Anti-Ice PMMA Surface Design and Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Performance Testing and Characterization
2.3. PMMA Hydrophobic Surface Preparation
2.4. Anti-Icing and Anti-Frost Performance Test
3. Results and Discussion
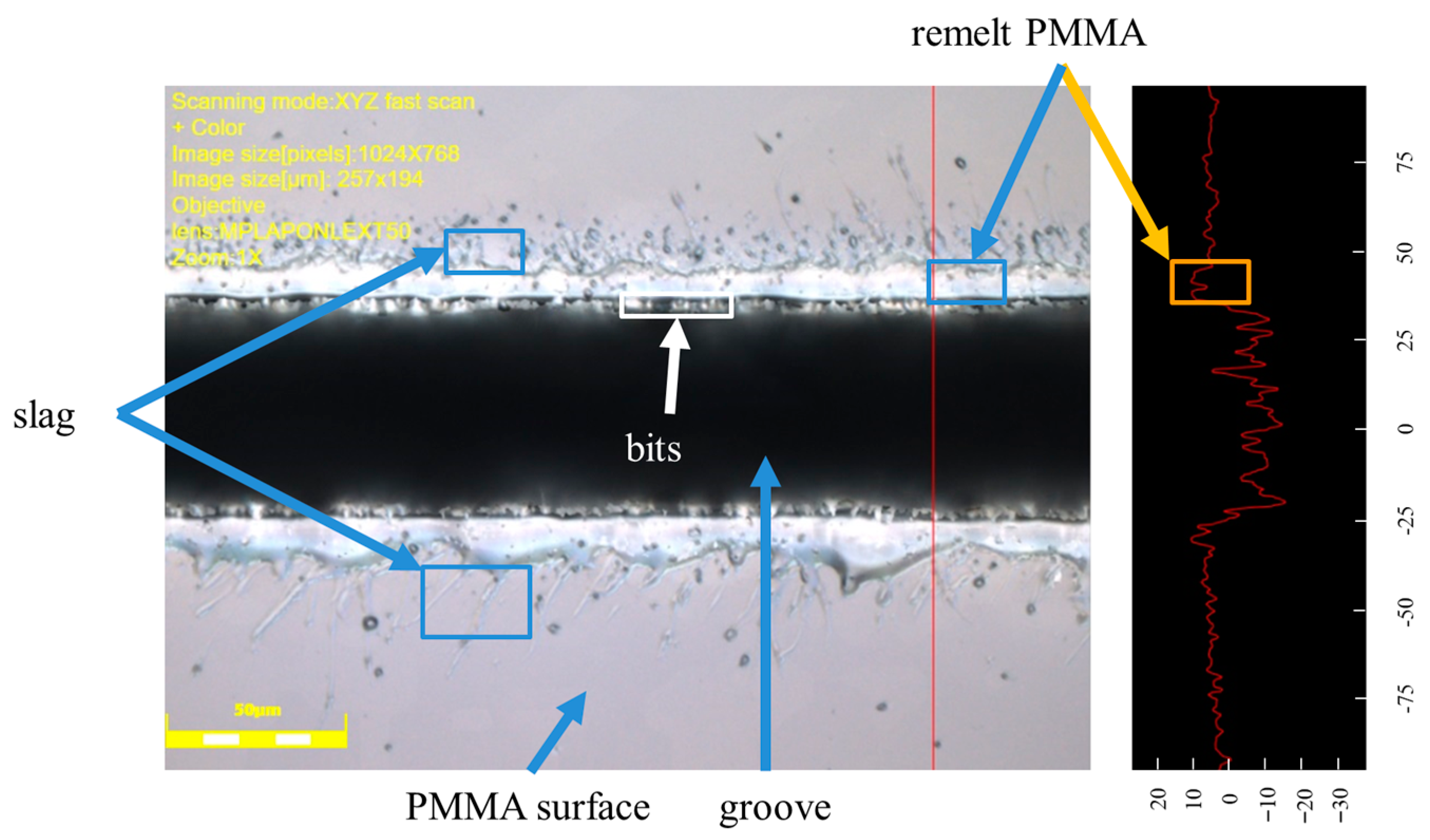
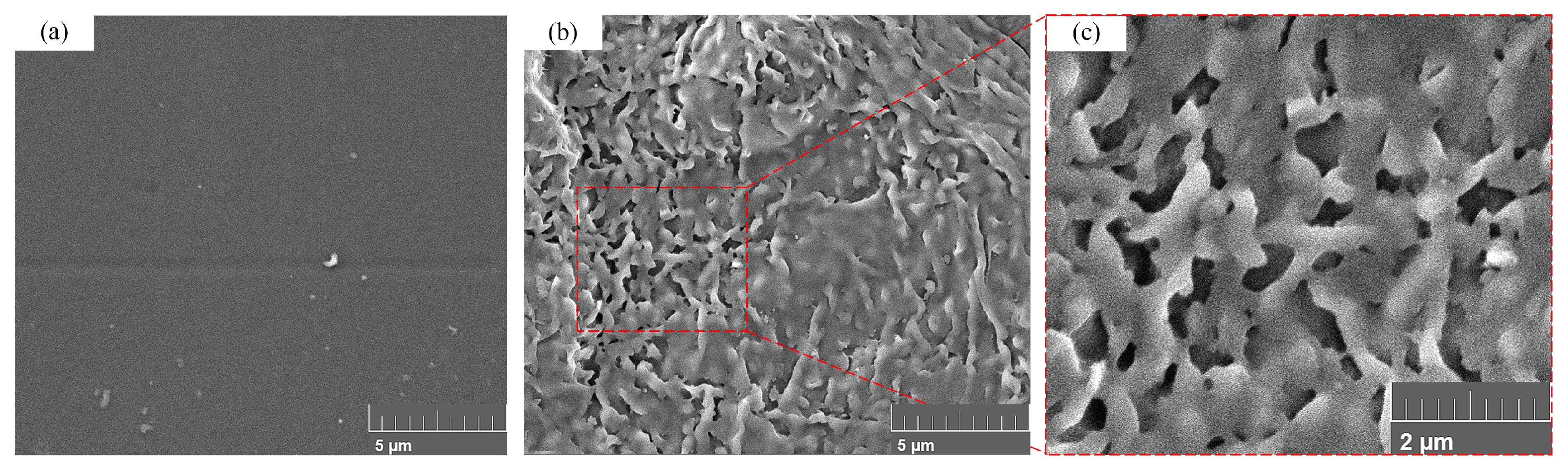
3.1. PMMA Processing Mechanism by Femtosecond Laser
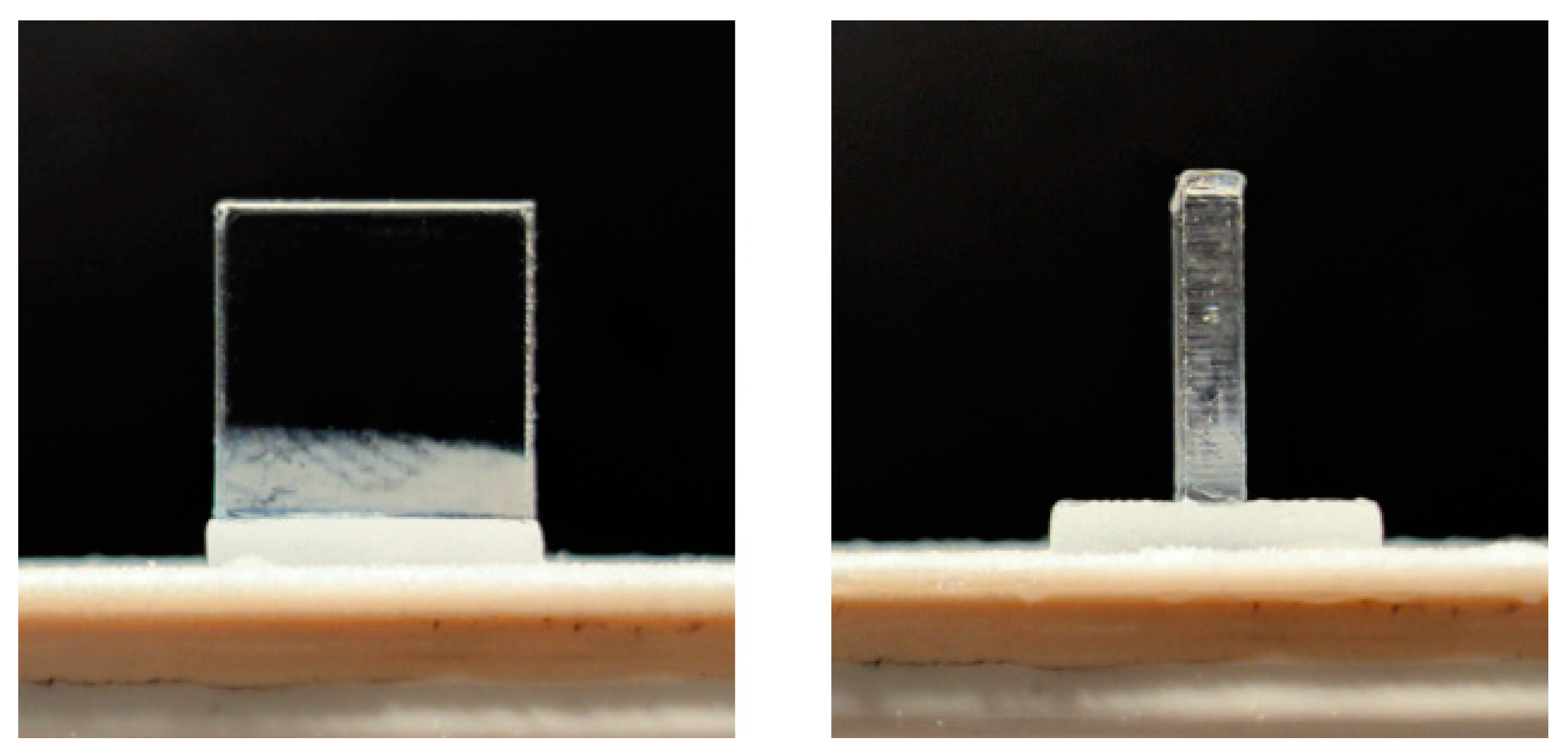
3.2. PMMA Ablation Threshold
3.3. Effect of Surface Microstructure on Hydrophobicity
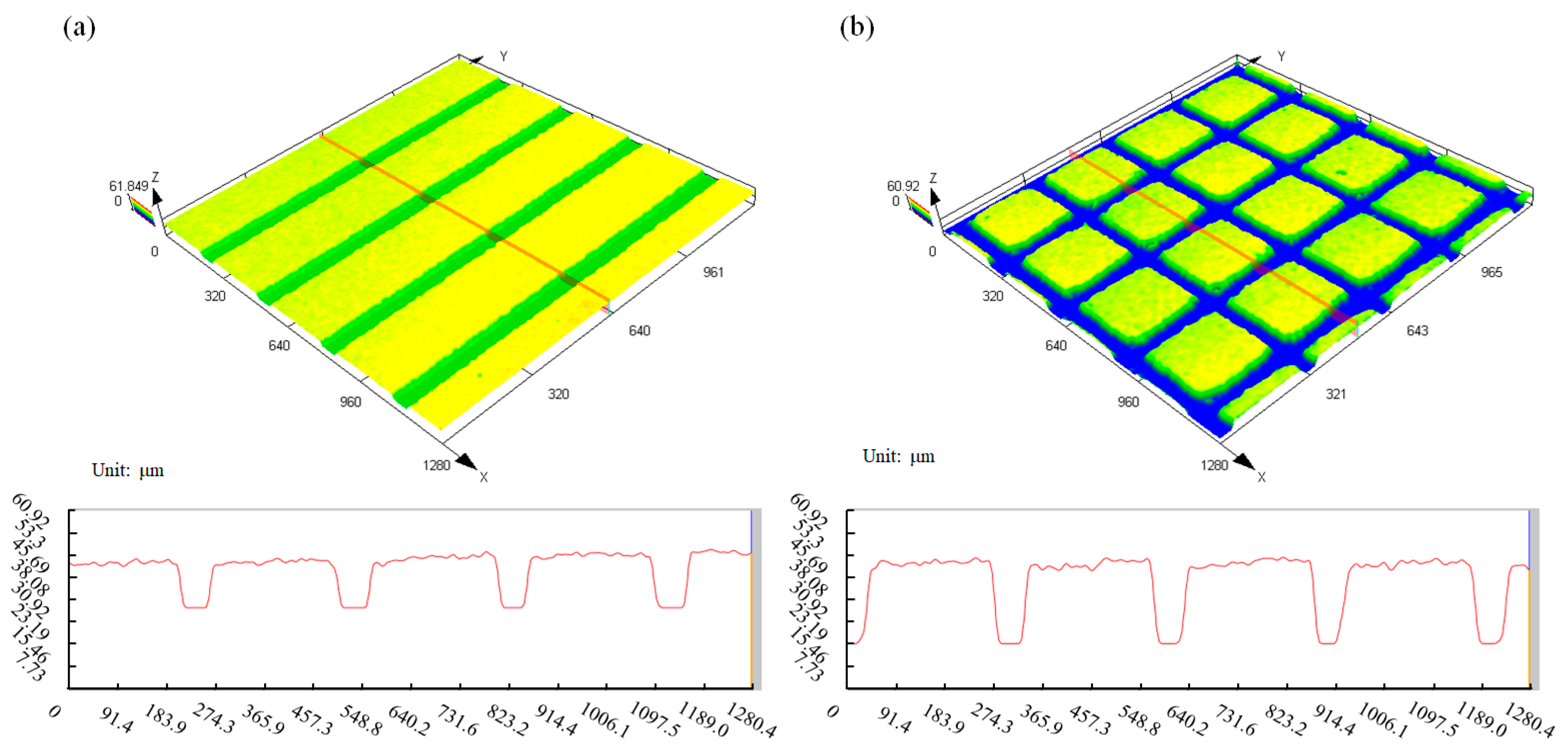
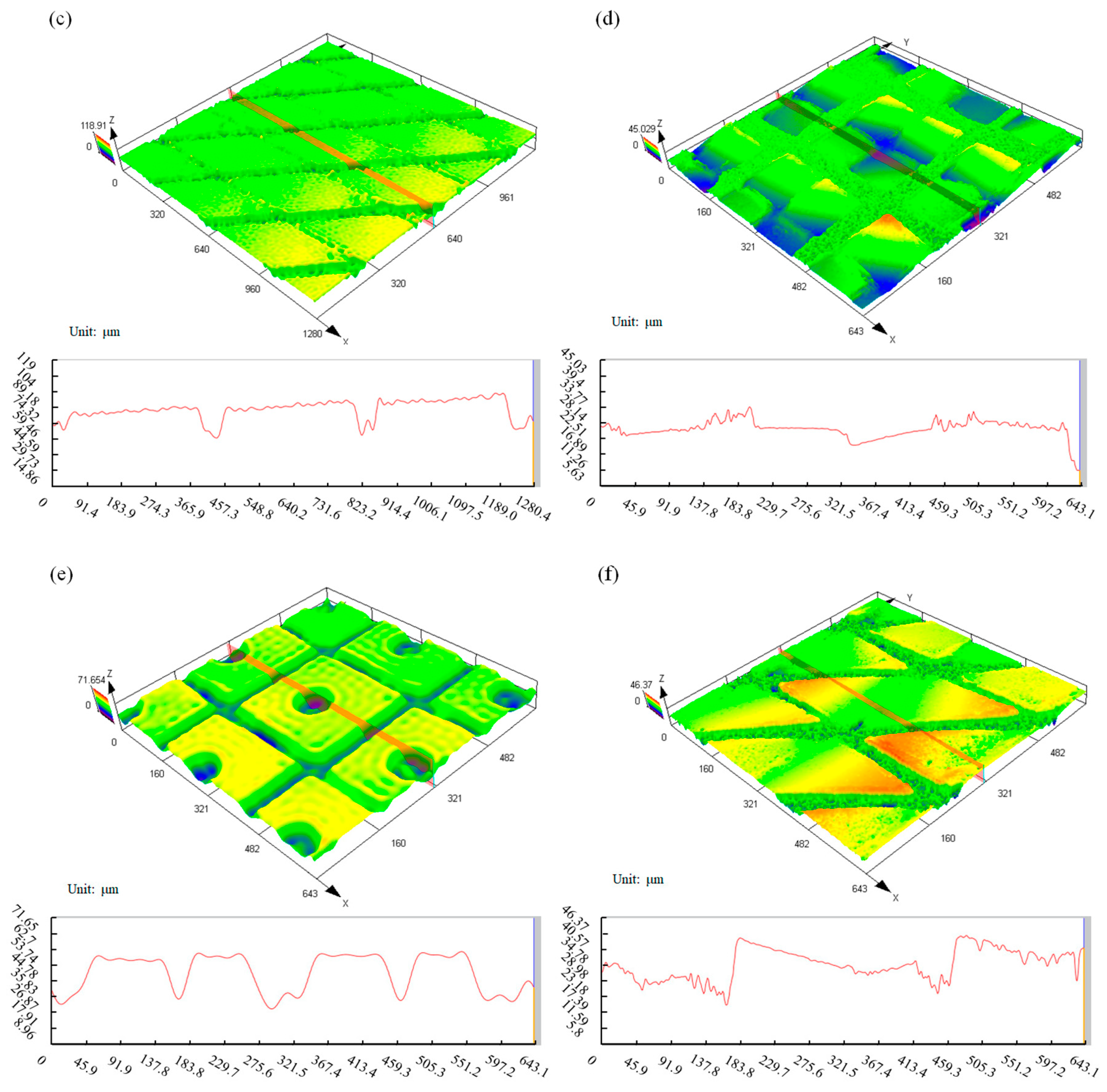
3.3.1. Microstructure Preparation
3.3.2. Low Surface Energy Treatment
3.3.3. Contact Angle Measurement
3.4. Anti-Icing Experiment Tests
3.4.1. Test for Delayed Frosting Performance
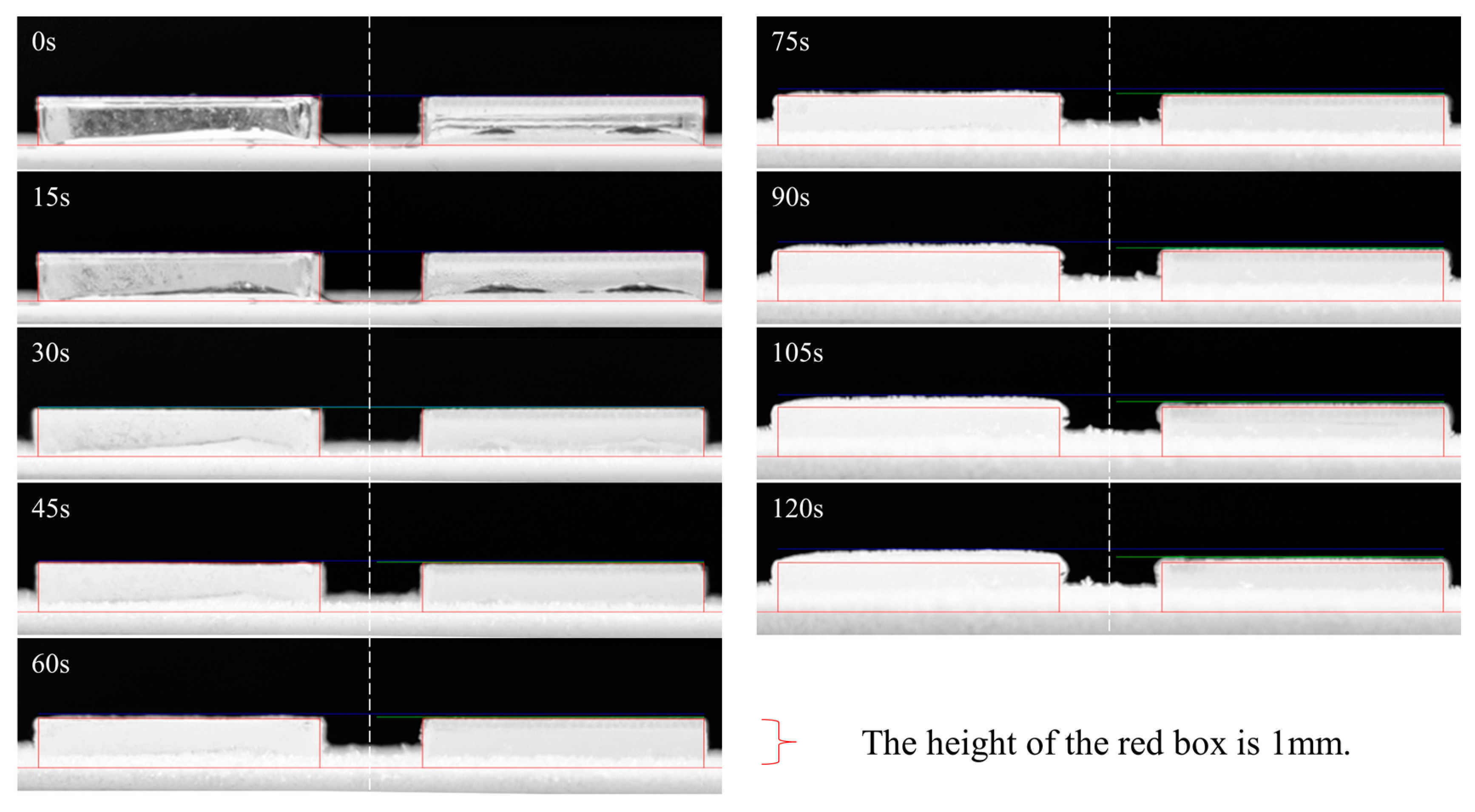
3.4.2. Antifrost Performance Test
3.5. Icicle Experiment
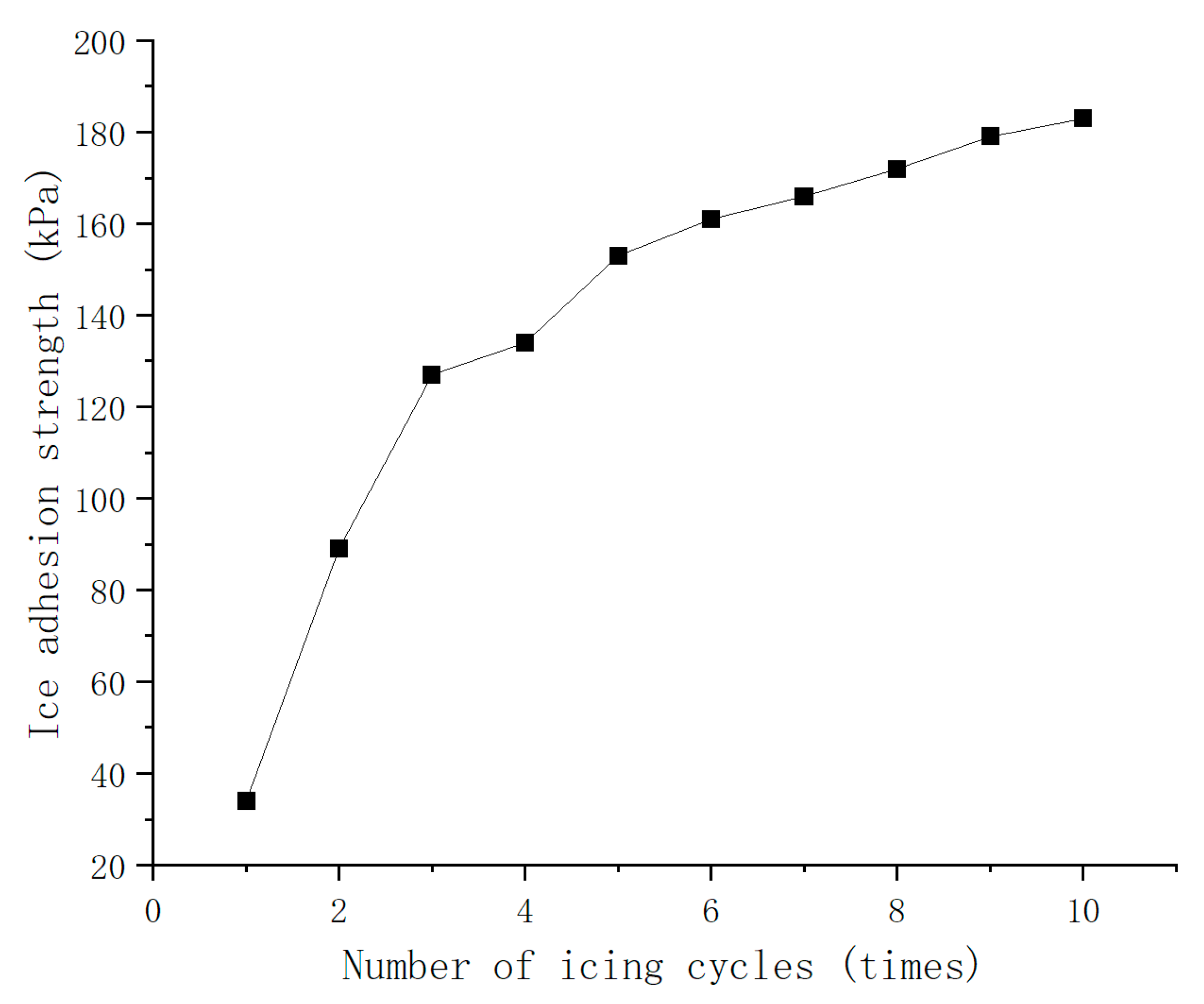
3.5.1. Adhesion Strength Test of Ice
3.5.2. Cyclic Frosting Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, P.; Yao, T.; Li, Z.; Wei, W.; Xie, Q.; Duan, W.; Han, H. A Superhydrophobic/Electrothermal Synergistically Anti-Icing Strategy Based on Graphene Composite. Compos. Sci. Technol. 2020, 198, 108307. [Google Scholar] [CrossRef]
