Kinetics of Vegetable Oils (Rice Bran, Sunflower Seed, and Soybean) Extracted by Pressurized Liquid Extraction in Intermittent Process
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Preparation
2.2. Average Particle Size
2.3. Pressurized Liquid Extraction in Intermittent Process
2.4. Mathematical Modeling of the Extraction Kinetics
2.4.1. Case Study 1: Semi-Continuous Process or General Extraction Curve
2.4.2. Case Study 2: PLE in Intermittent Process
3. Results and Discussion
3.1. Average Particle Size Determination
3.2. Pressurized Liquid Extraction in Intermittent Process
3.3. Process Kinetics and Modeling
3.3.1. Extraction Kinetics of the Semi-Continuous Process
3.3.2. PLE in Intermittent Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wen, C.; Shen, M.; Liu, G.; Liu, X.; Liang, L.; Li, Y.; Zhang, J.; Xu, X. Edible Vegetable Oils from Oil Crops: Preparation, Refining, Authenticity Identification and Application. Process Biochem. 2023, 124, 168–179. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 27 September 2023).
- Saunders, R.M. Rice Bran: Composition and Potential Food Uses. Food Rev. Int. 1985, 1, 465–495. [Google Scholar] [CrossRef]
- Jaski, J.M.; Abrantes, K.K.B.; Zanqui, A.B.; Stevanato, N.; da Silva, C.; Barão, C.E.; Bonfim-Rocha, L.; Cardozo-Filho, L. Simultaneous Extraction of Sunflower Oil and Active Compounds from Olive Leaves Using Pressurized Propane. Curr. Res. Food Sci. 2022, 5, 531–544. [Google Scholar] [CrossRef]
- Gasparetto, H.; de Castilhos, F.; Paula Gonçalves Salau, N. Recent Advances in Green Soybean Oil Extraction: A Review. J. Mol. Liq. 2022, 361, 119684. [Google Scholar] [CrossRef]
