Abstract
A review of the application of matrix solid-phase dispersion (MSPD) in the extraction of biologically active compounds and impurities from plants and food samples with a particular emphasis on conventional and new types of sorbents has been provided. An overview of MSPD applications for the isolation of organic residues from biological samples, determined using chromatographic and spectroscopic techniques, has been presented. In this study, procedural solutions that may extend MSDP applicability for the extraction such as vortex-assisted, ultrasound-assisted, microwave-assisted, and extraction with a magnetic sorbent have been discussed. Special attention has been paid to MSPD sorbents including modified silica, diatomite, magnesium silicate, alumina, carbon materials (carbon nanotubes, graphene oxide, graphene, or graphite), molecularly imprinted polymers, and cyclodextrin. An important aspect of the MSPD procedure is the use of high-purity and environmentally friendly solvents for extraction (e.g., deep eutectic solvents), with such criteria being the most important for modern analytical chemistry. Many advantages of MSPD are presented, such as high recoveries, the requirement for a smaller volume of solvent, and shorter procedure times than classical methods.
1. Introduction
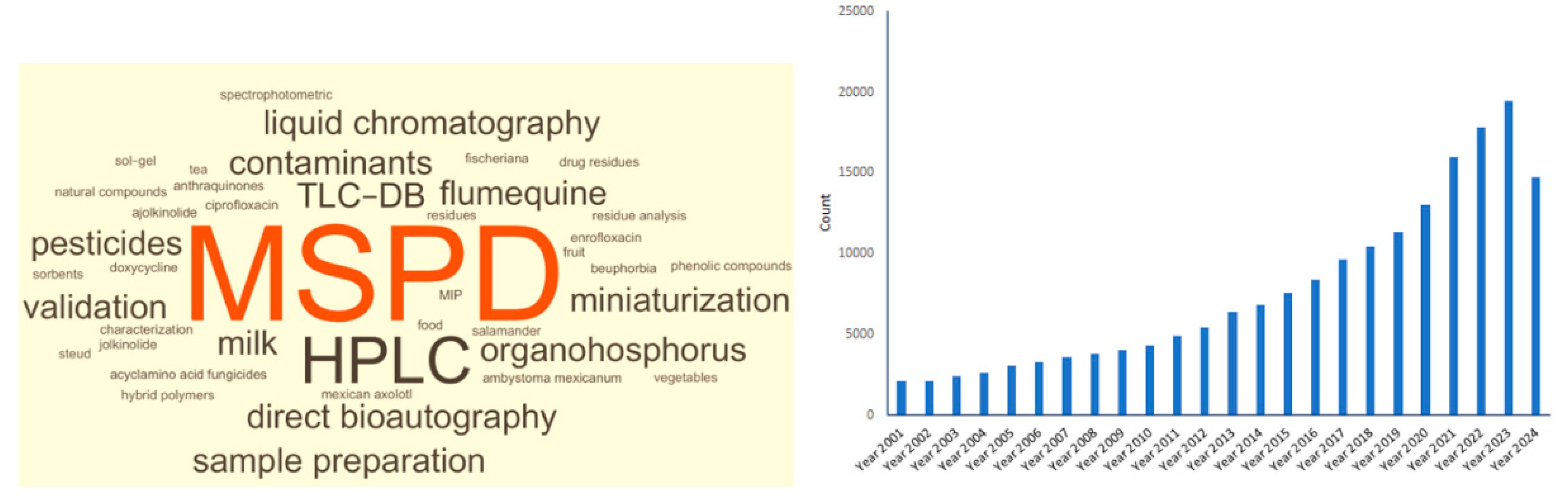
Currently, matrix solid-phase dispersion (MSPD) has found numerous applications in general as a sample preparation method for the extraction of biologically active compounds, naturally occurring constituents, and others from complex biological matrices, as well as for the fractionation of biological samples. Its simplicity of use and its flexibility contribute to it being more often chosen than other methods. Given the above, this method is one of the most commonly used in testing laboratories [1,2,3,4,5]. The interest in MSPD is not decreasing, as evidenced by the possibilities of its practical use and the number of available articles (Figure 1). That is why it is still one of the most popular sample preparation methods.
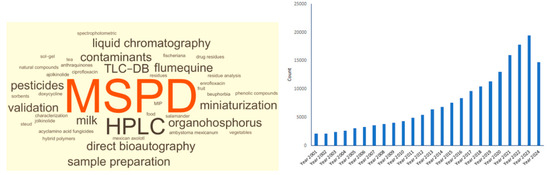
Figure 1.
Word cloud (also known as tag cloud, word collage or wordle) and timeline results by year for the catchword “matrix solid phase dispersion—MSPD” (source according to https://www.sciencedirect.com/, accessed on 27 May 2024).
A wide range of possibilities of MSPD usage are based on several principles involving forces applied to the sample by mechanical blending to produce complete sample disruption, as well as the interactions of the sample matrix with a solid support-bonded phase or the surface chemistry of other solid support materials [1]. Typical solid-phase extraction is the basic technique applied for the enrichment and separation of organic compounds from biological matrices. Currently, it also constitutes the basic method employed in routine laboratory practices [6,7,8,9]. The method has gained its popularity due to the broad range of sorbents used. To ensure the proper performance of the separation process, particular attention should be paid to the properties of sorbents used, particularly their chemical characteristics. Sorbents can differ from hydrophobic ones, of varied chain lengths, to polar ones, which covers virtually the whole range of samples prepared [8,9]. Considering the separation of analytes, specific and non-specific interactions between an analyte and the surface of a solid sorbent (active sites situated on a surface) as well as the liquid phase are of key importance. However, the influence of other substances present in a sample and the matrix itself on sorption processes cannot be neglected. Such effects can result in decreasing recoveries and low repeatability. In the extraction process that occurs in the liquid–solid system, the partitioning coefficient of the substance between liquid and solid phases results from the molecular interactions between the solvent and the sorbent surface. The resultant effect of their occurrence in a liquid–solid system is observed as the sorption of components from a solution. The selective sorption of particular substances from different matrices is feasible due to the presence of specific and non-specific interactions between components in a sample and active sites on the sorbent surface as well as interactions with the liquid phase. The extraction efficiency depends on the mechanism and even various mechanisms of interaction between the sorbent and analyte. The energy of such interactions is differentiated. In the case of non-ionic compounds, van der Waals forces with the transfer of charge and hydrogen bonds are of fundamental significance [8].
The knowledge in the field of extraction, which requires the use of solid sorbents, is based on four mechanisms. They refer to the following: reversed-phase-, normal-phase-, and polymer-based sorbents as well as ion exchange methods [8]. The first one involves nonpolar sorbents and a polar or moderately polar sample matrix (the liquid sample usually contains mid- to nonpolar analytes). In this case, the separation of organic analytes from the polar solution onto sorbents is the result of attractive forces (commonly called van der Waals or dispersion forces) between the carbon–hydrogen bonds of analytes and the functional groups on the sorbent’s surface. The retention of an analyte depends on its structure more than the interactions of the analyte’s functional groups with the surface of the sorbent. In the case of the normal phase system, the retention of analytes is due to interactions between their polar functional groups and polar groups on the sorbent surface. Here, hydrogen bonding and π-π interactions can be considered. The idea of using polymer-based sorbents comes from the retention of hydrophobic compounds containing hydrophilic groups. This enables the selective separation of hydrophobic organic compounds, where high-molecular-weight compounds, including proteins, are not retained and can be easily flushed. The last one, ion exchange solid-phase extraction, has been used in the analysis of solutions containing target compounds as ions. Bonded to the silica surface, aliphatic quaternary amine groups allow for the isolation of negatively charged compounds (anionic), while the silica with aliphatic sulfonic acid groups allows for the separation of positively charged cationic compounds. In this case, the retention mechanism is based on the electrostatic attraction of the compound’s charged functional group to the charged group of the bonded silica surface [8].
Taking into account the above-mentioned phenomena, matrix solid-phase dispersion (MSPD) is one of the methods that apply the extraction of analytes in the liquid–solid system [10,11,12,13,14,15,16,17]. The MSPD method has been widely used in the sample preparation, extraction, and fractionation of solid, semi-solid, and/or highly viscous biological samples. MSPD is a simple and cost-efficient sample preparation method that allows the reduction in organic solvent use, exclusion of sample component degradation, elimination of additional sample purification, and pre-concentration prior to the chromatographic analysis. The method enables full fractionation of the sample matrix components and allows the selective isolation of a single or a few compounds from the sample. MSPD involves the direct mechanical mixing of a sample with a sorbent which is based on silica attached mainly to octadecyl groups. The technique involves homogenization of a small amount of a sample. It is worth mentioning that during grinding, there are mechanical shearing forces that are present and cause the disruption of a tissue structure by dispersing the sample on the carrier sorbent surface by hydrophilic and hydrophobic interactions. During sample preparation with MSPD, a semi-dry and homogeneous mixture of a sorbent and a sample is prepared. After homogenization, the mixture is transferred to an SPE column where it is eluted with an appropriate eluent (a single organic solvent or a mixture). The MSPD application range is broad and it can thus be employed to perform the simultaneous extraction of numerous compounds from various matrices, e.g., essential oils from herbs [10], catechins and alkaloids from green tea leaves [11], rutin from black elderberry [12], phenolic compounds from tea fruit [13], pesticides from fruits [14,15,16], and free fatty acids from chocolate [17].
The aim of this study is to present the latest achievements resulting from the use of MSDP in scientific research as well as routine laboratory practices. Technical solutions that may extend its applicability are also discussed. Special attention is also paid to the sorbents used in MSPD, and these are not only based on modified silica but also a range of other materials, e.g., carbon materials and molecularly imprinted materials. The employment of appropriate solvents for elution constitutes another aspect for consideration. For such purposes, organic solvents are most frequently applied. Still, it should be pointed out that according to the requirements of “green chemistry”, the use of more eco-friendly solvents including deep eutectic solvents is recommended, which is also presented in this publication.
2. Routes of Sample Preparation by the MSPD Technique
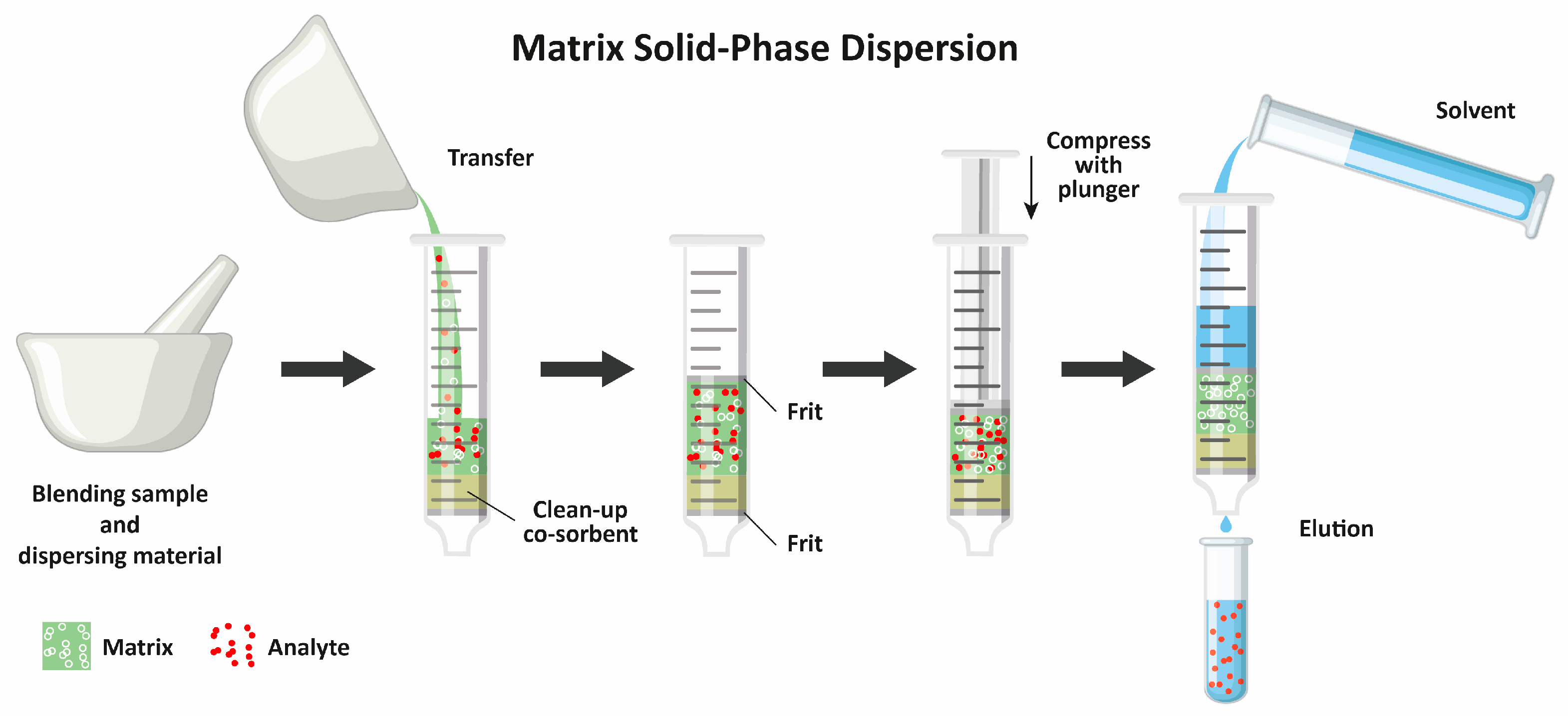
The MSPD procedures are implemented in two stages (Figure 2). In the first one, the sample is mixed with a solid sorbent, which enables the crushing of the matrix structure (plant- or animal-origin material). In the second one, the analytes are eluted from the sample with an appropriate solvent. Both stages can be performed using different techniques or methodologies. In the first MSPD stage, the sample (often cut into small pieces or pulverized) is ground in a mortar with selected solid materials using a pestle. The solid particles enable the disruption of plant and animal cells and the simultaneous absorption of the liquids together with the analytes. Among different sorbents, the most widely used are silica gel [18,19,20,21,22,23], octadecyl-modified silica gel [24,25,26,27,28], Florisil [29,30], and diatomaceous earth [31,32], which are sometimes mixed with other sorbents or drying agents [33,34,35]. However, apart from sorbents possessing active surfaces capable of interaction with the analytes, inert, nonporous materials are sometimes used, which can only crush the matrix, e.g., sand [36,37]. In all of the aforementioned studies, no solvent was added to the sample ground in the mortar. However, in some studies, the addition of solvent was also tested. For example, Wianowska and Dawidowicz added 1,4-dioxane [26], Wu et al. used deep eutectic solvent [38], and Chatzimitakos et al. applied acetonitrile [39]. Interestingly, there are also studies where no solid material was added in the first MSPD stage and only a specialized solvent (e.g., ionic liquid) was used without the need for crushing the sample in a mortar [40].
