Abstract
(1) Background: In this study, bioactive compounds (phenolics and betalains) extracted from beetroot were stabilized by encapsulation with maltodextrin and soy protein by the freeze drying method. Stability of bioactive compounds and bioactivities in a beetroot extract and encapsulates during 60 days of storage at 4 °C (without light) and at 25 °C (with and without light) were investigated. (2) Methods: Contents of bioactive compounds and bioactivity (antioxidant activity by DPPH, RP, and ABTS tests; anti-inflammatory and antihyperglycemic activity) were determined. Improvement in stability of bioactives’ content and bioactivity of prepared encapsulates in relation to the extract was observed after storage at room temperature under light conditions. (3) Results: Encapsulation with maltodextrin showed improvement in stability of all studied bioactive parameters, while an encapsulate with soy protein improved stability of bioactives and antioxidant activity compared to the extract. The encapsulated beetroot extract represents a promising food additive for functional foods due to their content of bioactive compounds and consequent bioactivities.
1. Introduction
Nowadays, beetroot is cultivated in many countries and frequently consumed in daily diets [1]. In 2018, the production of beetroot reached up to 275.49 million metric tons in the world [1].
Beetroot (Beta vulgaris L.) contains a number of bioactive compounds, including betalains (red-violet betacyanins and yellow-orange betaxanthins) and phenolic compounds (flavonoids and phenolic acids), that could exhibit health-promoting effects mostly due to their antioxidant properties [1,2,3,4]. In addition, natural betalain pigments can be used as natural colorants in the food industry [3]. However, there should be strategies to improve or enhance the stability of betalain and phenolic compounds in food products, because these bioactive compounds are very unstable under different conditions such as pH, light, temperature, and presence of oxygen [5,6]. Some of these factors can produce many changes in the chemical structure of bioactive compounds, leading to the loss of their activity and potential health benefits [5].
Recently, encapsulation has been intensively studied as a promising method for improving stability, to preserve the biological activity and to ensure controlled release of the active compounds [7,8]. In the encapsulation process, one material or a mixture of materials (core) are coated or entrapped within other materials or systems (wall material) [9]. Accordingly, by the encapsulation process, bioactive compounds (usually in the plant extracts) as core material can be stabilized using different matrices (proteins and carbohydrates) [7,8]. The selection of the carrier material is an important factor affecting various properties such as storage stability of encapsulates [7]. Because of their suitable properties, maltodextrin and soy protein are among the most commonly used food-grade wall materials for the encapsulation of phytochemicals [10]. In addition, the selection of appropriate encapsulation techniques (such as spray drying, freeze drying, etc.) is another critical issue requiring successful encapsulation [8]. Between various techniques, freeze drying, also known as lyophilization, is one of the most used processes for the protection of thermosensitive and unstable compounds, since dehydration at low temperature consists of eliminating water by the sublimation of the frozen product [11]. However, a limited number of studies had examined physicochemical characteristics of a beetroot extract encapsulation prepared by the lyophilization technique [6]. In addition, the stability characterization of encapsulated powders during storage has potential use as ingredients with significant bioactivities in the development of new functional food products. Therefore, the objective of this study was to evaluate the effect of storage conditions and choice of encapsulation material on the content of bioactive compounds (total betalains, betacyanins, betaxanthins, and total polyphenols) and bioactivity (antioxidant, anti-inflammatory, and antihyperglycemic activity) in beetroot encapsulates prepared by the freeze drying process. The content of bioactive compounds and bioactivity of beetroot encapsulates were compared with the values determined for the beetroot extract used for their preparation in order to evaluate the suitability of the encapsulation parameters.
2. Materials and Methods
2.1. Chemicals and Instruments
The Folin–Ciocalteau reagent, 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•), 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS•+), Trolox, and trichloroacetic acid were purchased from Sigma Chemical Co. (St. Louis, MO, USA), and ferric chloride was obtained from J.T. Baker (Deventer, Holland, The Netherlands). Soy protein isolates were purchased from Olimp Laboratories (Debica, Poland) and maltodextrin was purchased from Battery Nutrition Limited (London, UK). Other chemicals and solvents were of the highest analytical grade. Spectrophotometric measurements were performed with a Multiskan GO microplate reader (Thermo Fisher Scientific Inc., Waltham, MA, USA). The freeze dryer, model Christ Alpha 2-4 LSC, was from Martin Christ (Osterode am Harz, Germany).
2.2. Plant Material
Lyophilized beetroot material, obtained from Alfred Galke GmbH (Bad Grund, Germany), was ground to an average particle diameter of about 0.5 mm.
2.3. Extraction Procedure
In this study, extraction was performed using the Soxhlet apparatus ISOLAB NS29-32 (Merck KGaA, Darmstadt, Germany) following the procedure described by Borjan et al. [12]. Dried and ground material (20 g) was introduced into the tube, and 150 mL of 50% aqueous ethanol was added to the flask. Extraction was carried out in three cycles for approximately 2 h. The heating temperature was adjusted to the boiling point of the employed solvent. The extract was evaporated to dryness under reduced pressure. The weight of the beetroot extract (E) (average of triplicate analysis) was 10.2011 ± 0.4107 g.
2.4. Encapsulation Process
Two encapsulated beetroot extracts (E) with different wall materials, maltodextrin (M) and soy protein (SP), were prepared. For the preparation of encapsulates on the basis of maltodextrin (ME), 1.5 g of beetroot extract E dissolved in 40 mL of distilled water was mixed with 2 g of wall material (maltodextrin). For the preparation of encapsulates on the basis of soy protein (SPE), instead of maltodextrin, soy protein was used as wall material. The mixtures were homogenized (Heidolph DIAX 900, Heidolph Instruments GmbH, Kelheim, Germany) at 11,000 rpm for 5 min and additionally mixed using a laboratory shaker (Unimax 1010, Heidolph Instruments GmbH, Kelheim, Germany) at 300 rpm for 10 min, under light protection, at room temperature. The homogenized mixtures were frozen at −18 °C for 3 h and then freeze-dried at −40 °C for 48 h to ensure complete drying.
2.5. Chemical Characterization
The beetroot extract (E), encapsulates (ME and SPE), and wall materials (M and SP) used in the preparation of encapsulates were characterized in terms of bioactive compounds’ (phenolics and betalaines) contents and bioactivities (antioxidant, anti-inflammatory, and antihyperglycemic).
For the determination of total bioactive compounds and bioactivities, encapsulates (ME and SPE) were subjected to the extraction procedure by Sáenz et al. [13], with modification. In the first step, 200 mg of the sample was dispersed in 2 mL methanol/acetic acid/water (50:8:42, v/v/v). For 1 min, the mixture was vortexed and ultrasounded (ultrasonic bath Sonic 12GT, Vims elektrik, Loznica, Serbia) for 20 min, again vortexed for 1 min and ultrasounded for 20 min, and centrifuged (centrifuge Lace 24, Colo Lab Experts, Novo Mesto, Slovenia) for 5 min at 4000 rpm, and the supernatant was separated. In the second step, the residue was vortexed with 1 mL of the solvent mixture for 1 min, and after that extracted for 30 min with a laboratory shaker at 400 rpm. The mixture was ultrasounded for 20 min, and centrifuged for 5 min at 4000 rpm, and the supernatant was separated. In the third step, the residue was vortexed with 1 mL of the solvent mixture for 1 min, ultrasounded for 5 min, and centrifuged for 5 min at 4000 rpm, and the supernatant was separated. The supernatants of all three steps were combined and used for encapsulates’ chemical characterization. For the comparison of the content of bioactive compounds and bioactivity of encapsulates with wall materials, the same extraction procedure was performed on wall materials, i.e., on maltodextrin (M) and soy protein (SP), used in encapsulates’ preparation. For the determination of surface bioactive compounds, encapsulates (ME and SPE) were subjected to the extraction procedure by Carmona et al. [14], with modification. In total, 200 mg of the sample was dispersed in an ethanol/methanol (1:1, v/v) solution (2 mL), shaken for 1 min, and centrifuged at 4000 rpm for 5 min. After separation, in the supernatant, surface phenolics and betalains were determined spectrophotometrically. In order of characterization, beetroot extract E (200 mg) was dissolved in 10 mL of the solvent mixture, which was used for the extraction of total bioactive compounds from encapsulates.
For the determination of anti-inflammatory and antihyperglycemic activities, extracts obtained after the procedure by Sáenz et al. [13], as well as the solution of beetroot extracts, were evaporated to dryness under reduced pressure and dissolved in the appropriate buffer, i.e., in the 10 mM potassium phosphate buffer (pH 7.0) for the investigation of antihyperglycemic activity and in the buffered saline phosphate buffer (pH 6.4) for the investigation of anti-inflammatory activity.
Content of Bioactive Compounds
- Total phenolic content
Total phenolics content in the beetroot extract, encapsulated beetroot extract, and wall materials used for encapsulates’ preparation was determined spectrophotometrically using the Folin–Ciocalteau (FC) method adapted to the microscale [4]. Briefly, the reaction mixture was prepared in a 96-well microplate by mixing 15 μL of the sample, 170 μL of distilled water, 12 μL of the Folin–Ciocalteu reagent (2 M), and 30 μL of 20% sodium carbonate. After 1 h, room temperature, dark conditions, and the absorbance at 750 nm were measured using distilled water as a blank. The results were expressed as mg gallic acid equivalents (GAE) per 100 g of sample (DW).
- Total betalain content
Total betalains’ (total betacyanin and betaxanthin) content in the beetroot extract, encapsulated beetroot extract, and wall materials used for encapsulates’ preparation was estimated spectrophotometrically according to the method of Von Elbe et al. [15], adapted to the microscale. In brief, the sample was diluted with a phosphate buffer (0.05 M, pH 6.5) in a plate well, up to the final volume of 250 µL. The phosphate buffer was used as a blank. Wavelengths of 545 nm and 476 nm were used for the analysis of betacyanins and betaxanthin, while the wavelength of 600 nm was used for correction. Total betalains’ content was calculated as the sum of betacyanin and betaxanthin contents. The total content of betacyanin was expressed as mg betanin equivalents (BE) per 100 g of sample (DW). The total content of betaxanthin was expressed as mg vulgaxanthin-I (VE) per 100 g of sample (DW).