- Mgoma, S.T.; Basitere, M.; Mshayisa, V.V. Kinetics and Thermodynamics of Oil Extraction from South African Hass Avocados Using Hexane as a Solvent. S. Afr. J. Chem. Eng. 2021, 37, 244–251. [Google Scholar] [CrossRef]
- Yang, J.; Vardar, U.S.; Boom, R.M.; Bitter, J.H.; Nikiforidis, C.V. Extraction of Oleosome and Protein Mixtures from Sunflower Seeds. Food Hydrocoll. 2023, 145, 109078. [Google Scholar] [CrossRef]
- Sayasoonthorn, S.; Kaewrueng, S.; Patharasathapornkul, P. Rice Bran Oil Extraction by Screw Press Method: Optimum Operating Settings, Oil Extraction Level and Press Cake Appearance. Rice Sci. 2012, 19, 75–78. [Google Scholar] [CrossRef]
- Baümler, E.R.; Carrín, M.E.; Carelli, A.A. Extraction of Sunflower Oil Using Ethanol as Solvent. J. Food Eng. 2016, 178, 190–197. [Google Scholar] [CrossRef]
- Colivet, J.; Oliveira, A.L.; Carvalho, R.A. Influence of the Bed Height on the Kinetics of Watermelon Seed Oil Extraction with Pressurized Ethanol. Sep. Purif. Technol. 2016, 169, 187–195. [Google Scholar] [CrossRef]
- Ramos, P.R.; Vicentini-Polette, C.M.; Mazalli, M.R.; de Lima Caneppele, F.; de Oliveira, A.L. Pressurized Liquid Extraction of Soybean Oil Using Intermittent Process with Ethanol and Hexane as Solvent: Extraction Yield and Physicochemical Parameters Comparison. J. Food Process Eng. 2024, 47, e14562. [Google Scholar] [CrossRef]
