01 c |
| * DPPH | 0.13 ± 0.02 b | 0.18 ± 0.01 a | 0.17 ± 0.01 a |
| ** TPC | 8.27 ± 0.15 c | 9.95 ± 0.19 a | 9.63 ± 0.26 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Letizia, F.; Fusco, G.M.; Fratianni, A.; Gaeta, I.; Carillo, P.; Messia, M.C.; Iorizzo, M. Application of Lactiplantibacillus plantarum LP95 as a Functional Starter Culture in Fermented Tofu Production. Processes 2024, 12, 1093. https://doi.org/10.3390/pr12061093
Letizia F, Fusco GM, Fratianni A, Gaeta I, Carillo P, Messia MC, Iorizzo M. Application of Lactiplantibacillus plantarum LP95 as a Functional Starter Culture in Fermented Tofu Production. Processes. 2024; 12(6):1093. https://doi.org/10.3390/pr12061093
Chicago/Turabian StyleLetizia, Francesco, Giovanna Marta Fusco, Alessandra Fratianni, Ilenia Gaeta, Petronia Carillo, Maria Cristina Messia, and Massimo Iorizzo. 2024. "Application of Lactiplantibacillus plantarum LP95 as a Functional Starter Culture in Fermented Tofu Production" Processes 12, no. 6: 1093. https://doi.org/10.3390/pr12061093
APA StyleLetizia, F., Fusco, G. M., Fratianni, A., Gaeta, I., Carillo, P., Messia, M. C., & Iorizzo, M. (2024). Application of Lactiplantibacillus plantarum LP95 as a Functional Starter Culture in Fermented Tofu Production. Processes, 12(6), 1093. https://doi.org/10.3390/pr12061093