- Sarshar, M.A.; Song, D.; Swarctz, C.; Lee, J.; Choi, C.-H. Anti-Icing or Deicing: Icephobicities of Superhydrophobic Surfaces with Hierarchical Structures. Langmuir 2018, 34, 13821–13827. [Google Scholar] [CrossRef] [PubMed]
- Kravanja, G.; Godec, R.F.; Rozman, M.; Rudolf, R.; Ivanic, A. Biomimetic Superhydrophobic Concrete with Enhanced Anticorrosive, Freeze Thaw, and Deicing Resistance. Adv. Eng. Mater. 2022, 24, 2101445. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Ivanov, V.K.; Pashinin, A.S. Durable Icephobic Coating for Stainless Steel. ACS Appl. Mater. Interfaces 2013, 5, 2549–2554. [Google Scholar] [CrossRef]
- Dong, X.; Gao, S.; Huang, J.; Li, S.; Zhu, T.; Cheng, Y.; Zhao, Y.; Chen, Z.; Lai, Y. A Self-Roughened and Biodegradable Superhydrophobic Coating with UV Shielding, Solar-Induced Self-Healing and Versatile Oil–Water Separation Ability. J. Mater. Chem. A 2019, 7, 2122–2128. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Bai, H.; Cao, M. Advances in Bioinspired Superhydrophobic Surfaces Made from Silicones: Fabrication and Application. Polymers 2023, 15, 543. [Google Scholar] [CrossRef]