- HPLC analysis
Qualitative and quantitative analyses of flavonoids and phenolic acids in the extract, encapsulated beetroot extract, and wall materials used for encapsulates’ preparation were performed by the HPLC analysis according to Tumbas Šaponjac et al. [4]. Separation was performed on a Luna C-18 RP column, 5 mm, 250 × 4.6 mm, with a C18 guard column, 4 × 30 mm (both from Phenomenex, Torrance, CA, USA). Two mobile phases, A (acetonitrile) and B (1% formic acid), were used at flow rates of 1 mL min−1 with the following gradient profile: 0–10 min from 10 to 25% B; 10–20 min linear rise up to 60% B; and from 20 min to 30 min, linear rise up to 70% B, followed by 10-min reverse to initial 10% B with additional 5 min of equilibration time.
- Encapsulation efficiency
Encapsulation efficiency (EE) of bioactive compounds (phenolics or betalains) was calculated according to Carmona et al. [14], using Equation (1):
EE (%) = 100 × (Total phenolics or betalains − Surface phenolics or betalains)/Total phenolics or betalains
- Bioactivity analysis
Biological (antioxidant, anti-inflammatory, and antihyperglycemic) activities of the beetroot extract, encapsulated beetroot extract, and wall materials were determined spectrophotometrically by appropriate methods.
- Antioxidant activity
Antioxidant activities were estimated against the stable DPPH radicals (AADPPH) and ABTS+ radicals (AAABTS) and by reducing power (RP) ability. The DPPH assay, i.e., the ability of samples to scavenge DPPH radicals, was evaluated spectrophotometrically according to the method of Girones-Vilaplana et al. [16], adapted to the microscale. Briefly, 200 μL DPPH• solution in methanol (0.89 mM) was mixed with 8 μL of the sample in a microplate well and left in the dark at ambient temperature. Absorbance was measured at 515 nm after 50 min. Methanol was used as a blank. The ABTS assay, i.e., the ability of samples to scavenge ABTS+ radicals, was evaluated, employing a modified method according to Tumbas Šaponjac et al. [17], adapted to the microscale. Briefly, 200 μL ABTS+ radical solution prepared in 0.1 mol/L acetate buffer (pH 5.0) and activated with MnO2 was mixed with 8 μL of the corresponding diluted sample in a microplate well and left in the dark at ambient temperature. Absorbance was measured at 414 nm after 35 min. Water was used as the blank. Reducing power was analyzed according to Oyaizu’s method [18], additionally adapted for a microplate. In brief, 120 μL sample or 120 μL water (blank test), 120 μL sodium phosphate buffer, pH = 6.6, and 120 μL of 1% potassium ferricyanide were mixed and incubated in a water bath for 20 min at 50 °C. After cooling, 120 μL of 10% trichloroacetic acid was added and solutions were centrifuged at 3000 rpm for 10 min. After centrifugation, 80 μL of the supernatant was mixed with 80 μL of distilled water and 16 μL of 0.1% ferric chloride in the microplate well. Absorbances were measured immediately at 700 nm. The results were expressed in mg Trolox equivalent (TE) per 100 g of sample (DW).
- Anti-Inflammatory Activity
The anti-inflammatory activity (AIA) was determined by a protein denaturation bioassay using egg albumin (from fresh hen’s eggs), according to the method adopted by Ullah et al. [19]. Briefly, 2 mL of the sample was incubated with egg albumin from a fresh hen egg (0.2 mL) and 2.8 mL buffered saline phosphate (pH 6.4) at 37 °C for 15 min and then at 70 °C for 5 min. After cooling, the absorbance of the solution was measured at 660 nm. Results were expressed as the effect of 50 mg of the sample in the % inhibition of protein denaturation.
- Antihyperglycemic Activity
To examine in vitro antihyperglycemic activity (AHgA), α-glucosidase inhibitory potential was performed, using the method reported by Tumbas Šaponjac et al. [17]. Briefly, in each well, 100 μL of 2 mmol/L 4-nitrophenyl α-D glucopyranoside in 10 mmol/L potassium phosphate buffer (pH 7.0) and 20 μL of the samples, diluted in a buffer, were mixed. The reaction was initiated by the addition of 100 μL of the enzyme solution (56.66 mU/mL). The plates were incubated at 37 °C for 10 min. The absorbance of 4-nitrophenol released from 4-nitrophenyl α-D-glucopyranoside at 405 nm was measured. Results were expressed as the effect of 0.2 mg of the sample in the % inhibition of α -glucosidase activity.
2.6. Storage Stability Study
The beetroot extract (E) and encapsulates (ME and SPE) were stored at the following three different conditions: in glass bottles (light conditions) at room temperature (25 ± 5 °C), in amber bottles (dark conditions) at room temperature (25 ± 5 °C), and in amber bottles (dark conditions) at refrigeration temperature (4 ± 2 °C). The content of bioactive compounds and bioactivity of the samples stored under dark conditions at room temperature were evaluated for a period of 60 days and analyzed every 15 days. In addition, bioactive compounds and bioactivity of the samples stored under light conditions at room temperature and under dark conditions at refrigeration temperature were analyzed after 60 days of storage. Chemical characterization, i.e., the content of bioactive compounds and bioactivity of the samples, was determined as previously described.
The retention of bioactive parameters (bioactive compound or bioactivity) was calculated according to the following equation, Equation (2):
where bioactive parameters (content of bioactive compound or bioactivity) at t and t0 were values determined at different times, and at zero time of storage, respectively.
Retention of bioactive parameter (%) = [(Bioactive parameter at t)/(Bioactive parameter at t0)] × 100
2.7. Statistical Analysis
Statistical analyses were performed with the R programming language version 4.2.2 and RStudio version 2022.02.03 using ggplot2 [20], dplyr [21], and PerformanceAnalytics [22] packages. The distribution of the data was evaluated using the Shapiro–Wilk normality test. Because the data followed a non-normal distribution, the nonparametric Spearman correlation test was used for the correlation analysis, whereas a one-way Kruskal–Wallis analysis of variance was chosen for the comparison between the extract and encapsulations with post hoc Wilcoxon’s pairwise comparison. All experiments were performed in triplicate and results are presented as means ± standard deviation.
3. Results and Discussion
3.1. Content of Bioactive Compounds, Encapsulation Efficiencies, and Bioactivities
Content of bioactive compounds and bioactivities in the beetroot extract (E), encapsulates (ME and SPE), and wall materials, maltodextrin (M) and soy protein (SP), used for encapsulates’ preparation, are presented in Table 1.

Table 1.
Content of bioactive compounds and bioactivities of beetroot extract (E), encapsulates (ME and SPE), and wall materials, maltodextrin (M) and soy protein (SP), used for encapsulates’ preparation.
The contents of total phenolics by the FC method in E and encapsulates ME and SPE were found to be 869.14 mg GAE/100 g, 276.36 mg GAE/100 g, and 337.83 mg GAE/100 g, respectively (Table 1). Also, the contents of total phenolics were determined by the HPLC method in E (340.87 mg/100 g) and encapsulates ME (135.70 mg/100 g) and SPE (201.17 mg/100 g) (Table 1). Significantly higher contents of total phenolics determined by both, FC and HPLC, methods were determined in encapsulates in relation to wall materials used for their preparation (Table 1). Gallic acid and catechin were identified as the most abound individual phenolic compounds present in the beetroot extract. High contents of these compounds were retained in both encapsulates after the encapsulation process. Previously, gallic acid and catechin were determined in high amounts in beetroot pomace [4]. In comparison to the content of phenolics determined by HPLC, higher values of phenolics determined spectrophotometrically could be a consequence of interfering substances (particularly sugars, aromatic amines, ascorbic acid, etc.) present in extracts, which may lead to higher total phenolics determined by the Folin–Ciocalteu method [23]. In the study of Flores-Mancha et al. [6], freeze-dried pure beet juice contained the highest phenolics content (1235 mg GAE/100 g), lower in the encapsulate of pure beet juice with maltodextrin (609 mg GAE/100 g) and the lowest in the encapsulate with inulin (597 mg GAE/100 g). Antigo et al. [24] revealed content of phenolics of 488.87 mg GAE/100 g in maltodextrin-encapsulated beet powder obtained by the lyophilization method. Tumbas Šaponjac et al. [4] reported the content of 326.51 mg GAE/100 g in an encapsulated beetroot pomace extract with soy protein prepared by a freeze drying method. In another study, Tumbas Šaponjac et al. [25] determined the content of total phenolics of 150.71 mg GAE/100 g in encapsulated beetroot juice with soy protein prepared by the freeze drying method. Bazaria and Kumar [26] revealed the content of phenolics for spray-dried beetroot juice concentrate encapsulated with whey protein for different operational conditions in the range from 16.69 mg GAE/100 g to 25.89 mg GAE/100 g. Also, Bazaria and Kumar [27] encapsulated beetroot juice with maltodextrin of 10 and 20 dextrose equivalency (10DE and 20DE) and Arabic gum by spray drying. The highest total phenolic content (26.36 mg GAE/100 g) was found for the blend of AG and MD (DE20) [27].
The contents of betalains in E and encapsulates ME and SPE were determined to be 68.03 mg/100 g, 24.42 mg/100 g, and 23.28 mg/100 g, respectively (Table 1). As expected, the presence of betalains in wall materials was not found (Table 1). The contents of betaxanthins in beetroot extract E and encapsulates ME and SPE were 35.95 mg VE/100 g, 14.05 mg/100 g, and 14.06 mg VE/100 g, respectively. According to the content of betacyanins (10.37 mg BE/100 g in ME and 9.22 mg BE/100 g in SPE), encapsulates obtained in our study could be used in improvement in food color, since in most applications, an estimated quantity of less than 50 mg betanin/kg could produce the desired color of a product [28]. In the study of Tumbas Šaponjac et al. [4], similar content of betaxanthins (61.33 mg VE/100 g) to the content of betacyanins (60.52 mg BE/100 g) was reported in an encapsulated beetroot pomace extract. Also, the content of betaxanthins (261.55 mg VE/100 g) did not differ significantly from the content of betacyanins (259.73 mg BE/100 g) in an encapsulate of beetroot juice [25]. Flores-Mancha et al. [6] revealed that the content of total betalains in freeze-dried pure beet juice was 382.35 mg/100 g (219.17 mg/100 g of betacyanins and 163.17 mg/100 g of betaxanthins). Total betalains’ contents in encapsulates with maltodextrin and inulin were 15.71 mg/100 g and 10.11 mg/100 g, respectively [6]. The total betalain content in encapsulates of spray-dried beetroot juice concentrate with whey protein concentrate ranged from 261.94 to 272.54 mg/100 g [26]. Also, Bazaria and Kumar [27] reported that encapsulation with the blend of Arabic gum and maltodextrin (DE20) retained betalains (208.06 mg/100 g DW) to the highest degree. Do Carmo et al. [28] determined in spray-dried beetroot extract powders prepared with maltodextrin, inulin, whey protein, and their mixtures contents of betaxanthins and betacyanins in the range from 136.86 to 155.37 mg/100 g and from 211.93 to 230.10 mg/100 g, respectively. In the study of Janiszewska [29], beetroot juice was encapsulated using Arabic gum, maltodextrin, and a mixture of both (1:1) as a carrier by the spray drying technique, and the highest content of yellow pigments was found in the encapsulate with maltodextrin (61 mg/100 g), while the violet pigment content was highest in the Arabic gum encapsulate (129 mg/100 g). Pitalua et al. [30] reported for beetroot juice obtained by spray drying that content of betalains was 13.58 mg/100 g powder. In microcapsules of beetroot juice with Arabic gum obtained by spray drying, the betalains’ content was 11.98 mg/100 g powder [30].
