Development of a CaCO3 Precipitation Method Using a Peptide and Microwaves Generated by a Magnetron
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Peptide Synthesis
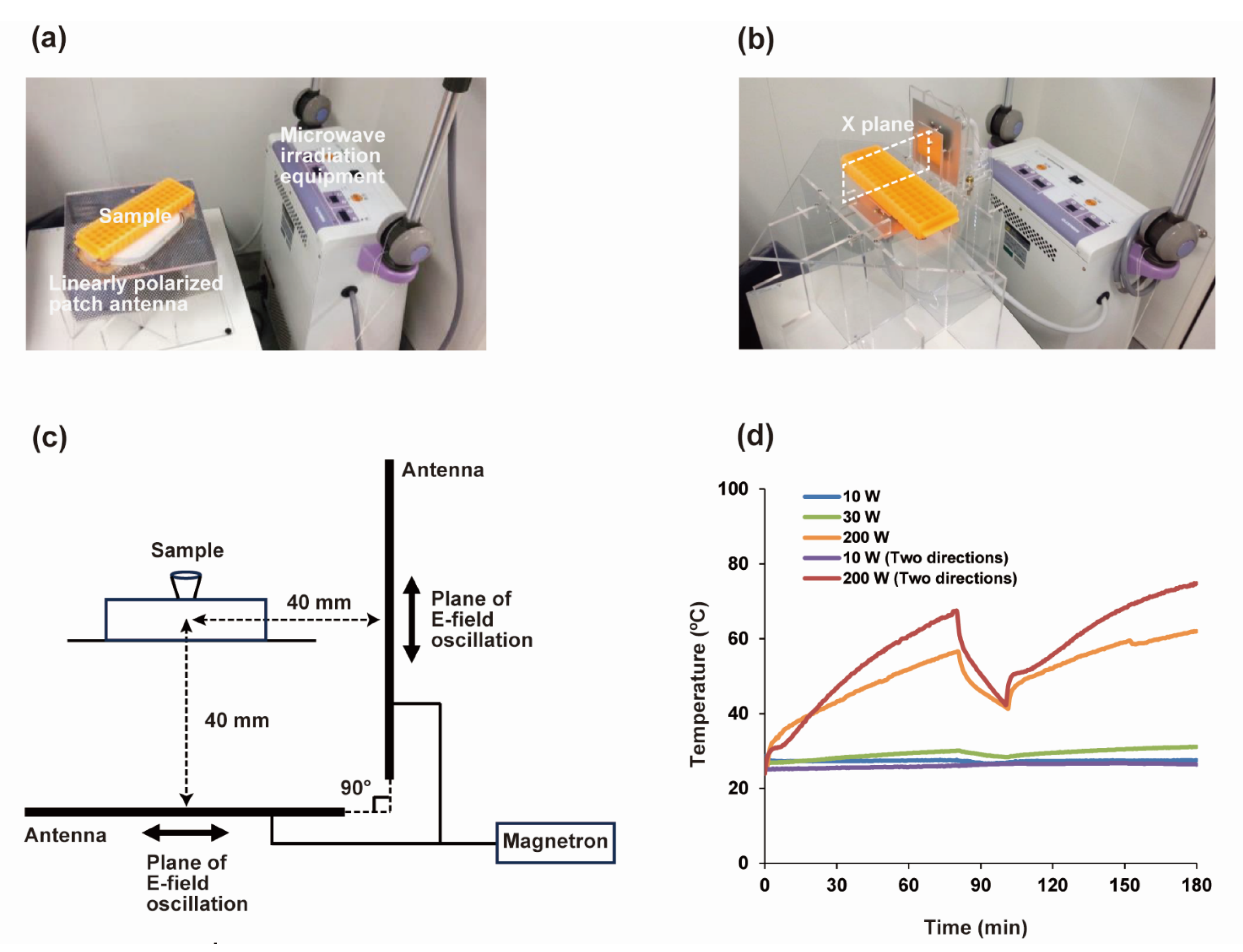
2.3. MW Irradiation Equipment
2.4. Measurements of E-Fields’ Strength
2.5. Measurements of Sample Temperature
2.6. CaCO3 Mineralization in the Presence and Absence of MW Irradiation
2.7. TEM and TEM-EELS Measurements
2.8. AFM Measurements
2.9. DLS Measurements
2.10. HPLC Analysis for Peptide Consumption Rates
3. Results and Discussion
3.1. Design of the CaCO3-Precipitating Peptide
3.2. Development of the MW Irradiating Equipment
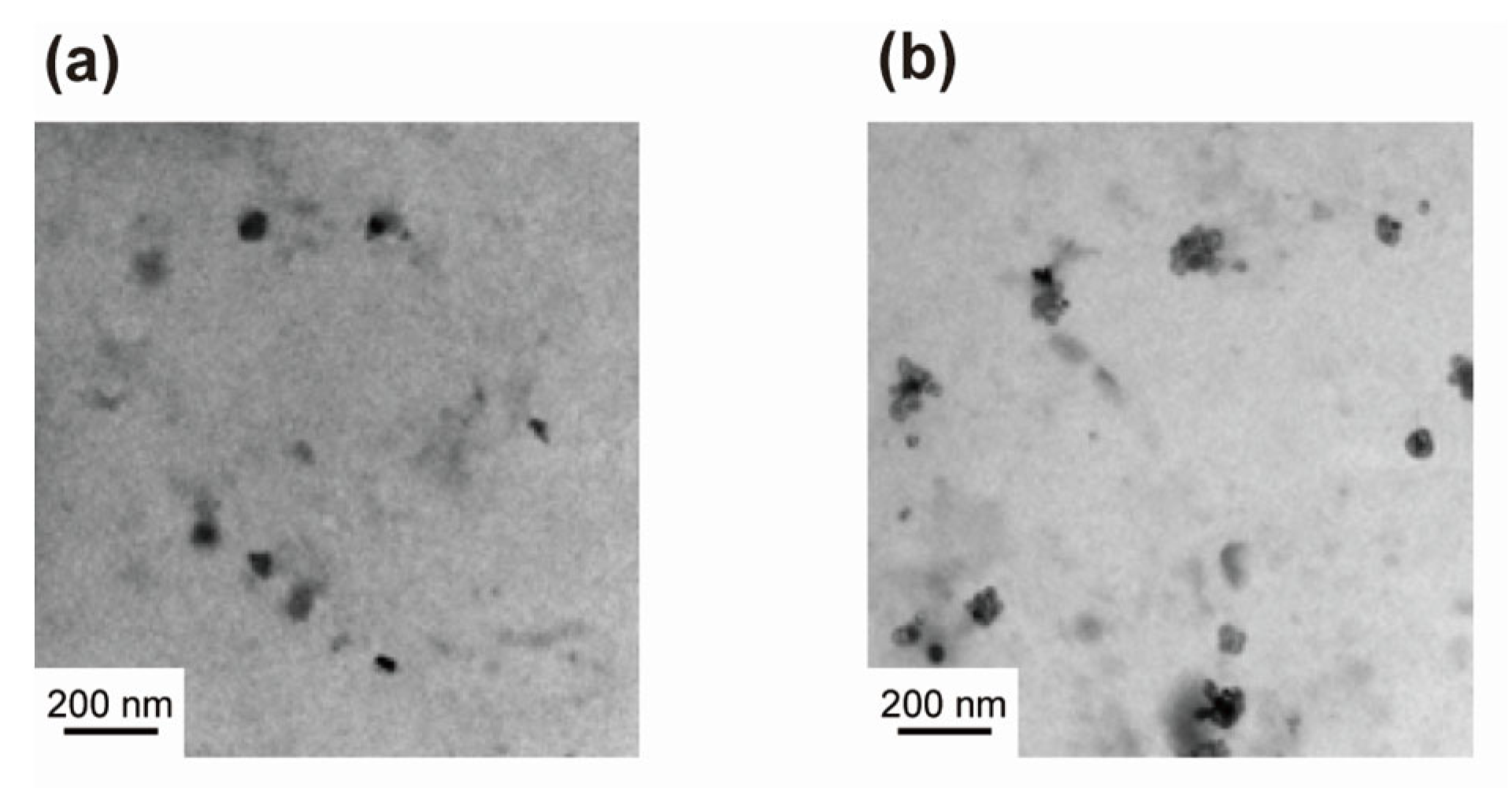
3.3. Morphology of the CaCO3 Precipitates Formed by MW Irradiation from One Direction
3.4. Morphology of CaCO3 Precipitates Formed under MW Irradiation from Two Directions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitchen, H.J.; Vallance, S.R.; Kennedy, J.L.; Tapia-Ruiz, N.; Carassiti, L.; Harrison, A.; Whittaker, A.G.; Drysdale, T.D.; Kingman, S.W.; Gregory, D.H. Modern microwave methods in solid-state inorganic materials chemistry: From fundamentals to manufacturing. Chem. Rev. 2014, 114, 1170–1206. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-J.; Chen, F. Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef] [PubMed]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A review of microwave synthesis of zinc oxide nanomaterials: Reactants, process parameters and morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, K.L.; Deng, K.; McGuire, S.C.; Tan, S.; Rui, N.; Zhang, L.; Rodriguez, J.A.; Wong, S.S. Microwave-assisted synthesis of Cu@IrO2 core-shell nanowires for low-temperature methane conversion. ACS Appl. Nano Mater. 2021, 4, 11145–11158. [Google Scholar] [CrossRef]
- De la Hoz, A.; Díaz-Ortiza, Á.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Ghosh, S.; Bhattacherjee, D.; Zyryanov, G.V.; Bagdi, A.K.; Hajra, A. Recent advances in microwave-assisted cross-coupling reactions. Asian J. Chem. 2022, 11, e202200179. [Google Scholar] [CrossRef]
- Tsukahara, Y.; Higashi, A.; Yamauchi, T.; Nakamura, T.; Yasuda, M.; Baba, A.; Wada, Y. In situ observation of nonequilibrium local heating as an origin of special effect of microwave on chemistry. J. Phys. Chem. C 2010, 114, 8965–8970. [Google Scholar] [CrossRef]