- Jalil, S.A.; Akram, M.; Bhat, J.A.; Hayes, J.J.; Singh, S.C.; ElKabbash, M.; Guo, C. Creating Superhydrophobic and Antibacterial Surfaces on Gold by Femtosecond Laser Pulses. Appl. Surf. Sci. 2020, 506, 144952. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.Y.; Gao, N.; Barthlott, W. Mimicking Natural Superhydrophobic Surfaces and Grasping the Wetting Process: A Review on Recent Progress in Preparing Superhydrophobic Surfaces. Adv. Colloid Interface Sci. 2011, 169, 80–105. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, X.; Tao, J.; Zhu, C.; Lai, Y.; Chen, Z. Icephobic Materials: Fundamentals, Performance Evaluation, and Applications. Prog. Mater. Sci. 2019, 103, 509–557. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, F.; Li, Y.; Chen, T.; Nwokolo, I.K.; Ahmed, S.; Han, E. Modified Graphene Micropillar Array Superhydrophobic Coating with Strong Anti-Icing Properties and Corrosion Resistance. Coatings 2024, 14, 247. [Google Scholar] [CrossRef]
- Neinhuis, C. Characterization and Distribution of Water-Repellent, Self-Cleaning Plant Surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the Sacred Lotus, or Escape from Contamination in Biological Surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farhadi, S.; Nose, K.; Du, X.W. Superhydrophobic Surfaces: Are They Really Ice-Repellent? Langmuir 2011, 27, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zheng, H.; Sheng, W.; Hao, X.; Zhang, X. Preparation and Anti-Icing Properties of Zirconia Superhydrophobic Coating. Molecules 2024, 29, 1837. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, Z.; Chen, M.; Liu, Z.; Jiang, S.; Wang, L. Preparation of Durable Superhydrophobic Coatings Based on Discrete Adhesives. Coatings 2024, 14, 463. [Google Scholar] [CrossRef]
- Huang, W.; Huang, J.; Guo, Z.; Liu, W. Icephobic/Anti-Icing Properties of Superhydrophobic Surfaces. Adv. Colloid Interface Sci. 2022, 304, 102658. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, Y.; Xu, W.; Liu, H.; Lu, Y. Inexpensive and Non-Fluorinated Superhydrophobic Concrete Coating for Anti-Icing and Anti-Corrosion. J. Colloid Interface Sci. 2019, 541, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; He, R.; Zhang, Q.; Zhan, X.; Chen, F. Magnetic Particle-Based Super-Hydrophobic Coatings with Excellent Anti-Icing and Thermoresponsive Deicing Performance. J. Mater. Chem. A 2015, 3, 21637–21646. [Google Scholar] [CrossRef]
- Xie, H.; Wei, J.; Duan, S.; Zhu, Q.; Yang, Y.; Chen, K.; Zhang, J.; Li, L.; Zhang, J. Non-Fluorinated and Durable Photothermal Superhydrophobic Coatings Based on Attapulgite Nanorods for Efficient Anti-Icing and Deicing. Chem. Eng. J. 2022, 428, 132585. [Google Scholar] [CrossRef]