- Rodrigues, L.d.C.; Bodini, R.B.; de L. Caneppele, F.; Dacanal, G.C.; Crevelin, E.J.; de Moraes, L.A.B.; de Oliveira, A.L. Pressurized Liquid Extraction (PLE) in an Intermittent Process as an Alternative for Obtaining Passion Fruit (Passiflora edulis) Leaf Hydroalcoholic Extract (Tincture). Processes 2023, 11, 2308. [Google Scholar] [CrossRef]
- Ferro, D.M.; Mayer, D.A.; Oliveira Müller, C.M.; Ferreira, S.R.S. Scale-up Simulation of PLE Process Applied to Recover Bio-Based Materials from Sida Rhombifolia Leaves. J. Supercrit. Fluids 2020, 166, 105033. [Google Scholar] [CrossRef]
- Comerlatto, A.; Voll, F.A.; Daga, A.L.; Fontana, É. Mass Transfer in Soybean Oil Extraction Using Ethanol/Isopropyl Alcohol Mixtures. Int. J. Heat Mass Transf. 2021, 165, 120630. [Google Scholar] [CrossRef]
- Czaikoski, K.; Mesomo, M.C.; De Scheer, A.P.; Dalla Santa, O.R.; Queiroga, C.L.; Corazza, M.L. Kinetics, Composition and Biological Activity of Eupatorium Intermedium Flower Extracts Obtained from ScCO2 and Compressed Propane. J. Supercrit. Fluids 2015, 97, 145–153. [Google Scholar] [CrossRef]
- Segovia, F.J.; Corral-Pérez, J.J.; Almajano, M.P. Avocado Seed: Modeling Extraction of Bioactive Compounds. Ind. Crops Prod. 2016, 85, 213–220. [Google Scholar] [CrossRef]
- Kabuba, J.; Huberts, R. Steam Extraction of Essential Oils: Investigation of Process Parameters. Can. J. Chem. Eng. 2009, 87, 915–920. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Ramos, P.E.; Cerqueira, M.A.; Cook, M.T.; Bourbon, A.I.; Khutoryanskiy, V.V.; Charalampoulos, D.; Teixeira, J.A.; Vicente, A.A. Development of an Immobilization System for in Situ Micronutrients Release. Food Res. Int. 2016, 90, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Chinnarasu, C.; Montes, A.; Pereyra, C.; Casas, L.; Fernández-Ponce, T.; Mantell, C.; Pattabhi, S.; Martínez De La Ossa, E. Preparation of Polyphenol Fine Particles Potent Antioxidants by a Supercritical Antisolvent Process Using Different Extracts of Olea Europaea Leaves. Korean J. Chem. Eng 2016, 33, 594–602. [Google Scholar] [CrossRef]
- Özkal, S.G.; Yener, M.E. Supercritical Carbon Dioxide Extraction of Flaxseed Oil: Effect of Extraction Parameters and Mass Transfer Modeling. J. Supercrit. Fluids 2016, 112, 76–80. [Google Scholar] [CrossRef]
- Pereira, C.G.; Meireles, M.A.A. Supercritical Fluid Extraction of Bioactive Compounds: Fundamentals, Applications and Economic Perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Cornelio-Santiago, H.P.; Mazalli, M.R.; Rodrigues, C.E.C.; de Oliveira, A.L. Extraction of Brazil Nut Kernel Oil Using Green Solvents: Effects of the Process Variables in the Oil Yield and Composition. J. Food Process Eng. 2019, 42, e13271. [Google Scholar] [CrossRef]
- Oliveira, N.A.d.; Cornelio-Santiago, H.P.; Fukumasu, H.; Oliveira, A.L. de Green Coffee Extracts Rich in Diterpenes—Process Optimization of Pressurized Liquid Extraction Using Ethanol as Solvent. J. Food Eng. 2018, 224, 148–155. [Google Scholar] [CrossRef]
- Amarante, R.C.A.; Oliveira, P.M.; Schwantes, F.K.; Morónmorón-Villarreyes, J.A. Oil Extraction from Castor Cake Using Ethanol: Kinetics and Thermodynamics. Ind. Eng. Chem. Res. 2014, 53, 6824–6829. [Google Scholar] [CrossRef]