- Horikoshi, S.; Watanabe, T.; Narita, A.; Suzuki, Y.; Serpone, N. The electromagnetic wave energy effect(s) in microwave-assisted organic syntheses (MAOS). Sci. Rep. 2018, 8, 5151. [Google Scholar] [CrossRef]
- Frasso, M.A.; Stiegman, A.E.; Dudley, G.B. Microwave-specific acceleration of a retro-Diels-Alder reaction. Chem. Commun. 2021, 56, 11247–11250. [Google Scholar] [CrossRef]
- Yamada, M.; Ohta, R.; Harada, K.; Takehara, T.; Haneoka, H.; Murakami, Y.; Suzuki, T.; Ohki, Y.; Takahashi, N.; Akiyama, T.; et al. Product selective reaction controlled by the combination of palladium nanoparticles, continuous microwave irradiation, and a co-existing solid; ligand-free Buchwald-Hartwig amination vs. aryne amination. Green Chem. 2021, 23, 8131–8137. [Google Scholar] [CrossRef]
- Matsukawa, Y.; Muranaka, A.; Murayama, T.; Uchiyama, M.; Takaya, H.; Yamada, Y.M.A. Microwave-assisted photooxidation of sulfoxides. Sci. Rep. 2021, 11, 20505. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.L.; Tofteng, A.P.; Malik, L.; Jensen, K.J. Microwave heating in solid-phase peptide synthesis. Chem. Soc. Rev. 2012, 41, 1826–1844. [Google Scholar] [CrossRef] [PubMed]
- Hojo, K.; Manabe, Y.; Uda, T.; Tsuda, Y. Water-based solid-phase peptide synthesis without hydroxy side chain protection. J. Org. Chem. 2022, 87, 11362–11368. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.; Zamanian, A.; Behnamghader, A.; Daliri-Joupari, M. Bone-like hydroxyapatite mineralization on the bio-inspired PDA nanoparticles using microwave irradiation. Surf. Interfaces 2019, 15, 38–42. [Google Scholar] [CrossRef]
- Sugawara, A.; Nishimura, T.; Yamamoto, Y.; Inoue, H.; Nagasawa, H.; Kato, T. Self-organization of oriented calcium carbonate/polymer composites: Effects of a matrix peptide isolated from the exoskeleton of a crayfish. Angew. Chem. Int. Ed. 2006, 45, 2876–2879. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, H.; Matsunaga, R.; Nishimura, T.; Yamamoto, Y.; Kajiyama, S.; Oaki, Y.; Akaiwa, K.; Inoue, H.; Nagasawa, H.; Tsumoto, K.; et al. CaCO3/Chitin hybrids: Recombinant acidic peptides based on a peptide extracted from the exoskeleton of a crayfish controls the structures of the hybrids. Faraday Discuss. 2012, 159, 483–494. [Google Scholar] [CrossRef]
