Adsorption and Diffusion Characteristics of CO2 and CH4 in Anthracite Pores: Molecular Dynamics Simulation
Abstract
:1. Introduction
2. Simulation Method
2.1. Molecular Model
2.2. Model Basis and Conditional Configuration
3. Results and Discussion
3.1. Interaction Energy and Adsorption Capacity
3.2. Effect of Chemical Functional Groups on Gas Adsorption
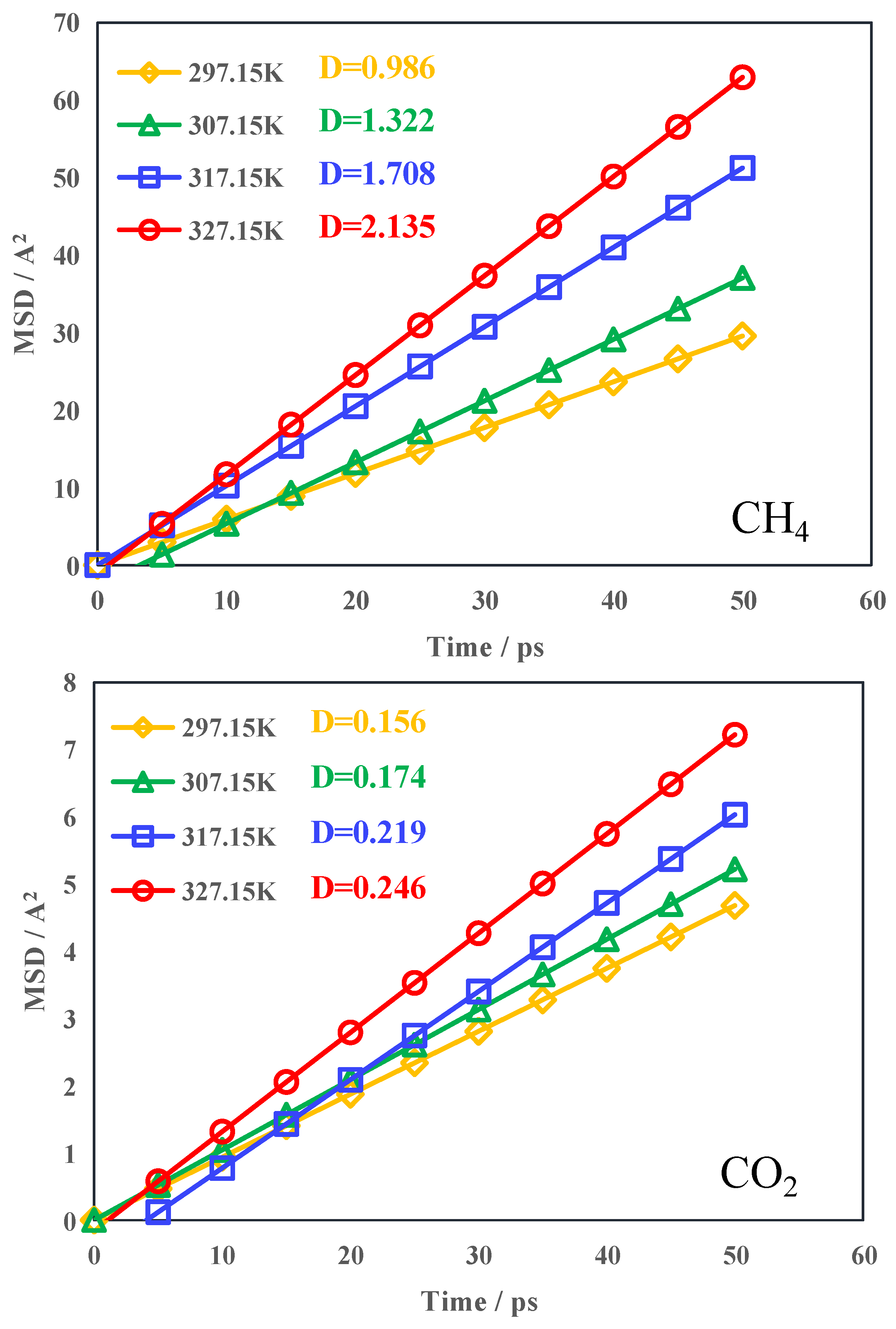
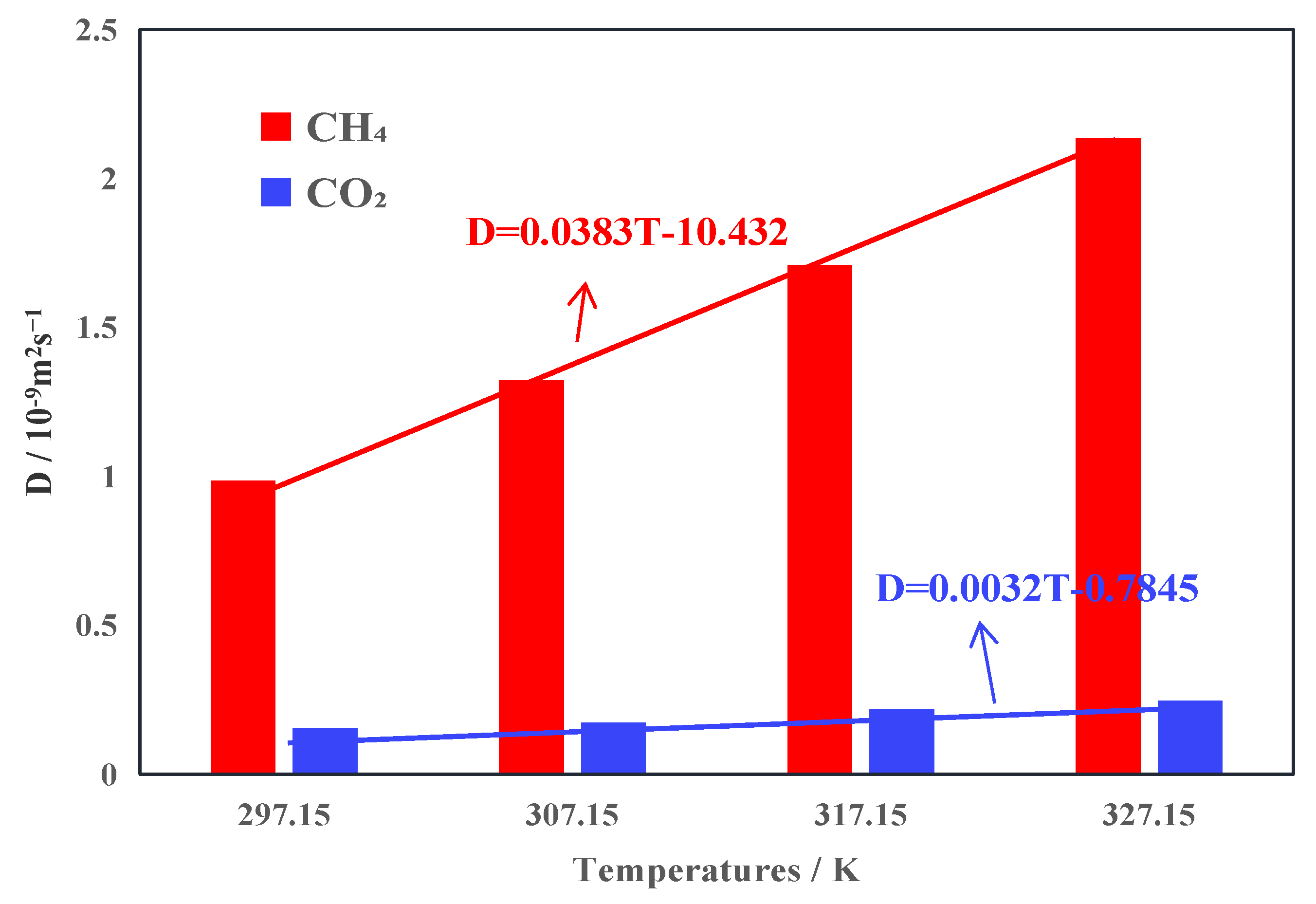
3.3. Gas Diffusivity Capacity
4. Conclusions
- The interaction between coal molecules and CO2 and CH4 increases with the increase in pressure, and the adsorption effect is enhanced. Under the same conditions, the adsorption of CO2 and CH4 is still dominated by van der Waals forces, and the electrostatic interaction is weak. At 307.15 K and 101.35 kPa, the interaction energies of coal adsorption of single-component CO2 and CH4 are −1273.92 and −761.53 kJ·mol−1, respectively.
- The interaction energy between anthracite molecules and CO2 is significantly higher compared to CH4, indicating that coal has a greater adsorption capacity for CO2 than for CH4. The distribution characteristics of gas in the pores before and after injection indicate that CO2 mainly adsorbs and displaces CH4 by occupying adsorption sites.
- Under the same conditions, the diffusivity of CH4 exceeds that of CO2; elevated temperatures result in an increase in gas diffusivity, facilitating the diffusion of gas out of the coal pores. With the increase in temperature, the growth rates of CO2 and CH4 diffusion coefficients are different, and the growth rate of the CH4 diffusion coefficient is greater than that of CO2. This shows that CO2-ECBM is suitable for coal seams with high temperatures.