- Guerrero, M.S.; Torres, J.S.; Nuñez, M.J. Extraction of Polyphenols from White Distilled Grape Pomace: Optimization and Modelling. Bioresour. Technol. 2008, 99, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Igbozulike, A.O.; Ndirika, V.I.O.; Simonyan, K.J. Influence of Drying Process Variables on the Effective Moisture Diffusivity and Activation Energy of African Oil Bean Seed. Sci. Afr. 2023, 22, e01895. [Google Scholar] [CrossRef]
- Pinheiro, M.N.C.; Castro, L.M.M.N. Effective Moisture Diffusivity Prediction in Two Portuguese Fruit Cultivars (Bravo de Esmolfe Apple and Madeira Banana) Using Drying Kinetics Data. Heliyon 2023, 9, e17741. [Google Scholar] [CrossRef]
- Salgin, U.; Doker, O.; Çalimi, A. Extraction of Sunflower Oil with Supercritical CO2: Experiments and Modeling. J. Supercrit. Fluids 2006, 38, 236–331. [Google Scholar] [CrossRef]


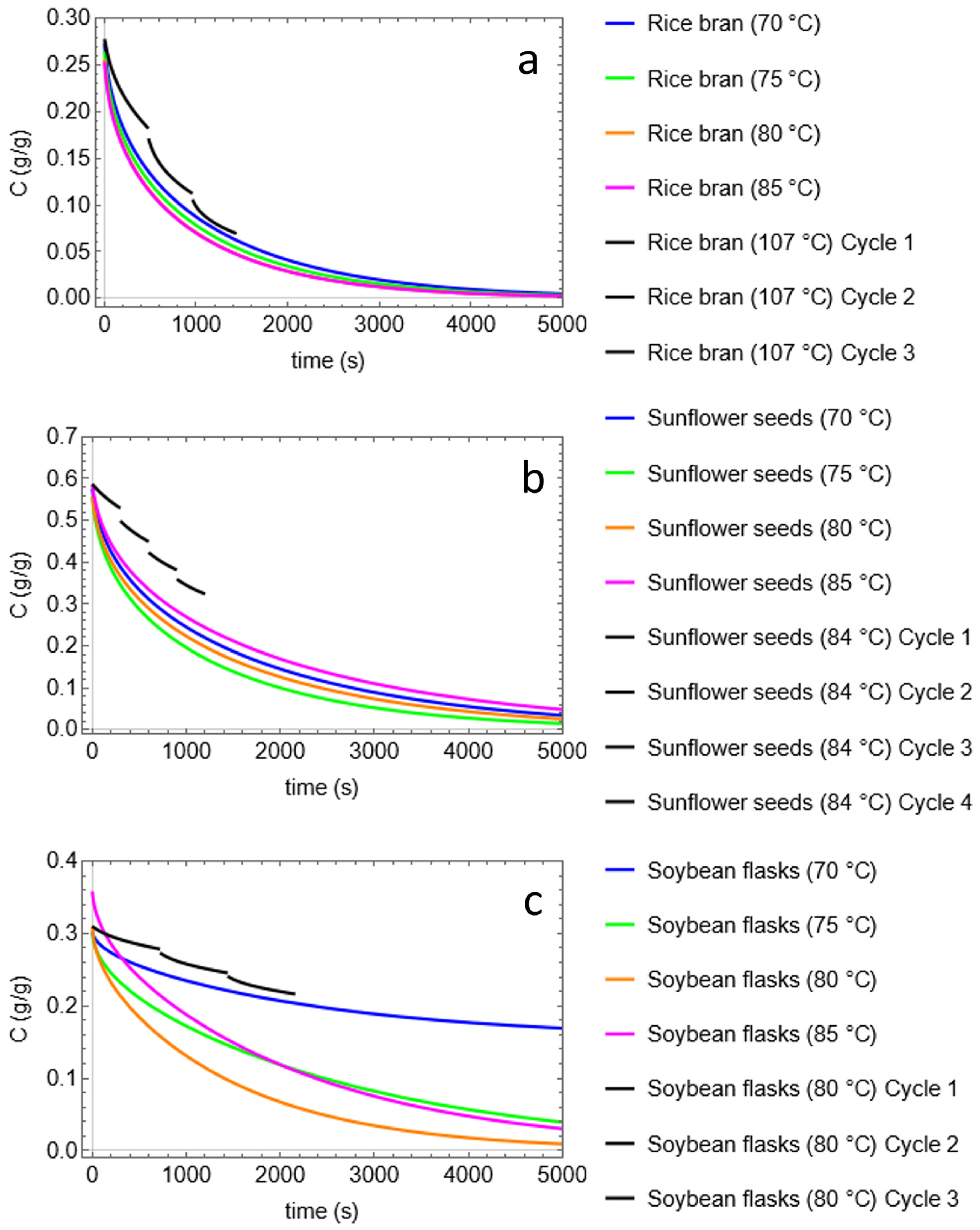
| Temperature (°C) | Static Time (min) | Cycles | Rinse Volume (%) * | Oil Recovery (%) | |
|---|---|---|---|---|---|
| Rice Bran | 107 | 8 | 3 | 60 | 130.42 |
| Sunflower seeds | 84 | 5 | 4 | 110 | 93.9 |
| Soybean flask | 80 | 12 | 3 | 60 | 86.16 |
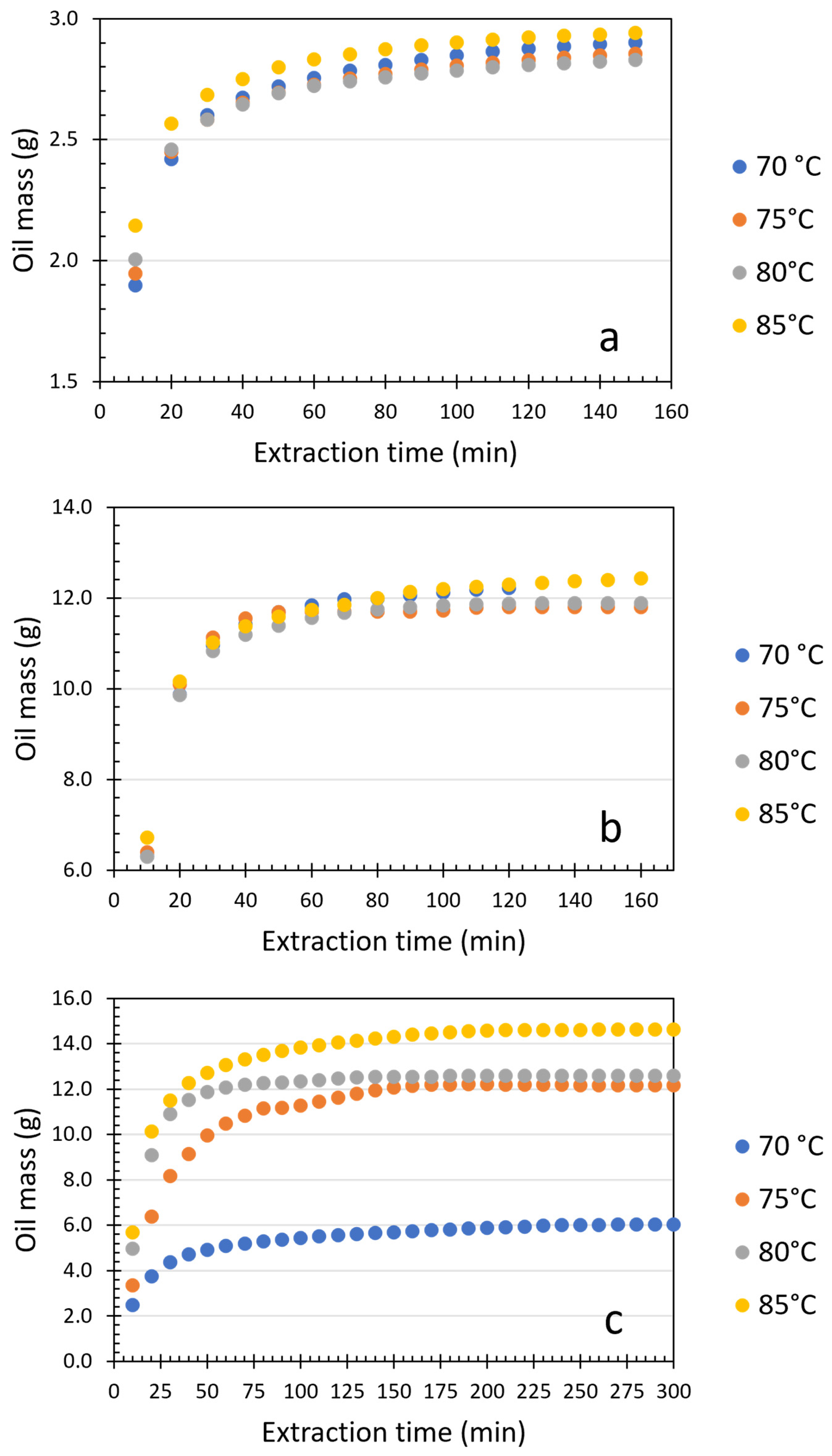
| Rice Bran | Sunflower Seed | Soybean Oil | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extraction Time (min) | 70 °C | 75 °C | 80 °C | 85 °C | 70 °C | 75 °C | 80 °C | 85 °C | 70 °C | 75 °C | 80 °C | 85 °C |
| 10 | 1.90 | 1.95 | 2.01 | 2.14 | 6.31 | 6.40 | 6.30 | 6.72 | 2.49 | 3.35 | 4.96 | 5.69 |
| 20 | 0.52 | 0.50 | 0.45 | 0.42 | 3.57 | 3.70 | 3.56 | 3.43 | 1.26 | 3.03 | 4.12 | 4.45 |
| 30 | 0.18 | 0.13 | 0.12 | 0.12 | 1.09 | 1.03 | 0.96 | 0.86 | 0.62 | 1.78 | 1.81 | 1.36 |