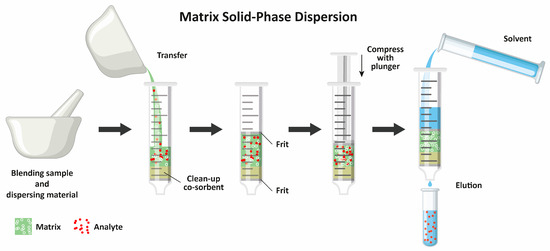
Figure 2.
Scheme of the MSPD procedure.
Alternatively, the sample may be weighed into a plastic test tube and crushed in the presence of a sorbent using steel balls shaken by hand or with a vortex [41,42]. A similar approach was proposed by Yang et al. who homogenized a powdered sample with an octadecyl-bonded silica (C18) sorbent in a centrifuge tube [43]. In this approach, the samples of leafy vegetables were subjected to preliminary blending and freeze-dried. However, such a simplified method cannot guarantee that the sorbent will disrupt plant cells like in the studies where metal balls were used.
In the second stage of MSPD, different procedures are possible depending on the type of the first stage. In the most widely used procedure, the solid mixture of the sample with the adsorbent is packed into an empty column, usually an empty SPE tube. The sample can be transferred from the mortar to the column as is, i.e., placed within two frits (or cotton plugs) without any other solid material [18,19,20,21,26,33,35,37,38,44,45,46], or a portion of a sorbent, a drying material, or sand can be added below and/or above the sample [24,29,32,36,47,48]. The analytes are then eluted from the sorbent, which may be performed in one step or with successive portions of different solvents used for cleanup and elution. Finally, the sample extract is centrifuged and/or filtered before injecting it into a chromatographic column. It must be noted that the procedure can be substantially minimized. Yuan et al. [49] ground only 3 mg of a sample and 2 mg of a sorbent in a mortar. The resulting mixture was transferred to a 200 µL pipette tip containing degreased cotton at the bottom. After covering the sample with another portion of degreased cotton, a 1 mL tip was assembled to enable the addition of 1 mL of a solvent. The extract was obtained by centrifugation and subjected to HPLC analysis. This procedure shows the great possibilities offered by MSPD in the analysis of relatively small samples; however, when developing each analytical method, one must remember to take a representative sample for analysis.
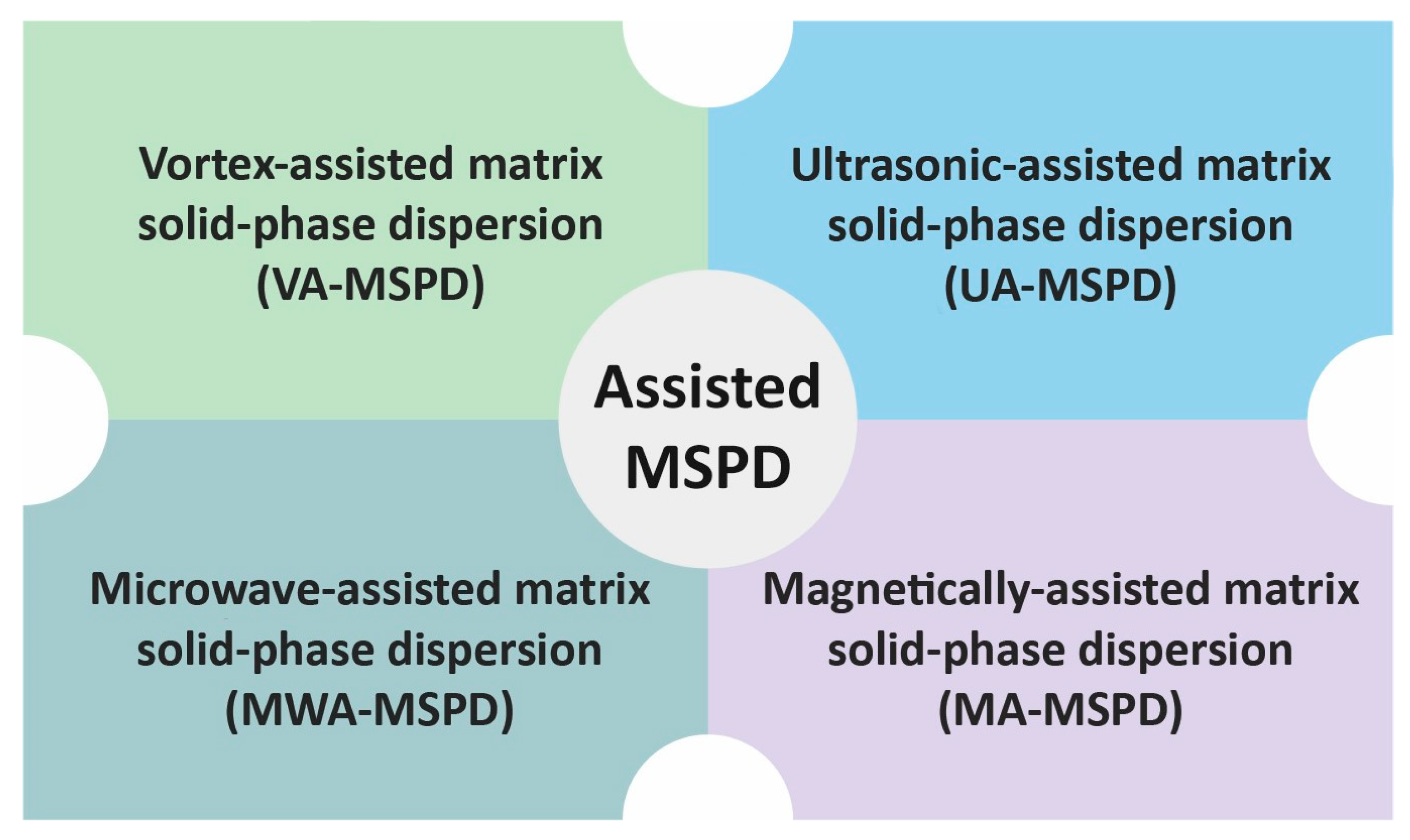
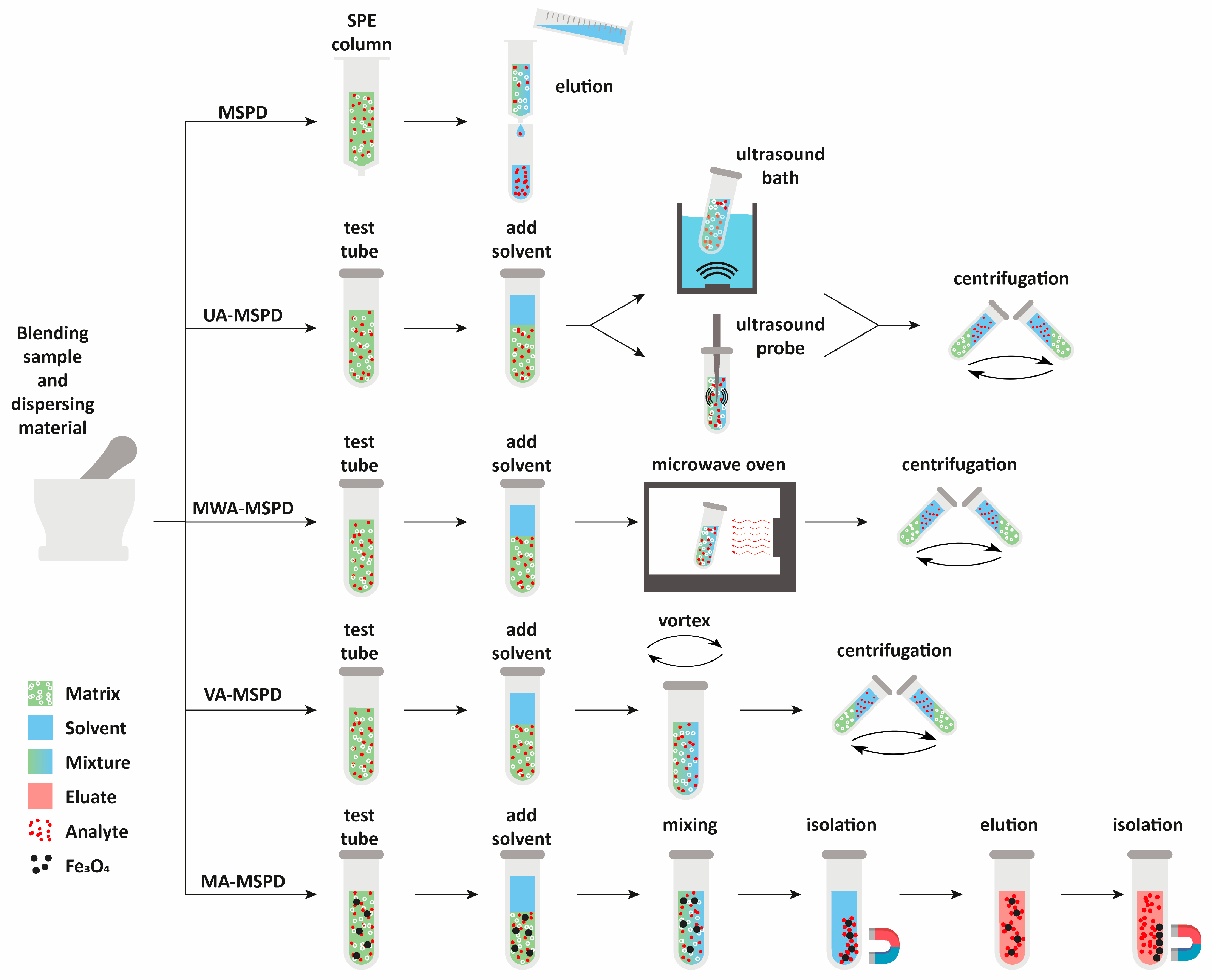
The sample from the first stage of MSPD may also be further processed without packing it in a column. Instead, the sample may be transferred to a test tube or a centrifuge tube and subjected to extraction with a solvent. For this purpose, efficient mass transfer between the solid material and solvent must be ensured. Such solvent extraction can be performed using various technical solutions that contribute to better process efficiency (Figure 3).
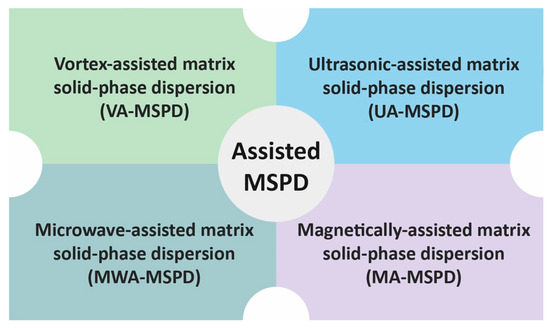
Figure 3.
Different technical solutions used in MSPD methods.
The sample may be subjected to vortex-assisted extraction (VA-MSPD) [25,30,31], ultrasound-assisted extraction (UA-MSPD) [22,23,34], microwave-assisted extraction (MWA-MSPD) [50,51,52], or magnetically assisted extraction (MA-MSPD) [39,53,54].
In vortex-assisted extraction, the sample is placed in a tube and then some solvent is added. The tube is vortexed so that the analytes can migrate into the liquid phase. Then, the solvent is separated by filtration [31] or by centrifugation [30]. The sample can also be subjected to sequential extraction with a few portions of the extracting solvent. Each added solvent portion is vortexed and centrifuged, and the separated portions of sample solutions are combined to ensure the complete extraction of analytes [25].
The extraction of analytes from a complex solid matrix such as plant material or food often requires the use of large amounts of solvents to increase recoveries in the MSPD technique. A good alternative seems to be the use of support in the form of an additional energy source, such as microwave energy or ultrasound. These treatments ensure an increase in extraction efficiency while reducing the volume of the eluent used, as well as shortening the extraction time.
Ultrasound-assisted extraction is often used to release bioactive compounds such as vitamins, polyphenols, polysaccharides, and other phytochemicals or emerging contaminants from plant material. The application of ultrasound is an effective way to enhance mass transfer processes because it causes cavitation and associated mechanisms such as the micro-motion of liquid jets, compression and decompression in the material with the subsequent disruption of cell walls, and high rates of heating and cooling. The mechanical effects of ultrasound-induced cavitation intensify the penetration of the solvent into the cell and improve mass transfer between the cell and the solvent, thanks to which intercellular materials are transferred to the solvent, which translates into a higher extraction efficiency.
Albero et al. [34] developed a sensitive and rapid method for determining 17 emerging contaminants in four vegetables (carrots, lettuce, onions, and tomatoes) in order to assess the potential significance of this exposure route for human health. In their research, they used two-stage ultrasound-assisted extraction. The pre-homogenized vegetable sample with Florisil and anhydrous magnesium sulfate was transferred into glass columns with Teflon frits and anhydrous magnesium sulfate. Then, ethyl acetate/methanol (9:1, v/v) containing 3% of ammonia solution was added. The column was sonicated in a water bath to speed up extraction. Then, the extracts were collected in tubes using a multiport vacuum manifold and evaporated. A second extraction step was performed using ethyl acetate/methanol (9:1, v/v) containing 3% of formic acid—ultrasonic-assisted extraction, elution, and evaporation. Finally, the sample was reconstituted in acetonitrile before derivatization in the gas chromatograph injector and analysis. As an alternative to a water bath, an ultrasonic probe may be used. In this approach, the solid material from the first MSPD stage is transferred into a centrifuge tube and an appropriate solvent is added. The ultrasonic probe is introduced to the sample to facilitate extraction and then the sample is centrifuged and the supernatant is taken for analysis [22,23].