- Weng, W.; Deng, Q.; Yang, P.; Yin, K. Femtosecond Laser-Chemical Hybrid Processing for Achieving Substrate-Independent Superhydrophobic Surfaces. J. Cent. South Univ. 2024, 31, 1–10. [Google Scholar] [CrossRef]
- Lee, S.-I.; Cheong, H.; Park, J.-W.; Kim, M.; Paik, H. Gloss Control of PMMA/ABS Co-Extrusion Sheet Using Cross-Linked PMMA Particles Br. Polym. Korea 2023, 47, 72–78. [Google Scholar] [CrossRef]
- Suriano, R.; Kuznetsov, A.; Eaton, S.M.; Kiyan, R.; Cerullo, G.; Osellame, R.; Chichkov, B.N.; Levi, M.; Turri, S. Femtosecond Laser Ablation of Polymeric Substrates for the Fabrication of Microfluidic Channels. Appl. Surf. Sci. 2011, 257, 6243–6250. [Google Scholar] [CrossRef]
- Luo, Y.; Jia, W.; Song, Y.; Liu, B.; Hu, M.; Chai, L.; Wang, C. High-Repetition-Rate Femtosecond Laser Micromachining of Poly(Methyl Methacrylate). Chin. Opt. Lett. 2015, 13, 070003. [Google Scholar]
- Will, M.; Nolte, S.; Chichkov, B.N.; Tünnermann, A. Optical Properties of Waveguides Fabricated in Fused Silica by Femtosecond Laser Pulses. Appl. Opt. 2002, 41, 4360. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Mei, X.; Jiang, G.; Lei, S.; Yang, C. Effect of Two Typical Focus Positions on Microstructure Shape and Morphology in Femtosecond Laser Multi-Pulse Ablation of Metals. Appl. Surf. Sci. 2008, 255, 2303–2311. [Google Scholar] [CrossRef]