Encapsulation efficiencies of phenolics in ME and SPE were determined to be 79.09 ± 0.50% and 69.64 ± 1.10%, respectively, while encapsulation efficiencies of betalains (betacyanins and betaxanthins) in both encapsulates, ME and SPE, were estimated as 100% (i.e., content of surface betacyanins and betaxanthins was below detection limit). Robert et al. [31] in encapsulated cactus pear (Opuntia ficus-indica) pulp with a soybean protein isolate by spray drying determined EE of phenolics, betacyanins, and betaxanthins (79.7%, 99.6%, and 98.1%, respectively). Saénz et al. [32] in the encapsulated pulp and ethanolic extracts of cactus pear (Opuntia ficus-indica) with maltodextrin by spray drying determined EE of phenolics, betacyanins, and betaxanthins (72.8%, 99.3%, and 96.5%, respectively). EE of phenolics in the encapsulated beetroot juice and extract with soy protein obtained at different encapsulation conditions ranged from 60.26% to 85.03% and from 47.79% to 92.47%, respectively [4,25]. EE of betacyanin and betaxanthin in the encapsulates of pulp and ultrafiltered cactus pear extracts prepared at different conditions of the encapsulation process with Capsul by spray drying reached values above 98% for both systems studied [33]. Also, EE of indicaxanthin in the encapsulates of yellow-orange cactus pear pulp prepared at different encapsulation conditions with maltodextrin as the encapsulating agent by spray drying was high (≥92%) [14]. High encapsulation efficiency values for betacyanins and betaxanthins indicate a strong betalain–polymer interaction regarding the type of used encapsulating agents [33].
Bioactivity of investigated samples was estimated based on antioxidant, antihyperglycemic, and anti-inflammatory activity assays (Table 1). Antioxidant activity determined by various tests (DPPH, RP, and ABTS) of beetroot extract E ranged from 882.04 to 1667.09 mg TE/100 g, while that of encapsulates ME and SPE was in the range from 338.17 to 712.55 mg TE/100 g and from 339.29 to 687.82 mg TE/100 g, respectively. Also, antioxidant activity was determined for wall materials used in the preparation of encapsulates (maltodextrin and soy protein). Antioxidant activity by DPPH, RP, and ABTS tests of maltodextrin was found to be below detection limits, 2.04 ± 0.07 mg TE/100 g and 44.62 ± 1.92 mg TE/100 g, respectively, while for soy protein, it was 46.75 ± 2.01 mg TE/100 g, 20.50 ± 0.27 mg TE/100 g, and 116.24 ± 3.51 mg TE/100 g, respectively. Tumbas Šaponjac et al. [4] reported that antioxidant activity by DPPH and RP tests of a beetroot pomace extract encapsulate was 1655.36 µmol TE/100 g (i.e., 414.32 mg TE/100 g) and 394.95 µmol TE/100 g (i.e., 98.85 mg TE/100 g), respectively. In another study, Tumbas Šaponjac et al. [4] reported that antioxidant activity by DPPH and RP tests of encapsulated beetroot juice was 1.02 mmol TE/100 g (i.e., 255.30 mg TE/100 g) and 1.81 mmol TE/100 g (i.e., 453.02 mg TE/100 g), respectively. In the study of do Carmo et al. [28], antioxidant activity by the DPPH test was in the range from 60.57% to 85.01% for spray-dried encapsulates of a beetroot extract prepared with various carrier agents (maltodextrin, inulin, and whey protein isolate and their mixtures). Anti-inflammatory activity of the SPE encapsulate was significantly higher in comparison to activity of soy protein (33.66 ± 0.73%), while this was not observed for the ME encapsulate in comparison to activity of maltodextrin (82.20 ± 0.09%) (Table 1). An increase in antihyperglycemic activity of the ME and SPE encapsulate in comparison to activity of wall materials, maltodextrin (30.54 ± 1.93%) and soy protein (7.12 ± 1.11%), was observed (Table 1). The evidence suggests that beetroot has health-promoting potential, including anti-inflammatory, antioxidant, and antidiabetic properties [34]. Our findings in terms of bioactive properties of the beetroot extract and encapsulates are in agreement with previous reports.
3.2. Storage Stability
Changes in the content of phenolics and betalains in the beetroot extract (E) and encapsulates (ME and SPE), stored for 60 days under various conditions, are shown in Figure 1 and Figure 2.
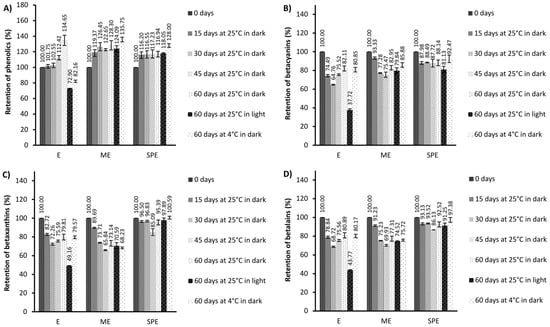
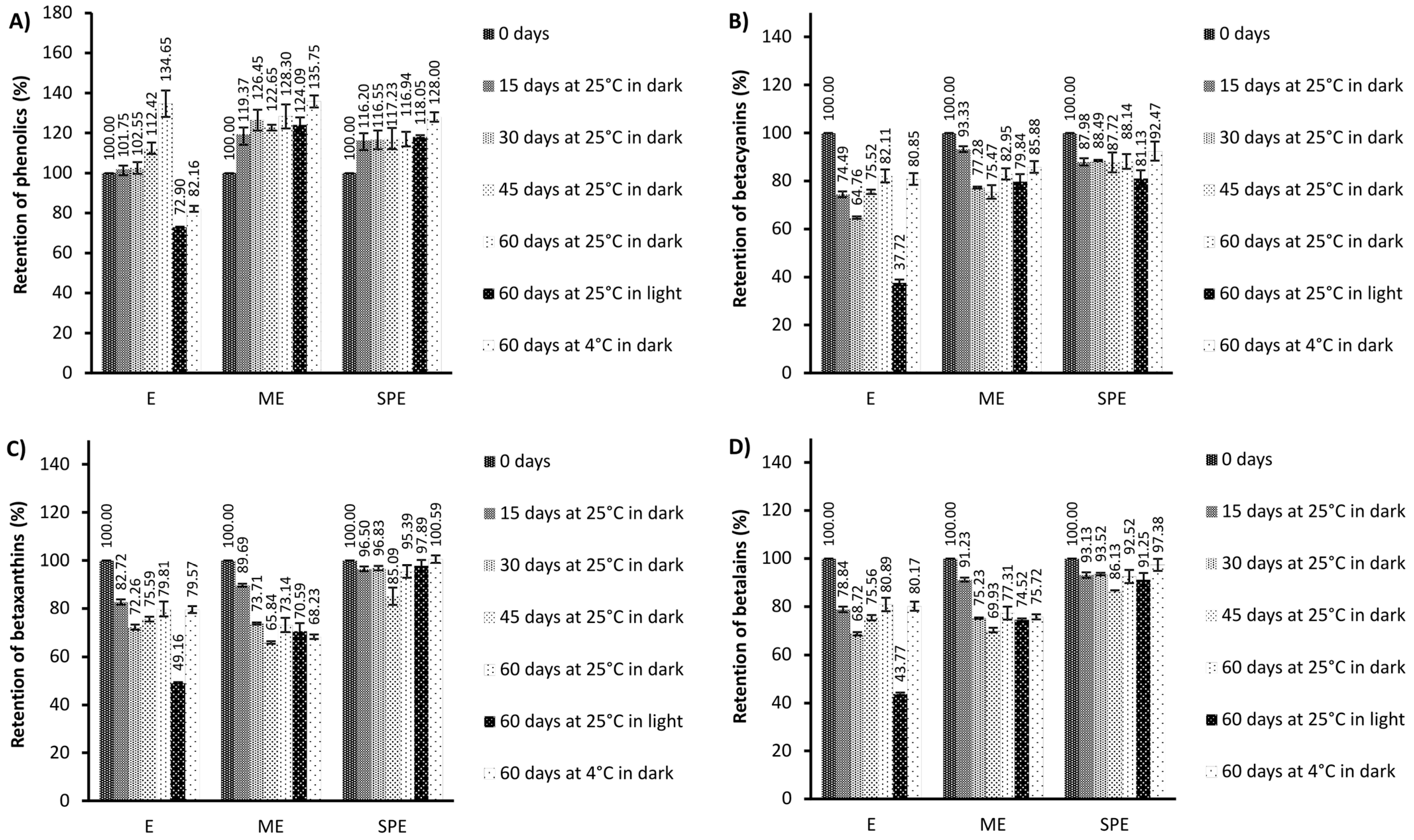
Figure 1.
Retention (%) of phenolics (A), betacyanins (B), betaxanthins (C), and betalains (D) during storage under different conditions of the extract (E) and encapsulates (ME and SPE). The bars represent the mean of three replicates and the error bars represent the standard deviations.