- Arakaki, A.; Shimizu, K.; Oda, M.; Sakamoto, T.; Nishimura, T.; Kato, T. Biomineralization-inspired synthesis of functional organic/inorganic hybrid materials: Organic molecular control of self-organization of hybrids. Org. Biomol. Chem. 2015, 13, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayanan, R.; Chi-Jin, E.O.; Loh, X.J.; Kini, R.M.; Valiyaveettil, S. Purification and characterization of a vaterite-inducing peptide, pelovaterin, from the eggshells of pelodiscus sinensis (Chinese soft-shelled turtle). Biomacromolecules 2005, 6, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Murai, K.; Kinoshita, T.; Nagata, K.; Higuchi, M. Mineralization of calcium carbonate on multifunctional peptide assembly acting as mineral source supplier and template. Langmuir 2016, 32, 9351–9359. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Lin, J.; Cai, C. The effect of a thermo-responsive polypeptide-based copolymer on the mineralization of calcium carbonate. J. Mater. Chem. 2012, 22, 3939–3947. [Google Scholar] [CrossRef]
- Elhadj, S.; Salter, E.A.; Wierzbicki, A.; De Yoreo, J.J.; Han, N.; Dove, P.M. Peptide controls on calcite mineralization: Polyaspartate chain length affects growth kinetics and acts as a stereochemical switch on morphology. Cryst. Growth Des. 2006, 6, 197–201. [Google Scholar] [CrossRef]
- Usui, K.; Ozaki, M.; Yamada, A.; Hamada, Y.; Tsuruoka, T.; Imai, T.; Tomizaki, K. Site-specific control of multiple mineralizations using a designed peptide and DNA. Nanoscale 2016, 8, 17081–17084. [Google Scholar] [CrossRef] [PubMed]
- Usui, K.; Yokota, S.; Ozaki, M.; Sakashita, S.; Imai, T.; Tomizaki, K. Modification of the N-terminus of a calcium carbonate precipitating peptide affects calcium carbonate mineralization. Protein Pept. Lett. 2018, 25, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Knight, B.M.; Edgar, K.J.; Yoreo, J.D.; Dove, P.M. Chitosan as a canvas for studies of macromolecular controls on CaCO3 biological crystallization. Biomacromolecules 2023, 24, 1078–1102. [Google Scholar] [CrossRef] [PubMed]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [PubMed]