Microwave energy was first used for extraction by Ganzler et al. in 1986 [55], showing its advantage over Soxhlet or shake-flask extraction methods. Microwave extraction uses electromagnetic waves that penetrate the raw material and interact with the molecules, transferring energy that heats the material, thereby accelerating the process. Although dried plant material is used in most cases in the extraction process, plant cells always contain trace amounts of moisture, which is heated during irradiation and then evaporates, generating pressure that affects the walls of plant cells, which leads to their disruption. This results in accelerating the mass transport process and improving extraction efficiency. Dos Santos et al. [51] developed an MWA-MSPD procedure for the extraction of 36 pesticides from fruit and vegetable samples. Three other modifications of MSPD were also tested in that study: VA-MSPD with the use of a vortex, UA-MSPD with the use of an ultrasonic bath, and UA–MSPD with the use of an ultrasound probe. According to the authors, the developed MWA-MSPD method is efficient, simple, cheap, robust, and environmentally friendly because they used inexpensive sand to homogenize the samples. Li et al. [50] were the first to develop a dynamic microwave extraction method coupled with MSPD for the extraction of six triazine herbicides from rice. The alumina sample was ground in a mortar and then quantitatively transferred to the extractor and placed in a microwave oven. The extraction solvent was introduced into the extractor using a peristaltic pump set to a flowrate of 2.0 mL min−1. Zhang et al. [52] also used a method based on dynamic microwave-assisted extraction coupled with MSPD in the extraction procedure. The method they developed allows extraction and purification to be performed in one stage simultaneously for 15 samples in 4 min. The MSPD elution procedure was performed under the influence of microwaves, which significantly improved the extraction efficiency, shortened the sample pretreatment time, and reduced the consumption of organic solvent.
Magnetic sorbent extraction often takes advantage of vortex- or ultrasound-assisted extraction. However, the mixture of the solid sample containing a magnetic sorbent after extraction is separated from the solvent using a magnetic field, so there is no need for filtration or centrifugation before the analysis. For example, Gholami et al. [53] synthesized a magnetic sorbent based on an Fe3O4 core covered with a silica layer and a molecularly imprinted polymer capable of selective extraction of the analytes. The milk sample that was blended in a mortar with the sorbent and Na2SO4 was transferred to a tube and a mixture of acetonitrile with water was added. After mixing the sample for 5 min, the solid material that adsorbed melamine was separated using a strong magnet and the liquid was decanted. The sorbent was redispersed in a mixture of methanol with ammonia and sonicated for melamine extraction into the solvent. The magnetic separation was repeated to obtain a liquid sample. A similar procedure was used by Chatzimitakos et al. [39] who ground in a mortar a dried vegetable sample with magnetic graphene oxide and acetonitrile. After the addition of water, the sample was transferred to a beaker and sonicated. The sorbent was separated with a magnet and washed with water twice using a magnet for separation. Finally, the analytes were eluted with acetonitrile and the solvent was separated from the sorbent using a magnet. The magnetically assisted MSPD was also combined with dispersive liquid–liquid microextraction to increase the enrichment factor of the method [54]. The authors synthesized a magnetic sorbent based on an Fe3O4 core and covered it with a polymeric layer of a sorbent. The milk sample was blended with the sorbent and Na2SO4 in a mortar. The resulting mixture was transferred to a beaker containing water and stirred mechanically. The sorbent was separated using a magnet and water was decanted. The analytes were then vortexed with 1 mL of methanol and eluted while the sorbent was again attracted by a magnet. Methanol was mixed with 90 µL of octanol and dispersed in water and the sample was sonicated until a stable cloudy solution was obtained. After microextraction was completed, centrifugation was used to separate the solvent containing the analytes from the sample.
There are also innovative solutions enabling dispersion in the solid phase by preparing an effervescent tablet. This technique is known as effervescence-assisted matrix solid-phase dispersion (EA-MSPD). Hu et al. [56] used this method in the extraction of four coumarins from Crotex fraxini. In this study, the carbon dioxide source was a mixture of 100 mg of sodium bicarbonate and 200 mg of sodium dihydrogen phosphate, which was combined with 25 mg of benzo-15-crown-5 as an adsorbent and 25 mg of powdered plant material. All ingredients were ground in a mortar and an effervescent tablet was formed using a tablet press. The tablet was dissolved in an aqueous solution, and carbon dioxide was released as a result of the effervescence reaction, making the sample better dispersed in the extraction solvent (100 mM sodium dodecyl sulfate), whereas Qian at al. [57] used 0.592 g of sodium carbonate and 0.658 g of oxalic acid as the effervescent mixture. In this study, a powdered sample and an effervescent mixture were ground using a ball milling machine.
To sum up, there are many technical solutions that have been proposed by researchers to improve the process of plant material extraction. Proposals to support MSPD extraction lead to an increase in process efficiency, shortening the time and minimizing the amount of reagents used. Figure 4 shows the methods of performing MSPD extraction.
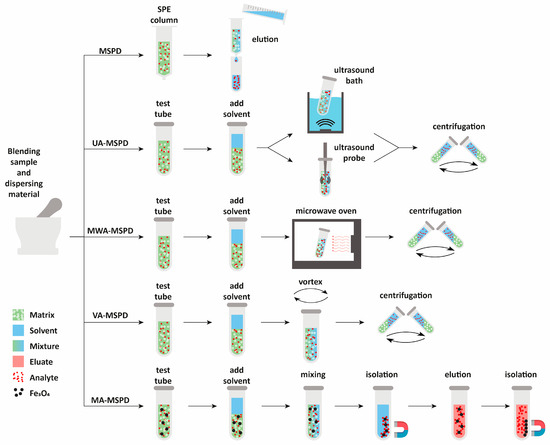
Figure 4.
The methods of performing MSPD extraction procedure. UA-MSPD—ultrasonic-assisted MSPD; MWA-MSPD—microwave-assisted MSPD; VA-MSPD—vortex-assisted MSPD; MA-MSPD—magnetically assisted MSPD.
3. Sorbents Used in MSPD
MSPD uses various sorbents which are placed in a mortar together with the sample. The mixture is then ground with a glass or porcelain piston. In this stage, the sorbent acts as an abrasive agent, which is intended to break the initial physical structure of the sample matrix and fragment it into small elements, and may also act as an adsorbent for the analytes of interest. The pre-prepared material is introduced into a glass or plastic column, which may contain an additional sorbent to clean the sample during elution. The sorbents used are different, starting from very simple ones, such as sand, whose role is only to fragment and disperse the sample, to very complex sorbents based on molecularly imprinted polymers used for specific analytes. In the lower part of the column, a frit or paper disc is placed, on which the dispersed sample or an additional sorption material and sample are placed. The frit or paper is placed again at the top of the column and a plunger is used to compress the column contents to remove air bubbles or prevent channeling of the eluent. The last step is to elute the analytes from the column and determine them. The sorbents used in MSPD are similar to those used in SPE for selective adsorption with simultaneous purification. The choice of sorbent used to homogenize the sample is guided by the nature of the material being analyzed (Table 1).
The MSPD technique is used in food analysis to determine both contaminating substances, such as pesticides or mycotoxins, as well as natural compounds present in plant samples (such as phenolic compounds). Nevertheless, following current literature reports, it can be concluded that scientists focused mainly on the characterization or determination of natural ingredients in plants and fruits.
Barker et al. [24], recognized as the inventors of the MSPD technique, used C18 (octadecyl-modified silica gel) as the sorbent in their research. Many scientists followed their example and used this sorbent in their research. Dias Soares and Fernandes used the C18 sorbent both as a dispersing and cleaning agent in the determination of acrylamide in processed cereal products, chocolates, and baby food. Interestingly, they used pure water as the eluent and not, like Barker et al., a complicated sequential elution with organic solvents [58]. The C18 sorbent was also successfully used to analyze flour samples for the presence of 14 mycotoxins. Elution was carried out using an acetonitrile/methanol mixture (1:1, v/v) with the addition of 1 mM ammonium formate [28].
Mansur et al. [59] tested four types of sorbents by analyzing buckwheat sprouts for their flavonoid content. The best results were obtained using the C18 sorbent, where the ratio of C18 sorbent to the sample was 2:1 (w/w) and elution was carried out with 5 mL of 80% ethanol. The developed MSPD extraction method was compared with two frequently used extraction techniques: homogenate-assisted extraction (HAE) and ultrasound-assisted extraction (UAE). However, the MSPD method proved to be more efficient and effective.
A frequently used abrasive is also silica gel. This material was used to grind safflower to determine hydroxysafflor yellow A and kaempferol. The ratio of silica gel to sample mass was selected to be 3:1. The sample ground in an agate mortar was transferred to a column with a cotton layer and the analytes were eluted with a methanol/water mixture (1:3, v/v) and then subjected to chromatographic analysis. The developed and optimized method was compared with two other extraction methods using the Soxhlet apparatus and ultrasound-assisted extraction. The MSPD procedure proved to be more convenient and less time-consuming, with reduced sample and solvent requirements [60]. A similar procedure was used to determine two phenolics and three terpenoids from the Euphorbia fischeriana. It was found that the optimal ratio of the mass of the sample to the mass of silica gel as the dispersing sorbent was 1:2. In this procedure, no additional purification sorbent was used in the column. Elution of the analytes was carried out with a water/ethanol mixture (3:7) and the analytes were determined using ultra-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry (UPLC/Q-TOF-MS) [20]. Silica gel was also used to prepare samples of Saururus chinensis, a plant well known in folk medicine in China and South Korea. Four amides (N-p-trans-coumaroyltyramine, aristolactam AII, sauristolactam, and aristolactam BII) were extracted from these samples and determined [21]. Recently, Zou et al. [61] proposed a rapid and effective strategy for the isolation of four anti-inflammatory flavonoids from Lindera aggregata (Sims) Kosterm Leaves by combining MSPD with high-speed counter-current chromatography (HSCCC). In that MSPD procedure also, only silica gel was used in the preparation of plant material.
It is worth mentioning here the work of Dong et al. who also used silica gel as a sorbent for grinding the plant sample. A novel micro-MSPD method was successfully used for the extraction of quaternary alkaloids from Phellodendri chinensis cortex. Amounts of 25 mg of silica gel and 25 mg of the sample were weighed into an agate mortar and then ground with a pestle for one minute to ensure proper fragmentation of the sample. After homogenization, the mixture was quantitatively transferred into a 1 mL plastic solid-phase extraction (SPE) column. The analytes were then eluted with 200 μL of methanol/water (9:1) containing 150 mM sodium hexane sulfonate at pH 4.5. A neutral ion pair was formed between the ion-pairing reagent (eluent) and positively charged alkaloids in this process, which was beneficial for the selective extraction of polar alkaloids [19].
There are few works that use naturally occurring sand as a grinding material compared to studies using silica gel. Both materials are silicon dioxide (SiO2) and the difference is that sand is a crystalline, nonporous form, while silica gel is non-crystalline and highly porous, which makes it a good adsorption material and drying agent. Therefore, sand can only act as an abrasive material on the sample. Castillo et al. developed a method for extracting bioactive compounds from the microalga Haematococcus pluvialis using sand as a material supporting sample homogenization, which makes this method inexpensive and environmentally friendly [36].
Another material of natural origin used in MSPD is diatomite (diatomaceous earth), specifically a sedimentary rock composed of approximately 80–90% diatomaceous shells. Quian et al. in their research presented a green analytical method for the rapid determination of ergosterol in edible mushrooms. The sample powder was dispersed in diatomaceous earth in a ratio of 1:5. The homogeneous mixture was transferred to an SPE column that contained the C18 sorbent. The mixture was first washed with 70% ethanol, and then the analytes were eluted with 100% ethanol [32]. Li et al. developed an ultra-fast and ecological method for the determination of loganic acid and gentiopicroside in Gentianae Macrophyllae Radix (GMR) by vortex-assisted matrix solid-phase dispersion extraction (VA-MSPD) and liquid chromatography–mass spectrometry (LC-MS). They used the same ratio of sample to dispersant (diatomaceous earth), i.e., 1:5. In that work, the homogenized material was not introduced into the column but was shaken with 0.5 mL of 15% ethanol for 0.5 min [31]. Chen et al. in their research used diatomaceous earth with the addition of silanol groups (Celite AZO). Such a sorbent has a larger specific surface area and lower surface activity [62].
Wianowska and Dawidowicz [26] conducted research in which they compared three different abrasive materials (C18, sand, silica gel) supporting sample homogenization without and with dispersing liquid. It turned out that the solventless MSPD method using the C18 sorbent was superior to those using sand or silica gel, ensuring satisfactory accuracy and precision.
Another routinely used sorbent in MSPD is Florisil, i.e., magnesium silicate. It is used both as a dispersing material and a purifying sorbent in the SPE column. Purdešová and Dömötorová used Florisil in the homogenization stage of an apple sample. The sample was transferred from a mortar to a column containing glass wool with anhydrous magnesium sulfate to dry it. Pesticides from the column were eluted with 60 mL of ethyl acetate. The eluate was collected and concentrated to dryness using a rotary vacuum evaporator, and the dry residue was dissolved in 1 mL of toluene [29]. While developing a method for determining pesticides in freeze-dried egg samples, dos Reis Souza et al. tested Florisil and the C18 sorbent as a dispersing and cleaning material in MSPD. Ultimately, the best results were obtained when Florisil was used as a dispersant and C18 as a cartridge column support [63]. Also, Silva et al. [64] undertook the determination of pesticides. The sample was coconut pulp, which is a by-product of the production of coconut oil and milk. Pesticides are widely used in various stages of coconut palm cultivation to control pests and diseases and thereby increase yields. As a result, this translates into a need to control the pesticide content in coconuts. This study assessed various method parameters, including the type of solid phase (C18, alumina, silica gel, and Florisil). Based on the results obtained, it was determined that the combination of C18 as an abrasive, Florisil as a cleaning layer in the SPE column, and n-hexane-saturated acetonitrile as an eluting solvent is a suitable procedure for the extraction of eight pesticides in a complex matrix such as coconut pulp. In addition to Florisil and the homogenized sample, the column contained 0.1 g of silanized glass wool as a first layer, and then 1.0 g of anhydrous Na2SO4.