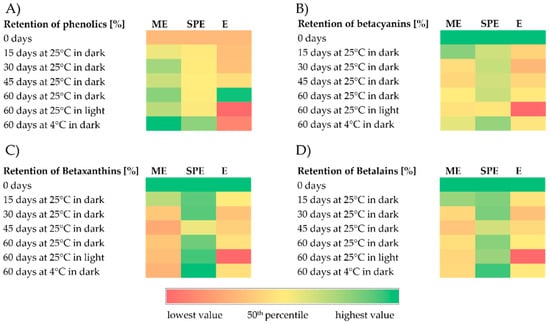
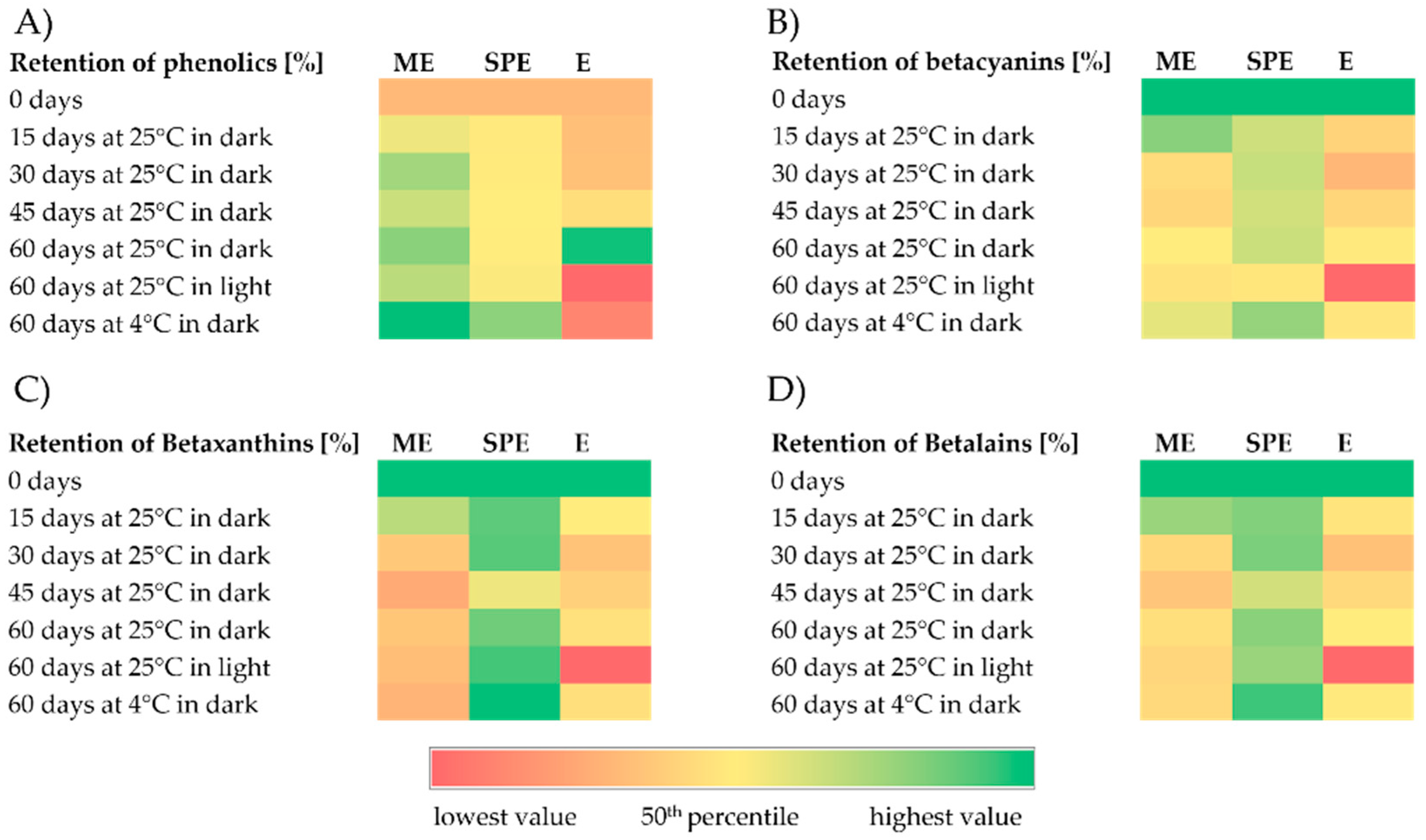
Figure 2.
Heatmaps representing retention (%) of phenolics (A), betacyanins (B), betaxanthins (C), and betalains (D) during storage under different conditions of encapsulates (ME and SPE) and extract (E).
According to the results, higher content of phenolic compounds in the extract and encapsulates in relation to zero-time values was found during 60 days of storage at 25 °C under dark conditions. The retention of phenolics in the extract stored during 60 days at 4 °C under dark conditions and at 25 °C under light conditions was lower in relation to retention in the encapsulates. Accordingly, at stated conditions, a significantly better preservation of phenolics was observed in encapsulates in comparison to the extract. Saénz et al. [32] followed the content of phenolic compounds and betalains in encapsulates during 44 days of accelerated storage and they found that the retention of phenolics was dependent on the wall materials used for encapsulate preparation. Also, they reported that the retention of phenolics after storage was 158% for the extract encapsulated with inulin; i.e., in accordance with our study, the increase in phenolics was observed. Polyphenol retention remained constant until the 28th day of accelerated storage and then increased in all three of the investigated systems [30]. An increased amount of polyphenols during storage could be because of hydrolysis of polyphenol glycosides and/or being condensed into aglycones, leading to a higher number of free hydroxyl groups [30].
During storage, lower content of betalains (betacyanins and betaxanthins) in the extract and encapsulates in relation to zero-time values was mostly found, with the exception of the retention of betaxanthins in the SPE encapsulate. A high retention of betaxanthins (>95%) in the SPE encapsulate at the end of the 60-day storage period was found at all investigated storage conditions. After storage at 4 °C and 25 °C under dark conditions, a better preservation of betalains (betacyanin and betaxanthin) can be observed in encapsulate SPE compared to encapsulate ME and extract E. In both encapsulates after storage at room temperature under light conditions, a significantly greater stability of betalains was observed compared to the extract, indicating a significant improvement achieved by encapsulation. Kumar and Giridhar [35] revealed that total betalain retention in the spray-dried encapsulates of betalain with maltodextrin was approximately 97% after 2 years of storage at 4 °C in a subdued light condition. In the study of Rodriguez et al. [9], betalain retention in a red dragon fruit peel extract, as well as in lyophilized maltodextrin–Arabic gum and maltodextrin–pectin encapsulates of the same extract, was greatest at 4 °C, lower at room temperature (27 °C) without light and lowest at room temperature with light. Similarly, in maltodextrin-encapsulated H. polyrhizus betalains, decreasing betalain retention with increasing storage temperature, especially in the presence of light, was observed [9,36]. Azeredo et al. [37] encapsulated red beetroot extracts prepared with different maltodextrin beetroot dry mass ratios by spray drying. Betacyanin loss was decreased by the maltodextrin/beetroot material ratio and was higher in translucent jars (from 27.92% to 45.55%) than in dark jars (from 17.57 to 21.72%) after storage for six months at room temperature under diffuse sunlight [37]. In the study of Gandia-Herrero et al. [38], the degradation of the pigment was increased considerably when encapsulates on the basis of maltodextrin prepared by spray drying were kept at 20 °C in the presence of light during 180 days, with only 30% of the initial miraxanthin V and 50% of the initial betanidin amount remaining. In our study, similar levels of betacyanins’ retention in encapsulates with maltodextrin were found relative to the study of Gandia-Herrero et al. [38] at almost the same storage conditions applied. In addition, our results of somewhat higher betaxanthin than betacyanin degradation, in the encapsulates with maltodextrin after 60 days of storage at 20 °C in the presence of light, are in accordance with the study of Gandia-Herrero et al. [38]. Spray-dried encapsulates of Opuntia lasiacantha extracts with maltodextrin as a matrix exhibited 86% betalain (i.e., betanin) retention after 24 weeks of storage at room conditions without light [39]. In the study by Cai and Corke [40], Amaranthus betacyanin extracts were spray-dried using a range of maltodextrins and starches as carriers, and the highest pigment retention after storage at 25 °C for 16 weeks was found with maltodextrins of 25 and 10 DE (97.3% and 88.7%) at 5% and 32% RH, respectively, which is similar to the retention (84.3%) reported for the commercial colorant from beetroot. The retention of betacyanins and betaxanthins in the encapsulated beetroot pomace prepared with soy protein by the freeze drying method was about 24% after 60 days of storage at room temperature (25 °C) in high-density polyethylene bags [4]. In the study of Janiszewska [29], no significant decrease in the yellow and violet betalain pigment content was seen after 6 months of storage of encapsulated beetroot juice prepared with Arabic gum, maltodextrin, and their mixture (1:1) as compared to their contents directly after the drying process. In the study of do Carmo et al. [28], lower stability of betalains during accelerated storage was found for powder produced without carrier agents, which emphasizes the importance of using these materials to obtain a beetroot extract powder with good stability. However, the degradation of betalain pigments (stored at higher temperatures) was reported by Saénz et al. [13] and Robert et al. [31] for spray-dried betalains from cactus pear. In the study by Saénz et al. [13], the retention of betalains, betacyanin, and indicaxanthin in various encapsulates after 44 days of accelerated storage ranged from 54% to 63% and from 50% to 80%, respectively. The betacyanin and betaxanthin retention after 56 days of accelerated storage was the highest with a blend of soy protein and maltodextrin (53 and 93%, respectively) and the lowest with soy protein (31% and 67%, respectively), showing that incorporated polysaccharides probably increased betalains’ stability because of their higher film-forming characteristics [31]. Ultraviolet and visible light absorption increases betalain reactivity to oxygen, which may lead to pigment degradation [9]. Encapsulating agents can reduce the interaction of the betalains with degradative factors such as light, temperature, and oxygen [9]. Vergara et al. [33] reported that the hydrolysis pathway was the main mechanism of betalain degradation during microparticle storage at 60 °C.
Antioxidant activity in the beetroot extract (E) and encapsulates (ME and SPE), determined by DPPH, RP, and ABTS tests during 60 days of storage under different conditions, is shown in Figure 3 and Figure 4.