- The presence of oxygen functional groups and aromatic hydrocarbons in anthracite coal facilitates the adsorption of gas molecules on the coal surface. The hydroxyl group significantly influences the adsorption of both CH4 and CO2, and of the other functional groups, the ethoxy group has the greatest impact on the adsorption of CH4, while the carbonyl group has a significant impact on the adsorption of CO2.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, T.A. Coalbed methane: A review. Int. J. CoalGeol. 2012, 101, 36–81. [Google Scholar] [CrossRef]
- Ying, L.M.; Chen, J.; Du, C.; Pang, L.X.; Wen, Y.J. The research progress of coal and gas co-mining. Adv. Mater. Res. 2012, 524, 489–493. [Google Scholar] [CrossRef]
- Wen, G.C.; Sun, H.T.; Li, R.F.; Fu, J.; Zhao, X.S. Assessment method and application of coalbed methane resources in coal mining stability area. J. China Coal Soc. 2018, 43, 160–167. [Google Scholar]
- Wang, Y.; Sun, Q.; Chen, F.; Wang, M. Multiscale Model for Hydrogen Transport and Storage in Shale Reservoirs. SPE J. 2024, 1–27. [Google Scholar] [CrossRef]
- Kong, D.; Gao, J.; Lian, P.; Zheng, R.; Zhu, W.; Xu, Y. Characteristics of gas-oil contact and mobilization limit during gas-assisted gravity drainage process. Adv. Geo-Energy Res. 2022, 6, 169–176. [Google Scholar] [CrossRef]
- Huo, B.; Jing, X.; Fan, C.; Han, Y. Numerical investigation of flue gas injection enhanced underground coal seam gas drainage. Energy Sci. Eng. 2019, 7, 3204–3219. [Google Scholar] [CrossRef]
- Fan, C.; Elsworth, D.; Li, S.; Chen, Z.; Luo, M.; Song, Y.; Zhang, H. Modelling and optimization of enhanced coalbed methane recovery using CO2/N2 mixtures. Fuel 2019, 253, 1114–1129. [Google Scholar] [CrossRef]