| 40 | 0.07 | 0.07 | 0.06 | 0.07 | 0.45 | 0.42 | 0.37 | 0.36 | 0.34 | 0.99 | 0.62 | 0.75 |
| 50 | 0.05 | 0.04 | 0.05 | 0.05 | 0.28 | 0.14 | 0.20 | 0.22 | 0.21 | 0.81 | 0.37 | 0.47 |
| 60 | 0.03 | 0.03 | 0.03 | 0.03 | 0.15 | 0.01 | 0.17 | 0.15 | 0.17 | 0.53 | 0.18 | 0.34 |
| 70 | 0.03 | 0.03 | 0.02 | 0.02 | 0.13 | 0.00 | 0.11 | 0.11 | 0.11 | 0.33 | 0.14 | 0.24 |
| 80 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.00 | 0.08 | 0.14 | 0.09 | 0.33 | 0.06 | 0.19 |
| 90 | 0.02 | 0.02 | 0.02 | 0.02 | 0.08 | 0.00 | 0.05 | 0.15 | 0.08 | 0.02 | 0.04 | 0.17 |
| 100 | 0.02 | 0.01 | 0.01 | 0.01 | 0.06 | 0.03 | 0.03 | 0.06 | 0.07 | 0.11 | 0.04 | 0.15 |
| 110 | 0.02 | 0.01 | 0.01 | 0.01 | 0.05 | 0.05 | 0.02 | 0.05 | 0.07 | 0.18 | 0.06 | 0.12 |
| 120 | 0.01 | 0.01 | 0.01 | 0.01 | 0.04 | 0.02 | 0.02 | 0.05 | 0.05 | 0.16 | 0.06 | 0.10 |
| 130 | 0.01 | 0.01 | 0.01 | 0.01 | - | - | - | 0.04 | 0.05 | 0.17 | 0.05 | 0.09 |
| 140 | 0.01 | 0.01 | 0.01 | 0.01 | - | - | - | 0.03 | 0.04 | 0.15 | 0.02 | 0.09 |
| 150 | 0.01 | 0.01 | 0.01 | 0.01 | - | - | - | 0.03 | 0.04 | 0.13 | 0.02 | 0.08 |
| 160 | - | - | - | - | - | - | - | - | 0.04 | 0.07 | 0.00 | 0.08 |
| 170 | - | - | - | - | - | - | - | - | 0.04 | 0.06 | 0.00 | 0.06 |
| 180 | - | - | - | - | - | - | - | - | 0.04 | 0.00 | 0.03 | 0.06 |
| 190 | - | - | - | - | - | - | - | - | 0.03 | 0.01 | 0.01 | 0.04 |
| 200 | - | - | - | - | - | - | - | - | 0.03 | 0.00 | 0.01 | 0.03 |
| 210 | - | - | - | - | - | - | - | - | 0.03 | -0.00 | 0.00 | 0.02 |
| 220 | - | - | - | - | - | - | - | - | 0.03 | 0.00 | 0.00 | 0.01 |