- Usui, K.; Ozaki, M.; Hirao, K.; Kosaka, T.; Endo, N.; Yoshida, S.; Yokota, S.; Arimoto, Y.; Osawa, R.; Nakanishi, N.; et al. Effect of linearly polarized microwaves on nanomorphology of calcium carbonate mineralization using peptides. Sci. Rep. 2023, 13, 12027. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.; White, P.D. Fmoc Solid Phase Peptide Synthesis; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Usui, K.; Togashi, H.; Endo, N.; Ozaki, M.; Arimoto, Y.; Uraka, T.; Osawa, R.; Minaki, K.; Nakanishi, N.; Umetani, T. Microwave irradiation systems for analyses of biomolecular behaviors. J. Jpn. Soc. Electromagn. Wave Energy Appl. 2017, 1, 17–24. (In Japanese) [Google Scholar]
- Tsubaki, S.; Matsuzawa, T.; Higuchi, T.; Fujii, S.; Wada, Y. Determining the influence of microwave-induced thermal unevenness on vanadium oxide catalyst particles. Chem. Eng. J. 2022, 433, 133603. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayamori, F.; Togashi, H.; Endo, N.; Ozaki, M.; Hirao, K.; Arimoto, Y.; Osawa, R.; Tsuruoka, T.; Imai, T.; Tomizaki, K.-y.; et al. Development of a CaCO3 Precipitation Method Using a Peptide and Microwaves Generated by a Magnetron. Processes 2024, 12, 1327. https://doi.org/10.3390/pr12071327
Kayamori F, Togashi H, Endo N, Ozaki M, Hirao K, Arimoto Y, Osawa R, Tsuruoka T, Imai T, Tomizaki K-y, et al. Development of a CaCO3 Precipitation Method Using a Peptide and Microwaves Generated by a Magnetron. Processes. 2024; 12(7):1327. https://doi.org/10.3390/pr12071327
Chicago/Turabian StyleKayamori, Fumihiro, Hiroyuki Togashi, Natsumi Endo, Makoto Ozaki, Kan Hirao, Yonejiro Arimoto, Ryuji Osawa, Takaaki Tsuruoka, Takahito Imai, Kin-ya Tomizaki, and et al. 2024. "Development of a CaCO3 Precipitation Method Using a Peptide and Microwaves Generated by a Magnetron" Processes 12, no. 7: 1327. https://doi.org/10.3390/pr12071327
APA StyleKayamori, F., Togashi, H., Endo, N., Ozaki, M., Hirao, K., Arimoto, Y., Osawa, R., Tsuruoka, T., Imai, T., Tomizaki, K.-y., Umetani, T., Nakanishi, N., & Usui, K. (2024). Development of a CaCO3 Precipitation Method Using a Peptide and Microwaves Generated by a Magnetron. Processes, 12(7), 1327. https://doi.org/10.3390/pr12071327