- Wang, Y.W.; Dai, Z.X.; Wang, G.S.; Chen, L.; Xia, Y.Z.; Zhou, Y.H. A hybrid physics-informed data-driven neural network for CO2 storage in depleted shale reservoirs. Pet. Sci. 2024, 21, 286–301. [Google Scholar] [CrossRef]
- Ranathunga, A.S.; Perera, M.S.A.; Ranjith, P.G.; Wei, C.H. An experimental investigation of applicability of CO2 enhanced coal bed methane recovery to low rank coal. Fuel 2017, 189, 391–399. [Google Scholar] [CrossRef]
- Kong, D.; Gao, Y.; Sarma, H.; Li, Y.; Guo, H.; Zhu, W. Experimental investigation of immiscible water-alternating-gas injection in ultra-high water-cut stage reservoir. Adv. Geo-Energy Res. 2021, 5, 139–152. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, Z.; Chen, L.; Shen, X.; Chen, F.; Soltanian, M.R. An integrated multi-scale model for CO2 transport and storage in shale reservoirs. Appl. Energy 2023, 331, 120444. [Google Scholar] [CrossRef]
- Pini, R.; Ottiger, S.; Storti, G.; Mazzotti, M. Pure and competitive adsorption of CO2, CH4 and N2 on coal for ECBM. Energy Procedia 2009, 1, 1705–1710. [Google Scholar] [CrossRef]
- Zheng, S.; Yao, Y.; Sang, S.; Liu, D.; Wang, M.; Liu, S. Dynamic characterization of multiphase methane during CO2-ECBM: An NMR relaxation method. Fuel 2022, 324, 124526. [Google Scholar] [CrossRef]
- Dutka, B.; Kudasik, M.; Topolnicki, J. Pore pressure changes accompanying exchange sorption of CO2/CH4 in a coal briquette. Fuel Process. Technol. 2012, 100, 30–34. [Google Scholar] [CrossRef]
- Majewska, Z.; Ceglarska-Stefańska, G.; Majewski, S.; Ziętek, J. Binary gas sorption/desorption experiments on a bituminous coal: Simultaneous measurements on sorption kinetics, volumetric strain and acoustic emission. Int. J. Coal Geol. 2009, 77, 90–102. [Google Scholar] [CrossRef]