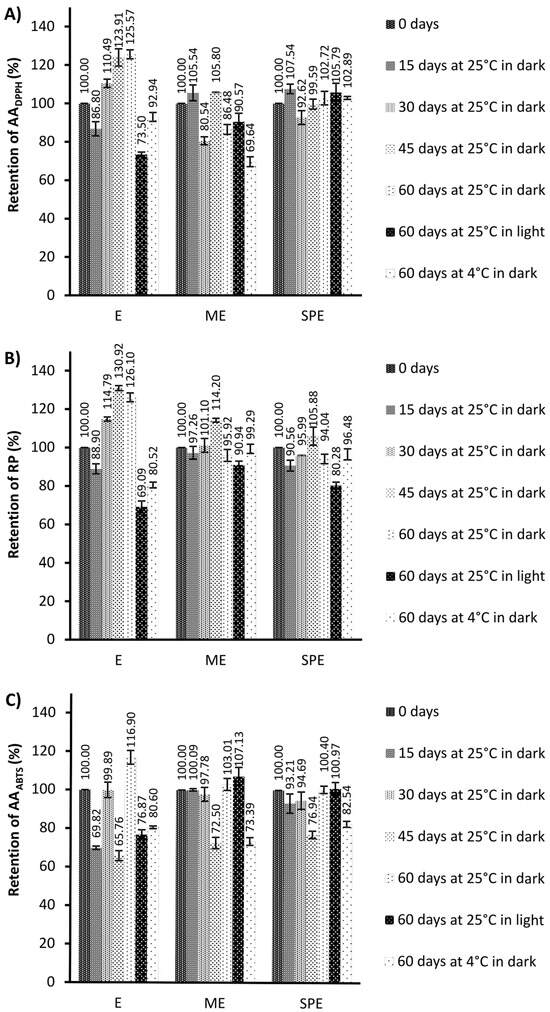
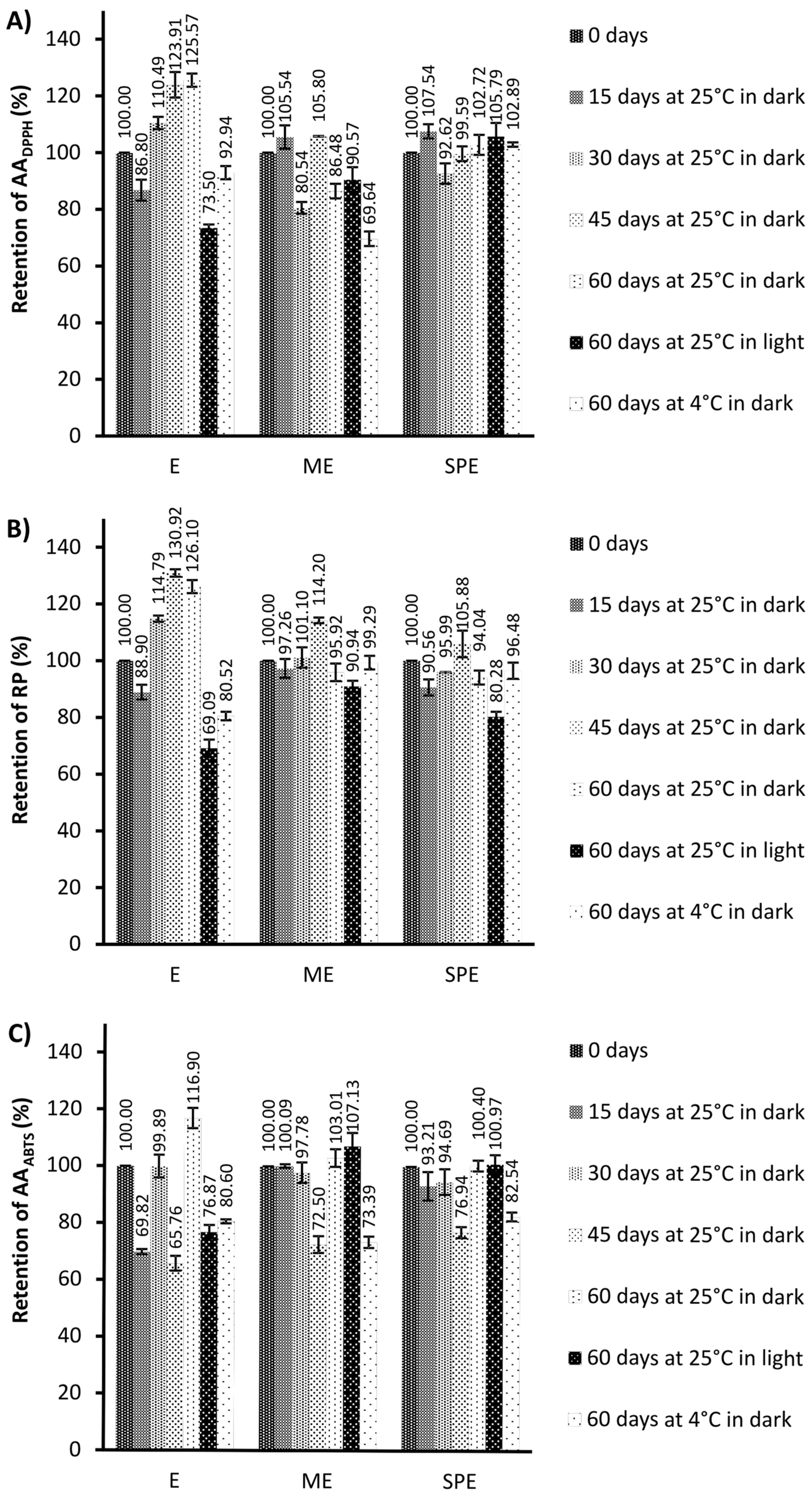
Figure 3.
The retention (%) of antioxidant activity against the stable DPPH radicals (AADPPH) (A), reducing power (RP) (B), and antioxidant activity against ABTS+ radicals (AAABTS) (C) during storage under different conditions of the extract (E) and encapsulates (ME and SPE). The bars represent the mean of three replicates and the error bars represent the standard deviations.
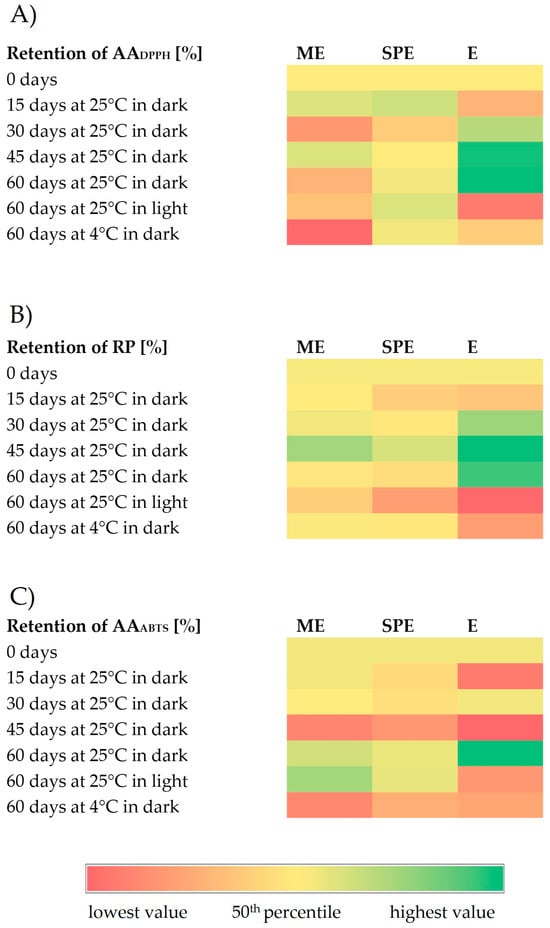
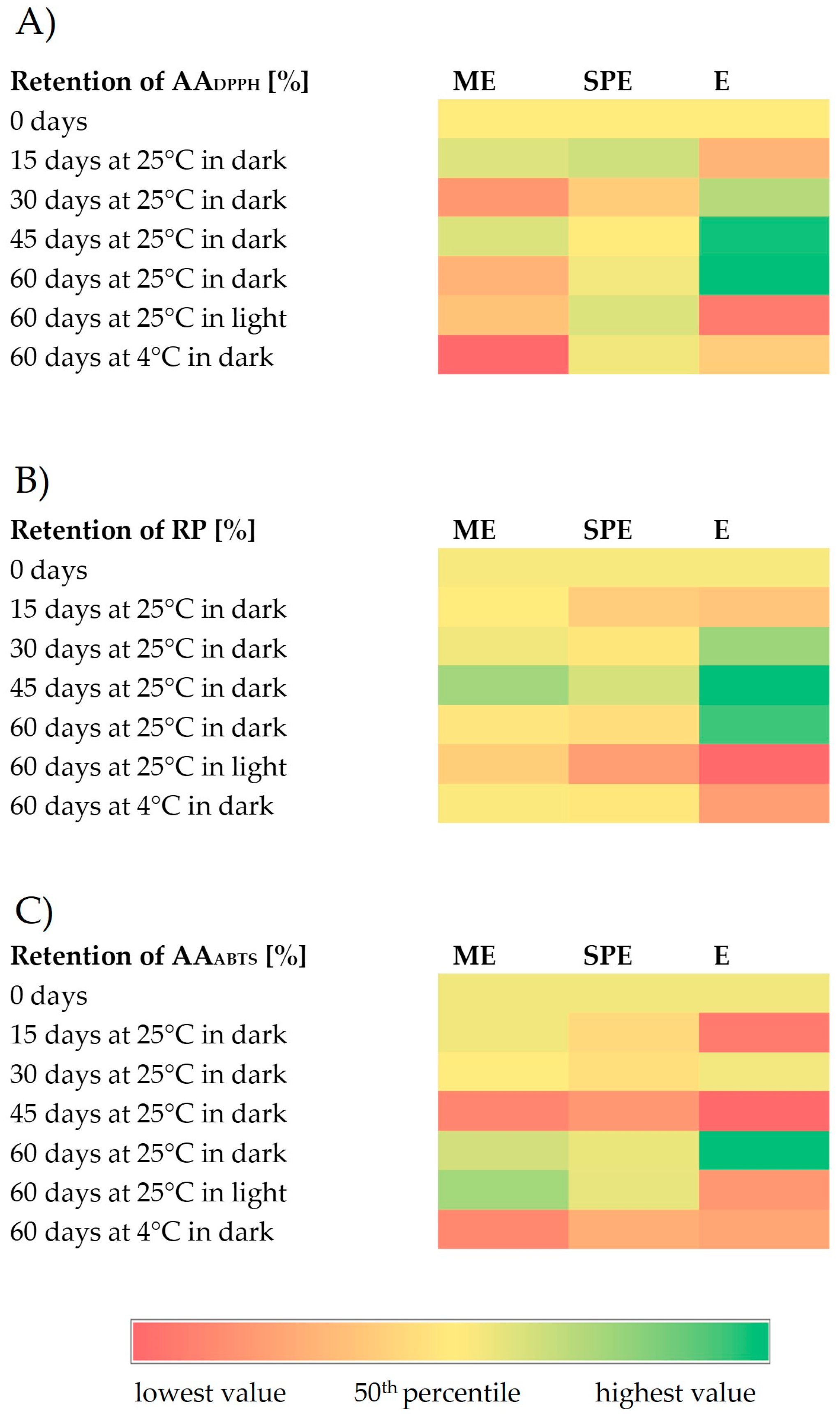
Figure 4.