Anhydrous sodium sulfate (Na2SO4) or anhydrous magnesium sulfate (MgSO4) is usually employed together with a sorbent in the matrix dispersion step, or used as a cleanup co-sorbent in order to eliminate residual water. These additives are particularly used when extracts are analyzed by gas chromatography (GC), and it is necessary to remove residual water. In some cases, they are the only agent used in the extraction step, as a cleaning and drying agent, without the use of another additional SPE sorbent [65]. There are works in which the above-mentioned salts and an additional sorbent, such as Florisil or sand, are added in the sample homogenization stage or as a cleanup sorbent in the SPE tube [47,48,66].
In MSPD extraction, proper dispersant selection is of paramount importance to achieve high extraction yields, since the dispersant is used not only as an adsorption separation material to adsorb the target analytes but also as a solid support material to break up and disperse the sample. Therefore, studies often test several popular dispersants before choosing the one that works best. Wang et al. [67] evaluated the performance of several popular dispersants, such as acid alumina, C18, silica gel, diatomaceous earth, and Florisil. Acid alumina was selected from that wide range of sorbents and was also introduced into the SPE column as an extract-cleaning agent.
Among the conventional polar sorbents, alumina (Al2O3) is worth mentioning, as it is still successfully used. This is evidenced by the recently published work by de Carvalho et al. [68], who tested three adsorbents (neutral alumina, silica gel, and C18) and ultimately chose natural alumina for the extraction of crystal violet and congo red dyes from shrimp samples. Wang et al. [69] used MCM-41 in their research. MCM-41 is an innovative nanostructured material, specifically mesoporous silica modified with alumina, characterized by a hexagonal ordered mesopore structure, large surface area, large pore volume, and excellent thermal stability (surface area: >900 m2/g, silica/alumina ratio: 100:1, pore size: 3.5 nm). An industrial MCM-41-miniaturized MSPD extraction coupled with the response surface methodology was explored to determine six flavonoids in Pollen typhae by ultra-high performance liquid chromatography connected to a photodiode array detector. He et al. [70] in their research tested seven types of adsorbents (Beta 40 (40, SiO2/Al2O3 ratio), Beta 100 (100, SiO2/Al2O3 ratio), ZSM-22 (70, SiO2/Al2O3 ratio), ZSM-35 (60, SiO2/Al2O3 ratio), ZSM-5 (300 nm), aluminum oxide-A, and C18). Ultimately, two zeolites Beta 40 and ZSM-22 were selected in the form of a mixture of sorbents for the simultaneous extraction and determination of eight compounds (two phenolic acids, two iridoid glycosides, vanillin, and three flavonoids) of different polarity from Viticis Fructus using high-performance liquid chromatography in combination with a diode array detector (HPLC-DAD). Chen et al. [71] prepared a new sorbent based on NaY zeolites in the pores of which they immobilized the ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate [C4MIM][PF6]. They used this material in the preparation of Rheum palmatum L. samples for the extraction of aloe-emodin, emodin, chrysophanol, and physcion. [C4MIM][PF6] was immobilized in micropores of the NaY zeolite particles by direct impregnation. Since such a modified sorbent has many pores filled with [C4MIM]PF6, the IL-NaY-based MSPD extraction method has many important advantages, such as reducing IL consumption, maintaining IL properties, and increasing mass transfer efficiency. The presence of urushiols in the bark of Toxicodendron vernicifluum was assessed using MSPD extraction in which ZSM-5 zeolite was used as the dispersant sorbent with magnetic properties. In this study, ZSM-5@Fe3O4 was compared with popularly used dispersants such as Florisil, C18, C8, and acid alumina. Similar recoveries were also obtained using C8. Nevertheless, the use of a sorbent with magnetic properties allowed the elimination of the popularly used second elution step from the SPE column [42].
Chatzimitakos et al. [39] also used a sorbent with magnetic properties in their research. This paper describes the use of magnetic graphene oxide as a convenient sorbent, improving the method of extraction and the determination of pesticides in vegetables. However, Fotouhi et al. [54] in their research examined modified magnetic graphene oxide. First, they obtained magnetic graphene oxide (GO) and then covered it with a layer of poly(indole-thiophene) (PIT). The synthesized sorbent was used in the determination of parabens in milk. MGO@PIT and Na2SO4 were added to the milk sample and mixed to form a dry powder. The mixture was then dispersed in ultrapure water. Due to the dissolution of Na2SO4 in water, only the magnetic sorbent remains in the solution, which can be easily separated using a magnet.
In MSPD extraction methods, not only were the traditional sorbents described so far used, but new, innovative, and highly advanced materials for the extraction of selected analytes are increasingly appearing. These include, among others, molecularly imprinted polymers (MIPs). The MIP-MSPD methodology for the simultaneous determination of five steroids in goat milk samples was proposed by Ganan et al. [33]. The molecularly imprinted polymer was synthesized by bulk polymerization using 17β-E2 as the template molecule. The following ratio of individual components was selected for polymerization, i.e., template (17β-E2)/functional monomer methacrylic acid (MAA)/cross-linking agent ethyleneglycol dimethacrylate (EGDMA) 1:30:150. The sample was prepared by introducing 200 µL of milk into the mortar and adding MIP-E2, Na2SO4, and washed sea sand. After homogenization, the sample was transferred to an SPE column and the analytes were eluted with 1 mL of acetonitrile. The MIP-E2 used as a dispersant in the MSPD procedure showed high affinity to steroids, and the obtained extracts were sufficiently cleaned to be directly analyzed.
In the work of Gholami et al. [53], a miniaturized MSPD procedure was presented in which the SPE column was eliminated. Instead, a silica-based magnetic sorbent coated with a molecularly printed polymer was used. In order to obtain a selective sorbent for the isolation of melamine from milk, first, Fe3O4 was obtained by co-precipitation from two- and three-valent iron salts, and then these particles were enclosed in a SiO2 structure using the sol–gel method. Afterward, NH2 groups were introduced onto its surface by adding dropwise 3-aminopropyltrimethoxysilane (APTMS) to the Fe3O4@SiO2 dispersion in toluene. The material was further modified to obtain a selective sorption material with molecular mapping. A pre-prepared mixture of melamine with methacrylic acid (MAA), ethylene glycol dimethylacrylate (EGDMA), and 2,2′-azobis(2-methylpropionitrile) initiator (AIBN) was added to the Fe3O4@SiO2-NH2 particles dispersed in the solution. For sample preparation, 30.0 mg of the resulting magnetic molecularly imprinted polymeric (M-MIP) sorbent was mixed with 150 µL of the milk sample and 400.0 mg of Na2SO4, and the whole was homogenized in a glass mortar. The blended mixture was transferred to a test tube and then 5.0 mL of deionized water/acetonitrile (1:1, v/v) was added and shaken to eliminate matrix interference and also dissolve Na2SO4. To optimize the extraction process, a central composite design (CCD) based on the response surface methodology (RSM) was used as an ideal method to assess the simultaneous influence of multiple parameters and their interactions, optimize multifaceted processes, and empirically establish polynomial relationships between the target response and independent parameters.
Another example of the use of selective MIP sorbents is the extraction of camptothecin from Camptotheca acuminate. In this study, carbon nanotubes were used as a support for the polymer. Camptothecin was used as a template, MAA as a functional monomer, and EGDMA as a crosslinker. The powdered plant material with the MIP sorbent was placed into a glass mortar and ground. The homogenized sample was loaded into a cartridge and rinsed with 10% aqueous methanol, and then the analyte was eluted with acetic acid/methanol (5:95, v/v) [72].
Yuan et al. [49] took advantage of the specific properties of graphene oxide to prepare a new poly(deep eutectic solvents)-surface-imprinted graphene oxide composite (PDESs-MIP/GO) with high selectivity towards phenolic acids, using deep eutectic solvents as monomers and crosslinkers. As is known, graphene oxide (GO) has the structure of two-dimensional sheets with a large specific surface area. There are a large number of various functional groups on its surface, such as hydroxyl, carboxyl, and epoxy. Therefore, it can be easily modified by reaction with other functional groups to improve its adsorption capacity. Hence, in this study, an imprinted layer of PDESs is grown on the GO surface to increase the adsorption interactions and adsorption capacity of materials and achieve improved selectivity. PDES-MIP/GO was prepared using sulfhydryl-functionalized GO as a carrier; two different DESs—one as a functional monomer and the other as a cross-linker; and epinephrine as a template. In the polymerization, epinephrine was selected as the dummy template.
The use of a template in the synthesis of molecularly imprinted polymers, which is the same compound as the analyte, involves the difficulty of washing it out of the polymer and the subsequent leakage of unwashed traces. This leakage will seriously affect the quantitative results of target analytes, especially for high-sensitivity detection. Dummy templates are used to eliminate this problem because their leakage will not affect the results. Analogs or compounds with the same structural fragments as the analytes are used as dummy templates. The materials prepared are called dummy molecularly imprinted polymers (D-MIPs). Zhang et al. [73] used dummy molecularly imprinted microspheres (D-MIMs) for the extraction of azole fungicides. D-MIM was prepared by Pickering emulsion polymerization. Alpha-(2,4-Dichlorophenyl)-1H-imidazole-1-ethanol (DCE) was used as the fragment dummy template. The pre-polymerization solution was obtained by dissolving the dummy template DCE, the monomer MAA, the crosslinker EGDMA, and the initiator AIBN in toluene. The SiO2 nanoparticles dispersed in a 0.2% aqueous solution of Triton X-100 were used as the aqueous dispersion phase. Then, a Pickering emulsion was obtained by mixing the pre-polymerization solution and the aqueous dispersion phase using a high-shear dispersion homogenizer (15,000 rpm for 2 min). The size of the imprinted microspheres was carefully optimized by changing the high-shear dispersing time and speed. The SiO2 nanoparticles remaining on the D-MIM surface were removed by soaking the sorbent in hydrofluoric acid overnight. The obtained polymer was ground with fish samples in a mortar, and then the homogenized powder was transferred to an SPE column containing 200 mg of D-MIM. After washing with water and acetonitrile, the azole fungicides were eluted with a mixture of methanol/TFA (98:2, v/v).
Another type of molecular mapping is a novel technique proposed by Arabi et al. [37], who developed the synthesis of dummy molecularly imprinted silica nanoparticles (D-MISNPs). They developed a new strategy for the sol–gel synthesis of a selective sorbent based on a single step using APTMS as the functional monomer, propanamide as the dummy template, and tetraethyl orthosilicate (TEOS) as the cross-linker. The sorbent obtained in this way was used as a dispersant and selective agent in the extraction of acrylamide using the MSPD technique.
In recent years, mesoporous molecular sieves have attracted great interest as sorbents due to their mesoscopic-scale ordered arrangement of mesopores, narrow pore size distribution, and large specific surface area and pore volume. Molecular sieves are crystalline metal aluminosilicates with a three-dimensional network connecting silica and aluminum oxide tetrahedra. Among the molecular sieves developed so far, KIT-6 should be mentioned, which is characterized by a three-dimensional, symmetric cubic Ia3d structure with two interpenetrating continuous channel networks. This material was used in MSPD extraction by Cao et al. [35]. Compared to common silica-based sorbents (C18 and activated silica gel), the proposed KIT-6 dispersant shows excellent adsorption capacity for bioactive flavonoids from toothpaste, plants, and saliva. Chu et al. [74] used the TS-1 mesoporous molecular sieve as a solid supporting material in micro-MSPD extraction. The developed method is characterized by good precision, satisfactory recovery, and low detection limits of lignans. The same material has also been successfully used to extract sesquiterpenoids from herbal medicines [75]. Du et al. [76] developed an ultrasound-enhanced matrix solid-phase dispersion microextraction applying mesoporous molecular sieve SBA-15 for the determination of multiple compounds in Fructus Psoralea.
It is also worth mentioning that carbon molecular sieves (CMSs), which exhibit a narrow pore size distribution, allow some molecules to be distinguished from others based on their size, shape, and state of adsorption equilibrium. The pore surface area of CMS is very small and its effective diameter is in the nanoscale range. CMS can be obtained from all kinds of carbon materials. This material was also validated in MSPD extraction by Du et al. [45]. In this study, seven dispersants (CMS, C18, mesoporous carbon, silica gel, carbon nanotubes, graphene oxide, and Florisil) were investigated for the extraction of polyphenols from pomegranate peel. Among them, the best results, proving good extraction efficiency of the analytes, were obtained by CMS. This could be attributed to the larger specific area and uniform channels of the CMS micropores, which provided a high adsorption capacity, seemingly ideal for dispersing target compounds.
The adsorption potential of carbon material (e.g., carbon nanotubes, graphene oxide, graphene, or graphite) was also checked in the MSPD technique [39,45,49,54,77,78,79]. Large amounts of graphene can be easily produced from graphite by chemical methods at a relatively low cost, and the resulting graphene is usually referred to as chemically converted graphene (CCG). This makes it possible to use graphene in adsorption applications. Liu et al. [79] in their study noted that grinding a solid sample with chemically converted graphene (CCG) powder achieved close contact and sufficient dispersion of the sample matrix due to the large surface area and flexible morphology of CCG nanoparticles, while Sun et al. [78] compared the adsorption capabilities of graphene-encapsulated silica (GES) with graphene, silica gel, C18 silica, neutral alumina, and diatomaceous earth. GES turned out to be the best adsorbent for the analytes proposed by the authors. This may be due to the large delocalized π–electron system and, therefore, GES can interact with the benzene ring compounds through strong π-π interactions.
Another interesting material is cyclodextrin (CD), which has various applications due to its low toxicity, excellent solubility in water, and good adsorption capacity. The most common α-, β-, and γ-cyclodextrins consist of 6, 7, or 8 glucose units, respectively, connected by α-1,4-glycosidic bonds, together forming a cyclic structure. Due to numerous hydroxyl groups, cyclodextrins have special chemical properties, which translates into their interactions with other chemical functional groups. β-cyclodextrin has been successfully used in the extraction of antioxidant ingredients from Mori Fructus [80], or isomers of chlorogenic acid and dicaffeoylquinic acid from plants [81]. The methylated derivative of β-CD can form a stronger complex with analytes and shows better solubility and lower toxicity than the unmethylated ones. Due to this, the usefulness of 2,6-dimethyl-β-cyclodextrin (DM-β-CD) in the extraction of terpenoids, crocins, quinic acid derivatives, and flavonoids in Gardeniae fructus was checked. The recoveries of the eight compounds ranged from 96.6 to 100% (RSD < 3.50%) [82].