- Szameit, A.; Dreisow, F.; Pertsch, T.; Nolte, S.; Tünnermann, A. Control of Directional Evanescent Coupling in Fs Laser Written Waveguides. Opt. Express 2007, 15, 1579. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, E.; Gogoll, S.; Sils, J.; Huisinga, M.; Johansen, H.; Kästner, G.; Reichling, M.; Matthias, E. Laser Damage of Alkaline-Earth Fluorides at 248 nm and the Influence of Polishing Grades. Appl. Surf. Sci. 1997, 109–110, 162–167. [Google Scholar] [CrossRef]
- Li, F.; Chen, X.; Lin, W.; Pan, H.; Jin, X.; Hua, X. Nanosecond Laser Ablation of Al-Si Coating on Boron Steel. Surf. Coat. Technol. 2017, 319, 129–135. [Google Scholar] [CrossRef]
- Bonse, J.; Wrobel, J.M.; Krüger, J.; Kautek, W. Ultrashort-Pulse Laser Ablation of Indium Phosphide in Air. Appl. Phys. A. 2001, 72, 89–94. [Google Scholar] [CrossRef]
- Lan, L.; Wang, H.; Zhu, L.; Di, Y.; Kang, J.; Qiu, J. Preparation and Wetting Mechanism of Laser-Etched Composite Self-Assembled 1H,1H,2H,2H-Perfluorodecyltriethoxysilane Superhydrophobic Surface Coating. Phys. Status Solidi A 2022, 219, 2100568. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, S.; Ge, Z.; Li, J.; Xie, J.; Xu, J.; Xie, Z.; Yu, K. Study on Delaying Frost Growth Performanceof Micro-Nanostructure SuperhydrophobicCopper Surfaces. Pol. J. Environ. Stud. 2023, 32, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Ickes, L.; Welti, A.; Hoose, C.; Lohmann, U. Classical Nucleation Theory of Homogeneous Freezing of Water: Thermodynamic and Kinetic Parameters. Phys. Chem. Chem. Phys. 2015, 17, 5514–5537. [Google Scholar] [CrossRef] [PubMed]





| Parameter | Unit | Value |
|---|---|---|
| Impulse Frequency | kHz | 100 |
| Pulse Width | fs | 400 |
| Wave Length | nm | 1030 |
| Average Power | W | 4 |
| Scanning Speed | mm/s | 1 |
| Number | Microstructure | Average Contact Angle (°) |
|---|---|---|
| 1 | Grating Structure | 132.3 ± 2 |
| 2 | Cylindrical Structure | 142.5 ± 0.6 |
| 3 | Parallelogram Structure | 137.8 ± 0.6 |
| 4 | Cross-Shape Secondary Structure | 149.9 ± 1.1 |
| 5 | Dot-Shape Secondary Structure | 153.6 ± 0.6 |
| 6 | Triangular Secondary Structure | 151.9 ± 0.5 |
| 7 | PMMA | 132.3 ± 2 |
| Number | Microstructure | Freezing Time (s) |
|---|---|---|
| 1 | Grating Structure | 198 |
| 2 | Cylindrical Structure | 243 |
| 3 | Parallelogram Structure | 233 |
| 4 | Cross-Shape Secondary Structure | 265 |
| 5 | Dot-Shape Secondary Structure | 276 |