Heatmaps representing retention (%) of antioxidant activity against stable DPPH radicals (AADPPH) (A), reducing power (RP) (B), and antioxidant activity against ABTS+ radicals (AAABTS) (C) during storage under different conditions of encapsulates (ME and SPE) and extract (E).
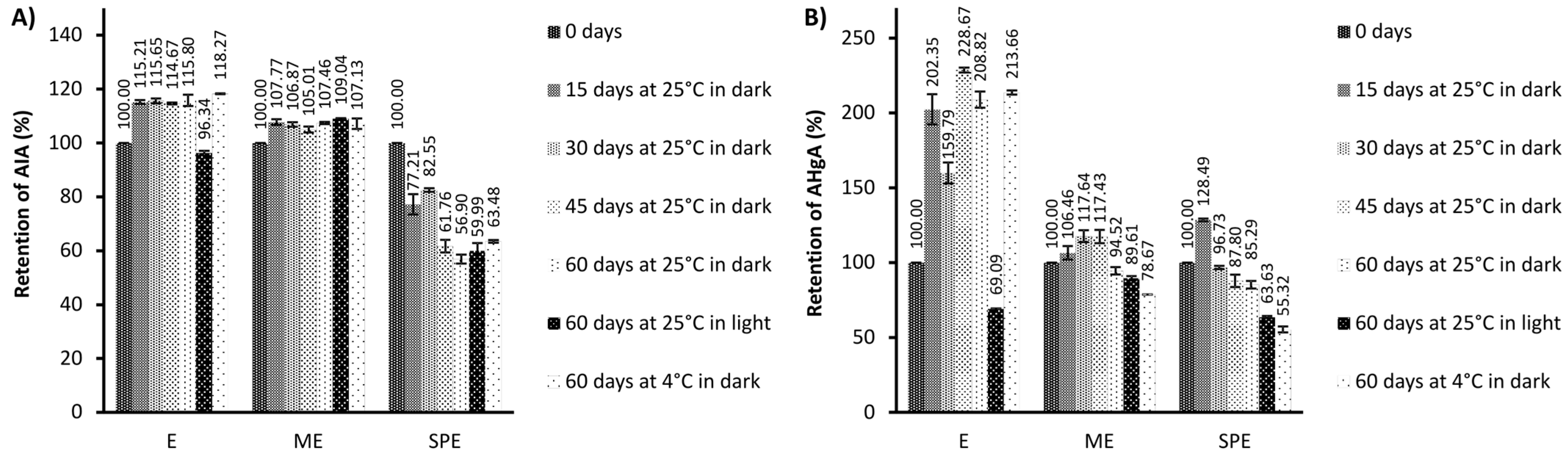
Generally, antioxidant activity in encapsulates has remained stable compared to the extracts after storage at 25 °C under light conditions. After storage at 4 °C under dark conditions, a significantly improved preservation of antioxidant activity was observed for encapsulate SPE in the DPPH test, and for both encapsulates in the RP test in relation to the preservation of antioxidant activity of the extract. After storage at 25 °C in dark conditions, a significant increase in antioxidant activity was observed for the extract, but not for encapsulates. Higher antioxidant activities could be the result of the formation of phenolics during storage as a consequence of the hydrolysis of polyphenol conjugates [30]. The changes in antioxidant activities may be explained by the alteration in the phenolic profiles [41]. Also, phenolic compounds can show a synergistic effect by supporting antioxidant activities of present phenolics [41]. The loss of original phenolics might be compensated for by new formed phenolics with the same or improved antioxidant activities [41,42]. In addition to the increase in phenolics, higher antioxidant activity of the extract during storage at 25 °C and dark conditions coincided with loss of betalains, which is in agreement with an observation of [30]. The degradation reaction of betalains produces D-glucoside cycle-DOPA (CDG) and betalamic acid (BA), which have an amine and aldehyde group that may be able to act as antioxidants [30]. During degradation, a Schiff’s base condensation of the amine of CDG occurs with the aldehyde, forming intermediate compounds in the Maillard reaction [30]. Anti-inflammatory and antihyperglycemic activity in the beetroot extract (E) and encapsulates (ME and SPE), during 60 days of storage under different conditions, is presented in Figure 5 and Figure 6.
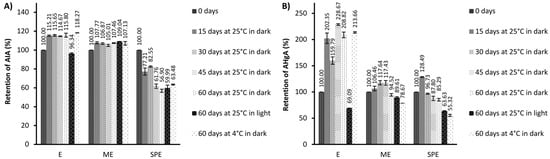
Figure 5.
The retention (%) of anti-inflammatory activity (AIA) (A) and antihyperglycemic activity (AHgA) (B) during storage under different conditions of the extract (E) and encapsulates (ME and SPE). The bars represent the mean of three replicates and the error bars represent the standard deviations.
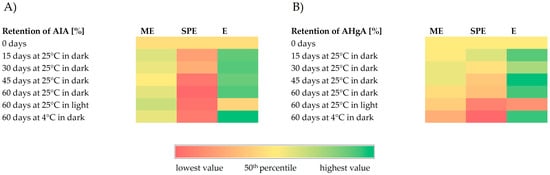
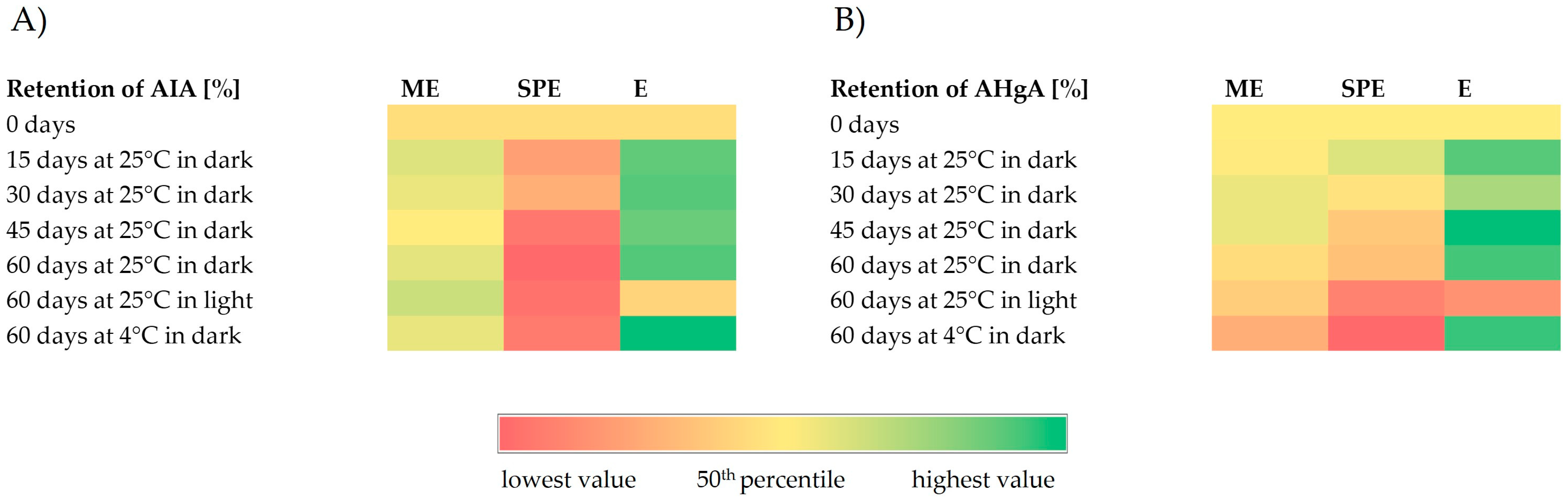
Figure 6.
Heatmaps representing retention (%) of anti-inflammatory activity (AIA) (A) and antihyperglycemic activity (AHgA) (B) during storage under different conditions of encapsulates (ME and SPE) and extract (E).
In general, anti-inflammatory activity of the beetroot extract and encapsulate ME was quite stable or slightly improved; meanwhile, a decrease in anti-inflammatory activity was observed for encapsulate SPE during storage at investigated conditions. Improvement in antihyperglycemic activity during storage at 25 °C and 4 °C in dark conditions could be observed for the beetroot extract, while at the same storage conditions for both encapsulates, a decrease in antihyperglycemic activity was found. Lower levels in antihyperglycemic activity were observed for the extract and encapsulates after storage at 25 °C in light conditions in comparison to the levels at zero storage time. Nevertheless, a higher retention of antihyperglycemic activity after storage at 25 °C in light conditions was found for encapsulate ME with respect to encapsulate SPE and extract E.
Based on the results (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6), in comparison to other storage conditions, in general, the highest improvement in the stability of the studied bioactive parameters (content of bioactive compounds and bioactivities) in encapsulates (ME and SPE) in relation to the extract (E) was observed after storage at room temperature and light conditions. The rate constant (k) and half-life time (t1⁄2) were calculated by the method of Cai and Corke [39] using the regression analysis of ln (retention) against storage time when plotted on a natural logarithmic scale. Approximation that the degradation of the parameters followed first-order kinetics was used to indicate improvement in bioactive parameters in the encapsulate in relation to the extract after storage at room temperature and light conditions (Table 2). It can be observed that the improvement in the stability of bioactive compounds (betalains and phenolics) and antioxidant activity (by each of the applied tests) of both encapsulates (ME and SPE) compared to the extract (E) was achieved. Improvement in the stability of anti-inflammatory and antihyperglycemic activity was observed for the ME encapsulate in relation to extract E, while for the SPE encapsulate, improvement in these bioactive parameters was not found.