Another cyclic compound acting in chemistry on a host–guest basis, just like cyclodextrins, is calixarene. It is a macrocyclic or cyclic oligomer based on phenols connected by methylene groups. Thanks to hydrophobic cavities, it can accommodate smaller molecules or ions in its structure. These abilities were exploited by Young et al. [44] in the extraction of organic acids from fruit. The class of cavitands also includes crown ethers (CEs). In the work of Hu et al. [56], a new extraction method was developed for coumarin compounds from the natural medicinal plant Crotex fraxini using the cavity and hydrophobic properties of CE as an adsorbent. In this study, an effervescent tablet containing carbon dioxide sources (sodium bicarbonate and sodium dihydrogen phosphate) and an adsorbent (benzo-15-crown-5) was prepared. The effervescent tablet was dissolved in an aqueous solution, and the effervescence reaction generated carbon dioxide, making the sample better dispersed in the 100 mM sodium dodecyl sulfate extraction solvent.
Metal oxides, such as ZnO [83] or TiO2, have recently been used as adsorbents in MSPD. In two of their works, Gómez-Mejía et al. used commercially available TiO2 nanoparticles to develop simple and economic methods of MSPD extraction. They used TiO2 nanoparticles in the extraction of polyphenols from brewer’s yeast residues [84] and phenolic compounds from grape and grape pomace wastes [85]. TiO2 and ZnO nanoparticles interact with phenolic compounds through their diol groups in a chelation-like manner, which contributes to their better isolation from the matrix and increased selectivity.
Recently, there have been works using waste material as an adsorbent. Such material has potential due to the possibility of waste management. Jiang et al. [86] in their research checked the possibility of using crab waste obtained from local restaurants as a sorbent in the MSPD technique. Crab is one of the ten most frequently consumed seafood products. However, only a small part of its mass (20–30%) is suitable for consumption and the rest constitutes waste, hence the idea to manage this bio-waste, which mainly consists of chitin (or chitosan). The advantages of using crab shells as an adsorbent include low cost, high mechanical strength, rigid structure, resistance to drastic conditions, and high biocompatibility. Using waste-containing chitosan is as effective as using pure chitin. The use of medium-molecular-weight chitosan as an adsorbent in the extraction and determination of natural phenols from olive fruits was verified. In the developed method, recoveries were over 80.06% [87]. Currently, a lot of bio-waste comes from the agricultural industry, including residues from the production of fruit juices, such as peels. Among numerous agricultural wastes, mangosteen fruits are gaining attention as a biosorbent. About 60% of mangosteen fruit by weight is its peel, which creates a significant amount of agricultural waste. Thanks to this, mangosteen peel can be a suitable raw material for the production of much cheaper and more efficient biosorbents due to the abundance and availability of this waste. Peng et al. [88] in their work carbonized mangosteen peels and obtained activated carbon, which they used as an adsorbent in the extraction of flavonoids from Dendrobium huoshanense.

Table 1.
Summary of studies presenting the use of extraction sorbents in 1st and 2nd stages of matrix solid-phase extraction.
Table 1.
Summary of studies presenting the use of extraction sorbents in 1st and 2nd stages of matrix solid-phase extraction.
| Analytes | Samples | Sorbent in 1st Stage | Sorbent in 2nd Stage | Detection | LOD [µg/mL] | Recovery [%] | RSD [%] | Ref. |
|---|---|---|---|---|---|---|---|---|
| Mycotoxins | Flour | C18 | - | LC-MS/MS | 0.0001–0.075 # | 69–90 | 3–14 | [28] |
| Acrylamide | Cereal products | C18 | - | GC/MS | 0.0052 # | - | 1–4 | [58] |
| Flavonoids | Buckwheat | C18 | - | HPLC DAD | 0.01–0.03 | 96–104 | 1–7 | [59] |
| HSYA and kaempferol | Carthamus tinctorius L. | Silica gel | Cotton | HPLC-DAD | 0.035 and 0.015 | 93–102 | 1.5–3.5 | [60] |
| Phenolics and terpenoids | Euphorbia fischeriana | Silica gel | Cotton | UPLC-DAD | 0.019–0.095 | 92–103 | 0.3–1.3 | [20] |
| Amides | Saururus chinensis | Silica gel | - | HPLC-DAD | 0.03–0.09 | 97–100 | 1.9–2.8 | [21] |
| Flavonoids | Lindera aggregata | Silica gel | Cotton | HPLC-UV | - | - | 1.3–4.0 | [61] |
| Alkaloids (berberine and phellodendrine | Phellodendri chinensis | Silica gel | - | UHPLC-DAD | 0.03–0.04 | 81–91 | 3.6–3.9 | [19] |
| Carotenoids | Haematococcus pluvialis | Sand | Sand | HPLC-DAD | - | ~100 | - | [36] |
| Ergosterol | Edible fungi (Cordyceps militaris and Cordyceps sinensis) | Diatomaceous earth | C18 | HPLC-DAD | 0.04 | 100 | 2.3 | [32] |
| Loganic acid and gentiopicroside | Gentianae Macrophyllae, Gentianae crassicaulis, Gentianae dahuricais | Diatomaceous earth | - | LC-MS | 0.02–0.04 | 96–107 | 3.0–3.6 | [31] |
| Echinacoside, specnuezhenide, oleuropein, and nuezhenoside G13 | Ligustri Lucidi | Celite AZO | Chitosan | HPLC-UV | 10–15 # | 91–99 | 2.2–4.8 | [62] |
| Essential oil components | Herbal plants | C18 | - | GC-FID | - | - | - | [26] |
| Pesticides | Apples | Florisil | Anhydrous MgSO4 | GC-MS | 0.07–5.03 $,* | 71–107 | 0.3–54 | [29] |
| Pesticides | Eggs | Florisil | C18 | GC-MS | 0.003–0.015 # | 62–95 | 0.1–18 | [63] |
| Pesticides | Coconut pulp | C18 | Na2SO4 and Florisil | GC-MS | 0.02–0.17 # | 25–99 | 2.7–15 | [64] |
| Plastic additives and contaminants | Mytilus galloprovincialis | Na2SO4, Florisil, and sand | Na2SO4 | HPLC-DAD | 0.06–6.00 $ | 79–104 | 0.8–6.9 | [47] |
| Mycotoxins | Flour | C18 | - | LC-MS/MS | 0.0001–0.075 # | 71–90 | 3–14 | [48] |
| Pesticides | Tomato | Alumina | Na2SO4 and Florisil | GC-MS | 0.01–0.02 # | 77–100 | 3.7–13 | [66] |
| Lipophilic constituents | Salvia miltiorrhiza Bunge | Acid alumina | Acid alumina | HPLC-DAD | 0.03–0.04 | 84–94 | 3.0–5.6 | [67] |
| Crystal violet and congo red | Shrimp | Neutral alumina | - | DICD | 0.004–0.03 | 86–109 | 3–8 | [68] |
| Flavonoids | Pollen typhae | MCM-41 (silica modified with alumina) | - | UPLC-DAD | 0.05–0.25 | 93–103 | <4.53 | [69] |
| 8 active constituents | Viticis Fructus | Mix of Beta 40 and ZSM-22 zeolites | - | HPLC-DAD | 0.5–5.5 # | 96–105 | <0.1–4.2 | [70] |
| 4 active constituents | Rheum palmatum L. | Ionic liquid-immobilized NaY zeolite | Cotton | HPLC-DAD | 0.014–0.059 | 73–98 | 1–6 | [71] |
| Urushiols | Toxicodendron vernicifluum | ZSM-5 zeolite/Fe3O4 | - | HPLC-DAD | 0.2–0.5 | 98–103 | 0.19–0.72 | [42] |
| Pesticides | Vegetables | MGO | - | GC-MS | 0.4–4.0 $,* | 89–106 | 5.1–8.5 | [39] |
| Parabens | Breast milk | PIT@ MGO and Na2SO4 | - | HPLC-UV | 0.025 | 83–92 | 5.1–7.5 | [54] |
| Steroids | Goat milk | MIP-E2, Na2SO4, and sea sand | - | MECK-DAD | 0.6–2.17 | 81–110 | ≤12 | [33] |
| Melamine | Milk | MMIP and Na2SO4 | - | HPLC-UV | 0.067 | 87–95 | <6.3 | [53] |
| Camptothecin | Camptotheca acuminate | MIP | - | HPLC-UV | 0.00013 | 96–103 | <5.5 | [72] |
| Anti-adipogenesis markers | Solidago decurrens Lour | PDESs-MIP/GO | Cotton | HPLC-DAD | 2.0–6.1 # | 94–116 | ≤9.8 | [49] |
| Azole fungicides | Fish | DMIM | DMIM | HPLC-DAD | 0.033–0.045 # | 89–101 | 1.6–4.8 | [73] |
| Acrylamide | Biscuit and bread | DMISNPs and sea sand | - | HPLC-UV | 0.016 and 0.015 # | 84–100 | 1.2–6.7 | [37] |
| Bioactive flavonoids | Toothpaste, plant, and saliva | Mesoporous molecular sieve KIT-6 and Na2SO4 | - | UPLC- QTOF/MS | 0.02–0.04 | 89–101 | 2.2–4.8 | [35] |
| Sesquiterpenes | Curcuma wenyujin | Molecular sieve TS-1 | - | MEEKC | 5–34 | 100–102 | 0.4–2.2 | [75] |
| 8 multiple ingredients | Fructus Psoraleae | Mesoporous molecular sieve SBA-15 | - | HPLC-UV | 0.008–0.300 | 97–104 | <3.7 | [76] |
| 8 active ingredients | Radix polygoni multiflori | Silica | - | HPLC-UV | 0.005–0.12 | 93–100 | <5.3 | [45] |
| Active and toxic compounds | Complex food matrices | Nanographite | - | UHPLC-MS | 0.0007–0.052 | 82–112 | 0.3–4.0 | [77] |
| Poly-methoxylated flavonoids | Leaves of Murraya panaculata (L.) Jack | Graphene-encapsulated silica | - | HPLC-UV | 0.004–0.012 | 93–102 | 2.1–4.8 | [78] |
| Polybrominated diphenyl ethers and their methoxylated and hydroxylated analogs | Tree bark and fish | Chemically converted graphene | Na2SO4 and Florisil | LC-MS/MS | 2–91 @ | 51–114 | - | [79] |
| 6 antioxidant ingredients | Mori Fructus | β-cyclodextrin | - | HPLC-UV | 0.03–0.12 | 94–98 | <5 | [80] |
| Isomers | Honeysuckle | β-cyclodextrin | - | HPLC-UV | 0.0016–0.0033 | 87–105 | 0.1–0.6 | [81] |
| terpenoids, crocins, quinic acid derivatives, and flavonoids | Gardeniae fructus | 2,6-dimethyl-β-cyclodextrin | - | HPLC-DAD | 0.02–0.30 | 97–100 | <3.5 | [82] |
| chloro-genic acid, protocatechuic acid, malic acid, caffeic acid, and gallic acid | Chaenomeles speciosa | Calix[8]arene | - | UHPLC-Q-TOF/MS | 0.0002–0.001 | 82–113 | ≤3.84 | [44] |
| Coumarins | Cortex fraxini | benzo-15-crown-5, NaHCO3, NaH2PO4 | - | HPLC-UV | 0.0016–0.0038 | 91–100 | 0.6–4.6 | [56] |
| Multiple active compounds | Honeysuckles | Nano-ZnO | - | HPLC-DAD | 0.0014–0.014 | 71–105 | <4.3 | [83] |
| Polyphenols | Brewing yeast | TiO2 NPs and diatomaceous earth | - | HPLC-DAD | - | - | - | [84] |
| Polyphenols | Grape residues | TiO2 NPs and diatomaceous earth | - | LC-DAD-MS | 0.05–62 # | - | 1.0–7.2 | [85] |
| 4 anthraquinones | Cassiae Semen | Crab shell powder | - | HPLC-UV | 0.4–2.0 | 91–106 | 1.0–4.0 | [86] |
| Natural phenols | Olive fruits | Chitosan | - | UHPLC-Q-TOF/MS | 0.07–0.36 # | >80 | 0.9–1.6 | [87] |
| Active compounds | Functional food | Carbonized biosorbent (mangosteen-peel-based activated carbon) | - | UHPLC-Q-TOF/MS | 0.004–0.16 # | 80–100 | 1.7–5.6 | [88] |
* LOQ—limit of quantification; $—µg/kg; #—µg/g; @—pg/g. UHPLC-QTOF/MS—ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry; HSYA—hydroxysafflor yellow A; C18—octadecyl silica; DICD—digital image colorimetric detection; PIT—poly(indole-thiophene); MGO—magnetic graphene oxide; MECK-DAD—micellar electrokinetic chromatography with a diode-array detector; MIP-E2—molecularly imprinted polymer with 7β-estradiol as the template; MMIPs—magnetic molecularly imprinted polymers; PDESs-MIP/GO—poly(deep eutectic solvents)-surface-imprinted graphene oxide composite; DMIMs—dummy molecularly imprinted microspheres; DMISNPs—dummy molecularly imprinted silica nanoparticles; MEEKC—microemulsion electrokinetic capillary chromatography; NPs—nanoparticles.
4. Solvents Used in MSPD
The analytes contained in a mixture of a sample with a sorbent must be extracted to obtain a liquid sample that can be injected into analytical equipment for quantitation. The extraction may be performed in one step or preceded by sample cleanup before elution of the analytes. The solvent used for cleanup may be the same as used for elution, e.g., acetonitrile [48], or a mixture thereof with water, e.g., when 70% ethanol is used for cleanup and 100% ethanol for elution of the analytes [32]. In most studies, however, completely different solvents are used for cleanup and elution, e.g., n-hexane was used for cleanup of a sample containing plant essential oil components adsorbed on the octadecyl silica sorbent (C18), and ethyl acetate was used for their elution [26] or acetonitrile was used for the cleanup of plastic additives and other contaminants adsorbed on Florisil, but methanol/acetonitrile (3:7, v/v) was used for their elution [47].