| 6 | Triangular Secondary Structure | 269 |
| 7 | PMMA | 193 |
| Number | Microstructure | Frost Layer Thickness (mm) |
|---|---|---|
| 1 | Grating Structure | 0.27 |
| 2 | Cylindrical Structure | 0.18 |
| 3 | Parallelogram Structure | 0.19 |
| 4 | Cross-Shape Secondary Structure | 0.12 |
| 5 | Dot-Shape Secondary Structure | 0.11 |
| 6 | Triangular Secondary Structure | 0.12 |
| 7 | PMMA | 0.30 |
| Number | Microstructure | Adhesion Strength of Ice (kPa) |
|---|---|---|
| 1 | Grating Structure | 232.2 ± 2.1 |
| 2 | Cylindrical Structure | 100.9 ± 0.3 |
| 3 | Parallelogram Structure | 155.6 ± 3.3 |
| 4 | Cross-Shape Secondary Structure | 66.4 ± 0.9 |
| 5 | Dot-Shape Secondary Structure | 33.7 ± 0.9 |
| 6 | Triangular Secondary Structure | 52.4 ± 0.4 |
| 7 | PMMA | 308.1 ± 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Feng, A. Anti-Ice PMMA Surface Design and Processing. Processes 2024, 12, 1322. https://doi.org/10.3390/pr12071322
Chen Y, Feng A. Anti-Ice PMMA Surface Design and Processing. Processes. 2024; 12(7):1322. https://doi.org/10.3390/pr12071322
Chicago/Turabian StyleChen, Yanming, and Aixin Feng. 2024. "Anti-Ice PMMA Surface Design and Processing" Processes 12, no. 7: 1322. https://doi.org/10.3390/pr12071322