Table 2.
Kinetic parameters of bioactive compounds and bioactivity degradation in extract and encapsulates during 60 days of storage at room temperature and light conditions.
3.3. Correlation Analysis
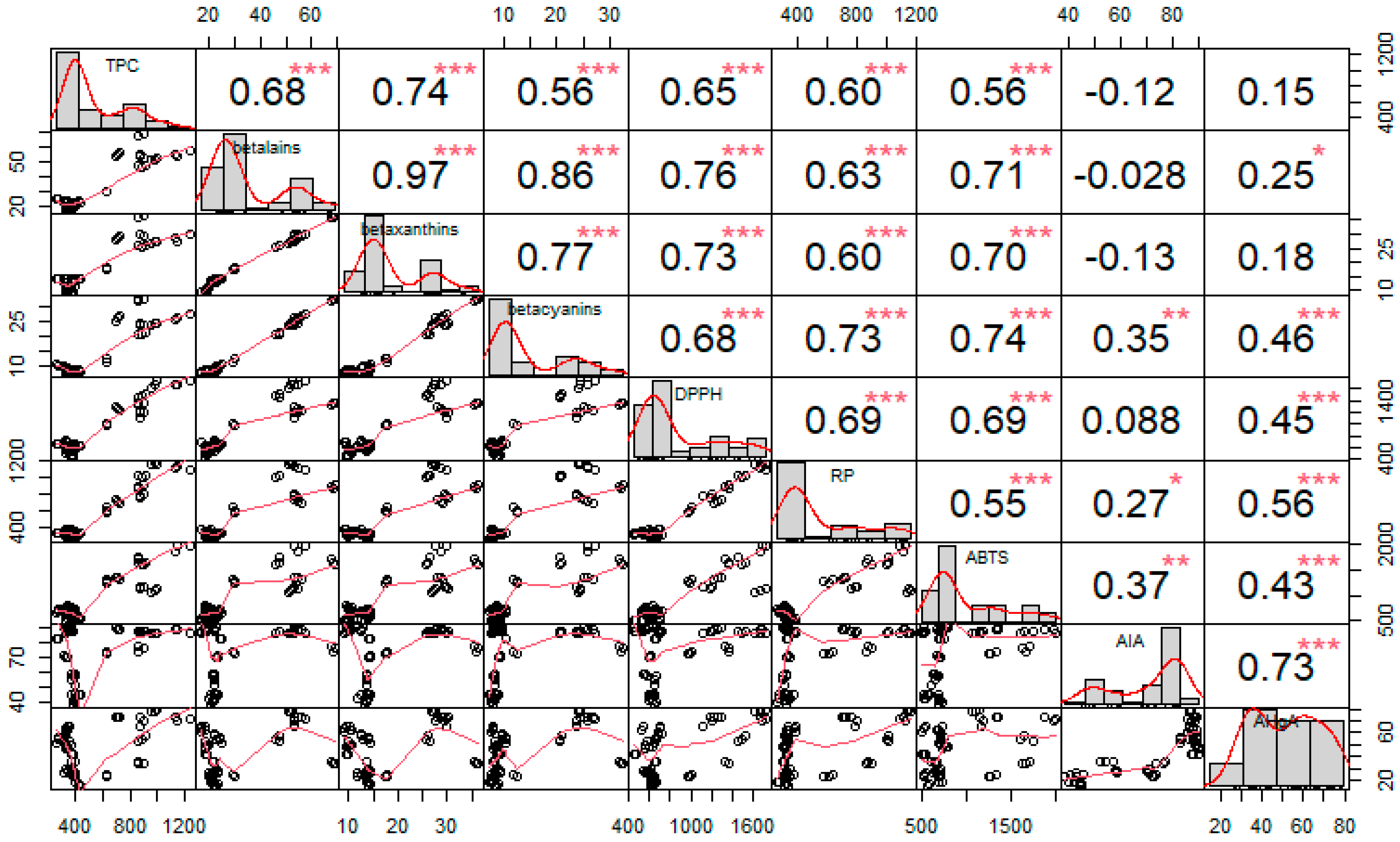
Spearman’s correlation tests were performed for the measured variables and are visualized in Figure 7. The bivariate correlation plots, correlation coefficients, and significance levels are given for each case. On the diagonal distribution of each plot is shown, above the diagonal, Spearman’s correlation values along with the significance levels (*—p ≤ 0.05, **—p ≤ 0.01, ***—p ≤ 0.001) and, below the diagonal, scatter plots with a fitted line being displayed.
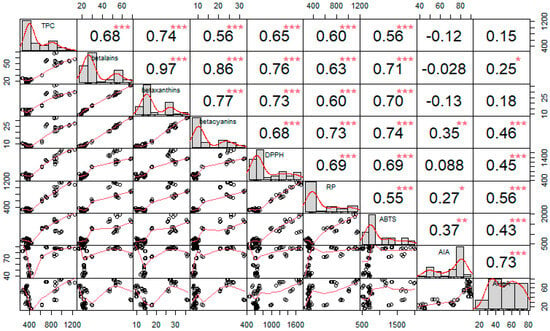
Figure 7.
The correlation matrix visualizing Spearman’s correlation tests between phenolics (TPC), betacyanins, betaxanthins, betalains, antioxidant activity against the stable DPPH radicals (DPPH), reducing power (RP), antioxidant activity against ABTS+ radicals (ABTS), anti-inflammatory activity (AIA), and antihyperglycemic activity (AHgA). The distribution is shown on the diagonal; above it, correlation coefficients and the significance levels (*—p ≤ 0.05, **—p ≤ 0.01, ***—p ≤ 0.001) are indicated for each case, while the scatter plots with a fitted line are displayed under the diagonal.
Total betalains, betaxanthins, and betacyanins showed a strong monotonic relationship with each other as Spearman’s correlation coefficients ranged from 0.77 to 0.97 (p < 0.001). They also exhibited moderate to strong correlations with measurements of antioxidant activity and total phenolic content, with Spearman’s correlation coefficients ranged from 0.56 to 0.76 (p < 0.001), suggesting that these components account for a large proportion of the compounds with antioxidant activity in the extract.
The correlations between the antioxidant methods were also confirmed and cross-validated the methods, as they all (ABTS, RP, and DPPH) showed moderate statistically significant correlation, with values of Spearman’s coefficient between 0.55 and 0.69 (p < 0.001).
In contrast, anti-inflammatory activity and blood glucose-lowering activity showed only weak to moderate correlations for some components. Anti-inflammatory activity showed weak to moderate statistically significant correlation with betacyanins and antioxidant activity measured by RP and ABTS methods (ρ = 0.27–0.37, p < 0.05). For antihyperglycemic activity, only total phenolic content and betaxanthins showed no statistical significant correlation, but Spearman’s coefficient values indicated weak to moderate correlations (ρ = 0.25–0.56, p < 0.05). In contrast, a strong correlation was found between anti-inflammatory and blood glucose-lowering effects (ρ = 0.73, p < 0.001), suggesting that the components studied in this study form only a part of these biological functions and that other components with this ability are present in the extract.
3.4. Kruskal–Wallis One-Way Analysis of Variance
The Kruskal–Wallis test confirmed significant differences (p < 0.05) between the extract and the encapsulations in terms of the retention of all measured values except antioxidant activity, measured by the RP and ABTS methods. The retention of total phenolic content, total betalains, betaxanthins, and betacyanins was higher in the encapsulations for most measurements. For most TPC measurements, the maltodextrin encapsulates had the highest retention. Post hoc Wilcoxon pairwise comparison showed significant differences between all samples for this variable (p < 0.05). For total betalains, soy protein encapsulates had the highest average retention values of 89.42%, followed by the maltodextrin encapsulates with 84.96%, compared to the average retention of the extracts of 73.64%. Also, the Wilcoxon post hoc pairwise comparison confirmed that encapsulates were statistically different from the extracts for betalains (p < 0.05), but not from each other (p = 0.12). In addition, soy protein encapsulates had statistically significantly higher retention than maltodextrin encapsulates and extracts for both betaxanthins and betacyanins (p < 0.05), while maltodextrin encapsulates were not statistically different from extracts for either measurement (p > 0.05). For anti-inflammatory and antihyperglycemic activity, the extract had statistically significantly higher retention than the two encapsulations, as confirmed by Wilcoxon’s pairwise comparison (p < 0.05).
4. Conclusions
In general, the highest improvement in the stability of bioactive parameters (content of bioactive compounds and bioactivity) of prepared encapsulates in relation to the extract was observed after storage at room temperature under light conditions. At these conditions, improvement in all studied bioactive parameters was achieved for the ME encapsulate, while in the case of encapsulate SPE, improvement in the stability of bioactive compounds and antioxidant activity compared to the extract was noticed. Also, the new knowledge about the extraction method revealed in this study could be applied to the field of plant extracts, and obtained beetroot encapsulates have potential to be used in the food industry. Due to the contents of bioactive compounds and bioactivities, they could be used as components for functional foods and for improvement in bioactive and red color characteristics of products.