The elution of adsorbed analytes is most often performed using organic solvents or their mixtures, which are widely applied in laboratories. For example, acetonitrile was used for the elution of pesticides from Florisil [41], methanol was applied for the elution of phenolic acids and flavonoids from the C18 sorbent [25] as well as for flavonoids from a molecular sieve [35], ethanol for isomeric triterpenoid acids from microcrystalline cellulose [46], ethyl acetate for pesticides from Florisil [29], acetonitrile saturated with n-hexane for pesticides from the C18 sorbent [64], water for acrylamide from the C18 sorbent [58], 15% ethanol for loganic acid and gentiopicroside from diatomaceous earth [31], 75% ethanol for multiple compounds contained in Fructus Psoraleae from a molecular sieve [76], or a mixture of methanol/tetrahydrofuran/water (4:3:3, v/v/v), which was used for the desorption of different compounds of various biological and pharmacological activities contained in Viticis Fructus from a zeolite-based sorbent [70].
The use of organic solvents for elution is sometimes not effective. However, there are analytes that change their form in acidic or basic media, so their extraction may be increased when an acid or a base is added to the extracting solvent. Chen et al. [42] mixed Toxicodendron vernicifluum bark powder sample with magnetic zeolite ZSM-5 in a tube using vortex and steel balls for crushing material. After removing the balls, 0.1% (v/v) trifluoroacetic acid in methanol was added to extract the urushiols in an ultrasonic bath. The solid particles were separated from the extract using a magnet. Yuan et al. [49] ground a Solidago decurrens Lour sample with a poly(deep eutectic solvent)-surface-imprinted graphene oxide composite adsorbent. After transferring the mixture to a pipette tip, the authors eluted anti-adipogenesis markers (protocatechuic acid, chlorogenic acid, and 3,5-O-dicaffeoylquinic acid) using a mixture of methanol/formic acid (9:1, v/v). Gholami et al. [53] analyzed milk samples for the presence of melamine. The samples were blended in a mortar with Na2SO4 and a magnetic MIP. After transferring to a tube, 5.0 mL of deionized water/acetonitrile (50:50, v/v) was added to eliminate interferences and dissolve Na2SO4. The sorbent was separated using a magnet and the solution was discarded. Melamine was extracted with 2 mL of methanol/ammonia solution (95:5, v/v) under sonication, and the resulting sample solution was separated with a magnet. The extraction mixture containing ammonia solution was also used by Albero et al. [34]. The authors homogenized vegetable samples in a glass mortar with Florisil and anhydrous magnesium sulfate. After transferring it to a glass tube containing anhydrous magnesium sulfate, they added ethyl acetate/methanol (9:1, v/v) containing 3% ammonia, sonicated the sample, and collected it by applying vacuum. The second portion of analytes was extracted by sonication with ethyl acetate/methanol (9:1, v/v) containing 3% formic acid and collected under vacuum. The procedure was complicated and included extraction in both basic and acidic conditions, but it allowed for extracting 17 compounds including some natural and synthetic endocrine-active compounds, nonsteroidal anti-inflammatory drugs, and other drugs.
Another solution for selective elution was proposed by Dong et al. [19] who extracted the analytes using a mixture of methanol/water (9:1) containing 150 mM sodium hexane sulfonate commonly used as a surfactant and emulsifier in personal care products. As a result, a neutral ion pair was formed between the ion-pairing reagent and positively charged alkaloids (berberine and phellodendrine). Hu et al. [56] dispersed a mixture of Cortex fraxini sample and a benzo-15-crown-5 sorbent in water and tested 3 different surfactants supporting extraction: Triton X-100 (a non-ionic surfactant), sodium dodecyl sulfate (an anionic surfactant), and 1-dodecyl-3-methylimidazolium bromide (a cationic surfactant). The highest extraction of 4 coumarins (esculin, fraxin, esculetin, and fraxetin) was obtained using 100 mM sodium dodecyl sulfate. Du et al. [89] blended a Forsythiae Fructus sample with Florisil and tested 3 non-ionic surfactants to extract six compounds of various polarities (caffeic acid, forsythoside A, phillyrin, quercetin, isorhamnetin, and arctigenin). Two surfactants were of the octylphenol ethoxylates family (Triton X-100 and Triton X-114), while the third one was alkyl ethoxylate (namely Genapol X-080). In the optimized procedure, Triton X-114 was selected. Chen et al. [90] extracted 5 terpenoids (curdione, isocurcumenol, furanodienone, germacrone, and furanodiene) from Radix Curcumae blended with cucurbituril. The authors tested 6 different surfactants as extractants: sodium dodecyl sulfate, N-dodecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate, 3-(N,N-dimethylmyristylammonio)propanesulfonate, 3-(N,N-dimethylpalmitylammonio)propanesulfonate, dodecyltrimethylammonium chloride, and Triton X-100. Finally, 3-(N,N-dimethylpalmitylammonio)propanesulfonate was selected, which is a zwitterionic surfactant—a unique type of surface-active agent due to the presence of both cationic and anionic groups attached to the same molecule.
The surface-active properties of some ionic liquid precursors are also utilized. The most often used compounds are 1-dodecyl-3-methylimidazolium bromide (abbreviated [Domim]Br or [C12mim]Br) [18,44] and 1-dodecyl-3-methyl-1H-imidazolium hydrogen sulfate (abbreviated [Domim]HSO4 or [C12mim]HSO4) [82,86,91], which can be considered as cationic surfactants. [C12mim]Br was used by Du et al. [18] for the extraction of 8 active ingredients from radix polygoni multiflori blended with silica. Apart from [C12mim]Br, several other ionic liquid precursors were tested in this study including 1-butyl-3-methylimidazolium methanesulfonate ([C4mim]MS]), 1-dodecyl-3-methylimidazolium trifluoromethanesulfonate ([C12mim]OTf, where OTf is the preferred abbreviation used for triflate, i.e., trifluoromethanesulfonate), 1-ethyl-3-methylimidazolium bromide ([C2mim]Br), 3-dodecyl-1-methyl-1H-imidazolium nitrate ([C12mim]NO3), and 1-dodecyl-3-methyl-1H-imidazolium hydrogen sulfate ([C12mim]HSO4). Yang et al. [44] also used [C12mim]Br. In their study, 5 organic acids were determined in fruit samples: chlorogenic, malic, protocatechuic, caffeic, and gallic. [C12mim]Br was used to extract these acids from the samples blended with a calixarene sorbent and was superior to several other solvents (1-ethyl-3-methylimidazolium bromide ([C2mim]Br), 1-hexyl-3-methylimidazolium bromide ([C6mim]Br), and 1-decyl-3-methylimidazolium bromide ([C10mim]Br). [C12mim]HSO4 was used by Wang et al. [82] for the determination of terpenoids, crocins, quinic acid derivatives, and flavonoids in Gardeniae fructus. The sample was blended in a mortar with 2,6-dimethyl-β-cyclodextrin and [C12mim]HSO4 was used for extraction in ultrasound-assisted MSPD. Other solvents were also tested including 1-dodecyl-3-methylimidazolium nitrate ([C12mim]NO3), 1-dodecyl-3-methylimidazolium hydrogen sulfate ([C12mim]HSO4), 1-dodecyl-3-methylimidazole chloride ([C12mim]Cl), 1-dodecyl-3-methylimidazolium bromide ([C12mim]Br), 1-tetradecyl-3-methylimidazolium bromide ([C14mim]Br), 1-butyl-3-methylimidazolium bromide ([C4mim]Br), 1-butyl-3-methylimidazolium trifluoromethanesulfonate ([C4mim]OTf), and 1-ethyl-3-methylimidazolium bromide ([C2mim]Br). Jiang et al. [86] used [C12mim]HSO4 for the extraction of 4 anthraquinones (aurantio-obtusin, chryso-obtusin, obtusifolin, and emodin) from Cassiae Semen. The seeds were ground in a mortar with a powdered crab shell and transferred to a centrifuge tube. A vortex-assisted extraction enabled the extraction of the analytes with [C12mim]HSO4. This extracting solvent was superior to other tested solvents (1-butyl-3-methylimidazolium hexafluorophosphate ([C2mim]PF6), 1-dodecyl-3-methylimidazole chloride ([C12mim]Cl), and 1-dodecyl-3-methyl-1-H-imidazolium nitrate ([C12mim]NO3)). [C12mim]HSO4 was also used by Du et al. [91] who extracted 5-HMF and iridoid glycosides from Fructus Corni. The sample was ground with silica in a mortar and the analytes were extracted by vortex-assisted MSPD. Apart from [C12mim]HSO4, the authors tested 13 other ionic liquid precursors and ionic liquids.
Contrary to the previous studies for the extraction of antioxidant ingredients from Mori Fructus, Du et al. [80] used 1-ethyl-3-methylimidazolium bromide ([C2mim]Br) that has a short ethyl side-chain. Derivatives with butyl and dodecyl side-chains were also tested but the authors noticed that too long a side-chain of the cation gives steric hindrance that was not good for the interaction with target compounds. A short side-chain was also beneficial for the extraction of active compounds from Platycladi Cacumen [92]. Myricitrin, isoquercitrin, quercitrin, amentoflavone, and hinokiflavone were extracted with high recovery, reaching about 100% using 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid ([C2mim]BF4). The same ionic liquid was also used in the study by Xu et al. [93] who used it for the extraction of bioactive flavonoid glycosides from lime fruit.
Apart from ionic liquids and their precursors, deep eutectic solvents (DESs) are also used for extraction in MSPD. These solvents, just like ionic liquids, can be designed for the intended application. DESs are composed of a hydrogen bond donor and a hydrogen bond acceptor. Both donor and acceptor may be synthesized to form DESs of required properties. However, recently, materials of natural origin were often used for the synthesis of deep eutectic solvents. The use of natural deep eutectic solvents (NADESs) brings the analytical methods closer to the green analytical chemistry requirements. Gu et al. [83] synthesized 6 different NADESs for the extraction of 7 active compounds from honeysuckles. As hydrogen bond donors, 3 compounds were tested: choline chloride, betaine, and L-proline. As hydrogen bond acceptors, 2 compounds were taken: ethylene glycol and laevulinic acid. For extraction of the analytes, a DES composed of betaine and ethylene glycol in a 1:2 mole ratio was selected. Other DESs were synthesized and tested by Ivanović et al. [30]. The authors made 4 DESs, each containing choline chloride as the hydrogen bond acceptor. Hydrogen bond donors were lactic acid, 1,2-propanediol, fructose with water, and urea. The best DES for the extraction of bioactive compounds from Helichrysum arenarium L. was composed of choline chloride/lactic acid in a molar ratio of 2:1.
The use of green solvents is a recent trend in analytical chemistry. Apart from NADESs, other safe extracting solvents (or solutions) are also used. For example, Chen et al. [62] used a solution of chitosan for the extraction of four bioactive compounds (specnuezhenide, oleuropein, echinacoside, and nuezhenoside G13) from Ligustri Lucidi Fructus. Zhu et al. [77] used an aqueous solution of cyclodextrin for the extraction of flavonoids and phenoxyacetic herbicides from Alpinia officinarum.
5. Conclusions
Available knowledge and collected references allow us to conclude that significant progress in extraction methods used in the isolation of residues of mainly organic substances in various biological, food, and plant material samples is still being developed. In this context, MSPD enables the simultaneous extraction of many substances with different physicochemical properties. Moreover, we have paid special attention to the fact that the use of MSPD and related methods (UA-MSPD, EA-MSPD, MWA-MSPD, VA-MSPD) appears to be very promising for the separation of various groups of compounds—from medium- to nonpolar analytes from complex biological matrices. A single procedure requires the use of one or many various sorbents, which are placed in a mortar together with the sample. Even sand, but also polar sorbents like various silica gels and alumina, and other, modified C18 sorbents, diatomite, and magnesium silicate (Florisil) can be used as abrasive materials. Moreover, some sorbents with magnetic properties, e.g., magnetic graphene oxide, can be applied. For the extraction of selected analytes, some highly advanced materials such as molecularly imprinted polymers are increasingly appearing. Of particular importance is the fact that, according to “green chemistry” requirements, the use of sorbents (e.g., cyclodextrin) characterized by low toxicity, excellent solubility in water, and good adsorption capacity, as well as some ionic liquid precursors due to their surface-active properties, is promising for the future.
Author Contributions
Conceptualization, A.Z.-G. and T.G.; writing—original draft preparation, A.Z.-G., T.G., M.L., and R.F.; writing—review and editing, A.Z.-G., T.G., and M.L.; visualization, R.F. and A.Z.-G.; supervision, A.Z.-G.; funding acquisition, A.Z.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education, grant number 0911/SBAD/2406.
Data Availability Statement
Not applicable.