- Topolnicki, J.; Kudasik, M.; Dutka, B. Simplified model of the CO2/CH4 exchange sorption process. Fuel Process. Technol. 2013, 113, 67–74. [Google Scholar] [CrossRef]
- Pini, R.; Ottiger, S.; Burlini, L.; Storti, G.; Mazzotti, M. CO2 storage through ECBM recovery: An experimental and modeling study. Energy Procedia 2009, 1, 1711–1717. [Google Scholar] [CrossRef]
- Sander, R.; Connell, L.D.; Pan, Z.; Camilleri, M.; Heryanto, D.; Lupton, N. Core flooding experiments of CO2 enhanced coalbed methane recovery. Int. J. Coal Geol. 2014, 131, 113–125. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, Q.; Jin, Z.; Xing, X.; Wang, S. Molecular simulation study of oil-water two-phase fluid transport in shale inorganic nanopores. Chem. Eng. Sci. 2021, 245, 116948. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Qu, Z.; Yin, Y.; Kang, Q.; Yu, B.; Tao, W.Q. Modeling of multi-scale transport phenomena in shale gas production—A critical review. Appl. Energy 2020, 262, 114575. [Google Scholar] [CrossRef]
- Dang, Y.; Zhao, L.; Lu, X.; Xu, J.; Sang, P.; Guo, S.; Zhu, H.; Guo, W. Molecular simulation of CO2/CH4 adsorption in brown coal: Effect of oxygen-, nitrogen-, and sulfur-containing functional groups. Appl. Surf. Sci. 2017, 423, 33–42. [Google Scholar] [CrossRef]
- Yao, J.; Greenkorn, R.A.; Chao, K.C. Monte Carlo simulation of the grand canonical ensemble. Mol. Phys. 1982, 46, 587–594. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Becker, E.D.; Lupkowski, M.; van Swol, F. Molecular dynamics and Monte Carlo simulations in the grand canonical ensemble: Local versus global control. J. Chem. Phys. 1993, 98, 4897–4908. [Google Scholar] [CrossRef]