| 230 | - | - | - | - | - | - | - | - | 0.03 | 0.00 | 0.00 | 0.01 |
| 240 | - | - | - | - | - | - | - | - | 0.03 | 0.00 | 0.00 | 0.00 |
| 250 | - | - | - | - | - | - | - | - | 0.01 | 0.00 | 0.00 | 0.00 |
| 260 | - | - | - | - | - | - | - | - | 0.01 | 0.00 | 0.00 | 0.00 |
| 270 | - | - | - | - | - | - | - | - | 0.01 | 0.00 | 0.00 | 0.00 |
| 280 | - | - | - | - | - | - | - | - | 0.00 | 0.00 | 0.00 | 0.00 |
| 290 | - | - | - | - | - | - | - | - | 0.00 | 0.00 | 0.00 | 0.00 |
| 300 | - | - | - | - | - | - | - | - | 0.00 | 0.00 | 0.00 | 0.00 |
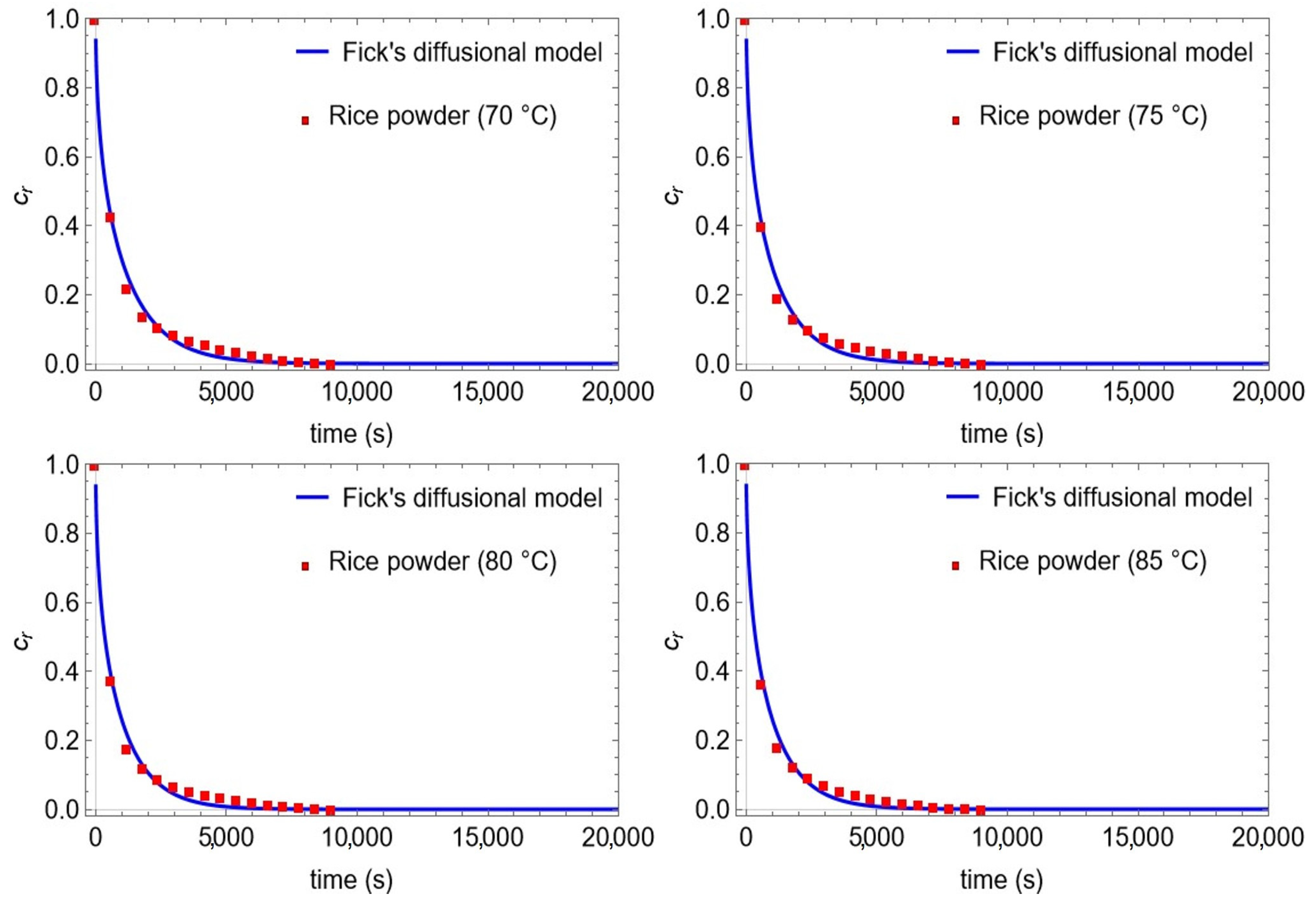
| Properties | Rice Bran (Spherical) | |||
|---|---|---|---|---|
| T (°C) | 70 | 75 | 80 | 85 |
| min (g) | 10 | 10 | 10 | 10 |
| mout (g) | 7.10 | 7.20 | 7.28 | 7.31 |
| Cin (goil/gmat) | 0.29 | 0.281 | 0.272 | 0.269 |
| Ceq (goil/gmat) | 0 | 0 | 0 | 0 |
| dm (×10−3 m) | 0.84 | 0.84 | 0.84 | 0.84 |
| Deff (×10−12 m2/s) | 13.09 ± 0.66 | 14.38 ± 0.80 | 15.70 ± 0.86 | 15.55 ± 0.85 |
| R2 | 0.9929 | 0.9919 | 0.9925 | 0.9924 |
| Properties | Sunflower seeds (spherical) | |||
| T (°C)) | 70 | 75 | 80 | 85 |
| min (g) | 20 | 20 | 20 | 20 |
| mout (g) | 7.57 | 8.2 | 8.12 | 7.57 |
| Cin (goil/gmat) | 0.611 | 0.59 | 0.592 | 0.621 |
| Ceq (goil/gmat) | 0 | 0 | 0 | 0 |
| dm (×10−3 m) | 0.88 | 0.88 | 0.88 | 0.88 |
| Deff (×10−12 m2/s) | 9.37 ± 1.00 | 12.60 ± 1.84 | 10.41 ± 1.15 | 8.10 ± 0.54 |
| R2 | 0.9713 | 0.9548 | 0.9702 | 0.9829 |
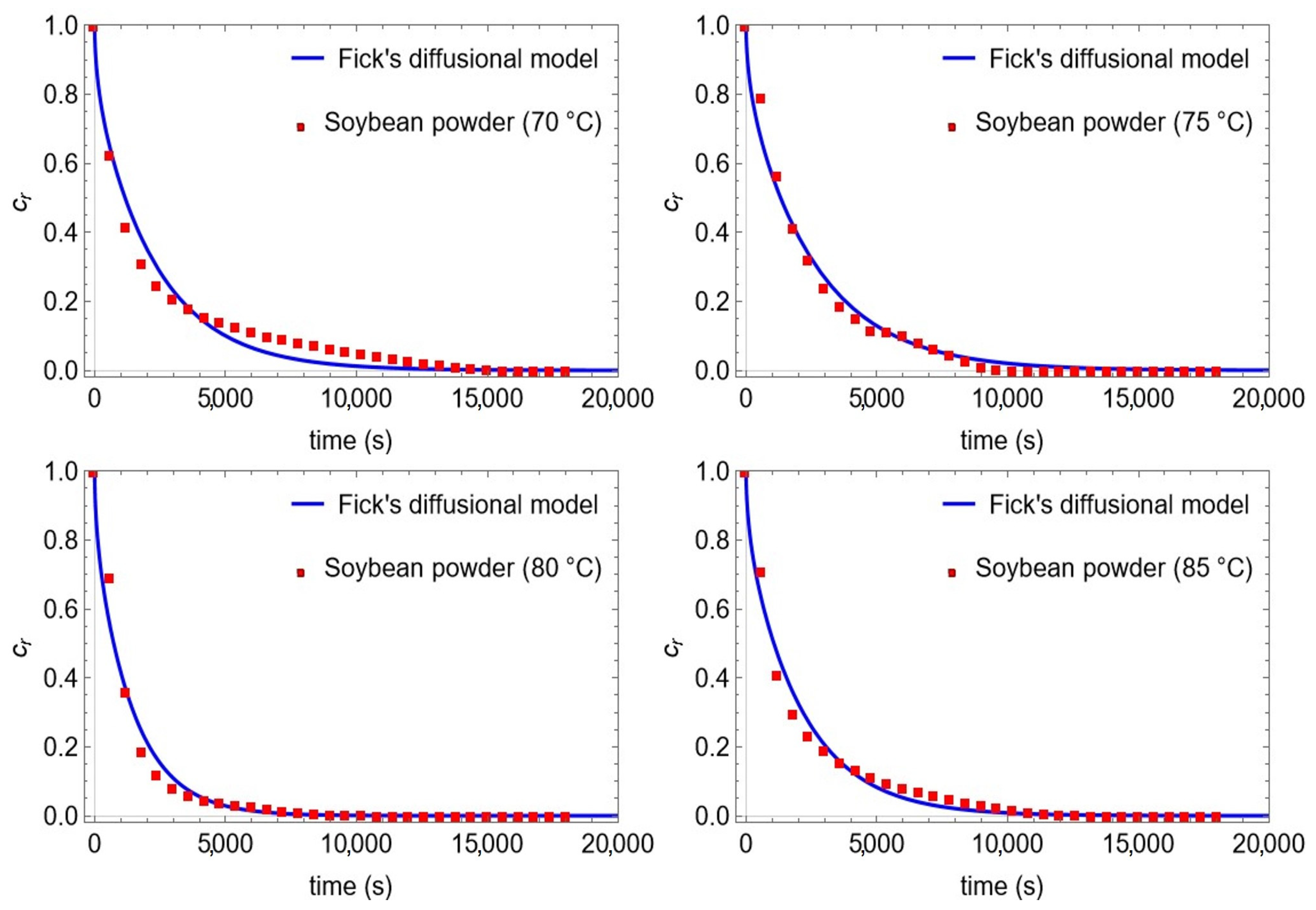
| Properties | Roasted soybean (plates) | |||
| T (°C)) | 70 | 75 | 80 | 85 |