Acknowledgments
M. Ligor is a member of the Toruń Center of Excellence “Towards Personalized Medicine” operating under the Excellence Initiative-Research University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barker, S.A. Matrix Solid Phase Dispersion (MSPD). J. Biochem. Biophys. Methods 2007, 70, 151–162. [Google Scholar] [CrossRef]
- Karasová, G.; Branšteterová, E.; Lachová, M. Matrix Solid Phase Dispersion as an Effective Preparation Method for Food Samples and Plants before HPLC Analysis—A Riview. Czech J. Food Sci. 2003, 21, 219–234. [Google Scholar] [CrossRef]
- Ahmed, F.E. Analyses of Pesticides and Their Metabolites in Foods and Drinks. TrAC Trends Anal. Chem. 2001, 20, 649–661. [Google Scholar] [CrossRef]
- Furusawa, N. A Toxic Reagent-Free Method for Normal-Phase Matrix Solid-Phase Dispersion Extraction and Reversed-Phase Liquid Chromatographic Determination of Aldrin, Dieldrin, and DDTs in Animal Fats. Anal. Bioanal. Chem. 2004, 378, 2004–2007. [Google Scholar] [CrossRef]
- Ramos, L. Current Trends in the Determination of Organic Compounds in Foodstuffs Using Matrix Solid Phase Dispersion. TrAC Trends Anal. Chem. 2024, 172, 117601. [Google Scholar] [CrossRef]
- Liška, I.; Krupčíik, J.; Leclercq, P.A. The Use of Solid Sorbents for Direct Accumulation of Organic Compounds from Water Matrices—A Review of Solid-phase Extraction Techniques. J. High. Resolut. Chromatogr. 1989, 12, 577–590. [Google Scholar] [CrossRef]
- Mavumengwana-Khanyile, B.; Katima, Z.; Songa, E.A.; Okonkwo, J.O. Recent Advances in Sorbents Applications and Techniques Used for Solid-Phase Extraction of Atrazine and Its Metabolites Deisopropylatrazine and Deethylatrazine: A Review. Int. J. Environ. Anal. Chem. 2019, 99, 1017–1068. [Google Scholar] [CrossRef]
- Żwir-Ferenc, A.; Biziuk, M. Solid Phase Extraction Technique—Trends, Opportunities and Applications. Pol. J. Environ. Stud. 2006, 15, 677–690. [Google Scholar]
- Buszewski, B.; Szultka, M. Past, Present and Future of Soils Phase Extraction: A Review. Crit. Rev. Anal. Chem. 2012, 42, 198–213. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Rado, E. Matrix Solid-Phase Dispersion (MSPD) in Chromatographic Analysis of Essential Oils in Herbs. J. Pharm. Biomed. Anal. 2010, 52, 79–85. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D. PLE in the Analysis of Plant Compounds: Part I. The Application of PLE for HPLC Analysis of Caffeine in Green Tea Leaves. J. Pharm. Biomed. Anal. 2005, 37, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Wianowska, D. Application of the MSPD Technique for the HPLC Analysis of Rutin in Sambucus nigra L.: The Linear Correlation of the Matrix Solid-Phase Dispersion Process. J. Chromatogr. Sci. 2009, 47, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Karasová, G.; Lehotay, J. MSPD Extraction of Phenolic Compounds from Fruit-green Tea Using Various Non-polar Sorbents. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 2837–2845. [Google Scholar] [CrossRef]
- Freitas, S.; Serafim, F.; Lanças, F. Determination of Target Pesticide Residues in Tropical Fruits Employing Matrix Solid-Phase Dispersion (MSPD) Extraction Followed by High Resolution Gas Chromatography. J. Braz. Chem. Soc. 2018, 29, 1148–2018. [Google Scholar] [CrossRef]
- Fernandes, V.C.; Domingues, V.F.; Delerue-Matos, C.; Mateus, N. Determination of Pesticides in Fruit and Fruit Juices by Chromatographic Methods. An Overview. J. Chromatogr. Sci. 2011, 49, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.M.; Picó, Y.; Mañes, J. Analysis of Pesticide Residues in Fruit and Vegetables by Matrix Solid Phase Dispersion (MSPD) and Different Gas Chromatography Element-Selective Detectors. Chromatographia 1995, 41, 685–692. [Google Scholar] [CrossRef]
- Perret, D.; Gentili, A.; Marchese, S.; Sergi, M.; Caporossi, L. Determination of Free Fatty Acids in Chocolate by Liquid Chromatography with Tandem Mass Spectrometry. Rapid Commun. Mass. Spectrom. 2004, 18, 1989–1994. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Chen, Y.; Li, J.; Tang, G.; Tian, F.; He, J.; Chang, Y. Quantification of Eight Active Ingredients in Crude and Processed Radix Polygoni Multiflori Applying Miniaturized Matrix Solid-phase Dispersion Microextraction Followed by UHPLC. J. Sep. Sci. 2018, 41, 3486–3495. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yang, J.; Zheng, X.; Chen, Y.; Ye, L.; Wang, B.; Zheng, H.; Cao, J.; Wang, Q.; Hu, Y. Ion Pair Assisted Micro Matrix Solid Phase Dispersion Extraction of Alkaloids from Medical Plant. Electrophoresis 2020, 41, 123–130. [Google Scholar] [CrossRef]
- Li, W.; Lin, Y.; Wang, Y.; Hong, B. Development of a Matrix Solid-Phase Dispersion Extraction Combined with UPLC/Q-TOF-MS for Determination of Phenolics and Terpenoids from the Euphorbia Fischeriana. Molecules 2017, 22, 1524. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Cui, M.; Chen, J.; Li, X.; Chen, Y. Simultaneous Determination of Four Amides in Saururus Chinensis by Matrix Solid Phase Dispersion and High-Performance Liquid Chromatography Method. J. Food Drug Anal. 2018, 26, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Rashidipour, M.; Heydari, R. Ultrasonic-Assisted Matrix Solid-Phase Dispersion and High-Performance Liquid Chromatography as an Improved Methodology for Determination of Oleuropein from Olive Leaves. Anal. Bioanal. Chem. Res. 2018, 5, 307–316. [Google Scholar]
- Heydari, R.; Darabi Bazvand, M.R. Ultrasound-Assisted Matrix Solid-Phase Dispersion Coupled with Reversed-Phase Dispersive Liquid–Liquid Microextraction for Determination of Vitamin C in Various Matrices. Food Anal. Methods 2019, 12, 1949–1956. [Google Scholar] [CrossRef]
- Barker, S.A.; Long, A.R.; Short, C.R. Isolation of Drug Residues from Tissues by Solid Phase Dispersion. J. Chromatogr. A 1989, 475, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Senes, C.E.R.; Rodrigues, C.A.; Nicácio, A.E.; Boeing, J.S.; Maldaner, L.; Visentainer, J.V. Determination of Phenolic Acids and Flavonoids from Myrciaria Cauliflora Edible Part Employing Vortex-Assisted Matrix Solid-Phase Dispersion (VA-MSPD) and UHPLC-MS/MS. J. Food Compos. Anal. 2021, 95, 103667. [Google Scholar] [CrossRef]
- Wianowska, D.; Dawidowicz, A.L. Can Matrix Solid Phase Dispersion (MSPD) Be More Simplified? Application of Solventless MSPD Sample Preparation Method for GC–MS and GC–FID Analysis of Plant Essential Oil Components. Talanta 2016, 151, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.-J.; Pang, G.-F.; Shu, H.-R.; Fan, C.-L.; Liu, Y.-M.; Feng, J.; Wu, Y.-P.; Chang, Q.-Y. Simultaneous Determination of 346 Multiresidue Pesticides in Grapes by PSA-MSPD and GC-MS-SIM. J. Agric. Food Chem. 2010, 58, 9428–9453. [Google Scholar] [CrossRef] [PubMed]
- Rubert, J.; Soler, C.; Mañes, J. Evaluation of Matrix Solid-Phase Dispersion (MSPD) Extraction for Multi-Mycotoxin Determination in Different Flours Using LC–MS/MS. Talanta 2011, 85, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Purdešová, A.; Dömötorová, M. MSPD as Sample Preparation Method for Determination of Selected Pesticide Residues in Apples. Acta Chim. Slovaca 2017, 10, 41–46. [Google Scholar] [CrossRef]
- Ivanović, M.; Krajnc, P.; Mlinarič, A.; Islamčević Razboršek, M. Natural Deep Eutectic Solvent-Based Matrix Solid Phase Dispersion (MSPD) Extraction for Determination of Bioactive Compounds from Sandy Everlasting (Helichrysum arenarium L.): A Case of Stability Study. Plants 2022, 11, 3468. [Google Scholar] [CrossRef]
- Li, W.; Qian, Z.; Lei, Q.; Lian, Y.; Zou, Y.; Wang, Y.; Lan, D. An Ultra-Rapid and Eco-Friendly Method for Determination of Loganic Acid and Gentiopicroside from Gentianae Macrophyllae Radix by Vortex-Assisted Matrix Solid-Phase Dispersion Extraction and LC-MS. J. Pharm. Biomed. Anal. 2023, 222, 115085. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Wu, Z.; Li, C.; Tan, G.; Hu, H.; Li, W. A Green Liquid Chromatography Method for Rapid Determination of Ergosterol in Edible Fungi Based on Matrix Solid-Phase Dispersion Extraction and a Core–Shell Column. Anal. Methods 2020, 12, 3337–3343. [Google Scholar] [CrossRef] [PubMed]
- Gañán, J.; Morante-Zarcero, S.; Gallego-Picó, A.; Garcinuño, R.M.; Fernández-Hernando, P.; Sierra, I. Evaluation of a Molecularly Imprinted Polymer for Determination of Steroids in Goat Milk by Matrix Solid Phase Dispersion. Talanta 2014, 126, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Albero, B.; Sánchez-Brunete, C.; Miguel, E.; Tadeo, J.L. Application of Matrix Solid-Phase Dispersion Followed by GC–MS/MS to the Analysis of Emerging Contaminants in Vegetables. Food Chem. 2017, 217, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Cao, J.; Ye, L.; Xu, J.; Hu, S.; Peng, L. Synthesis and Application of Mesoporous Molecular Sieve for Miniaturized Matrix Solid-phase Dispersion Extraction of Bioactive Flavonoids from Toothpaste, Plant, and Saliva. Electrophoresis 2015, 36, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.; Pereira, S.; Otero, A.; Fiol, S.; Garcia-Jares, C.; Lores, M. Matrix Solid-Phase Dispersion as a Greener Alternative to Obtain Bioactive Extracts from Haematococcus pluvialis. Characterization by UHPLC-QToF. RSC Adv. 2020, 10, 27995–28006. [Google Scholar] [CrossRef] [PubMed]
- Arabi, M.; Ghaedi, M.; Ostovan, A. Development of Dummy Molecularly Imprinted Based on Functionalized Silica Nanoparticles for Determination of Acrylamide in Processed Food by Matrix Solid Phase Dispersion. Food Chem. 2016, 210, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, X.; Yang, Y.; Liu, Y.; Chen, X. Development of a Deep Eutectic Solvent-Based Matrix Solid Phase Dispersion Methodology for the Determination of Aflatoxins in Crops. Food Chem. 2019, 291, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Chatzimitakos, T.G.; Karali, K.K.; Stalikas, C.D. Magnetic Graphene Oxide as a Convenient Nanosorbent to Streamline Matrix Solid-Phase Dispersion towards the Extraction of Pesticides from Vegetables and Their Determination by GC–MS. Microchem. J. 2019, 151, 104247. [Google Scholar] [CrossRef]
- De Silva, S.; Ocaña-Rios, I.; Cagliero, C.; Gostel, M.R.; Johnson, G.; Anderson, J.L. Isolation of DNA from Plant Tissues Using a Miniaturized Matrix Solid-Phase Dispersion Approach Featuring Ionic Liquid and Magnetic Ionic Liquid Solvents. Anal. Chim. Acta 2023, 1245, 340858. [Google Scholar] [CrossRef]
- Kemmerich, M.; Demarco, M.; Bernardi, G.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Balls-in-Tube Matrix Solid Phase Dispersion (BiT-MSPD): An Innovative and Simplified Technique for Multiresidue Determination of Pesticides in Fruit Samples. J. Chromatogr. A 2020, 1612, 460640. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, H.; Qi, Z.; Xue, X.; Wang, C. Vortex-Blending Matrix Solid-Phase Dispersion and UPLC-Q-TOF/MS Were Proposed to Extract and Examine the Urushiols from Toxicodendron Vernicifluum Bark. J. Pharm. Biomed. Anal. 2024, 242, 116066. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-J.; Chung, W.-H.; Ding, W.-H. Optimization of Double-Vortex-Assisted Matrix Solid-Phase Dispersion for the Rapid Determination of Paraben Preservative Residues in Leafy Vegetables. RSC Adv. 2020, 10, 35557–35564. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dong, X.; Hu, Y.-H.; Wang, Q.-Y.; Wang, S.-L.; Cao, J.; Zhang, H.-H. Calixarene and Ionic Liquid Assisted Matrix Solid-Phase Dispersion Microextraction of Organic Acids from Fruit. J. Chromatogr. A 2019, 1602, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Huang, J.; Wang, B.; Wang, C.; Wang, Q.; Hu, Y.; Yi, L.; Cao, J.; Peng, L.; Chen, Y.; et al. Carbon Molecular Sieve Based Micro-matrix-solid-phase Dispersion for the Extraction of Polyphenols in Pomegranate Peel by UHPLC-Q-TOF/MS. Electrophoresis 2018, 39, 2218–2227. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Peng, L.-Q.; Xu, J.-J. Microcrystalline Cellulose Based Matrix Solid Phase Dispersion Microextration for Isomeric Triterpenoid Acids in Loquat Leaves by Ultrahigh-Performance Liquid Chromatography and Quadrupole Time-of-Flight Mass Spectrometry. J. Chromatogr. A 2016, 1472, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Cañadas, R.; Garrido Gamarro, E.; Garcinuño Martínez, R.M.; Paniagua González, G.; Fernández Hernando, P. Occurrence of Common Plastic Additives and Contaminants in Mussel Samples: Validation of Analytical Method Based on Matrix Solid-Phase Dispersion. Food Chem. 2021, 349, 129169. [Google Scholar] [CrossRef] [PubMed]
- Garrido Gamarro, E.; Soliz Rojas, D.L.; Garcinuño Martínez, R.M.; Paniagua González, G.; Fernández Hernando, P. Occurrence of Common Plastic Additives and Contaminants in Raw, Steamed and Canned Mussel Samples from Different Harvesting Areas Using MSPD-HPLC Methodology. Food Res. Int. 2024, 181, 114109. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, Y.; Zhang, Y.; Yin, J.; Han, Y.; Han, D.; Yan, H. Miniaturized Centrifugation Accelerated Pipette-Tip Matrix Solid-Phase Dispersion Based on Poly(Deep Eutectic Solvents) Surface Imprinted Graphene Oxide Composite Adsorbent for Rapid Extraction of Anti-Adipogenesis Markers from Solidago Decurrens Lour. J. Chromatogr. A 2024, 1715, 464599. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Z.; Li, N.; Wang, K.; Zang, S.; Jiang, J.; Yu, A.; Zhang, H.; Li, X. Dispersant-Assisted Dynamic Microwave Extraction of Triazine Herbicides from Rice. Anal. Methods 2016, 8, 3788–3794. [Google Scholar] [CrossRef]