- Mahjoory, M.; Shahgholi, M.; Karimipour, A. The effects of initial temperature and pressure on the mechanical properties of reinforced calcium phosphate cement with magnesium nanoparticles: A molecular dynamics approach. Int. Commun. Heat Mass Transf. 2022, 135, 106067. [Google Scholar] [CrossRef]
- Mahjoory, M.; Shahgholi, M.; Karimipour, A. Investigation on the size and percentage effects of magnesium nanoparticles on thermophysical properties of reinforced calcium phosphate bone cement by molecular dynamic simulation. Heliyon 2023, 9, e18835. [Google Scholar] [CrossRef] [PubMed]
- Brochard, L.; Vandamme, M.; Pellenq, R.J.M.; Fen-Chong, T. Adsorption-induced deformation of microporous materials: Coal swelling induced by CO2–CH4 competitive adsorption. Langmuir 2012, 28, 2659–2670. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Hong, L.; Wang, J.; Zheng, D. Molecular simulation of gas adsorption characteristics and diffusion in micropores of lignite. Fuel 2020, 269, 117443. [Google Scholar] [CrossRef]
- Long, H.; Lin, H.F.; Yan, M.; Bai, Y.; Tong, X.; Kong, X.G.; Li, S.G. Adsorption and diffusion characteristics of CH4, CO2, and N2 in micropores and mesopores of bituminous coal: Molecular dynamics. Fuel 2021, 292, 120268. [Google Scholar] [CrossRef]
- Tao, T.; Wang, S.; Qu, Y.; Cao, D. Displacement of shale gas confined in illite shale by flue gas: A molecular simulation study. Chin. J. Chem. Eng. 2021, 29, 295–303. [Google Scholar] [CrossRef]
- Kim, C.; Devegowda, D. Molecular dynamics study of fluid-fluid and solid-fluid interactions in mixed-wet shale pores. Fuel 2022, 319, 123587. [Google Scholar] [CrossRef]