| min (g) | 40 | 40 | 40 | 40 |
| mout (g) | 29.27 | 30.92 | 30.33 | 30.38 |
| Cin (goil/gmat) | 0.305 | 0.305 | 0.315 | 0.365 |
| Ceq (goil/gmat) | 0.154 | 0 | 0 | 0 |
| dm (×10−3 m) | 0.68 | 0.68 | 0.68 | 0.68 |
| Deff (×10−12 m2/s) | 19.61 ± 0.86 | 17.25 ± 0.55 | 31.29 ± 1.50 | 21.41 ± 0.76 |
| R2 | 0.9801 | 0.9902 | 0.9829 | 0.9881 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, P.R.; Sponchiado, J.; Echenique, J.V.F.; Dacanal, G.C.; Oliveira, A.L.d. Kinetics of Vegetable Oils (Rice Bran, Sunflower Seed, and Soybean) Extracted by Pressurized Liquid Extraction in Intermittent Process. Processes 2024, 12, 1107. https://doi.org/10.3390/pr12061107
Ramos PR, Sponchiado J, Echenique JVF, Dacanal GC, Oliveira ALd. Kinetics of Vegetable Oils (Rice Bran, Sunflower Seed, and Soybean) Extracted by Pressurized Liquid Extraction in Intermittent Process. Processes. 2024; 12(6):1107. https://doi.org/10.3390/pr12061107
Chicago/Turabian StyleRamos, Paulo Rodolfo, Joyce Sponchiado, João Victor Febrônio Echenique, Gustavo César Dacanal, and Alessandra Lopes de Oliveira. 2024. "Kinetics of Vegetable Oils (Rice Bran, Sunflower Seed, and Soybean) Extracted by Pressurized Liquid Extraction in Intermittent Process" Processes 12, no. 6: 1107. https://doi.org/10.3390/pr12061107
APA StyleRamos, P. R., Sponchiado, J., Echenique, J. V. F., Dacanal, G. C., & Oliveira, A. L. d. (2024). Kinetics of Vegetable Oils (Rice Bran, Sunflower Seed, and Soybean) Extracted by Pressurized Liquid Extraction in Intermittent Process. Processes, 12(6), 1107. https://doi.org/10.3390/pr12061107