- dos Santos, E.O.; Gonzales, J.O.; Ores, J.C.; Marube, L.C.; Caldas, S.S.; Furlong, E.B.; Primel, E.G. Sand as a Solid Support in Ultrasound-Assisted MSPD: A Simple, Green and Low-Cost Method for Multiresidue Pesticide Determination in Fruits and Vegetables. Food Chem. 2019, 297, 124926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ding, J.; Hou, J.; Zhao, L.; Chen, Y.; Ding, L. Dynamic Microwave Assisted Extraction Coupled with Matrix Solid Phase Dispersion for the Determination of Chlorfenapyr and Abamectin in Rice by LC-MS/MS. Microchem. J. 2017, 133, 404–411. [Google Scholar] [CrossRef]
- Gholami, H.; Arabi, M.; Ghaedi, M.; Ostovan, A.; Bagheri, A.R. Column Packing Elimination in Matrix Solid Phase Dispersion by Using Water Compatible Magnetic Molecularly Imprinted Polymer for Recognition of Melamine from Milk Samples. J. Chromatogr. A 2019, 1594, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Fotouhi, M.; Seidi, S.; Shanehsaz, M.; Naseri, M.T. Magnetically Assisted Matrix Solid Phase Dispersion for Extraction of Parabens from Breast Milks. J. Chromatogr. A 2017, 1504, 17–26. [Google Scholar] [CrossRef]
- Ganzler, K.; Salgó, A.; Valkó, K. Microwave Extraction: A Novel Sample Preparation Method for Chromatography. J. Chromatogr. A 1986, 371, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-H.; Wang, Q.-Y.; Ye, L.-H.; Yang, J.; Dong, X.; Zheng, H.; Zhou, J.; Cao, J. Effervescent Salt and Crown Ether-Assisted Matrix Solid-Phase Dispersion Extraction of Coumarins from Cortex Fraxini. Ind. Crops Prod. 2019, 141, 111752. [Google Scholar] [CrossRef]
- Qian, Z.; Huang, D.; He, Z.; He, Q.; Tan, G.; Huang, Q.; Sun, Y.; Li, W. Rapid Determination of Three Organic Acids in Polygonum Vivipari Rhizoma via One Marker by HPLC-UV at Equal Absorption Wavelength and Effervescence-Assisted Matrix Solid-Phase Dispersion. Int. J. Anal. Chem. 2023, 2023, 5546053. [Google Scholar] [CrossRef]
- Dias Soares, C.M.; Fernandes, J.O. MSPD Method to Determine Acrylamide in Food. Food Anal. Methods 2009, 2, 197–203. [Google Scholar] [CrossRef]
- Mansur, A.R.; Kim, K.J.; Kim, D.-B.; Yoo, M.; Jang, H.W.; Kim, D.-O.; Nam, T.G. Matrix Solid-Phase Dispersion Extraction Method for HPLC Determination of Flavonoids from Buckwheat Sprouts. LWT 2020, 133, 110121. [Google Scholar] [CrossRef]
- Hong, B.; Wang, Z.; Xu, T.; Li, C.; Li, W. Matrix Solid-Phase Dispersion Extraction Followed by High Performance Liquid Chromatography-Diode Array Detection and Ultra Performance Liquid Chromatography–Quadrupole–Time of Flight–Mass Spectrometer Method for the Determination of the Main Compounds from Carthamus tinctorius L. (Hong-Hua). J. Pharm. Biomed. Anal. 2015, 107, 464–472. [Google Scholar] [CrossRef]
- Zou, Y.; Lv, Y.; Peng, X.; Tong, S.; Chu, C. A Novel Strategy by Combining “Magnified” Matrix Solid Phase Dispersion Extraction with High-Speed Countercurrent Chromatography for the Rapid and Efficient Isolation of Flavonoids Isomers with Anti-Inflammatory Effect from Lindera Aggregata (Sims) Kosterm Leaves. Sustain. Chem. Pharm. 2023, 33, 101073. [Google Scholar] [CrossRef]
- Chen, S.; Du, K.; Li, J.; Chang, Y. A Chitosan Solution-Based Vortex-Forced Matrix Solid Phase Dispersion Method for the Extraction and Determination of Four Bioactive Constituents from Ligustri Lucidi Fructus by High Performance Liquid Chromatography. J. Chromatogr. A 2020, 1609, 460509. [Google Scholar] [CrossRef] [PubMed]
- dos Reis Souza, M.R.; Oliveira Moreira, C.; de Lima, T.G.; Aquino, A.; Silveira Dórea, H. Validation of a Matrix Solid Phase Dispersion (MSPD) Technique for Determination of Pesticides in Lyophilized Eggs of the Chicken Gallus Gallus Domesticus. Microchem. J. 2013, 110, 395–401. [Google Scholar] [CrossRef]
- Dantas Silva, M.G.; Aquino, A.; Silveira Dórea, H.; Navickiene, S. Simultaneous Determination of Eight Pesticide Residues in Coconut Using MSPD and GC/MS. Talanta 2008, 76, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.A.; Albero, B.; Tadeo, J.L. Matrix Solid Phase Dispersion. In Solid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 531–549. [Google Scholar]
- Menezes Filho, A.; Navickiene, S.; Dórea, H.S. Development of MSPD Method for the Determination of Pesticide Residues in Tomato by GC-MS. J. Braz. Chem. Soc. 2006, 17, 874–879. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, S.; Zhang, Q.; He, S.; Li, Q.; Hu, J.; Zhang, H. Matrix Solid-Phase Dispersion Coupled with High-Performance Liquid Chromatography Diode Array Detection for Simultaneous Determination of Four Lipophilic Constituents from Salvia Miltiorrhiza Bunge. J. Chromatogr. Sci. 2017, 55, 316–326. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, J.P.; Ribeiro, I.S.; de Sousa Pereira, M.V.; de Araujo Andrade, T.; de Jesus, J.R. Multivariate Optimization, in Conjugation with Matrix Solid-Phase Dispersion and Digital Image Detection, Allows Residual Dye Determination from Shrimp Samples. Adv. Sample Prep. 2024, 9, 100105. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Yang, X.; Gao, X.; Wang, H.; Chang, Y. A Rapid and Efficient Extraction Method Based on Industrial MCM-41-miniaturized Matrix Solid-phase Dispersion Extraction with Response Surface Methodology for Simultaneous Quantification of Six Flavonoids in Pollen typhae by Ultra-high-performance Liquid Chromatography. J. Sep. Sci. 2019, 42, 2426–2434. [Google Scholar] [CrossRef]
- He, G.; Li, J.; Pang, X.; Wang, H.; Jin, H.; He, J.; Fang, S.-M.; Chang, Y.-X. A Beta/ZSM-22 Zeolites-Based-Mixed Matrix Solid-Phase Dispersion Method for the Simultaneous Extraction and Determination of Eight Compounds with Different Polarities in Viticis Fructus by High-Performance Liquid Chromatography. Molecules 2019, 24, 3423. [Google Scholar] [CrossRef]
- Chen, C.; Fu, Z.; Zhou, W.; Chen, Q.; Wang, C.; Xu, L.; Wang, Z.; Zhang, H. Ionic Liquid-Immobilized NaY Zeolite-Based Matrix Solid Phase Dispersion for the Extraction of Active Constituents in Rheum palmatum L. Microchem. J. 2020, 152, 104245. [Google Scholar] [CrossRef]
- Liu, H.; Hong, Y.; Chen, L. Molecularly Imprinted Polymers Coated on Carbon Nanotubes for Matrix Solid Phase Dispersion Extraction of Camptothecin from Camptotheca Acuminate. Anal. Methods 2015, 7, 8100–8108. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, X.; Wang, M.; Wang, Y.; Wu, Q.; Ji, L.; Li, Q.; Yang, J.; Zhou, Q. Dummy Molecularly Imprinted Microspheres Prepared by Pickering Emulsion Polymerization for Matrix Solid-Phase Dispersion Extraction of Three Azole Fungicides from Fish Samples. J. Chromatogr. A 2020, 1620, 461013. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Wei, M.; Wang, S.; Zheng, L.; He, Z.; Cao, J.; Yan, J. Micro-Matrix Solid-Phase Dispersion Coupled with MEEKC for Quantitative Analysis of Lignans in Schisandrae Chinensis Fructus Using Molecular Sieve TS-1 as a Sorbent. J. Chromatogr. B 2017, 1063, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Chu, C.; Wang, S.; Yan, J. Quantitative Analysis of Sesquiterpenes and Comparison of Three Curcuma wenyujin Herbal Medicines by Micro Matrix Solid Phase Dispersion Coupled with MEEKC. Electrophoresis 2018, 39, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Li, J.; Gao, X.; Chang, Y. Ultrasound-Enhanced Matrix Solid-Phase Dispersion Micro-Extraction Applying Mesoporous Molecular Sieve SBA-15 for the Determination of Multiple Compounds in Fructus Psoraleae. Sustain. Chem. Pharm. 2020, 15, 100198. [Google Scholar] [CrossRef]
- Zhu, S.-C.; Shi, Y.; Jin, H.-F.; Cao, J.; Ye, L.-H. Nanographite-Assisted Matrix Solid Phase Dispersion Microextraction of Active and Toxic Compounds from Complex Food Matrices Using Cyclodextrin Aqueous Solution as Elution Solvent. Food Chem. 2023, 417, 135894. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, X.; Yang, J.; Li, L.; Jin, Y.; Shi, X. Graphene-Encapsulated Silica as Matrix Solid-Phase Dispersion Extraction Sorbents for the Analysis of Poly-Methoxylated Flavonoids in the Leaves of Murraya panaculata (L.) Jack. J. Sep. Sci. 2015, 38, 2132–2139. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shi, J.; Sun, J.; Wang, T.; Zeng, L.; Zhu, N.; Jiang, G. Graphene-Assisted Matrix Solid-Phase Dispersion for Extraction of Polybrominated Diphenyl Ethers and Their Methoxylated and Hydroxylated Analogs from Environmental Samples. Anal. Chim. Acta 2011, 708, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Chen, Y.; Li, J.; Tian, F.; Gao, X.; Chang, Y. Determination of Antioxidant Ingredients in Mori fructus Employing Ionic Liquid-assisted Miniaturized Matrix Solid-phase Dispersion Extraction via Ultra-performance Liquid Chromatography. J. Food Biochem. 2019, 43, e12807. [Google Scholar] [CrossRef]
- Xu, J.-J.; Cao, J.; Peng, L.-Q.; Cao, W.; Zhu, Q.-Y.; Zhang, Q.-Y. Characterization and Determination of Isomers in Plants Using Trace Matrix Solid Phase Dispersion via Ultrahigh Performance Liquid Chromatography Coupled with an Ultraviolet Detector and Quadrupole Time-of-Flight Tandem Mass Spectrometry. J. Chromatogr. A 2016, 1436, 64–72. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Gao, X.; Chang, Y. Ionic Liquid Vortex-simplified Matrix Solid-phase Dispersion for the Simultaneous Determination of Terpenoids, Crocins, Quinic Acid Derivatives and Flavonoids in Gardeniae fructus by UHPLC. J. Sep. Sci. 2019, 42, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-X.; Cao, J.; Ye, L.-H. Nano-ZnO-Assisted Matrix Solid Phase Dispersion Microextraction Combined with an in Situ DES-Based Aqueous Two-Phase System for the Enrichment of Multiple Active Compounds in Honeysuckles. Ind. Crops Prod. 2022, 188, 115708. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Determination of Phenolic Compounds in Residual Brewing Yeast Using Matrix Solid-Phase Dispersion Extraction Assisted by Titanium Dioxide Nanoparticles. J. Chromatogr. A 2019, 1601, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mejía, E.; Mikkelsen, L.H.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. A Combined Approach Based on Matrix Solid-Phase Dispersion Extraction Assisted by Titanium Dioxide Nanoparticles and Liquid Chromatography to Determine Polyphenols from Grape Residues. J. Chromatogr. A 2021, 1644, 462128. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Zhang, H.; Liu, C.; Tang, Y.; Chu, C. New Vortex-Synchronized Matrix Solid-Phase Dispersion Method for Simultaneous Determination of Four Anthraquinones in Cassiae Semen. Molecules 2019, 24, 1312. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.-Q.; Li, Q.; Chang, Y.; An, M.; Yang, R.; Tan, Z.; Hao, J.; Cao, J.; Xu, J.-J.; Hu, S.-S. Determination of Natural Phenols in Olive Fruits by Chitosan Assisted Matrix Solid-Phase Dispersion Microextraction and Ultrahigh Performance Liquid Chromatography with Quadrupole Time-of-Flight Tandem Mass Spectrometry. J. Chromatogr. A 2016, 1456, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.-Q.; Zhang, Y.; Yan, T.-C.; Gu, Y.-X.; Yue, Z.-X.; Cao, J. Carbonized Biosorbent Assisted Matrix Solid-Phase Dispersion Microextraction for Active Compounds from Functional Food. Food Chem. 2021, 365, 130545. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Li, J.; Tian, F.; Chang, Y. Non-Ionic Detergent Triton X-114 Based Vortex- Synchronized Matrix Solid-Phase Dispersion Method for the Simultaneous Determination of Six Compounds with Various Polarities from Forsythiae Fructus by Ultra High-Performance Liquid Chromatography. J. Pharm. Biomed. Anal. 2018, 150, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhen, X.; Yu, Y.; Shi, M.; Cao, J.; Zheng, H.; Ye, L. Cucurbituril and Zwitterionic Surfactant-based Matrix Solid-phase Dispersion Microextraction to Simultaneously Determine Terpenoids from Radix Curcumae. J. Sep. Sci. 2021, 44, 1361–1370. [Google Scholar] [CrossRef]
- Du, K.; Li, J.; Bai, Y.; An, M.; Gao, X.; Chang, Y. A Green Ionic Liquid-Based Vortex-Forced MSPD Method for the Simultaneous Determination of 5-HMF and Iridoid Glycosides from Fructus Corni by Ultra-High Performance Liquid Chromatography. Food Chem. 2018, 244, 190–196. [Google Scholar] [CrossRef]
- Ding, M.; Li, J.; Zou, S.; Tang, G.; Gao, X.; Chang, Y. Simultaneous Extraction and Determination of Compounds with Different Polarities from Platycladi Cacumen by AQ C18-Based Vortex-Homogenized Matrix Solid-Phase Dispersion with Ionic Liquid. Front. Pharmacol. 2019, 9, 1532. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-J.; Yang, R.; Ye, L.-H.; Cao, J.; Cao, W.; Hu, S.-S.; Peng, L.-Q. Application of Ionic Liquids for Elution of Bioactive Flavonoid Glycosides from Lime Fruit by Miniaturized Matrix Solid-Phase Dispersion. Food Chem. 2016, 204, 167–175. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).