- Xiang, J.H.; Zeng, F.G.; Bin, L.I.; Zhang, L.; Li, M.F.; Liang, H.Z. Construction of macromolecular structural model of anthracite from Chengzhuang coal mine and its molecular simulation. J. Fuel Chem. Technol. 2013, 41, 391–400. [Google Scholar] [CrossRef]
- Jones, J.E. On the determination of molecular fields—II. From the equation of state of a gas. Proc. R. Soc. Lond. Ser. A Contain. Pap. A Math. Phys. Character 1924, 106, 463–477. [Google Scholar]
- Bai, Y.; Lin, H.F.; Li, S.G.; Yan, M.; Long, H. Molecular simulation of N2 and CO2 injection into a coal model containing adsorbed methane at different temperatures. Energy 2021, 219, 119686. [Google Scholar] [CrossRef]
- Jing, T.; Zhang, J.; Zhu, M.; Zhao, W.; Zhou, J.; Yin, Y. Methane Adsorption in Anthracite Coal under Different Pressures and Temperatures—A Study Combining Isothermal Adsorption and Molecular Simulation. Geofluids 2023, 2023, 8528359. [Google Scholar] [CrossRef]
- Park, S.; Sposito, G. Monte Carlo simulation of total radial distribution functions for interlayer water in Li-, Na-, and K-montmorillonite hydrates. J. Phys. Chem. B 2000, 104, 4642–4648. [Google Scholar] [CrossRef]
- Dong, X.; Liu, Q.; Cui, L.; Yu, Y.; Zhang, M. Molecular simulation and experimental study on propylene dehumidification through a PVA–PAA blend membrane. J. Mater. Chem. A 2014, 2, 16687–16696. [Google Scholar] [CrossRef]
- Liu, X.Q.; He, X.; Qiu, N.X.; Yang, X.; Tian, Z.Y.; Li, M.J.; Xue, Y. Molecular simulation of CH4, CO2, H2O and N2 molecules adsorption on heterogeneous surface models of coal. Appl. Surf. Sci. 2016, 389, 894–905. [Google Scholar] [CrossRef]