Author Contributions
Conceptualization, M.K.M.; methodology, S.S. and D.B.; software, V.P.; validation, V.P., M.K.M. and G.Ć.; formal analysis, S.S. and D.B.; investigation, V.P.; writing—original draft preparation, M.K.M. and J.V.; writing—review and editing, M.K.M. and J.V.; visualization, G.Ć.; supervision, Ž.K.; project administration, M.K.M. and J.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Project No. 337-00-18/2022-01/12). Special thanks are given to the Slovenian Research Agency (ARRS) for financial support of research programme P2-0046: Separation processes and product design and bilateral project BI-RS/20-21-004.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fu, Y.; Shi, J.; Xie, S.Y.; Zhang, T.Y.; Soladoye, O.P.; Aluko, R.E. Red beetroot betalains: Perspectives on extraction, processing, and potential health benefits. J. Agric. Food Chem. 2020, 68, 11595–11611. [Google Scholar] [CrossRef]
- Rahimi, P.; Abedimanesh, S.; Namin, S.A.M.; Ostadrahimi, A. Betalains, the nature-inspired pigments, in health and diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 2949–2978. [Google Scholar] [CrossRef]
- Flores-Mancha, M.A.; Ruíz-Gutiérrez, M.G.; Sánchez-Vega, R.; Santellano-Estrada, E.; Chávez-Martínez, A. Characterization of betabel extract (Beta vulgaris) encapsulated with maltodextrin and inulin. Molecules 2020, 25, 5498. [Google Scholar] [CrossRef]
- Tumbas Šaponjac, V.; Čanadanović-Brunet, J.; Ćetković, G.; Jakišić, M.; Djilas, S.; Vulić, J.; Stajčić, S. Encapsulation of beetroot pomace extract: RSM optimization, storage and gastrointestinal stability. Molecules 2016, 21, 584. [Google Scholar] [CrossRef]
- Gómez-López, I.; Lobo-Rodrigo, G.; Portillo, M.P.; Cano, M.P. Characterization, stability, and bioaccessibility of betalain and phenolic compounds from Opuntia stricta var. Dillenii fruits and products of their industrialization. Foods 2021, 10, 1593. [Google Scholar] [CrossRef]
- Flores-Mancha, M.A.; Ruíz-Gutiérrez, M.G.; Sánchez-Vega, R.; Santellano-Estrada, E.; Chávez-Martínez, A. Effect of encapsulated beet extracts (Beta vulgaris) added to yogurt on the physicochemical characteristics and antioxidant activity. Molecules 2021, 26, 4768. [Google Scholar] [CrossRef]
- Bartosz, T.; Irene, T. Polyphenols encapsulation—Application of innovation technologies to improve stability of natural products. Phys. Sci. Rev. 2016, 1, 20150005. [Google Scholar] [CrossRef]
- Castro-Enríquez, D.D.; Montaño-Leyva, B.; Del Toro-Sánchez, C.L.; Juaréz-Onofre, J.E.; Carvajal-Millan, E.; Burruel-Ibarra, S.E.; Tapia-Hernández, J.A.; Barreras-Urbina, C.G.; Rodríguez-Félix, F. Stabilization of betalains by encapsulation—A review. J. Food Sci. Technol. 2020, 57, 1587–1600. [Google Scholar] [CrossRef]
- Rodriguez, E.B.; Vidallon, M.L.P.; Mendoza, D.J.R.; Dalisay, K.A.M.; Reyes, C.T. Stabilization of betalains from the peel of red dragon fruit [Hylocereus polyrhizus (Weber) Britton & Rose] through biopolymeric encapsulation. Philipp. Agric. 2015, 98, 382–391. [Google Scholar]
- Labuschagne, P. Impact of wall material physicochemical characteristics on the stability of encapsulated phytochemicals: A review. Food Res. Int. 2018, 107, 227–247. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef]
- Borjan, D.; Šeregelj, V.; Andrejč, D.C.; Pezo, L.; Šaponjac, V.T.; Knez, Ž.; Vulić, J.; Marevci, M.K. Green techniques for preparation of red beetroot extracts with enhanced biological potential. Antioxidants 2022, 11, 805. [Google Scholar] [CrossRef]
- Saénz, C.; Tapia, S.; Chávez, J.; Robert, P. Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica). Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]
- Carmona, J.C.; Robert, P.; Vergara, C.; Sáenz, C. Microparticles of yellow-orange cactus pear pulp (Opuntia ficus-indica) with cladode mucilage and maltodextrin as a food coloring in yogurt. LWT-Food Sci. Technol. 2021, 138, 110672. [Google Scholar] [CrossRef]
- Von Elbe, J.H. Betalains Volume 2: Pigments, colorants, flavors, texture, and bioactive food components. In Handbook of Food Analytical Chemistry; Wrolstad, R.E., Acree, T.E., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Smith, D., Sporns, P., Eds.; John Wiley and Sons, Inc.: New York, NY, USA, 2003; F.3.1.1.–F.3.1.7.; pp. 123–131. [Google Scholar]
- Gironés-Vilaplana, A.; Mena, P.; Moreno, D.A.; García-Viguera, C. Evaluation of sensorial, phytochemical and biological properties of new isotonic beverages enriched with lemon and berries during shelf life. J. Food Sci. Agric. 2014, 94, 1090–1100. [Google Scholar] [CrossRef]
- Tumbas Šaponjac, V.; Gironés-Vilaplana, A.; Djilas, S.; Mena, P.; Cetković, G.; Moreno, D.A.; Canadanović-Brunet, J.; Vulić, J.; Stajčić, S.; Krunić, M. Anthocyanin profiles and biological properties of caneberry (Rubus spp.) press residues. J. Sci. Food Agric. 2014, 94, 2393–2400. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of product of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Ullah, H.M.A.; Zaman, S.; Juhara, F.; Akter, L.; Tareq, S.M.; Masum, E.H.; Bhattacharjee, R. Evaluation of antinociceptive, in-vivo & in-vitro anti-inflammatory activity of ethanolic extract of Curcuma zedoaria rhizome. BMC Complement. Altern. Med. 2014, 14, 346. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. RStudio Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K. RStudio Dplyr: A Grammar of Data Manipulation; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Peterson, B.G.; Carl, P.; Boudt, K.; Bennett, R.; Ulrich, J.; Zivot, E.; Cornilly, D.; Hung, E.; Lestel, M.; Balkissoon, K.; et al. PerformanceAnalytics: Econometric Tools for Performance and Risk Analysis; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Antigo, J.L.D.; Bergamasco, R.C.; Madrona, G.S. Effect of pH on the stability of red beet extract (Beta vulgaris L.) microcapsules produced by spray drying or freeze drying. Food Sci. Technol. 2018, 38, 72–77. [Google Scholar] [CrossRef]
- Tumbas Šaponjac, V.T.; Čanadanović-Brunet, J.M.; Ćetković, G.S.; Jakišić, M.V.; Vulić, J.J.; Stajčić, S.M.; Šeregelj, V.N. Optimisation of beetroot juice encapsulation by freeze-drying. Pol. J. Food Nutr. Sci. 2020, 70, 25–34. [Google Scholar] [CrossRef]
- Bazaria, B.; Kumar, P. Effect of dextrose equivalency of maltodextrin together with Arabic gum on properties of encapsulated beetroot juice. J. Food Meas. Charact. 2016, 11, 156–163. [Google Scholar] [CrossRef]
- Khan, M.I. Stabilization of betalains: A review. Food Chem. 2016, 197, 1280–1285. [Google Scholar] [CrossRef]
- Do Carmo, E.L.D.; Teodoro, R.A.R.; Félix, P.H.C.; Fernandes, R.V.B.; Oliveira, É.R.; Veiga, T.R.L.A.; Borges, S.V.; Botrel, D.A. Stability of spray-dried beetroot extract using oligosaccharides and whey proteins. Food Chem. 2018, 30, 51–59. [Google Scholar] [CrossRef]
- Janiszewska, E. Microencapsulated beetroot juice as a potential source of betalain. Powder Technol. 2014, 264, 190–196. [Google Scholar] [CrossRef]
- Pitalua, E.; Jimenez, M.; Vernon-Carter, E.J.; Beristain, C.I. Antioxidative activity of microcapsules with beetroot juice using gum Arabic as wall material. Food Bioprod. Process. 2010, 88, 253–258. [Google Scholar] [CrossRef]
- Robert, P.; Victoria, T.; Paula, G.; Vergara, C.; Saenz, C. The encapsulation of purple cactus pear (Opuntia ficus-indica) pulp by using polysaccharide-proteins as encapsulating agents. LWT-Food Sci. Technol. 2015, 60, 1039–1045. [Google Scholar] [CrossRef]
- Saénz, C.; Gómez, H.; Fabry, A.M.; Cancino, B.; Vergara, C.; Paz, R. Soft-drinks prepared with pulp, ultrafiltrated and nanofiltrated purple cactus pear microparticles: Betalains stability. Acta Hortic. 2015, 1067, 343–348. [Google Scholar] [CrossRef]
- Vergara, C.; Saavedra, J.; Sáenz, C.; García, P.; Robert, P. Microencapsulation of pulp and ultrafiltered cactus pear (Opuntia ficus-indica) extracts and betanin stability during storage. Food Chem. 2014, 157, 246–251. [Google Scholar] [CrossRef]
- Karimzadeh, L.; Sohrab, G.; Hedayati, M.; Ebrahimof, S.; Emami, G.; Razavion, T. Effects of concentrated beetroot juice consumption on glycemic control, blood pressure, and lipid profile in type 2 diabetes patients: Randomized clinical trial study. Iran. J. Med. Sci. 2022, 192, 1143–1153. [Google Scholar] [CrossRef]
- Kumar, S.S.; Giridhar, P. Stabilization of bioactive betalain pigment from fruits of Basella rubra L. through maltodextrin encapsulation. Madridge J. Food Technol. 2017, 2, 73–77. [Google Scholar] [CrossRef]
- Woom, K.; Wongm, F.; Chuam, H.; Tangm, P. Stability of spray-dried pigment of red dragon fruit [Hylocereus polyrhizus (Weber) Britton and Rose] as a function of organic acid additives and storage conditions. Philipp. Agric. Sci. 2011, 94, 264–269. [Google Scholar]
- Azeredo, H.M.C.; Santos, A.N.; Souza, A.C.R.; Mendes, K.C.B.; Andrade, M.I.R. Betacyanin stability during processing and storage of a microencapsulated red beetroot extract. Am. J. Food Technol. 2007, 2, 307–312. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Cabanes, J.; Escribano, J.; García-Carmona, F.; Jiménez-Atiénzar, M. Encapsulation of the most potent antioxidant betalains in edible matrixes as powders of different colors. J. Agric. Food Chem. 2013, 61, 4294–4302. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Corke, H. Production and properties of spray-dried Amaranthus betacianin pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Díaz-Sánchez, F.; López, E.M.S.; Kerstupp, S.F.; Ibarra, R.V.; Scheinvar, L. Colorant extract from red prickly pear (Opuntia lasiacantha) for food application. Electron. J. Environ. Agric. Food Chem. 2006, 5, 1330–1337. [Google Scholar]
- Tsali, A.; Goula, A.M. Valorization of grape pomace: Encapsulation and storage stability of its phenolic extract. Powder Technol. 2018, 340, 194–207. [Google Scholar] [CrossRef]
- Moser, P.; Telis, V.R.N.; de Andrade Neves, N.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Storage stability of phenolic compounds in powdered BRS Violeta grape juice microencapsulated with protein and maltodextrin blends. Food Chem. 2017, 214, 308–318. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).