- Xu, H.; Tang, D.; Zhao, J.; Li, S.; Tao, S. A new laboratory method for accurate measurement of the methane diffusion coefficient and its influencing factors in the coal matrix. Fuel 2015, 158, 239–247. [Google Scholar] [CrossRef]
- Saghafi, A.; Faiz, M.; Roberts, D. CO2 storage and gas diffusivity properties of coals from Sydney Basin, Australia. Int. J. Coal Geol. 2007, 70, 240–254. [Google Scholar] [CrossRef]

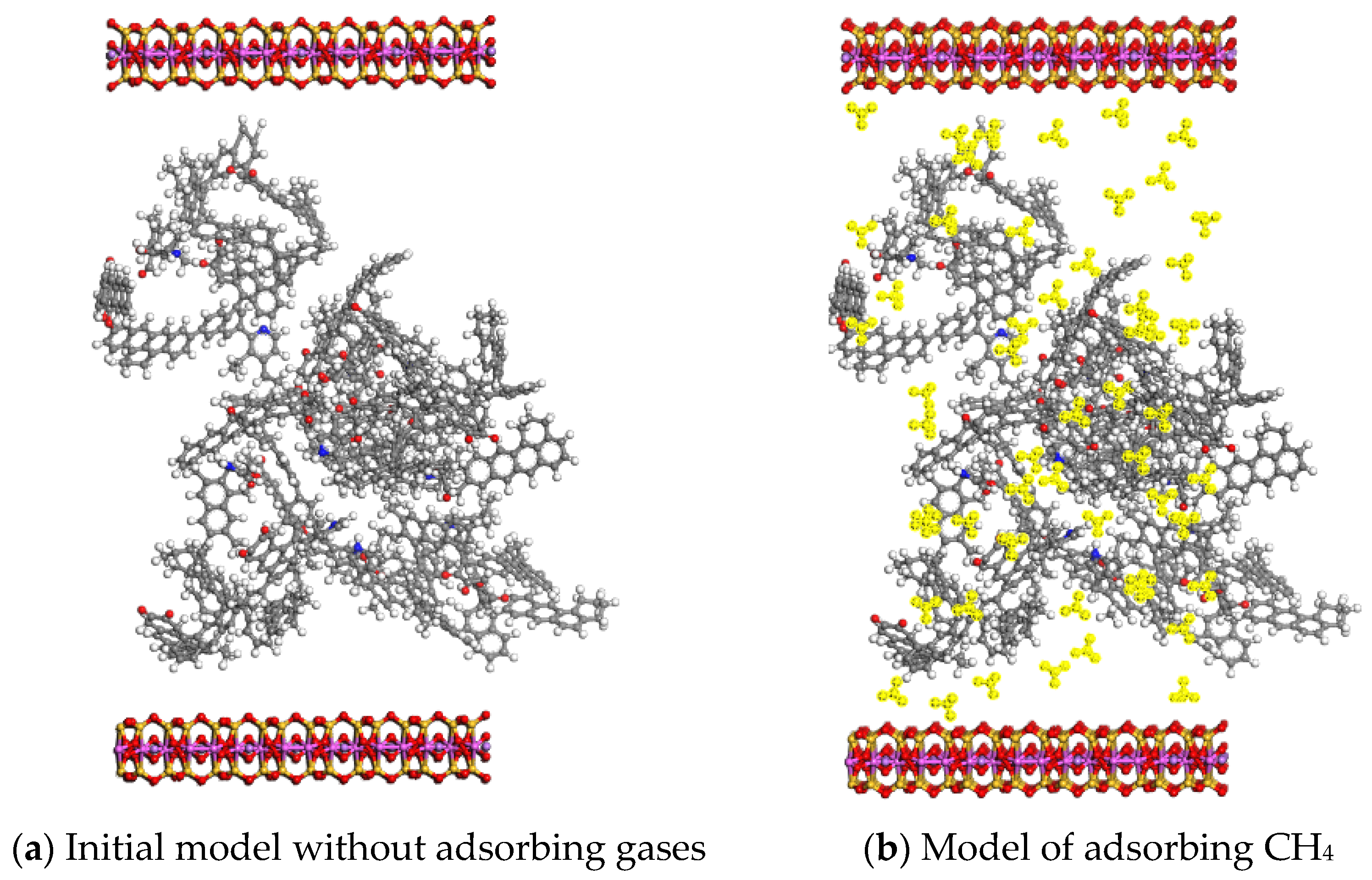
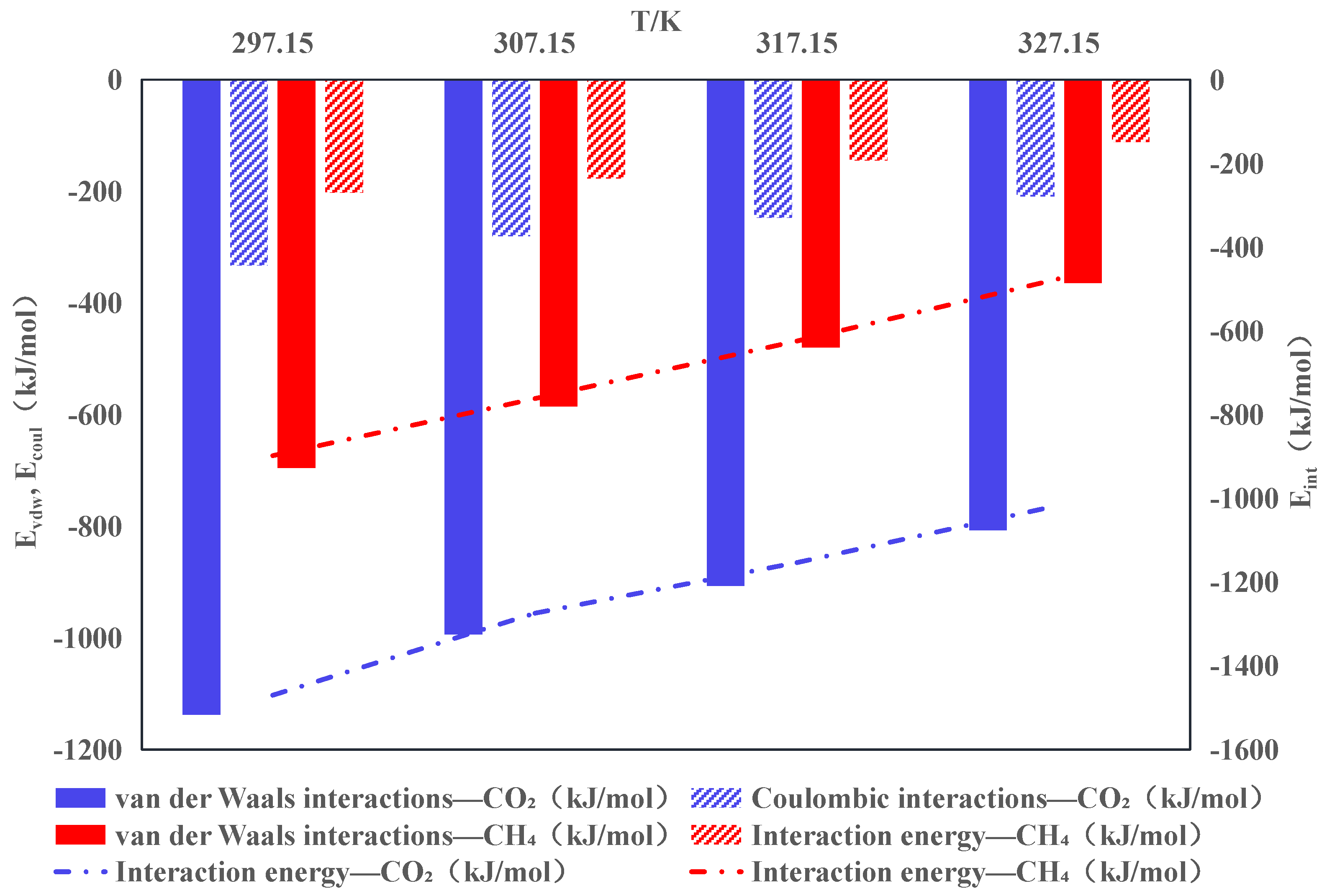


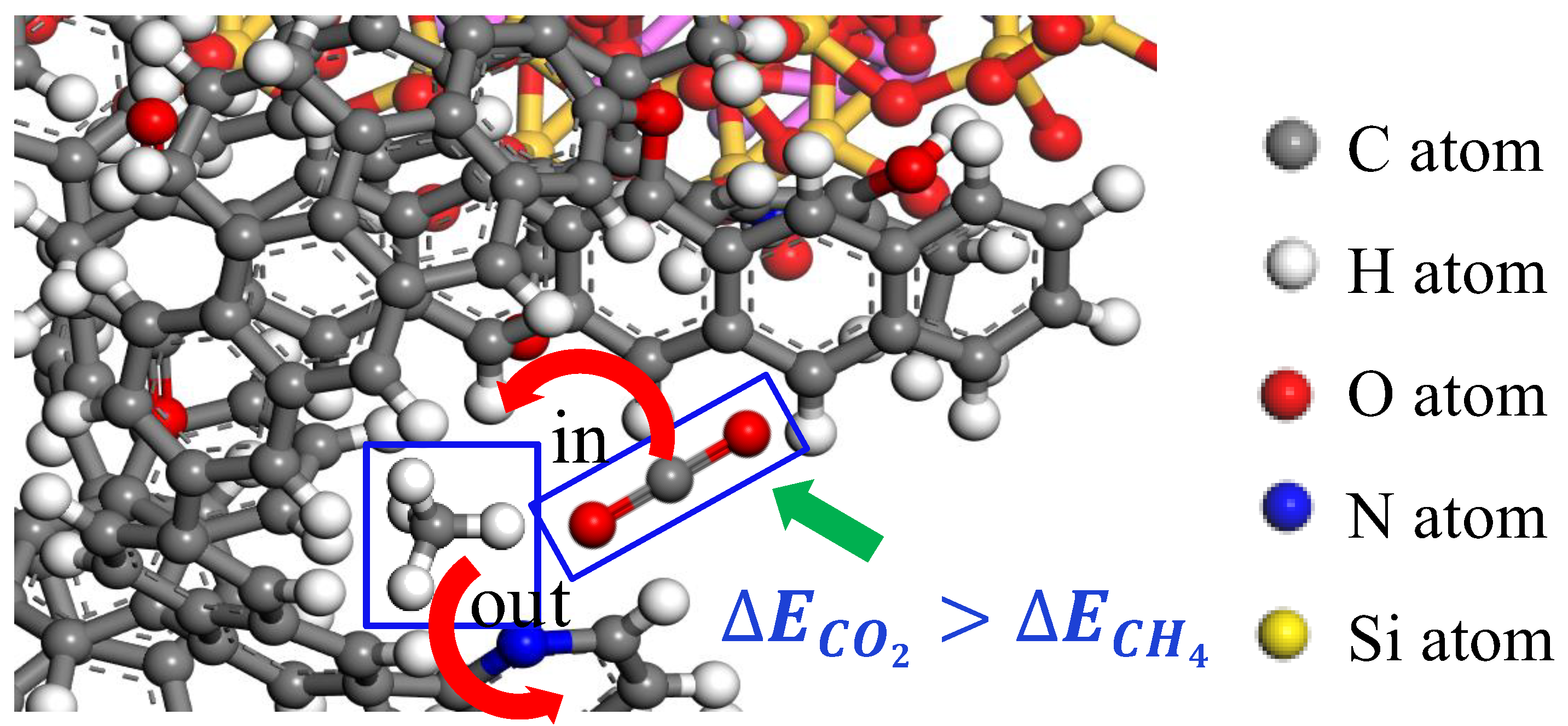

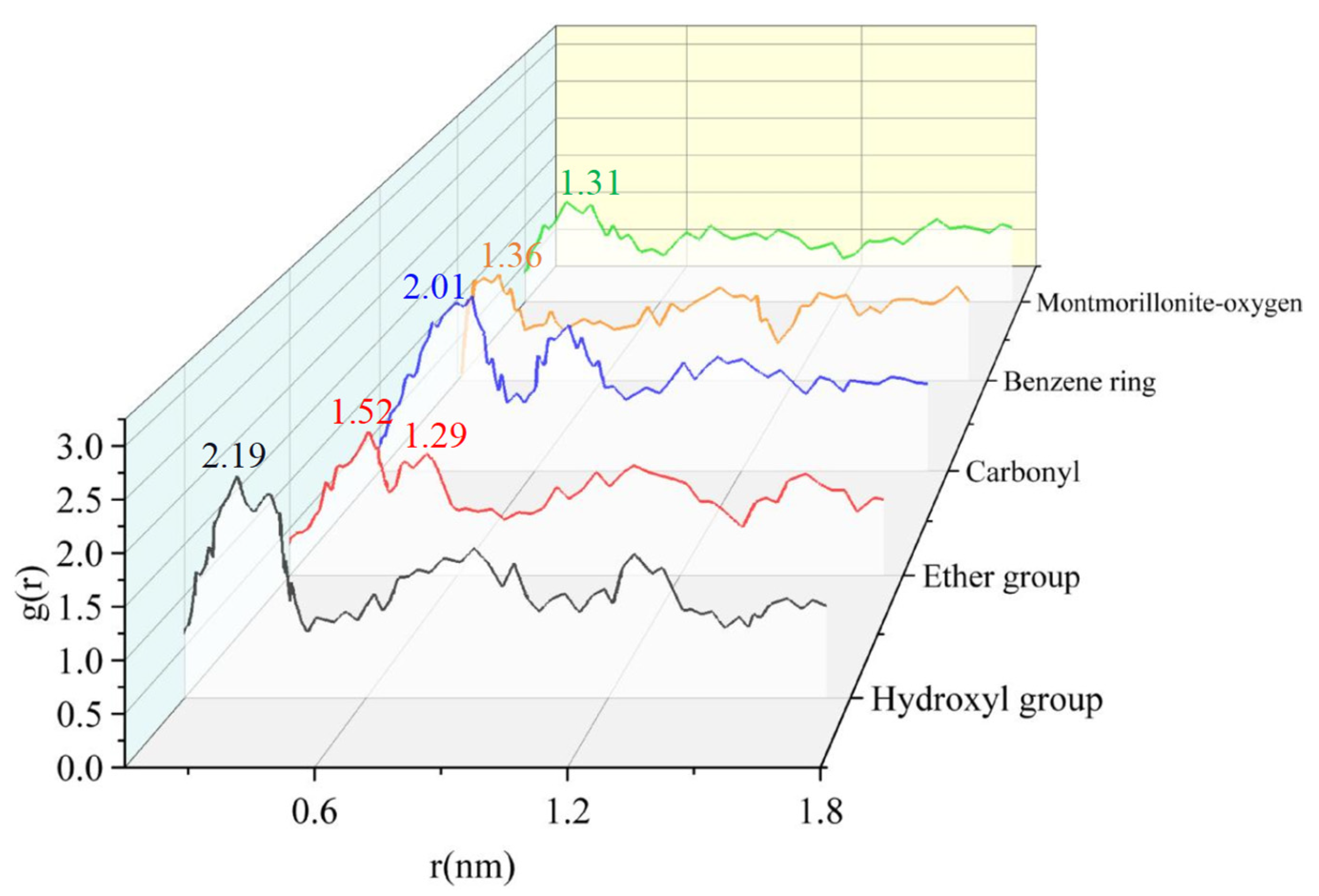
| Setting | Parameter | Setting | Parameter |
|---|---|---|---|
| Forcefield | COMPASSII | Cutoff distance | 12.5 Å |
| Charges | Forcefield assigned | Ensemble | NVT |
| Quality | Medium | Temperature control method | Nose |
| Electrostatic | Ewald | Pressure control method | Berendsen |
| Van der Waals | Atom-based | Simulation time | 50 ps |
| Ewald accuracy | 0.001 kcal/mol | Time step | 1 fs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Wang, Y.; Chen, X. Adsorption and Diffusion Characteristics of CO2 and CH4 in Anthracite Pores: Molecular Dynamics Simulation. Processes 2024, 12, 1131. https://doi.org/10.3390/pr12061131
Gao Y, Wang Y, Chen X. Adsorption and Diffusion Characteristics of CO2 and CH4 in Anthracite Pores: Molecular Dynamics Simulation. Processes. 2024; 12(6):1131. https://doi.org/10.3390/pr12061131
Chicago/Turabian StyleGao, Yufei, Yaqing Wang, and Xiaolong Chen. 2024. "Adsorption and Diffusion Characteristics of CO2 and CH4 in Anthracite Pores: Molecular Dynamics Simulation" Processes 12, no. 6: 1131. https://doi.org/10.3390/pr12061131





